Abstract
Toxoplasma gondii is a ubiquitous zoonotic pathogen belonging to apicomplexan parasites. Infection in humans and animals may cause abortion and other severe symptoms under certain circumstances, leading to great economical losses and public health problems. T. gondii was first discovered in China in 1955 and the corresponding work was published in 1957. Since then, a lot of work has been done on this parasite and the diseases it causes. This review summarizes the major progress made by Chinese scientists over the last 60 years, and gives our perspectives on what should be done in the near future. A wide variety of diagnostic approaches were designed, including the ones to detect T. gondii specific antibodies in host sera, and T. gondii specific antigens or DNA in tissue and environmental samples. Further work will be needed to translate some of the laboratory assays into reliable products for clinic uses. Epidemiological studies were extensively done in China and the sero-prevalence in humans increased over the years, but is still below the world average, likely due to the unique eating and cooking habits. Infection rates were shown to be fairly high in meat producing animals such as, pigs, sheep, and chickens, as well as in the definitive host cats. Numerous subunit vaccines in the form of recombinant proteins or DNA vaccines were developed, but none of them is satisfactory in the current form. Live attenuated parasites using genetically modified strains may be a better option for vaccine design. The strains isolated from China are dominated by the ToxoDB #9 genotype, but it likely contains multiple subtypes since different ToxoDB #9 strains exhibited phenotypic differences. Further studies are needed to understand the general biology, as well as the unique features of strains prevalent in China.
Keywords: Toxoplasma gondii, epidemiology, China, vaccine, genotype
Introduction
Toxoplasma gondii is an obligate intracellular parasitic protozoan widely distributed around the world. One-third of the world's population was estimated to be infected by this parasite (Dubey, 2010). In addition to humans, it can also infect more than 200 species of animals and causes toxoplasmosis in them (Gao, 2014). As an opportunistic pathogen, T. gondii infection rarely causes severe symptoms in healthy humans and most other hosts. The main risks are congenital infections during pregnancy, which may cause abortion, stillbirth, developmental defects, and other serious diseases to the fetus. Congenital infections and subsequent complications are common in humans and farm animals such as, pigs and sheep, leading to great economical losses and social problems (Dubey, 2009).
One important reason for the wide spread of T. gondii is its complex life cycle, which consists a sexual phase and an asexual phase. Sexual reproduction occurs in definitive hosts, the felids. After infection, they shed oocysts in feces to contaminate water and environment and pass the infection to other hosts if oocysts are ingested. In intermediate hosts, the parasites propagate asexually and they can be transmitted between intermediate hosts through predation. A large portion of human toxoplasmosis cases were thought to be derived from consumption of undercooked meat that was infected (Montoya and Liesenfeld, 2004).
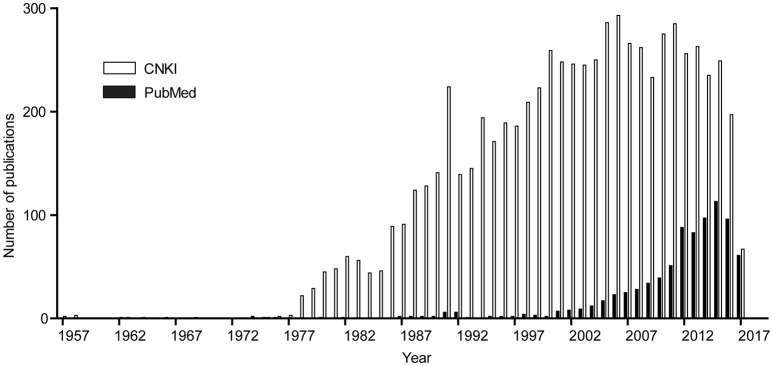
China has a long history of T. gondii research, which dates back to 1955 when Yu et al first isolated T. gondii from cats and rabbits in Fujian Province. This work was published in the Chinese journal Acta Microbiologica Sinica in 1957 (Yu et al., 1957). After this discovery, more and more work has been done to understand the epidemiology and biology of this parasite in China. When the key words “Toxoplasma” (or “toxoplasmosis”) and “China” were used to search the PubMed database (English articles) and the China National Knowledge Infrastructure (CNKI) database (Chinese articles), we estimated the number of publications on Toxoplasma or toxoplasmosis in each year from the Chinese research community and the results are summarized in Figure 1. There were only sporadic reports published before 1978, but since then the research activities became more active (Figure 1). There are at least two reasons for that: first, during late 1970s, many pigs in Shanghai and the nearby area were reported to have a fatal syndrome called “high fever with unknown reason,” and later it was shown to be caused by T. gondii infection (Ren and Bao, 1982), which promoted studies on this parasite; second, the implementation of the Economic Reform and Open up policies in 1978 in general boosted scientific researches. In this review, we collected all the studies published in English and Chinese journals and summarized the work that have been done on Toxoplasma and toxoplasmosis in China over the last 60 years. A perspective was also outlined for areas of research in the future.
Figure 1.
Number of papers published by scientists in China each year since 1957. The key word “Toxoplasma” and affiliation “China” were used to search the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) for papers published by scientists in China in international journals. The keywords “Toxoplasma” or “toxoplasmosis” or the Chinese equivalent of the two were used to search the Chinese library CNKI (http://www.cnki.net/) to estimate the papers in Chinese journals. Subsequently the numbers of papers from the two searches were reported by year.
Development of diagnostic approaches
Because of the lack of unique symptoms or traits, diagnosis of toxoplasmosis is challenging. This is particularly true for human infections during pregnancy, since the risks of which depend on when exactly the infection is acquired. Most European and North American countries have reference laboratories for the diagnosis and risk assessments. This concept has been introduced into China, but it never really works the way as it should, since there is no bona fide reference labs operating at the national level. Nonetheless, over the last 40 years, Chinese scientists have done a lot of work to develop diagnostic strategies to detect T. gondii infections. They can be mainly grouped into three types: serological tests detecting T. gondii specific antibodies post infection, immunological methods detecting T. gondii specific antigen, and nucleic acids based pathogen detection. Representative methods for each type were listed in Table 1.
Table 1.
Diagnostic methods developed by scientists in China.
| Detecting | Methods | Sensitivity and specificity (host) | References |
|---|---|---|---|
| T. gondii specific Antibodies |
Indirect-ELISA, using: Truncated recombinant SAG1 Multi-epitope peptide derived from SAG1, SAG2 and SAG3 GRA1, GRA7 MIC3 |
93.9% (31/33) positive concordance compared with Western blot (100%) (human) The total concordance was 93.2 and 95.7% for the detection of IgG and IgM antibodies, respectively, compared with commercial ELISA tests (human) Highly sensitive (93.2%) and specific (94.0%) (dog) Higher positive detections than MAT in pigs and goats |
Wu et al., 2009 Dai et al., 2013 Wang Z. et al., 2014 Zhang et al., 2010 |
| Dynamic flow immunochromatographic Test (DFICT) using SAG1 and SAG2 | The lowest detectable limit was 1:320 dilution of positive serum (dog and cat) | Jiang W. et al., 2015 | |
| Latex agglutination tests (LAT) using MIC3 | After 8 days infection, T. gondii specific antibodies could be detected (pig) | Jiang et al., 2008 | |
| Indirect hemagglutination assays (IHA) using soluble parasite antigen sensitized sheep red blood cells | T. gondii specific antibodies could be detected on the 7th days after initial infection (rabbit) | Chen et al., 1980 | |
| Piezoelectric immunoagglutination assay using total antigen of the RH strain | The detectable limit was 1:5,500 dilution of positive serum (rabbit) | Wang et al., 2004 | |
| Fast dipstick dye immunoassay (DDIA) using total soluble antigen of the RH strain | Sensitivity and specificity of IgG were 100 and 96%, respectively, while sensitivity and specificity of IgM were 100 and 94%, respectively (human) | Jin et al., 2005 | |
| T. gondii Antigens | Modified rapid sandwich ELISA using rabbit polyclonal antibodies against soluble T. gondii antigens | Detected circulating antigens at the concentration of 31.2 ng/mL, and no cross-reaction with other protozoa (human and rabbit) | Chen R. et al., 2008 |
| Immunochromatographic strip using sheep antisera against excretory/secretory antigens of T. gondii circulating antigens | Circulating antigens could be detected from day 2 to day 14 (or even longer) after parasite infection (pig, goat, and sheep) | Wang et al., 2011 | |
| T. gondii DNA | LAMP targeting 529 bp repeat sequence, SAG1 gene or B1 gene | Superior sensitivity than the conventional PCR, can detected T. gondii at the concentration of 2–3 parasites/mL, highly specific (pig, sheep, mouse, rabbit) | Yang et al., 2008; Lin et al., 2012; Lai et al., 2014 |
| RT-LAMP targeting 18s RNA gene | Showed higher sensitivity than RT-PCR with the detection limit of 1 tachyzoite in 1 g pork (pig) | Qu et al., 2013b | |
| RT-PCR targeting 529 bp repeat sequence | The sensitivity was as higher (1 fg DNA), compared to conventional PCR (100 fg) (pig, sheep, cattle, and dog) | Lin et al., 2011 |
A large collection of serological tests have been designed, using a wide variety of antigens. Most often, these tests were developed to detect T. gondii specific IgM or IgG antibodies in the sera of humans and animals. Although different formats of serological tests were designed, enzyme-linked immunosorbent assays (ELISA) and agglutination tests were the most common. In general, ELISA tests are more sensitive than agglutination tests, but they often require a species specific secondary antibody that recognize the IgM or IgG antibody of the animals to be tested. Agglutination tests are superior to ELISA in that they do not need any secondary antibodies, therefore are suitable for on-site diagnosis. Among the different ELISA formats, indirect ELISAs with native or recombinant T. gondii antigens to catch specific antibodies from infected hosts are the most common (Wu et al., 2009; Dai et al., 2013; Wang Z. et al., 2014). Many different antigens have been tried for this purpose, which included native antigens such as, the whole tachyzoite soluble extract and excretory-secretory antigens, as well as recombinant antigens such as, surface proteins (SAG1, SAG2, etc.), secretory proteins from different organelles (MIC3, GRA1, GRA7, etc.), and others (listed in Table 1 with corresponding references). In addition to single antigens, chimeric antigens containing epitopes from multiple proteins have also been tried and thought to be superior to single antigens (Dai et al., 2013; Wang Z. et al., 2014; Feng et al., 2016). To bypass the requirement of species specific secondary antibodies in indirect ELISAs, protein A or protein G from Staphylococcal aureus were used to functionally replace those secondary antibodies, due to their high binding affinities to immunoglobulins from a wide range of animals. Such ELISAs were called AG-ELISAs (Zhang et al., 2010). Besides indirect ELISAs, other formats of ELISAs, such as, double-sandwich ELISA were also developed (Lu et al., 2006). Although there was no solid and direct comparison between these different types of ELISA tests, from literature it seems like SAG and GRA7 based indirect ELISAs are the most reliable. The sensitivity and specificity were claimed to be nearly 100% and above 96%, respectively (Lu et al., 2006; Sun et al., 2015).
Like ELISAs, agglutination tests were also designed in a number of different ways (Table 1). Traditional agglutination tests (called modified agglutination tests) use fixed tachyzoites as agglutogen, which are widely used but difficult to standardize, due to the variations during tachyzoites preparation (Al-Adhami et al., 2016; Feng et al., 2016). To improve, different recombinant antigens derived from T. gondii were used to coat and sensitize red blood cells or latex particles, which were then used as agglutogen. Such testes are called indirect hemagglutination assays (IHA) and latex agglutination tests (LAT), respectively (Feng et al., 2016). The antigens used to sensitize agglutination particles were more or less the same as the ones used in indirect ELISAs (Chen et al., 1980; Yu, 2001; Jiang et al., 2008). In addition to agglutination tests and ELISAs, there are other serological methods reported, such as, indirect fluorescent assays (IFA) (Wang Y. B. et al., 2012). More recently, rapid diagnostic methods using immune colloidal gold technique were developed (Feng et al., 2016).
Although numerous T. gondii antibody detecting methods were designed in the lab, the majority of them were not subject to extensive evaluation to determine their value of clinic use. This is particularly the case for the assays to detect animal toxoplasmosis. Because of the high demand for TORCH (Toxoplasma, Rubella virus, Cytomegalovirus, and Herpes simplex virus) screens, China Food and Drug Administration (CFDA) issued certificates to a number of manufactures to make Toxoplasma specific IgM and IgG detecting kits for the diagnosis of human toxoplasmosis. However, published work assessing their sensitivity, specificity and Youden index indicated that, except for a few, the majority of these domestic kits were not of high enough quality to be useful in clinic (He et al., 2008). Especially for IgM detecting kits, the Youden index for many of them were close to zero (Jiang et al., 2003), therefore were of no value for clinic use. For the diagnosis of animal toxoplasmosis, so far there is no method certified by the China Institute of Veterinary Drug Control. Therefore, efforts are still needed to translate some of the laboratory assays to reliable and commercially available products for the diagnosis of toxoplasmosis in China. For the same reason, the sero-prevalence results discussed below only represent rough estimates of T. gondii infections in China.
In addition to the antibody detecting methods, there were also reports using immunological methods to detect T. gondii antigens in sera or other tissue extracts. For instance, a rapid modified ELISA was developed to test T. gondii antigens in humans, which included 5,428 sera, 548 cerebrospinal fluid and two breast milk samples. The lowest concentration of antigen detected was as low as 31.2 ng/mL without cross-reaction with antigens from other protozoa, trematodes, or nematodes (Chen R. et al., 2008). It is worth noting that detecting T. gondii antigens by immunological methods has limited success, due to the low levels of antigens exist and limited sensitivity of the tests.
To increase the sensitivity of pathogen detection, molecular biology methods based on nucleic acids detection were developed in recent years. Most of them were based on specific amplification of T. gondii DNA sequences, which include: conventional PCR, loop-mediated isothermal amplification (LAMP), nested-PCR, and real-time PCR (RT-PCR) etc. (Lin et al., 2012; Qu et al., 2013b). The target sequences for amplification often include the 529 bp repeat fragment, ITS-1, B1 gene, etc. Among these tests, LAMP is the most sensitive and does not require expensive equipments (Yang et al., 2008; Lin et al., 2012; Lai et al., 2014), therefore is suitable for on-site uses. However, it can be contaminated easily and give high rates of false positive reports.
Sero-prevalence studies in humans
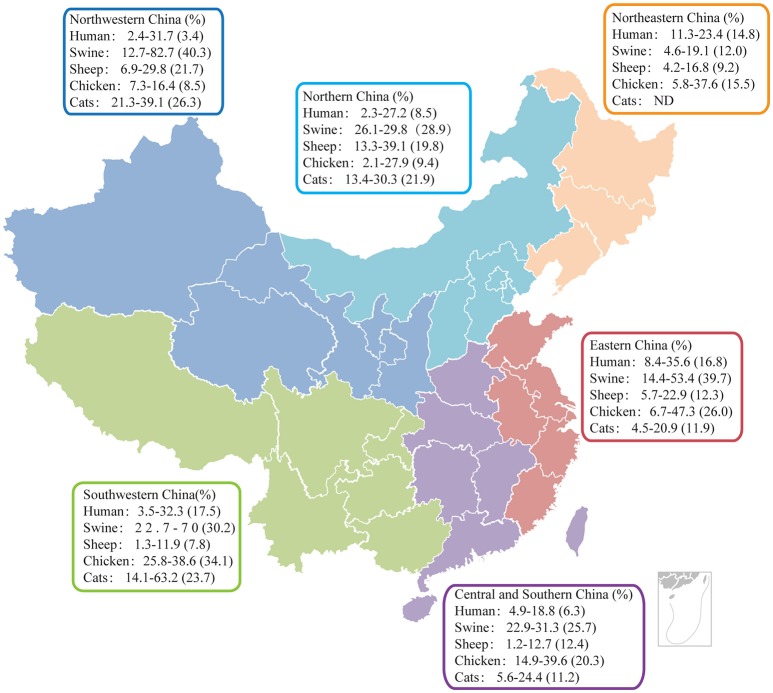
As an opportunistic pathogen, T. gondii posts health threats to the public, particularly to individuals with reduced immune functions. China has a large susceptible population: the number of AIDS patients increased from 650,000 in 2005 to 780,000 in 2011 (Wu, 2011; Ding et al., 2017), the number of women at childbearing age was estimated to be around 375.8 million in 2013 (Ding et al., 2017). Over the last a few decades, epidemiological surveys have been frequently done to monitor the prevalence of T. gondii in China. The average infection rate in humans is close to 10% but it varies significantly between different geographic regions and different populations (Xiao et al., 2010; Li et al., 2011; Sun et al., 2013; Cong et al., 2015a; Li H. L. et al., 2015; Wang L. et al., 2015; Wang S. et al., 2015), etc. Some of the representative results are shown in Figure 2. It is worth noting that the sero-prevalence of human toxoplasmosis in China is lower than the world average (10 vs. 25–30%), which is likely due to the unique eating habits (boil before eating/drinking) of Chinese people (Howe and Sibley, 1995; Yu, 2000).
Figure 2.
Results of representative serological studies to estimate the prevalence of T. gondii in humans and animals in China. Data collected from both Chinese and English publications after the year 2010 were analyzed and graphed on the map of China, which was divided into six regions for data presentation. Sero-prevalence range and average (in parentheses) for each animal in each region are shown. ND, Not determined.
Years of sero-prevalence studies seem to suggest that T. gondii infection rates in humans increased over the last 30 years in China. For example, the overall infection rate was 5.2% in the first national survey implemented between 1988 and 1992 (Yu et al., 1994), then rose to about 8% between 2001 and 2004 (Zhou et al., 2008). Possible reasons for such change include increased meat consumption and pet cat numbers as a consequence of economy development. At present, it is estimated that 10% of humans in China are infected, but the distribution is not even. For example, due to the unique culture and eating habits, people from certain ethnic groups have significant higher infection rates than others. Thirty percent of the Bai people in Yunnan province was sero-positive, compared to 10% in Han people in the same region, which is likely due to the raw pork eating culture of the Bai people (Li H. L. et al., 2015). For the same reason, the prevalences in Miao (25.4%), Buyi (25.3%), and Mongol (17.1%) ethnic groups are also higher than other groups (Xu et al., 2005). In addition, people with certain careers are also at higher risk, herdsmen, veterinary, meat processing workers, and slaughterhouse workers tend to be more frequently infected than general public (Chen et al., 2005; Huo et al., 2007; Yu et al., 2007).
Serological tests targeting special groups of people were also frequently done, the best known of which is the TORCH examination in pregnant women. TORCH test is highly recommended by many hospitals, and is mandatory in some cities in China. It should be pointed out that the Toxoplasma examination part of TORCH is over simplified, only testing the presence of T. gondii IgM and IgG antibodies in a single test to inform the infection status. Since IgM may last more than a year, IgM positive women do not necessarily acquire the infection during pregnancy (Feng et al., 2016). As such, medical decisions made from a single IgM/IgG test are not always appropriate. Nevertheless, routine TORCH screens found that on average 0.3% of pregnant women were IgM positive, and 5.3% were IgG positive for T. gondii (Ding et al., 2009, 2015; Yang et al., 2014; Liu T. et al., 2017). A certain portion (although the exact number is not clear) of the IgM positive women were predicted to be infected after conception, which is high likely to lead to poor obstetric outcomes, or increase the risk congenital toxoplasmosis to newborns (Zhou et al., 2011a; Peng et al., 2015; Guan, 2017). Surveys also suggested that sero-prevalence of T. gondii in infertile couples is higher than that in healthy couples (Zhou et al., 2002), although the relationship between T. gondii infection and infertility is currently unknown. Sero-prevalence studies in hospitalized patients suggested that infection rates in AIDS patients, cancer patients, and patients in intensive care units were almost two-fold of that of health people (Xiao et al., 2010; Jiang C. et al., 2015; Zhang et al., 2015). The exact reasons for the higher sero-prevalence in such patients are currently unknown, but these results imply that patients with compromised immune functions may pick up infections and sero-convert more frequently. A recent meta-analysis on AIDS patients worldwide also showed the same trend (Liu L. et al., 2017; Liu T. et al., 2017).
Animal toxoplasmosis
China has one of the highest biodiversities in the world, with a wide variety of landscapes and animal populations. Using mostly indirect ELISAs and agglutination tests, sero-prevalence of T. gondii in farm and wild animals was estimated over the years. In general, the prevalence in animals was much higher than that in humans. Representative results from epidemiological studies performed after the year 2010 were summarized in Figure 2, which provide a rough estimate for the current status of animal toxoplasmosis in China.
Among the major animals monitored, pigs were the most frequently infected by T. gondii. Roughly 30–50% of pigs raised in China are sero-positive for T. gondii (Yu et al., 2011; Wu et al., 2012; Xu Y. et al., 2014), and this number can reach 70% in some areas and farms (Li Y. N. et al., 2015). Due to the facts that China is the biggest pig-raising country (more than one billion pigs were raised and butchered each year) and pork-consuming country in the world, high T. gondii prevalence in pigs lead to serious economic lossess and public health concerns. First of all, As mentioned above, pigs are susceptible to T. gondii infection. In late 1970s and early 1980s, the “high fever with unknown reason” syndrome caused by T. gondii killed a large number of pigs in Yangtze River delta (Shanghai, Jiangsu, and Zhejiang) (Ren and Bao, 1982). Even nowadays, abortions caused by congenital swine toxoplasmosis are still very common, leading to huge economic losses (Fan, 2017; Wu, 2017). In addition, high T. gondii prevalence in pigs also increases the risks of human infection through meat consumption (Wang H. et al., 2012), since pork is the main meat Chinese people consume.
Among other meat producing animals, sheep and chickens are at the top of the list in terms of T. gondii infection rates. Northern and northwestern parts of China are the main regions for sheep production. Sero-prevalence in sheep is also the highest in these regions, reaching 20% (Figure 2), likely as a consequence of the widespread of T. gondii oocysts in the environment (Yang et al., 2017). In other regions, infection rates in sheep are about 10% on average (Wu et al., 2011a; Xu P. et al., 2014; Zou et al., 2015; Li et al., 2016; Zhang N. et al., 2016). Given the fact that among all farm animals, sheep are probably the most susceptible to T. gondii infection, economic losses caused by abortions and other diseases from sheep toxoplasmosis are predicted to be huge, but there is no reliable estimate so far. In addition, lamb meat is the major meat used in barbecue in China. Because of the unique processing style, barbecue often does not kill all pathogens in the meat. As such, high prevalence of T. gondii in sheep may make sheep an important source for human infections. Interestingly, T. gondii is also fairly prevalent in chickens, particularly in the south of China, over 20% of chickens are sero-positive (Zhao et al., 2012; Liu R. Z. et al., 2013). Beef is another major meat people consumed in China, but the sero-prevalence of T. gondii in cattle (below 10% on average) is significantly lower than that in pigs or chickens (Yu et al., 2006; Zhu et al., 2017). In addition, cattle are generally fairly resistant to T. gondii infection. Therefore, beef is probably not a significant source of human infections.
Cats, as definitive hosts for T. gondii, play a critical role in its transmission. Pet cats in particular, are important sources for human infections due to their intimate association with humans. Sero-prevalence studies, using mostly ELISAs and agglutination tests, suggested that cats were also infected with T. gondii with relatively high frequencies. The infection rates vary significantly, depending on the regions and cat types. In general, stray cats were much more frequently infected than pet cats (Ding et al., 2017). In one study, 43 stray cats in Shanghai examined by IHA were found to be infected at 100% (Chen, 2010). Judging from reported results, the average sero-positivity of housed cats were somewhere between 15 and 25% (Zhang et al., 2009; Wu et al., 2011c; Ding et al., 2017). But it should be pointed out that, unlike animals (pigs, chickens, etc.) that are concentrated in farms, cats are more randomly distributed and sero-prevalence studies in cats from selected regions may not reflect the overall picture. In our opinion, the sero-positivity was likely underestimated, due to the fact that most of the studies did not include samples from rural areas, where the prevalence is predicted to be higher than urban regions. There were also sporadic reports on the sero-prevalence of T. gondii in pet dogs. Generally speaking, infection in pet dogs is common but is not as frequent as in pet cats, and is around 10% on average (Wu et al., 2011b; Yang et al., 2013; Gao Y. M. et al., 2016).
There are also studies reporting sero-prevalence of T. gondii in wild animals such as, rodents, bats, and gulls etc. (Yuan et al., 2013a; Zhang Y. et al., 2013; Miao et al., 2014). However, these studies are often regional and static. Nonetheless, these sporadic studies suggested that wild animals were frequently infected. For example, sero-prevalence in rodents like Microtus fortis was as high as 50% in Jilin province (Zhang Y. et al., 2013), and is 29% in Hunan province (Zhang et al., 2004). Similarly, infection rates in bats and black-headed gulls are around 20% (Yuan et al., 2013a; Miao et al., 2014). These results, along with the epidemiological studies in farm animals and pets, indicated that T. gondii is present in the environments and food chain in China, at similar frequencies as in other parts of the world (Dos Santos et al., 2010; De Berardinis et al., 2017). However, human infection rates in China are lower than the world average, which likely ascribe to the unique eating habits of the Chinese people.
T. gondii in environments
High prevalence of T. gondii in animals is correlated with the wide spreading of parasites in environments, most likely in the form of oocysts. Indeed, when nucleic acids based detection methods were used to assess the presence of T. gondii DNA, environmental samples were examined as positive at fairly high frequencies. In 2012, two studies looked at the presence of T. gondii DNA in soil samples collected from pig farms and public parks in central China by PCR and LAMP, and found T. gondii contamination in 16–23% of the samples from parks, and 21–38% of the samples from pig farms, depending the methods used (Du et al., 2012a,b). Similarly, more than 9,000 soil samples collected from northeastern China were analyzed by real-time quantitative PCR and found over 30% of them to be positive (Gao X. et al., 2016). Presumably the detected T. gondii specific DNA was derived from oocysts, however it remains unknown how often viable oocysts can be isolated from such environmental samples. Given the environmental resistance of oocysts, a significant portion of the DNA positive samples were likely to contain viable oocysts, which may explain the high infection rates in animals. On the other hand, the PCR based methods to detect oocysts in environments are very sensitive to contaminations, extra caution needs to be taken in interpreting such data. Alternative methods such as, oocyst enrichment and isolation are necessary to estimate the spread of oocysts in environmental samples.
Genotypes of T. gondii strains circulating in China
Numerous efforts were taken to understand the genotypes and population structures of the T. gondii strains prevalent in China. Generally, two methods were used for this purpose. Either isolated live strains and genotyped them, or extracted DNA from infected tissues and then used the DNA samples for genotyping, with the latter being much more common. Either way, the isolates were often genotyped by PCR-RFLP based on 11 genetic markers (SAG1, 5′-SAG2 and 3′-SAG2, alternative SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1, and an apicoplast locus Apico; Su et al., 2006). Consistent with the large territory and great biodiversities of hosts in China, many different genotypes were discovered, which were summarized in Table 2. It is obvious that the dominating genotype in China is ToxoDB #9 (also called Chinese I or China I), moren than half of the isolates belong to this genotype (Li Y. N. et al., 2015). ToxoDB #9 is the dominating strain type in almost all examined hosts (Table 2). The next common one is ToxoDB #10 (Type I), which is the classic type 1 genotype (Zhou et al., 2009; Wang et al., 2013). However, it should be pointed out that most studies only reported the detection of DNA corresponding to ToxoDB #10 strains, only a few live type I parasites (TgHuZS2 and TgCtxz1) were isolated and preserved (Wang et al., 2013). Therefore, the actual frequency of such strains in China remain to be determined. ToxoDB #9 and #10 strains probably account for 90% of the strains examined in China. This situation is similar to North America and Europe in that a few genotypes dominated the population (Minot et al., 2012). However, unlike the clonal nature of the type I, II, and III strains in north America and Europe, there are probably multiple subtypes in the ToxoDB #9 group, since different ToxoDB #9 isolates have quite different properties (such as, acute virulence in mice). Using multilocus sequence typing, a most recent study suggested that ToxoDB #9 isolates could be divided into at least two (potentially more) groups: Chinese I and Chinese III, with Chinese III being more closely related to classic type 1 strains (Gao et al., 2017). In addition, the dominating ToxoDB #9 strains seem to be distinct from the strains isolated from other parts of the world. Using three independent sets of polymorphic DNA markers to estimate the phylogenetic distances among 950 isolates collected from all over the world, the TgCtPRC04 strain representing ToxoDB #9 isolated from China was separated from other strains and formed a unique haplogroup (Su et al., 2012). Whole genome sequencing was done on another ToxoDB #9 strain TgCtPRC2 (which was isolated from a cat and also belonged to the same haplogroup as TgCtPRC04) and the data of which was deposited in ToxoDB, which facilitated the biological studies of ToxoDB #9 strains.
Table 2.
Genotypes of T. gondii parasites prevalent in China.
| Host | Tissue origin | Type (Number) | Frequency | References |
|---|---|---|---|---|
| Cancer patients | Diseased tissues | ToxoDB #9 (8) ToxoDB #10 (9) |
ToxoDB #9: 65% ToxoDB #10: 35% |
Cong et al., 2015b |
| Serum samples | ToxoDB #9 (9) | Wang L. et al., 2015 | ||
| Pigs | Hilar lymph nodes | ToxoDB #10 (5 ToxoDB #3 (1) ToxoDB #9 (21) |
ToxoDB #9: 83% ToxoDB #10: 11% ToxoDB #3: 4% Others: 2% |
Zhou et al., 2010; Zhou et al., 2011b; Jiang H. H. et al., 2013; Jiang W. et al., 2013; Jiang et al., 2016 |
| Retail meat | ToxoDB #9 (1) ToxoDB #213 (1) |
Wang H. et al., 2012 | ||
| Blood, heart and brain | ToxoDB #9 (3) ToxoDB #3 (1) ToxoDB #9 (13) |
Li Y. N. et al., 2015; Wang D. et al., 2016 |
||
| Cats | Brain, tongue, Heart, liver, Blood, feces | ToxoDB #9 (29) ToxoDB #225 (2) ToxoDB #1 (4) ToxoDB #2 (1) ToxoDB #3 (1) ToxoDB #20 (1) ToxoDB #17 (1) |
ToxoDB #9: 74% ToxoDB #1: 10% ToxoDB #225: 5% Others: 11% |
Qian et al., 2012; Liu R. Z. et al., 2013; Liu T. et al., 2013; Wang et al., 2013; Li et al., 2014; Tian et al., 2014; Li Y. N. et al., 2015; Yang et al., 2015; Cong et al., 2016a |
| RODENTS | ||||
| Microtus fortis | Lung | ToxoDB #10 (2) ToxoDB #9 (2) |
ToxoDB #9: 53% ToxoDB #10: 35% Others: 12% |
Zhang et al., 2014 |
| Qinghai vole Plateau pika Tibetan ground-tit | Brain | ToxoDB #10 (4) New genotype (2) |
Zhang X. X. et al., 2013 | |
| Rats and mice | Brain | ToxoDB#9 (7) | Yan et al., 2014 | |
| OTHERS | ||||
| Wild birds | Breast, muscle | ToxoDB #10 (1) ToxoDB #1 (2) |
ToxoDB #9: 44% ToxoDB #10: 37% ToxoDB #3: 7% ToxoDB #2: 5% Others: 7% |
Huang et al., 2012 |
| Pet birds | Brain | ToxoDB #3 (1) | Cong et al., 2014 | |
| heart | ToxoDB#9 (1) ToxoDB#2 (1) ToxoDB#10 (1) |
Chen et al., 2015 | ||
| House sparrows | Heart, brain, lung | ToxoDB #3 (1) New genotype (1) |
Cong et al., 2013 | |
| Domestic rabbit | Brain, spleen, liver | ToxoDB #2 (1) | Zhou et al., 2013 | |
| Bats | Lung, heart, liver, spleen, stomach, intestine or kidney | ToxoDB #10 (4) ToxoDB #9 (1) |
Jiang et al., 2014 | |
| liver | ToxoDB#10 (3) ToxoDB#9 (5) |
Qin et al., 2014 | ||
| Black goats | Liver, lung, lymph nodes | ToxoDB#10 (7) ToxoDB#9 (1) |
Miao et al., 2015 | |
| Sika deer | Liver, lung, muscle | ToxoDB#9 (6) | Cong et al., 2016b | |
| Farmed minks | Brain | ToxoDB#9 (5) ToxoDB#3 (1) |
Zheng et al., 2016 | |
Vaccine developments
Starting in 1990s, with the rapid progress of molecular biology techniques, a significant amount of T. gondii research work was on vaccine developments, particularly subunit vaccines. Some of the representative studies were summarized in Table 3, which shows that there are mainly three types of vaccines tried: recombinant protein vaccines, DNA vaccines, and live vaccines. The core antigens used for recombinant protein vaccines and DNA vaccines are very similar, mostly surface proteins (SAGs) and proteins from secretory organelles such as, dense granules (GRAs), rhoptries (ROPs), and micronemes (MICs) (see Table 3 for examples and references). These two types of vaccines only differ in the way the core antigens being delivered: for recombinant protein vaccines, the core antigens were administrated in the form of purified proteins, often along with chemical adjuvant to boost the immune response; DNA vaccines were administrated in the form of DNA (plasmids or viral vectors) that expressed the core antigens in hosts, sometimes using cytokine (IL-12, IL-15, etc.) expressing constructs as adjuvants (Zhang et al., 2007; Cui et al., 2008; Chen et al., 2014a). Most of these vaccines elicited Th1 immune responses and had some degree of protections against parasite infections, increasing survival rates, and/or time or reducing cyst burdens (Yuan et al., 2011b; Zheng et al., 2017). Nonetheless, none of these subunit vaccines in the current form offered enough protection to be used in clinic.
Table 3.
T. gondii vaccine candidates designed by scientists in China.
| Antigens/parasite strains | Effect | References | |
|---|---|---|---|
| DNA and vector vaccines | SAG1 | Increased survival time to 20.38 ± 3.38 days vs. control (13.25 ± 1.16 days) | Chen et al., 1999 |
| SAG1, SAG2 linked to A2/B subunits of cholera toxin | 40% survival rate in immunized mice | Cong et al., 2005 | |
| SAG1, ROP2 with IL-12 as adjuvant | Increased survival time to 22 days vs. control (4–8 days) | Zhang et al., 2007 | |
| SAG1-ROP2-SAG2 co-deliveried with IL-12 | Increased survival rate | Cui et al., 2008 | |
| SAG1, GRA1, GRA2, GRA4 antigen segments | The survival rate of BALB/c and C57BL/6 mice were 100 and 40%, respectively | Liu et al., 2009 | |
| SAG2C, SAG2D, SAG2X | Reduction of cyst burden (77%) in the brain | Zhang M. et al., 2013 | |
| MIC3 (suicidal vector pSCA1) | Increased the survival time to 15 days vs. control mice (5 days) | Fang et al., 2009 | |
| MIC3 (recombinant pseudorabies virus rPRV) | The survival rates of BALB/c mice injected with rPRV-MIC3 alone was 50% | Nie et al., 2011 | |
| MIC3, SAG1 (baculovirus vaccine BV-MIC3+BV-SAG1) | 50% of the mice survived | Fang et al., 2012 | |
| MIC6 | Increased survival time to 13.3 ± 1.2 days vs. control mice (7 days) | Peng et al., 2009 | |
| MIC8 | Increased survival time to 10.3 ± 0.9 days vs. control mice (5 days) | Liu M. M. et al., 2010; Liu Y. et al., 2010 |
|
| MIC11 | Increased the survival time to 15 days vs. control mice (8–10 days) | Tao et al., 2013 | |
| MIC13 | Increased survival time to 21.3 ± 11.3 days vs. control mice (5–10 days) and reduced number of cysts in brain of mice (57.14%) | Yuan et al., 2013b | |
| ROP9 | Increased survival time to 12.9 ± 2.9 days vs. control mice (6 days) | Chen et al., 2014b | |
| ROP16 | Increased survival time to 21.6 ± 9.9 days vs. control mice (7 days) | Yuan et al., 2011a | |
| ROP18 | Increased survival time to 27.9 ± 15.1 days vs. control mice (7 days) | Yuan et al., 2011b | |
| ROM1 | Increased survival time to 12.5 ± 0.7 days vs. control mice (5 days) | Li et al., 2012 | |
| GRA6 with levamisole as adjuvant | 53.3% survival | Sun et al., 2011 | |
| eIF4A | Increased survival time to 23.0 ± 5.5 days compared to control mice (7 days) | Chen et al., 2013 | |
| MIC3, GRA1 | Increased survival time to 12–19 days (15.7 ± 1.88) vs. control group survived for 3–5 days (4.5 ± 0.38) | Gong et al., 2016 | |
| MIC3, ROP18 | Increased survival time to 14–19 days vs. control mice (7 days) | Qu et al., 2013a | |
| Aspartic protease 1 | Increased the survival time to 16 days vs. control (7 days) | Zhao et al., 2013 | |
| CDPK1 with a plasmid encoding IL-15 and IL-21 | Increased survival time to 19.2 ± 5.1 days vs. control (6 days) and reduced the number of brain cysts (72.7%) | Chen et al., 2014a | |
| NTPase-II (pDREP) | 71.4% reduction in brain cysts | Zheng et al., 2017 | |
| Recombinant protein vaccines | ROP5 | Increased survival time to 12.1 ± 3.4 days vs. control (6 days) | Zheng et al., 2013 |
| ROP17 | Lower liver and brain parasite burdens (59.17 and 49.08%, respectively) and increased survival time by 50% | Wang H. L. et al., 2014 | |
| ROP18, ROP38 encapsulated in poly (lactide-co-glycolide) | 81.3% reduction of tissue cysts | Xu et al., 2015 | |
| Live vaccines | T. gondii temperature-sensitive mutant | Induced protective immunity in an ocular toxoplasmosis model | Lu et al., 2009 |
| Eimeria tenalla sporozoites expressing TgSAG1 | Induced Th1 immune response and prolonged survival of mice | Tang et al., 2016 |
Live vaccines were thought to be the most effective against T. gondii, with the good example of “Toxovax,” although this vaccine is also not perfect (Buxton and Innes, 1995; Burrells et al., 2015). Design of T. gondii vaccines using live parasites were also tried in China, but only with limited success. For example, A temperature-sensitive mutant of RH (ts-4) was tested as a live vaccine for ocular toxoplasmosis in a mouse model. After intracameral inoculation, this strain did provide protective immunity against wild type parasite infection (Lu et al., 2009). However, the strain by itself also caused ocular pathology, preventing its use as a vaccine. Live vaccines were also designed using other parasite species as antigen delivery vectors. For example, transgenic Eimeria tenella expressing TgSAG1 was tested as vaccine to protect T. gondii infection in chickens and mice. Although it did elicit a Th1-dominant immune response that restricted T. gondii proliferation, the protective immunity it offered was limited (Tang et al., 2016). More recently, with the wide use of CRISPR/CAS9 based genome editing system, generating gene deletion mutants to be used as live vaccines became popular, which is also a feasible approach given the success of uracil auxotrophs (Fox and Bzik, 2002).
Molecular basis for pathogenesis
Before the year 2000, the majority of T. gondii research in China is about epidemiology and vaccine design, very little was done toward the understanding of parasite biology or mechanisms of pathogenesis. After entering the new century, basic research became more active. By looking at the papers Chinese scientists published during the last 15 years, it is obvious that the vast majority of the limited work was about host-parasite interactions, or what influence parasites had on host and host cells. Quite a few transcriptomic and proteomic studies examined the gene expression and protein abundance changes of the hosts upon parasite infection. For examples, RNA-Seq was used to look at the gene expression changes in cat liver, mouse liver and pig peripheral blood mononuclear cells (PBMCs) after infection (He et al., 2016a; Zhou et al., 2016a,b). Similarly, iTRAQ-based proteomic analysis on mice liver suggested that the relative abundances of hundreds of proteins were changed upon parasite infection, many of which were involved in key metabolic pathways (He et al., 2016b). These omics studies did generate a large amount of data that were informative, but follow-up studies are required to figure out the biological significances of these data. More recently, with the development of genome editing techniques, increasing amount of work started to address the biological properties of the parasite. Using the CRISPR/Cas9 technology, leucine aminopeptidase, calcium-dependent protein kinases, and rhoptry proteins were inactivated to check the functions of these genes during parasite growth and development (Zheng et al., 2015; Wang J. L. et al., 2016, 2017). Today, studies in China cover most fields of T. gondii research. In this review, we only focused on the studies of ToxoDB #9 strains, which are almost specific to China.
As mentioned above, strains isolated from China are dominated by the ToxoDB #9 genotype. However, strains belonging to this genotype may display different properties. TgCtwh3 and TgCtwh6 are probably the best characterized ToxoDB #9 strains, and they show different virulence in mice, with TgCtwh3 being significantly more virulent than TgCtwh6 (Wang et al., 2013). In addition, TgCtwh3 was shown to induce alternative activation of mouse macrophages with STAT6 phosphorylation, whereas the TgCtwh6 elicited classical activation of mouse macrophages with nuclear translocation of NF-κB (Zhang A. M. et al., 2013). In order to understand why the two strains with the same genotype had these differences, both strains were subject to whole genome sequencing and compared to that of type 1 strain GT1. Although single nucleotide polymorphisms (SNPs) and insertions/deletions (indels) were found in hundreds of genes in TgCtwh3 and TgCtwh6 strains, these two strains were genetically more similar to each other than to GT1. GRA3 and RON3 were shown to have drastic expression differences between the two strains, but whether or not they contribute to the phenotypic difference is still unknown (Cheng et al., 2015). A recent study using multilocus sequence typing suggested that ToxoDB #9 strains could be divided into multiple sub-groups. However, using this typing method, TgCtwh3 and TgCtwh6 were still grouped together, although they display different phenotypes. As the authors of this work implied, perhaps genotyping using neutral genetic markers is not very useful in predicting pathogenic phenotypes (Gao et al., 2017). Therefore, further studies are required to dissect the underlying molecular mechanisms for such phenotypic differences, as a way to better understand the biology of ToxoDB #9 strains.
Chinese herbal medicines for T. gondii intervention
For decades, inhibitors of folic acid metabolism such as, pyrimethamine and sulfonamide were the primary drugs to treat acute toxoplasmosis. But due to strong side effects and other limitations, these drugs are far from ideal. Developing new anti-Toxoplasma interventions is a long lasting task in the field and scientists in China contributed to this effort. Noticeable achievements include the studies of Chinese herbal medicine against T. gondii (Table 4). As early as 1990, the effect of artemisinin and its derivatives on T. gondii growth was tested in vitro, and found that at 0.4 μg/ml artemisinin blocked plaque formation on host cell monolayers (Ke et al., 1990). Follow-up work was done to test the effect of artemisinin in controlling toxoplasmosis in vivo in animal models (Shen et al., 2003; Yang and Wan, 2006). The results were not exactly the same from different studies but all seemed to show that artemisinin was not as effective as expected, and some derivatives were more potent than the others (Dunay et al., 2009).
Table 4.
Effects of herbal medicines or their active components on Toxoplasma gondii.
| Active ingredients | Dosage | Effect | References |
|---|---|---|---|
| Artemisinin | 0.4 μg/mL (5 days) 1.3 μg/mL (14 days) | Inhibit plaque formation in vitro Eliminate all parasites in vitro | Ke et al., 1990 |
| Ginkgolic acids | 167.1 μg/ml | No visible parasites in HFF cells after 48 h of exposure to ginkgolic acids (isolated from the Ginkgo biloba sarcotesta) | Chen R. et al., 2008 |
| Inontus obliquus polysaccharide | 3 mg/10 g/d | Decreased testicular spermatogenic cell pathology damage caused by Toxoplasma infection | Liu Y. et al., 2010 |
| Radix glycyrrhizae | 5 g/Kg | While combined with sulfachloropyrazine-sodium (SPZ), the survival rate of mice was up to 50% | Jiang W. et al., 2013 |
| Oxymatrine, matrine | 100 mg/Kg | Decreased the number of tachyzoite (45.2 and 53.8%) and increased survival rate of mice to 67% | Zhang X. et al., 2016 |
In addition to artemisinin, which was isolated from Artemisia annua, extracts from many other herbs such as, Glycyrrhiza, Scutellaria, and Ginkgo biloba etc, were also reported to have anti-Toxoplasma effects (Chen S. X. et al., 2008; Gong et al., 2011). However, in most cases, the active compounds in these extracts were not known and deserve further research. There are a few occasions where the active compounds were reported. For example, oxymatrine (OM) and matrine (ME), the main alkaloids in the Sophora leguminous plants, were shown to restrict T. gondii growth both in vivo and in vitro: both OM and ME showed high anti-T. gondii activity and low toxicity to Hela cells than spiramycin (SPY); treating infected mice with oxymatrine or matrine increased the survival rates, although not to 100% (Zhang X. et al., 2016). This shows the potential of using natural products to treat toxoplasmosis, but obviously we need to dig further to find out and optimize the active components.
Conclusions and perspective
Since the first isolation and discovery of T. gondii in China in 1955, Chinese scientists have been working on this parasite for over 60 years. Tremendous amount of work has been done on the development of diagnostic strategies, subunit vaccines, and epidemiology surveys. It is clear that T. gondii is widespread in China. In particular, pigs and chickens, the main meat source for Chinese citizens, as well as cats, a popular pet people keep, are among the most frequently infected animals, which greatly increase the chance of human infections. But thanks to the unique eating/cooking habits, human infection rates in China are below the world average. A large number of vaccine candidates in different forms were designed, but frankly speaking, none of them is good enough to be used as a vaccine in the current form. Years of research in the vaccine design field seems to suggest that live attenuated parasites may be the best way to achieve good T. gondii vaccines. With the improvements of genetic modification techniques in T. gondii, work on designing live attenuated vaccines becomes more common, significant progress in this direction is expected.
In terms of diagnostics, although lots of efforts have been taken and a variety of assays were developed, very few of them were translated into standardized commercial products or technical standards. As a consequence, many veterinary clinics use home-made protocols for T. gondii diagnosis. For the toxoplasmosis examination in humans during pregnancy, well-established reference laboratories are still lacking in China. Most hospitals use a single IgM-IgG test to judge the risk, which is not reliable and may lead to false positive results and unnecessary termination of pregnancy. In addition, diagnostic products, especially IgM detecting kits, from domestic manufacturers are not yet ideal for clinic uses. Therefore, efforts are needed to translate the laboratory assays to standardized products for accurate and consistent diagnosis. Reference laboratories should be established to help local clinics and hospitals to get reliable test results for human samples. In addition to these, new diagnostic strategies, which can not only tell the infection status but also inform the source of infection (oocysts vs. tachyzoites/bradyzoites; Santana et al., 2015), may be needed for better risk factor association analysis.
On the basic research side, although so far the contribution from Chinese scientists to the understanding of biology, as well as mechanisms of pathogenesis of T. gondii is limited, it is obvious that the research activities are really booming after the year 2000. Currently, there are groups working on the host mechanisms that restrict parasite infections (Qin et al., 2017), parasite mechanisms of immune evasion (Xue et al., 2017), molecular basis for T. gondii invasion (Wang Y. et al., 2014; Wang M. et al., 2017), growth and differentiation (Shen et al., 2016), etc. Besides these common research interests shared by the whole community, studies addressing the population structure of strains prevalent in China, the unique biological properties of ToxoDB #9 strains, and interplays between local prevalent strains and animal hosts are also required. It is expected that, after some years, breakthroughs in related fields will be made by our Chinese colleagues.
Author contributions
MP, CL, JZ, and BS reviewed the literature and wrote the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Min Hu and Yanqin Zhou from the College of Veterinary Medicine, Huazhong Agricultural University for their critical reading and comments of the manuscript. We also thank all the scientists whose work was included in the paper but was not cited due to limited space.
Footnotes
Funding. This work was supported the National Key Research and Development Program of China (project 2017YFD0501304), National Natural Science Foundation of China (project No. 31572508), and the Fundamental Research Funds for the Central Universities in China (Project 2662015PY104).
References
- Al-Adhami B. H., Simard M., Hernández-Ortiz A., Boireau C., Gajadhar A. A. (2016). Development and evaluation of a modified agglutination test for diagnosis of Toxoplasma infection using tachyzoites cultivated in cell culture. Food Waterborne Parasitol. 2, 15–21. 10.1016/j.fawpar.2015.12.001 [DOI] [Google Scholar]
- Burrells A., Benavides J., Canton G., Garcia J. L., Bartley P. M., Nath M., et al. (2015). Vaccination of pigs with the S48 strain of Toxoplasma gondii–safer meat for human consumption. Vet. Res. 46:47. 10.1186/s13567-015-0177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D., Innes E. A. (1995). A commercial vaccine for ovine toxoplasmosis. Parasitology 110(Suppl.), S11–S16. 10.1017/S003118200000144X [DOI] [PubMed] [Google Scholar]
- Chen J. (2010). Epidemiological Study of Toxoplasmosis of Dogs and Cats in Shanghai. Master, Shanghai Jiaotong University. (Translated from Chinese). [Google Scholar]
- Chen J., Huang S. Y., Li Z. Y., Yuan Z. G., Zhou D. H., Petersen E., et al. (2013). Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine 31, 1734–1739. 10.1016/j.vaccine.2013.01.027 [DOI] [PubMed] [Google Scholar]
- Chen J., Li Z. Y., Huang S. Y., Petersen E., Song H. Q., Zhou D. H., et al. (2014a). Protective efficacy of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) adjuvated with recombinant IL-15 and IL-21 against experimental toxoplasmosis in mice. BMC Infect. Dis. 14:487. 10.1186/1471-2334-14-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhou D. H., Li Z. Y., Petersen E., Huang S. Y., Song H. Q., et al. (2014b). Toxoplasma gondii: protective immunity induced by rhoptry protein 9 (TgROP9) against acute toxoplasmosis. Exp. Parasitol. 139, 42–48. 10.1016/j.exppara.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Chen R., Lin X., Hu L., Chen X., Tang Y., Zhang J., et al. (2015). Genetic characterization of Toxoplasma gondii from zoo wildlife and pet birds in Fujian, China. Iran. J. Parasitol. 10, 663–668. [PMC free article] [PubMed] [Google Scholar]
- Chen R., Lu S., Lou D., Lin A., Zeng X., Ding Z., et al. (2008). Evaluation of a rapid ELISA technique for detection of circulating antigens of Toxoplasma gondii. Microbiol. Immunol. 52, 180–187. 10.1111/j.1348-0421.2008.00020.x [DOI] [PubMed] [Google Scholar]
- Chen S. X., Wu L., Jiang X. G., Feng Y. Y., Cao J. P. (2008). Anti-Toxoplasma gondii activity of GAS in vitro. J. Ethnopharmacol. 118, 503–507. 10.1016/j.jep.2008.05.023 [DOI] [PubMed] [Google Scholar]
- Chen X. G., Fung M. C., Ma X., Peng H. J., Shen S. M., Liu G. Z. (1999). Baculovirus expression of the major surface antigen of Toxoplasma gondii and the immune response of mice injected with the recombinant P30. Southeast Asian J. Trop. Med. Public Health 30, 42–46. [PubMed] [Google Scholar]
- Chen Y. M., Li G. D., Li B. C. (1980). Indirect heamagglutination assay of experimental toxoplasmosis. J. Vet. Technol. 4, 10–16. (Translated from Chinese). [Google Scholar]
- Chen Z. Y., Li A. M., Lin G. C., Wang X. Z. (2005). Serological investigation of Toxoplasma infection among different populations of people in Guizhou Province. Acta Academiae Med Zunyi 28, 382–383. (Translated from Chinese). [Google Scholar]
- Cheng W., Liu F., Li M., Hu X., Chen H., Pappoe F., et al. (2015). Variation detection based on next-generation sequencing of type Chinese 1 strains of Toxoplasma gondii with different virulence from China. BMC Genomics 16:888. 10.1186/s12864-015-2106-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H., Gu Q. M., Jiang Y., He S. Y., Zhou H. Y., Yang T. T., et al. (2005). Oral immunization with a live recombinant attenuated Salmonella typhimurium protects mice against Toxoplasma gondii. Parasite Immunol. 27, 29–35. 10.1111/j.1365-3024.2005.00738.x [DOI] [PubMed] [Google Scholar]
- Cong W., Dong W., Bai L., Wang X. Y., Ni X. T., Qian A. D., et al. (2015a). Seroprevalence and associated risk factors of Toxoplasma gondii infection in psychiatric patients: a case-control study in eastern China. Epidemiol. Infect. 143, 3103–3109. 10.1017/S0950268814003835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W., Huang S. Y., Zhou D. H., Zhang X. X., Zhang N. Z., Zhao Q., et al. (2013). Prevalence and genetic characterization of Toxoplasma gondii in house sparrows (Passer domesticus) in Lanzhou, China. Korean J. Parasitol. 51, 363–367. 10.3347/kjp.2013.51.3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W., Liu G. H., Meng Q. F., Dong W., Qin S. Y., Zhang F. K., et al. (2015b). Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 359, 307–313. 10.1016/j.canlet.2015.01.036 [DOI] [PubMed] [Google Scholar]
- Cong W., Meng Q. F., Blaga R., Villena I., Zhu X. Q., Qian A. D. (2016a). Toxoplasma gondii, Dirofilaria immitis, feline immunodeficiency virus (FIV), and feline leukemia virus (FeLV) infections in stray and pet cats (Felis catus) in northwest China: co-infections and risk factors. Parasitol. Res. 115, 217–223. 10.1007/s00436-015-4738-y [DOI] [PubMed] [Google Scholar]
- Cong W., Meng Q. F., Song H. Q., Zhou D. H., Huang S. Y., Qian A. D., et al. (2014). Seroprevalence and genetic characterization of Toxoplasma gondii in three species of pet birds in China. Parasit. Vectors 7:152. 10.1186/1756-3305-7-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W., Qin S. Y., Meng Q. F., Zou F. C., Qian A. D., Zhu X. Q. (2016b). Molecular detection and genetic characterization of Toxoplasma gondii infection in sika deer (Cervus nippon) in China. Infect. Genet. Evol. 39, 9–11. 10.1016/j.meegid.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Cui Y. L., He S. Y., Xue M. F., Zhang J., Wang H. X., Yao Y. (2008). Protective effect of a multiantigenic DNA vaccine against Toxoplasma gondii with co-delivery of IL-12 in mice. Parasite Immunol. 30, 309–313. 10.1111/j.1365-3024.2008.01025.x [DOI] [PubMed] [Google Scholar]
- Dai J. F., Jiang M., Qu L. L., Sun L., Wang Y. Y., Gong L. L., et al. (2013). Toxoplasma gondii: enzyme-linked immunosorbent assay based on a recombinant multi-epitope peptide for distinguishing recent from past infection in human sera. Exp. Parasitol. 133, 95–100. 10.1016/j.exppara.2012.10.016 [DOI] [PubMed] [Google Scholar]
- De Berardinis A., Paludi D., Pennisi L., Vergara A. (2017). Toxoplasma gondii, a foodborne pathogen in the swine production Chain from a European perspective. Foodborne Pathog. Dis. [Epub ahead of print]. 10.1089/fpd.2017.2305 [DOI] [PubMed] [Google Scholar]
- Ding H., Gao Y. M., Deng Y., Lamberton P. H., Lu D. B. (2017). A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit. Vectors 10:27. 10.1186/s13071-017-1970-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Liu H. L., Han J. H., Chen W., Xu R. N., Li X. (2009). Serum TORCH test results of 7650 pregnant women. Pract. Prev. Med. 16, 160–162. 10.3969/j.issn.1007-4287.2009.10.057. (Translated from Chinese). [DOI] [Google Scholar]
- Ding R., Zhang Z. X., Zeng H., Chen M., Wang L. S. (2015). Analysis of TORCH test results of 6027 cases of pregnant women and newborns. Int. J. Lab. Med. 36, 485–486. 10.3969/j.issn.1673-4130.2015.04.022. (Translated from Chinese). [DOI] [Google Scholar]
- Dos Santos T. R., Nunes C. M., Luvizotto M. C., De Moura A. B., Lopes W. D., Da Costa A. J., et al. (2010). Detection of Toxoplasma gondii oocysts in environmental samples from public schools. Vet. Parasitol. 171, 53–57. 10.1016/j.vetpar.2010.02.045 [DOI] [PubMed] [Google Scholar]
- Du F., Feng H. L., Nie H., Tu P., Zhang Q. L., Hu M., et al. (2012a). Survey on the contamination of Toxoplasma gondii oocysts in the soil of public parks of Wuhan, China. Vet. Parasitol. 184, 141–146. 10.1016/j.vetpar.2011.08.025 [DOI] [PubMed] [Google Scholar]
- Du F., Zhang Q., Yu Q., Hu M., Zhou Y., Zhao J. (2012b). Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet. Parasitol. 187, 53–56. 10.1016/j.vetpar.2011.12.036 [DOI] [PubMed] [Google Scholar]
- Dubey J. P. (2009). Toxoplasmosis in pigs–the last 20 years. Vet. Parasitol. 164, 89–103. 10.1016/j.vetpar.2009.05.018 [DOI] [PubMed] [Google Scholar]
- Dubey J. P. (2010). Toxoplasmosis of Animals and Humans. Boca Raton, FL: CRC press. [Google Scholar]
- Dunay I. R., Chan W. C., Haynes R. K., Sibley L. D. (2009). Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob. Agents Chemother. 53, 4450–4456. 10.1128/AAC.00502-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y. H. (2017). A case of abortion caused by porcine toxoplasmosis. Heilongjiang J. Anim. Reprod. 25. 10.3969/j.issn.1005-2739.2017.01.025. (Translated from Chinese). [DOI] [Google Scholar]
- Fang R., Feng H., Hu M., Khan M. K., Wang L., Zhou Y., et al. (2012). Evaluation of immune responses induced by SAG1 and MIC3 vaccine cocktails against Toxoplasma gondii. Vet. Parasitol. 187, 140–146. 10.1016/j.vetpar.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Fang R., Nie H., Wang Z., Tu P., Zhou D., Wang L., et al. (2009). Protective immune response in BALB/c mice induced by a suicidal DNA vaccine of the MIC3 gene of Toxoplasma gondii. Vet. Parasitol. 164, 134–140. 10.1016/j.vetpar.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Feng J. X., Zhao Y. K., Meng F. P., Wu Z. M., Liu Z., Li N., et al. (2016). Research progress in diagnosis of toxoplasmosis. Infect. Dis. Inform. 29, 139–143. 10.3969/j.issn.1007-8134.2016.03.004. (Translated from Chinese). [DOI] [Google Scholar]
- Fox B. A., Bzik D. J. (2002). De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415, 926–929. 10.1038/415926a [DOI] [PubMed] [Google Scholar]
- Gao G. (2014). Recent advances in clinical characteristicks and diagnosis and treatment of toxoplasmosis. Chin. J. Pathog. Biol. 9, 848–851. 10.13350/j.cjpb.14092. (Translated from Chinese). [DOI] [Google Scholar]
- Gao J. M., Xie Y. T., Xu Z. S., Chen H., Hide G., Yang T. B., et al. (2017). Genetic analyses of Chinese isolates of Toxoplasma gondii reveal a new genotype with high virulence to murine hosts. Vet. Parasitol. 241, 52–60. 10.1016/j.vetpar.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Gao X., Wang H., Wang H., Qin H., Xiao J. (2016). Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Sci. Total Environ. 568, 1086–1091. 10.1016/j.scitotenv.2016.06.165 [DOI] [PubMed] [Google Scholar]
- Gao Y. M., Ding H., Lamberton P. H., Lu D. B. (2016). Prevalence of Toxoplasma gondii in pet dogs in mainland China: a meta-analysis. Vet. Parasitol. 229, 126–130. 10.1016/j.vetpar.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Gong G. H., Hou J. F., Jiang W., Liu Y. C., Chen Y. J., Jing Z. Y., et al. (2011). Analysis of anti-Toxoplasma gondii drugs of seven kinds of drugs. Anim. Husbandy Vet. Med. 43, 9–10. (Translated from Chinese). [Google Scholar]
- Gong P., Cao L., Guo Y., Dong H., Yuan S., Yao X., et al. (2016). Toxoplasma gondii: protective immunity induced by a DNA vaccine expressing GRA1 and MIC3 against toxoplasmosis in BALB/c mice. Exp. Parasitol. 166, 131–136. 10.1016/j.exppara.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Guan L. B. (2017). Analysis of TORCH test results in spontaneous abortion patients. Syst. Med. 2, 7–9. 10.19368/j.cnki.2096-1782.2017.05.007 [DOI] [Google Scholar]
- He J. J., Ma J., Elsheikha H. M., Song H. Q., Huang S. Y., Zhu X. Q. (2016a). Transcriptomic analysis of mouse liver reveals a potential hepato-enteric pathogenic mechanism in acute Toxoplasma gondii infection. Parasit. Vectors 9:427. 10.1186/s13071-016-1716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. J., Ma J., Elsheikha H. M., Song H. Q., Zhou D. H., Zhu X. Q. (2016b). Proteomic profiling of mouse liver following acute Toxoplasma gondii infection. PLoS ONE 11:e0152022. 10.1371/journal.pone.0152022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. Y., Jiang S. F., Ma X. B., Qiu Q. W. (2008). Evaluation of sixteen kinds of kits inner and abroad available for detecting antibodies of Toxoplasma gondii. Sci. Travel Med. 14, 43–45. 10.3969/j.issn.1006-7159.2008.03.022. (Translated from Chinese). [DOI] [Google Scholar]
- Howe D. K., Sibley L. D. (1995). Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172, 1561–1566. 10.1093/infdis/172.6.1561 [DOI] [PubMed] [Google Scholar]
- Huang S. Y., Cong W., Zhou P., Zhou D. H., Wu S. M., Xu M. J., et al. (2012). First report of genotyping of Toxoplasma gondii isolates from wild birds in China. J. Parasitol. 98, 681–682. 10.1645/GE-3038.1 [DOI] [PubMed] [Google Scholar]
- Huo S. L., Song Z. Z., Zhang B., Han S., Bai L., Wu C. P. (2007). A Serological Investigation of Toxoplasmosis in inner Mongolia. J. Med. Pest Control 23, 894–896. 10.3969/j.issn.1003-6245.2007.12.006. (Translated from Chinese). [DOI] [Google Scholar]
- Jiang C., Li Z., Chen P., Chen L. (2015). The seroprevalence of Toxoplasma gondii in Chinese population with cancer: a systematic review and meta-analysis. Medicine 94:e2274. 10.1097/MD.0000000000002274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. H., Huang S. Y., Zhou D. H., Zhang X. X., Su C., Deng S. Z., et al. (2013). Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasit. Vectors 6:227. 10.1186/1756-3305-6-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. H., Qin S. Y., Wang W., He B., Hu T. S., Wu J. M., et al. (2014). Prevalence and genetic characterization of Toxoplasma gondii infection in bats in southern China. Vet. Parasitol. 203, 318–321. 10.1016/j.vetpar.2014.04.016 [DOI] [PubMed] [Google Scholar]
- Jiang H. H., Wang S. C., Huang S. Y., Zhao L., Wang Z. D., Zhu X. Q., et al. (2016). Genetic characterization of Toxoplasma gondii isolates from pigs in Jilin Province, Northeastern China. Foodborne Pathog. Dis. 13, 88–92. 10.1089/fpd.2015.2043 [DOI] [PubMed] [Google Scholar]
- Jiang S. F., Zhang S. Y., Pan C. E., He Y. Y., Wei M. X. (2003). Evaluation of five commercial available kits for detecting antibodies of Toxoplasma gondii. Chin. J. Zoonoses 19, 97–99. 10.3969/j.issn.1002-2694.2003.01.030. (Translated from Chinese). [DOI] [Google Scholar]
- Jiang T., Gong D., Ma L. A., Nie H., Zhou Y., Yao B., et al. (2008). Evaluation of a recombinant MIC3 based latex agglutination test for the rapid serodiagnosis of Toxoplasma gondii infection in swines. Vet. Parasitol. 158, 51–56. 10.1016/j.vetpar.2008.07.035 [DOI] [PubMed] [Google Scholar]
- Jiang W., Chen Y. J., Zhang H. J., Liu Y. C., Jing Z. Y., Xue J. X., et al. (2013). Treatment effects of Sulfachloropyrazine-sodium with Scutellaria Baicalensis and Glycyrrhiza against acute murine toxoplasomsis. Chin. J. Anim. Vet. Sci. 40, 2022–2028. 10.11843/j.issn.0366-6964.2013.12.024. (Translated from Chinese). [DOI] [Google Scholar]
- Jiang W., Liu Y., Chen Y., Yang Q., Chun P., Yao K., et al. (2015). A novel dynamic flow immunochromatographic test (DFICT) using gold nanoparticles for the serological detection of Toxoplasma gondii infection in dogs and cats. Biosens. Bioelectron. 72, 133–139. 10.1016/j.bios.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Chang Z. Y., Ming X., Min C. L., Wei H., Sheng L. Y., et al. (2005). Fast dipstick dye immunoassay for detection of immunoglobulin G (IgG) and IgM antibodies of human toxoplasmosis. Clin. Diagn. Lab. Immunol. 12, 198–201. 10.1128/CDLI.12.1.198-201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke O. Y., Krug E. C., Marr J. J., Berens R. L. (1990). Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob. Agents Chemother. 34, 1961–1965. 10.1128/AAC.34.10.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. F., Huang H. P., Gu J. B., Situ C. M., Chen R. L., Zhong J. M., et al. (2014). The establishment of the toxoplasma detection method: LAMP. J. Trop. Med. 14, 1035–1037. (Translated from Chinese). [Google Scholar]
- Li F., Wang S. P., Wang C. J., He S. C., Wu X., Liu G. H. (2016). Seroprevalence of Toxoplasma gondii in goats in Hunan province, China. Parasite 23:44. 10.1051/parasite/2016053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. L., Dong L., Li Q., Zhang L., Chen J., Zou F. C., et al. (2015). Seroepidemiology of Toxoplasma gondii infection in Bai and Han ethnic groups in southwestern China. Epidemiol. Infect. 143, 881–886. 10.1017/S0950268814001551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Han Q., Gong P., Yang T., Ren B., Li S., et al. (2012). Toxoplasma gondii rhomboid protein 1 (TgROM1) is a potential vaccine candidate against toxoplasmosis. Vet. Parasitol. 184, 154–160. 10.1016/j.vetpar.2011.08.014 [DOI] [PubMed] [Google Scholar]
- Li M., Mo X. W., Wang L., Chen H., Luo Q. L., Wen H. Q., et al. (2014). Phylogeny and virulence divergency analyses of Toxoplasma gondii isolates from China. Parasit. Vectors 7:133. 10.1186/1756-3305-7-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Cui L., Zhao J., Dai P., Zong S., Zuo W., et al. (2011). Seroprevalence of Toxoplasma gondii infection in female sterility patients in China. J. Parasitol. 97, 529–530. 10.1645/GE-2680.1 [DOI] [PubMed] [Google Scholar]
- Li Y. N., Nie X., Peng Q. Y., Mu X. Q., Zhang M., Tian M. Y., et al. (2015). Seroprevalence and genotype of Toxoplasma gondii in pigs, dogs and cats from Guizhou province, Southwest China. Parasit. Vectors 8:214. 10.1186/s13071-015-0809-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. B., Zhang H. S., Cao J., Zhou Y. Z., Zhang Y. L., Li G. Q., et al. (2011). The establishment and preliminary application of the toxoplasma detection method: real-time PCR. Anim. Husbandry Vet. Med. 43, 20–23. (Translated from Chinese). [Google Scholar]
- Lin Z., Zhang Y., Zhang H., Zhou Y., Cao J., Zhou J. (2012). Comparison of loop-mediated isothermal amplification (LAMP) and real-time PCR method targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Vet. Parasitol. 185, 296–300. 10.1016/j.vetpar.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Liu L., Liu L. N., Wang P., Lv T. T., Fan Y. G., Pan H. F. (2017). Elevated seroprevalence of toxoplasma gondii in AIDS/HIV patients: a meta-analysis. Acta Trop. 176, 162–167. 10.1016/j.actatropica.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Liu M. M., Yuan Z. G., Peng G. H., Zhou D. H., He X. H., Yan C., et al. (2010). Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 106, 1079–1084. 10.1007/s00436-010-1742-0 [DOI] [PubMed] [Google Scholar]
- Liu R. Z., Chen Z. Q., Zhang C. F., Zhong W. C., Chen M. X. (2013). Serological investigation of Toxoplasma gondii infection in parts of Guangdong province. Poultry Poultry Dis. Control 7–9. (Translated from Chinese). [Google Scholar]
- Liu S., Shi L., Cheng Y. B., Fan G. X., Ren H. X., Yuan Y. K. (2009). Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 105, 267–274. 10.1007/s00436-009-1393-1 [DOI] [PubMed] [Google Scholar]
- Liu T., Meng X. F., Hou X. X. (2017). Analysis of TORCH test results of 7122 women of childbearing age in Luoyang, Henan province. J. Henan Univ. Sci. Technol. 35, 63–65. 10.15926/j.cnki.issn1672-688x.2017.01.021. (Translated from Chinese). [DOI] [Google Scholar]
- Liu T., Zhang Q., Liu L., Xu X., Chen H., Wang H., et al. (2013). Trophoblast apoptosis through polarization of macrophages induced by Chinese Toxoplasma gondii isolates with different virulence in pregnant mice. Parasitol. Res. 112, 3019–3027. 10.1007/s00436-013-3475-3 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ju Y. L., Liu L. B., Li J. J., Wang Y. C. (2010). Effects of Inontus obliquus plosaccharide against acute Toxoplasma gondii infection on apoptosis of testicular gem cell in male mice. J. Anhui Agric. Sci. 38, 10474–10475. 10.3969/j.issn.0517-6611.2010.19.196. (Translated from Chinese). [DOI] [Google Scholar]
- Lu B., Wu S., Shi Y., Zhang R., Zou L., Gao S., et al. (2006). Toxoplasma gondii: expression pattern and detection of infection using full-length recombinant P35 antigen. Exp. Parasitol. 113, 83–90. 10.1016/j.exppara.2005.12.014 [DOI] [PubMed] [Google Scholar]
- Lu F., Huang S., Kasper L. H. (2009). The temperature-sensitive mutants of Toxoplasma gondii and ocular toxoplasmosis. Vaccine 27, 573–580. 10.1016/j.vaccine.2008.10.090 [DOI] [PubMed] [Google Scholar]
- Miao Q., Han J. Q., Xiang X., Yuan F. Z., Liu Y. Z., Duan G., et al. (2014). Prevalence of antibody to Toxoplasma gondii in black-headed gulls (Chroicocephalus ridibundus), Dianchi Lake, China. J. Wildl. Dis. 50, 717–719. 10.7589/2014-01-016 [DOI] [PubMed] [Google Scholar]
- Miao Q., Huang S. Y., Qin S. Y., Yu X., Yang Y., Yang J. F., et al. (2015). Genetic characterization of Toxoplasma gondii in Yunnan black goats (Capra hircus) in southwest China by PCR-RFLP. Parasit. Vectors 8:57. 10.1186/s13071-015-0673-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot S., Melo M. B., Li F., Lu D., Niedelman W., Levine S. S., et al. (2012). Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc. Natl. Acad. Sci. U.S.A. 109, 13458–13463. 10.1073/pnas.1117047109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J. G., Liesenfeld O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- Nie H., Fang R., Xiong B. Q., Wang L. X., Hu M., Zhou Y. Q., et al. (2011). Immunogenicity and protective efficacy of two recombinant pseudorabies viruses expressing Toxoplasma gondii SAG1 and MIC3 proteins. Vet. Parasitol. 181, 215–221. 10.1016/j.vetpar.2011.04.039 [DOI] [PubMed] [Google Scholar]
- Peng G. H., Yuan Z. G., Zhou D. H., He X. H., Liu M. M., Yan C., et al. (2009). Toxoplasma gondii microneme protein 6 (MIC6) is a potential vaccine candidate against toxoplasmosis in mice. Vaccine 27, 6570–6574. 10.1016/j.vaccine.2009.08.043 [DOI] [PubMed] [Google Scholar]
- Peng Y. Y., Zhu G. M., Fan H. N., Lin Y. L., Li Y. (2015). Analysis of TORCH test in spontaneous abortion patients. Adv. Modern Biomed. 15, 3707–3710. 10.13241/j.cnki.pmb.2015.19.028. (Translated from Chinese). [DOI] [Google Scholar]
- Qian W., Wang H., Su C., Shan D., Cui X., Yang N., et al. (2012). Isolation and characterization of Toxoplasma gondii strains from stray cats revealed a single genotype in Beijing, China. Vet. Parasitol. 187, 408–413. 10.1016/j.vetpar.2012.01.026 [DOI] [PubMed] [Google Scholar]
- Qin A., Lai D. H., Liu Q., Huang W., Wu Y. P., Chen X., et al. (2017). Guanylate-binding protein 1 (GBP1) contributes to the immunity of human mesenchymal stromal cells against Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 114, 1365–1370. 10.1073/pnas.1619665114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S. Y., Cong W., Liu Y., Li N., Wang Z. D., Zhang F. K., et al. (2014). Molecular detection and genotypic characterization of Toxoplasma gondii infection in bats in four provinces of China. Parasit. Vectors 7:558. 10.1186/s13071-014-0558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D., Han J., Du A. (2013a). Evaluation of protective effect of multiantigenic DNA vaccine encoding MIC3 and ROP18 antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 112, 2593–2599. 10.1007/s00436-013-3425-0 [DOI] [PubMed] [Google Scholar]
- Qu D., Zhou H., Han J., Tao S., Zheng B., Chi N., et al. (2013b). Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) as a diagnostic tool of Toxoplasma gondii in pork. Vet. Parasitol. 192, 98–103. 10.1016/j.vetpar.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Ren X. P., Bao J. Z. (1982). The outbreak of toxoplasmosis cases in pig in China. Anim. Husbandry Vet. Med. (Translated from Chinese). [Google Scholar]
- Santana S. S., Gebrim L. C., Carvalho F. R., Barros H. S., Barros P. C., Pajuaba A. C., et al. (2015). CCp5A protein from Toxoplasma gondii as a serological marker of oocyst-driven infections in humans and domestic animals. Front. Microbiol. 6:1305. 10.3389/fmicb.2015.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Yuan Y., Cheng J., Pan M., Xia N., Zhang W., et al. (2016). Activation of chronic toxoplasmosis by transportation stress in a mouse model. Oncotarget 7, 87351–87360. 10.18632/oncotarget.13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. J., Zhan X. M., Yang S. J., He A., Zheng H. X., Zhang R. L., et al. (2003). Efficacy of artesunate against toxoplasmosis. Chin. J. Zoonoses 19, 128–129. 10.3969/j.issn.1002-2694.2003.05.041. (Translated from Chinese). [DOI] [Google Scholar]
- Su C., Khan A., Zhou P., Majumdar D., Ajzenberg D., Darde M. L., et al. (2012). Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U.S.A. 109, 5844–5849. 10.1073/pnas.1203190109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Zhang X., Dubey J. P. (2006). Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol. 36, 841–848. 10.1016/j.ijpara.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Sun X. M., Zou J., Elashram Saeed A. A., Yan W. C., Liu X. Y., Suo X., et al. (2011). DNA vaccination with a gene encoding Toxoplasma gondii GRA6 induces partial protection against toxoplasmosis in BALB/c mice. Parasit. Vectors 4:213. 10.1186/1756-3305-4-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Lu H., Jia B., Chang Z., Peng S., Yin J., et al. (2013). A comparative study of Toxoplasma gondii seroprevalence in three healthy Chinese populations detected using native and recombinant antigens. Parasit. Vectors 6:241. 10.1186/1756-3305-6-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wang Z., Li J., Wei F., Liu Q. (2015). Evaluation of an indirect ELISA using recombinant granule antigen GRA1, GRA7 and soluble antigens for serodiagnosis of Toxoplasma gondii infection in chickens. Res. Vet. Sci. 100, 161–164. 10.1016/j.rvsc.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Tang X., Yin G., Qin M., Tao G., Suo J., Liu X., et al. (2016). Transgenic Eimeria tenella as a vaccine vehicle: expressing TgSAG1 elicits protective immunity against Toxoplasma gondii infections in chickens and mice. Sci. Rep. 6:29379. 10.1038/srep29379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q., Fang R., Zhang W., Wang Y., Cheng J., Li Y., et al. (2013). Protective immunity induced by a DNA vaccine-encoding Toxoplasma gondii microneme protein 11 against acute toxoplasmosis in BALB/c mice. Parasitol. Res. 112, 2871–2877. 10.1007/s00436-013-3458-4 [DOI] [PubMed] [Google Scholar]
- Tian Y. M., Huang S. Y., Miao Q., Jiang H. H., Yang J. F., Su C., et al. (2014). Genetic characterization of Toxoplasma gondii from cats in Yunnan Province, Southwestern China. Parasit. Vectors 7:178. 10.1186/1756-3305-7-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liu Y., Jiang T., Zhang G., Yuan G., He J., et al. (2016). Seroprevalence and genotypes of Toxoplasma gondii isolated from pigs intended for human consumption in Liaoning province, northeastern China. Parasit. Vectors 9:248. 10.1186/s13071-016-1525-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L., Zhang T. E., Yin L. T., Pang M., Guan L., Liu H. L., et al. (2014). Partial protective effect of intranasal immunization with recombinant Toxoplasma gondii rhoptry protein 17 against toxoplasmosis in mice. PLoS ONE 9:e108377. 10.1371/journal.pone.0108377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Lei C., Li J., Wu Z., Shen G., Yu R. (2004). A piezoelectric immunoagglutination assay for Toxoplasma gondii antibodies using gold nanoparticles. Biosens. Bioelectron. 19, 701–709. 10.1016/S0956-5663(03)00265-3 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang T., Luo Q., Huo X., Wang L., Liu T., et al. (2012). Prevalence and genotypes of Toxoplasma gondii in pork from retail meat stores in Eastern China. Int. J. Food Microbiol. 157, 393–397. 10.1016/j.ijfoodmicro.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Wang J. L., Huang S. Y., Li T. T., Chen K., Ning H. R., Zhu X. Q. (2016). Evaluation of the basic functions of six calcium-dependent protein kinases in Toxoplasma gondii using CRISPR-Cas9 system. Parasitol. Res. 115, 697–702. 10.1007/s00436-015-4791-6 [DOI] [PubMed] [Google Scholar]
- Wang J. L., Li T. T., Elsheikha H. M., Chen K., Zhu W. N., Yue D. M., et al. (2017). Functional characterization of rhoptry kinome in the virulent Toxoplasma gondii RH strain. Front. Microbiol. 8:84. 10.3389/fmicb.2017.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chen H., Liu D., Huo X., Gao J., Song X., et al. (2013). Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 8:e53483. 10.1371/journal.pone.0053483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., He L. Y., Meng D. D., Chen Z. W., Wen H., Fang G. S., et al. (2015). Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasit. Vectors 8:162. 10.1186/s13071-015-0778-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao S., Du N., Fu J., Li Z., Jia H., et al. (2017). The moving junction protein RON4, although not critical, facilitates host cell invasion and stabilizes MJ members. Parasitology 144, 1490–1497. 10.1017/S0031182017000968 [DOI] [PubMed] [Google Scholar]
- Wang S., Lan C., Zhang L., Zhang H., Yao Z., Wang D., et al. (2015). Seroprevalence of Toxoplasma gondii infection among patients with hand, foot and mouth disease in Henan, China: a hospital-based study. Infect. Dis. Poverty 4:53. 10.1186/s40249-015-0088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. B., Yang X. D., Yang G. R. (2012). A survey of toxoplasmosis. Chin. J. Trop. Med. 12, 497–500. 10.13604/j.cnki.46-1064/r.2012.04.026. (Translated from Chinese). [DOI] [Google Scholar]
- Wang Y. H., Li X. R., Wang G. X., Yin H., Cai X. P., Fu B. Q., et al. (2011). Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitol. Int. 60, 105–107. 10.1016/j.parint.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Wang Y., Fang R., Yuan Y., Hu M., Zhou Y., Zhao J. (2014). Identification of host proteins interacting with the integrin-like A domain of Toxoplasma gondii micronemal protein MIC2 by yeast-two-hybrid screening. Parasit. Vectors 7:543. 10.1186/s13071-014-0543-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ge W., Huang S. Y., Li J., Zhu X. Q., Liu Q. (2014). Evaluation of recombinant granule antigens GRA1 and GRA7 for serodiagnosis of Toxoplasma gondii infection in dogs. BMC Vet. Res. 10:158. 10.1186/1746-6148-10-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Lv R., Sun X., Shu F., Zhou Z., Nie K., et al. (2012). Seroprevalence of Toxoplasma gondii antibodies from slaughter pigs in Chongqing, China. Trop. Anim. Health Prod. 44, 685–687. 10.1007/s11250-011-9965-3 [DOI] [PubMed] [Google Scholar]
- Wu K., Chen X. G., Li H., Yan H., Yang P. L., Lun Z. R., et al. (2009). Diagnosis of human toxoplasmosis by using the recombinant truncated surface antigen 1 of Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 64, 261–266. 10.1016/j.diagmicrobio.2009.02.009 [DOI] [PubMed] [Google Scholar]
- Wu S. M., Danba C., Huang S. Y., Zhang D. L., Chen J., Gong G., et al. (2011a). Seroprevalence of Toxoplasma gondii infection in Tibetan sheep in Tibet, China. J. Parasitol. 97, 1188–1189. 10.1645/GE-2912.1 [DOI] [PubMed] [Google Scholar]
- Wu S. M., Huang S. Y., Fu B. Q., Liu G. Y., Chen J. X., Chen M. X., et al. (2011b). Seroprevalence of Toxoplasma gondii infection in pet dogs in Lanzhou, Northwest China. Parasit. Vectors 4:64. 10.1186/1756-3305-4-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. M., Zhu X. Q., Zhou D. H., Fu B. Q., Chen J., Yang J. F., et al. (2011c). Seroprevalence of Toxoplasma gondii infection in household and stray cats in Lanzhou, northwest China. Parasit. Vectors 4:214. 10.1186/1756-3305-4-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. H. (2017). A case of the diagnosis, treatment and experience in porcine toxoplasmosis. Livestock Poult. Industry 28, 65–68. 10.19567/j.cnki.1008-0414.2017.04.039. (Translated from Chinese). [DOI] [Google Scholar]
- Wu Z. Y. (2011). The new situation and challenges of prevention and control of AIDS in China. Chin. J. Public Health 27, 1505–1507. (Translated from Chinese). [Google Scholar]
- Xiao Y., Yin J., Jiang N., Xiang M., Hao L., Lu H., et al. (2010). Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect. Dis. 10:4. 10.1186/1471-2334-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. Q., Chen Y. D., Sun F. H., Cai L., Fang Y. Y., Wang L. P., et al. (2005). A national survey on current status of the important parasitic diseases and control strategies. Chin. J. Parasitol. Parasite Dis. 23, 332–340. (Translated from Chinese). [PubMed] [Google Scholar]
- Xu P., Li X., Guo L., Li B., Wang J., Yu D., et al. (2014). Seroprevalence of Toxoplasma gondii infection in Liaoning cashmere goat from northeastern China. Parasite 21:22. 10.1051/parasite/2014023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li R. C., Liu G. H., Cong W., Zhang X. X., Yu X. L., et al. (2014). Seroprevalence of Toxoplasma gondii infection in sows in Hunan province, China. Sci. World J. 2014:347908. 10.1155/2014/347908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang N. Z., Wang M., Dong H., Feng S. Y., Guo H. C., et al. (2015). A long-lasting protective immunity against chronic toxoplasmosis in mice induced by recombinant rhoptry proteins encapsulated in poly (lactide-co-glycolide) microparticles. Parasitol. Res. 114, 4195–4203. 10.1007/s00436-015-4652-3 [DOI] [PubMed] [Google Scholar]
- Xue J., Jiang W., Chen Y., Gong F., Wang M., Zeng P., et al. (2017). Thioredoxin reductase from Toxoplasma gondii: an essential virulence effector with antioxidant function. FASEB J. [Epub ahead of print]. 10.1096/fj.201700008R [DOI] [PubMed] [Google Scholar]
- Yan C., Liang L. J., Zhang B. B., Lou Z. L., Zhang H. F., Shen X., et al. (2014). Prevalence and genotyping of Toxoplasma gondii in naturally-infected synanthropic rats (Rattus norvegicus) and mice (Mus musculus) in eastern China. Parasit. Vectors 7:591. 10.1186/s13071-014-0591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. P., Wan H. J. (2006). Research progress of artemisinin and its derivatives against Toxoplasma gondii. Int. J. Med. Parasit. Dis. 33, 160–162. 10.3760/cma.j.issn.1673-4122.2006.03.012. (Translated from Chinese). [DOI] [Google Scholar]
- Yang N., Mu M., Li H., Hu J., Gao W., Yang S., et al. (2013). Seroprevalence of Toxoplasma gondii infection in pet dogs in Shenyang, northeastern China. J. Parasitol. 99, 176–177. 10.1645/GE-3211.1 [DOI] [PubMed] [Google Scholar]
- Yang Q. L., Zhang R. S., Wu H. P., Zhang Y. K., Wang K. G. (2008). The detection of Toxoplasma gondii with loop-mediated isothermal amplification technology. Chin. J. Parasitol. Parasit. Dis. 26, 304–306. 10.3969/j.issn.1000-7423.2008.04.014. (Translated from Chinese). [DOI] [PubMed] [Google Scholar]
- Yang Y. B., Li S. Q., Du T., Liang H. S., Chen Q. L. (2014). Retrospective analysis of TORCH of 2642 infertile women in Guangzhou area. J. Trop. Med. 14, 1453–1455. (Translated from Chinese). [Google Scholar]
- Yang Y., Feng Y., Yao Q., Wang Y., Lu Y., Liang H., et al. (2017). Seroprevalence, Isolation, Genotyping, and Pathogenicity of Toxoplasma gondii Strains from Sheep in China. Front. Microbiol. 8:136. 10.3389/fmicb.2017.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ying Y., Verma S. K., Cassinelli A. B., Kwok O. C., Liang H., et al. (2015). Isolation and genetic characterization of viable Toxoplasma gondii from tissues and feces of cats from the central region of China. Vet. Parasitol. 211, 283–288. 10.1016/j.vetpar.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Yu E. S. (2000). China Toxoplasmosis. Hong Kong: Asian Medicine Press; 1–11. (Translated from Chinese). [Google Scholar]
- Yu E. S. (2001). Evaluation and suggestion on domestic commercial available kits for the diagnosis of toxoplasmosis. Chin. J. Zoonoses 17, 5–6. 10.3969/j.issn.1002-2694.2001.03.001. (Translated from Chinese). [DOI] [Google Scholar]
- Yu E. S., Chen T. S., Lin S. C. (1957). The occurrence of Toxoplasma in cats and rabbits in Fukien, China. Acta Microbiol. Sin. 5, 101–110. (Translated from Chinese). [Google Scholar]
- Yu H. J., Zhang Z., Liu Z., Qu D. F., Zhang D. F., Zhang H. L., et al. (2011). Seroprevalence of Toxoplasma gondii infection in pigs, in Zhejiang Province, China. J. Parasitol. 97, 748–749. 10.1645/GE-2713.1 [DOI] [PubMed] [Google Scholar]
- Yu J. H., Liu Q., Xia Z. F. (2006). Epidemiological investigations of infections with Neospora caninum and Toxoplasma gondii in cattle and water buffaloes. Vet. Sci. China 36, 247–251. 10.3969/j.issn.1673-4696.2006.03.017. (Translated from Chinese). [DOI] [Google Scholar]
- Yu P. L., Chen J. S., Zhang H. X., Zhang C., Wang C. X., Xu M. X. (2007). The serological survy of Toxoplasma infection in Wuhan city. Chin. J. Zoonoses 23, 393–394. 10.3969/j.issn.1002-2694.2007.04.023. (Translated from Chinese). [DOI] [Google Scholar]
- Yu S. H., Xu L. Q., Jiang Z. X., Xu S. H., Han J. J., Zhu Y. G., et al. (1994). Report on the first national wide survey of the distribution of human parasites in China I: regional distribution of parasite species. Chin. J. Parasitol. Parasite Dis. 12, 241–247. (Translated from Chinese). [PubMed] [Google Scholar]
- Yuan Z. G., Luo S. J., Dubey J. P., Zhou D. H., Zhu Y. P., He Y., et al. (2013a). Serological evidence of Toxoplasma gondii infection in five species of bats in China. Vector Borne Zoonotic Dis. 13, 422–424. 10.1089/vbz.2012.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. G., Ren D., Zhou D. H., Zhang X. X., Petersen E., Li X. Z., et al. (2013b). Evaluation of protective effect of pVAX-TgMIC13 plasmid against acute and chronic Toxoplasma gondii infection in a murine model. Vaccine 31, 3135–3139. 10.1016/j.vaccine.2013.05.040 [DOI] [PubMed] [Google Scholar]
- Yuan Z. G., Zhang X. X., He X. H., Petersen E., Zhou D. H., He Y., et al. (2011a). Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin. Vaccine Immunol. 18, 119–124. 10.1128/CVI.00312-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. G., Zhang X. X., Lin R. Q., Petersen E., He S., Yu M., et al. (2011b). Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine 29, 6614–6619. 10.1016/j.vaccine.2011.06.110 [DOI] [PubMed] [Google Scholar]
- Zhang A. M., Shen Q., Li M., Xu X. C., Chen H., Cai Y. H., et al. (2013). Comparative studies of macrophage-biased responses in mice to infection with Toxoplasma gondii ToxoDB #9 strains of different virulence isolated from China. Parasit. Vectors 6:308 10.1186/1756-3305-6-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang Z., Fang R., Nie H., Feng H., Zhou Y., et al. (2010). Use of protein AG in an enzyme-linked immunosorbent assay for serodiagnosis of Toxoplasma gondii infection in four species of animals. Clin. Vaccine Immunol. 17, 485–486. 10.1128/CVI.00479-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhou D. H., Zhou P., Lun Z. R., Chen X. G., Lin R. Q., et al. (2009). Seroprevalence of Toxoplasma gondii infection in stray and household cats in Guangzhou, China. Zoonoses Public Health 56, 502–505. 10.1111/j.1863-2378.2008.01209.x [DOI] [PubMed] [Google Scholar]
- Zhang J., He S., Jiang H., Yang T., Cong H., Zhou H., et al. (2007). Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol. Res. 101, 331–338. 10.1007/s00436-007-0465-3 [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhao L., Song J., Li Y., Zhao Q., He S., et al. (2013). DNA vaccine encoding the Toxoplasma gondii bradyzoite-specific surface antigens SAG2CDX protect BALB/c mice against type II parasite infection. Vaccine 31, 4536–4540. 10.1016/j.vaccine.2013.07.065 [DOI] [PubMed] [Google Scholar]
- Zhang N., Wang S., Wang D., Li C., Zhang Z., Yao Z., et al. (2016). Seroprevalence of Toxoplasma gondii infection and risk factors in domestic sheep in Henan province, central China. Parasite 23:53. 10.1051/parasite/2016064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Y., Jiang S. F., He Y. Y., Pan C. E., Zhu M., Wei M. X. (2004). Serologic prevalence of Toxoplasma gondii in field mice, Microtus fortis, from Yuanjiang, Hunan Province, People's Republic of China. J. Parasitol. 90, 437–438. 10.1645/GE-168R [DOI] [PubMed] [Google Scholar]
- Zhang X. X., Huang S. Y., Zhang Y. G., Zhang Y., Zhu X. Q., Liu Q. (2014). First report of genotyping of Toxoplasma gondii in free-living Microtus fortis in northeastern China. J. Parasitol. 100, 692–694. 10.1645/13-381.1 [DOI] [PubMed] [Google Scholar]
- Zhang X. X., Lou Z. Z., Huang S. Y., Zhou D. H., Jia W. Z., Su C., et al. (2013). Genetic characterization of Toxoplasma gondii from Qinghai vole, Plateau pika and Tibetan ground-tit on the Qinghai-Tibet Plateau, China. Parasit. Vectors 6:291. 10.1186/1756-3305-6-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jin L., Cui Z., Zhang C., Wu X., Park H., et al. (2016). Antiparasitic effects of oxymatrine and matrine against Toxoplasma gondii in vitro and in vivo. Exp. Parasitol. 165, 95–102. 10.1016/j.exppara.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Zhang Y. B., Cong W., Li Z. T., Bi X. G., Xian Y., Wang Y. H., et al. (2015). Seroprevalence of Toxoplasma gondii infection in patients of intensive care unit in China: a hospital based study. Biomed Res. Int. 2015:908217. 10.1155/2015/908217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu D., Cao L., Gao Y., Xia X., Zhang Z., et al. (2013). High prevalence of Toxoplasma gondii infection in Microtus fortis by seminested PCR from Jilin Province, Northeastern China. J. Parasitol. 99, 580–582. 10.1645/GE-3195.1 [DOI] [PubMed] [Google Scholar]
- Zhao G., Shen B., Xie Q., Xu L. X., Yan R. F., Song X. K., et al. (2012). Detection of Toxoplasma gondii in free-range chickens in China based on circulating antigens and antibodies. Vet. Parasitol. 185, 72–77. 10.1016/j.vetpar.2011.10.031 [DOI] [PubMed] [Google Scholar]
- Zhao G., Zhou A., Lu G., Meng M., Sun M., Bai Y., et al. (2013). Identification and characterization of Toxoplasma gondii aspartic protease 1 as a novel vaccine candidate against toxoplasmosis. Parasit. Vectors 6:175. 10.1186/1756-3305-6-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Lu S., Tong Q., Kong Q., Lou D. (2013). The virulence-related rhoptry protein 5 (ROP5) of Toxoplasma gondii is a novel vaccine candidate against toxoplasmosis in mice. Vaccine 31, 4578–4584. 10.1016/j.vaccine.2013.07.058 [DOI] [PubMed] [Google Scholar]
- Zheng J., Jia H., Zheng Y. (2015). Knockout of leucine aminopeptidase in Toxoplasma gondii using CRISPR/Cas9. Int. J. Parasitol. 45, 141–148. 10.1016/j.ijpara.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Zheng L., Hu Y., Hua Q., Luo F., Xie G., Li X., et al. (2017). Protective immune response in mice induced by a suicidal DNA vaccine encoding NTPase-II gene of Toxoplasma gondii. Acta Trop. 166, 336–342. 10.1016/j.actatropica.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Zheng W. B., Zhang X. X., Ma J. G., Li F. C., Zhao Q., Huang S. Y., et al. (2016). Molecular Detection and Genetic Characterization of Toxoplasma gondii in Farmed Minks (Neovison vison) in Northern China by PCR-RFLP. PLoS ONE 11:e0165308. 10.1371/journal.pone.0165308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. X., Zhou D. H., Liu G. X., Suo X., Zhu X. Q. (2016a). Transcriptomic analysis of porcine PBMCs infected with Toxoplasma gondii RH strain. Acta Trop. 154, 82–88. 10.1016/j.actatropica.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou C. X., Zhu X. Q., Elsheikha H. M., He S., Li Q., Zhou D. H., et al. (2016b). Global iTRAQ-based proteomic profiling of Toxoplasma gondii oocysts during sporulation. J. Proteomics 148, 12–19. 10.1016/j.jprot.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Zhou P., Chen N., Zhang R. L., Lin R. Q., Zhu X. Q. (2008). Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 24, 190–196. 10.1016/j.pt.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Zhou P., Chen Z., Li H. L., Zheng H., He S., Lin R. Q., et al. (2011a). Toxoplasma gondii infection in humans in China. Parasit. Vectors 4:165. 10.1186/1756-3305-4-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Nie H., Zhang L. X., Wang H. Y., Yin C. C., Su C., et al. (2010). Genetic characterization of Toxoplasma gondii isolates from pigs in China. J. Parasitol. 96, 1027–1029. 10.1645/GE-2465.1 [DOI] [PubMed] [Google Scholar]
- Zhou P., Sun X. T., Yin C. C., Yang J. F., Yuan Z. G., Yan H. K., et al. (2011b). Genetic characterization of Toxoplasma gondii isolates from pigs in southwestern China. J. Parasitol. 97, 1193–1195. 10.1645/GE-2851.1 [DOI] [PubMed] [Google Scholar]
- Zhou P., Zhang H., Lin R. Q., Zhang D. L., Song H. Q., Su C., et al. (2009). Genetic characterization of Toxoplasma gondii isolates from China. Parasitol. Int. 58, 193–195. 10.1016/j.parint.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Zhou Y. H., Lu Y. J., Wang R. B., Song L. M., Shi F., Gao Q. F., et al. (2002). Survey on infection of Toxoplasma gondii in infertile couples in Suzhou countryside. National J. Androl. 8, 350–352. 10.3969/j.issn.1009-3591.2002.05.012. (Translated from Chinese). [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhang H., Cao J., Gong H., Zhou J. (2013). Isolation and genotyping of Toxoplasma gondii from domestic rabbits in China to reveal the prevalence of type III strains. Vet. Parasitol. 193, 270–276. 10.1016/j.vetpar.2012.11.031 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Sun Y. Y., Li K., Zhou Z. Y., Wang Z. Y. (2017). Research progress on the infection of Toxoplasma gondii and its influencing factors in cattle and sheep in China. Prog. Vet. Med. 38, 107–110. (Translated from Chinese). [Google Scholar]
- Zou F., Yu X., Yang Y., Hu S., Chang H., Yang J., et al. (2015). Seroprevalence and risk factors of Toxoplasma gondii infection in buffaloes, sheep and goats in yunnan province, Southwestern China. Iran. J. Parasitol. 10, 648–651. [PMC free article] [PubMed] [Google Scholar]