Abstract
Numerous epidemiological studies indicate that cancer will be responsible for millions of deaths in one year. Although multiple therapeutic strategies exist, and vast research efforts are being focused on developing newer and better regimens, cancer-related morbidity and mortality remain high. Metastasis and recurrence are prominent causes of treatment failure in cancers. Moreover, early diagnosis and treatment initiation are difficult to achieve in clinical practice. Fortunately, targeted therapy, which exerts its function at the molecular level, has proved to be greatly beneficial in several human diseases including cancers. The Wnt signaling pathway is a crucial regulator of embryogenesis and development in humans, and its dysfunction has been implicated in the incidence and development of cancers and other diseases. The Dickkopf family (Dkks) is a widely studied Wnt signaling pathway antagonist and plays multiple roles in human physiological and pathological process through both Wnt pathway-dependent and -independent manners. However, the precise roles of Dkks in tumorigenesis and the causal mechanisms have not been clearly elucidated. We discuss the pleiotropic roles of Dkks, with a specific focus on the underlying mechanisms, in cancer biology. We review recent literature to explore the potential use of Dkks as a tumor diagnosis biomarker and therapeutic target.
Keywords: Cancer, Wnt, Dkks, mechanism, diagnosis, biomarker
Introduction
Cancer is the second leading cause of death worldwide, second only to cardiovascular diseases. For men, prostate and lung cancer are the most common and the leading causes of cancer death all over the world, respectively, while for women, breast cancer has the highest incidence and mortality rate. Interestingly, although the stomach cancer, esophageal cancer, and chronic myeloid leukemia incidence rates are decreasing rapidly, the corresponding death rates remain increasing [1]. Traditional diagnostic methods, such as α-fetoprotein (AFP), a gold standard in liver cancer diagnosis, are rapidly proving ineffective in the early detection of liver cancer [2]. Moreover, although diverse therapeutic strategies are being employed in the treatment of cancers, patient prognosis remains obscure [3]. Targeted therapy is a new approach to facilitate the treatment of cancer, and is based on the concept of targeting specific cancer-related genes and their products. In addition, targeted therapy is an important advance in the field of modern molecular biology and has yielded promising results in clinical practice. For instance, Trastuzumab, a monoclonal antibody with a selective effect on the human epidermal factor receptor 2 (HER2), has proved efficacious in the treatment of Her2-positive breast cancer [4,5]. Collectively, targeted therapy is a crucial driving force and an essential technology for precise tumor treatment.
The Wnt signaling pathway is critical in development and homeostasis of the human body, while its dysfunction has been implicated in the development and progression of cancer [6,7]. The Dickkopf family (Dkks) is a canonical Wnt signaling pathway antagonist, and play varied roles in human physiology. Notably, aberrant function of the Dkks has been reported in several cancers [8-10]. Herein, we summarize the available research data on the roles of Dkks in cancer biology, delineate the potential mechanisms, and try to explain some of the seemingly contradictory findings. Through this review, we aim to establish a bridge between basic research and clinical practice of cancer therapy.
Wnt signaling pathway & Dkks
Wnt signaling pathway is a well-conserved and vital regulatory cascade in human embryogenesis, including growth, differentiation, and apoptosis [11,12]. Dysfunction of this pathway has been reported in a multitude of human diseases, especially in cancers [13-17]. The Wnt signaling pathway consists of the β-catenin-dependent canonical pathway and the β-catenin-independent non-canonical pathway, which covers the planar cell polarity pathway (PCP) and the Wnt/Ca2+ pathway [18]. The canonical Wnt pathway is more widely studied, and then we have a relatively clear understanding of the ligands and downstream effectors [19]. Wnts comprise a group of secreted lipid-modified cysteine-rich signaling glycoproteins that are 350-400 amino acids in length. And then the Wnt protein family has 19 members and are highly conserved across species [20]. Frizzled proteins, with a conserved cysteine-rich domain and ten known members in the human, are a family of G protein-coupled receptors that function as receptors of the Wnt and other signaling pathways [21,22]. Wnt proteins activate frizzled family receptors (Frz) on the responder cells and initiate at least three different signaling pathways, including the canonical and the non-canonical β-catenin pathway. The binding of Wnts to a receptor complex composed of members of the seven transmembrane Frz; the serpentine co-receptors; and the low-density lipoprotein receptor-related protein 5/6 (LRP5/6), transduces upstream signaling, which subsequently activates the canonical Wnt signaling pathway [23,24]. β-catenin is the core signaling element in this cascade, and its activation and nuclear translocation play an essential role in the canonical Wnt signaling pathway. Several Wnt antagonists, such as the Wnt inhibitory factor (WIF), Cerberus, and the secreted frizzled-related protein (sFRP), block the binding of Wnt to the frizzled receptors, while Dkks inhibit the Wnt/LRP5/6 interaction [25]. Mutations in the Wnt signaling elements have been linked to a range of human diseases, and the balance between antagonistic and agonistic effectors play a crucial role in Wnt signaling pathway regulation [26-28].
The Dkks family has rarely been studied systematically in relation to cancer research. The gene family encode secreted glycoproteins that are hallmark antagonists of the canonical Wnt signaling pathway, and is composed of five members: Dkk1-4, and Dkkll (soggy) [29]. The Dkks glycoproteins, except for soggy, contain an N-terminal signal peptide and two conserved cysteine-rich domains separated by a linker region. The amino-terminal cysteine-rich domain (Cys-1) is unique to each Dkks, whereas the carboxyl-terminal cysteine-rich domain (Cys-2) is highly conserved among all Dkks members. Additionally, the sgy-domain is found only in Dkk3 and Dkkl1. The function of these structural domains vis-à-vis their role in the mechanism of Dkks action have not been clarified in details and will be important to further our understanding of the Dkks (Figure 1). Not all members of the Dkks family, however, antagonize the Wnt/β-catenin signaling. While Dkk1, Dkk2, and Dkk4 have been shown to antagonize Wnt-mediated β-catenin stabilization by binding to Wnt co-receptors, Dkk3, in contrast, does not bind cell surface co-receptors [30]. Although Dkks members play diverse roles in the Wnt signaling pathway, several studies have noted that other pathways may also be involved in the role of Dkks [9,31].
Figure 1.
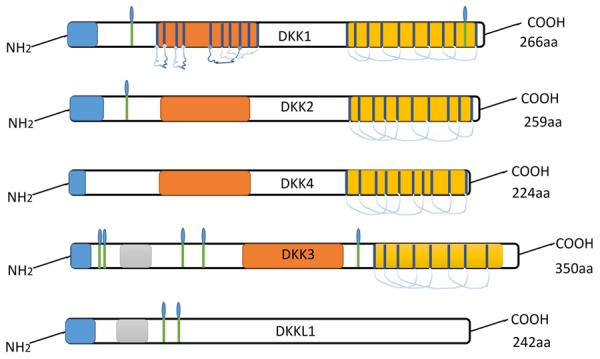
Ideograph of Dkk proteins.
Dkks gene expression pattern in cancer
Although an increasing number of studies are being conducted to elucidate the roles of Dkks in cancer, contradictory reports can be noted. Inconsistencies exist in the reported expression levels of Dkks members in similar tumors. The expression of Dkks gene family is tightly regulated during embryogenesis, both spatially and temporally. Furthermore, interactions between the encoding gene products also play crucial roles in an organism’s development.
Dkk1 has been demonstrated to inhibit Wnt signal transduction through two potential mechanisms: (1) Dkk1 can restrain Wnt signaling by binding to and antagonizing LRP5/6, and (2) Kremen1/2 (Krm1/2) can form a ternary complex with Dkk1 and LRP6, leading to a rapid internalization and clearance of the Wnt co-receptor LRP6 from the cell membrane, consequently blocking Wnt signaling [32]. Dkk1 upregulation has been reported in human hilar cholangiocarcinoma (HCCA) [33]; intrahepatic cholangiocarcinoma (ICC) [34]; chondrosarcoma [35]; hepatocellular carcinoma [36]; bladder cancer [37]; breast cancer [10,28]; pancreatic cancer [38]; multiple myeloma [39]; prostate cancer [40]; esophageal squamous cell carcinoma [41]; and laryngeal squamous cell carcinoma [42], while its downregulation has been noted in papillary thyroid cancer [43]; colorectal adenocarcinoma [44]; cervical squamous cell carcinoma [45]; and lung cancer [46] (Figure 2). Interestingly, Shi et al. [33] have shown that a higher expression of Dkk1 in HCCA was correlated with hilar lymph node metastasis, and have further noted that a loss of Dkk1 expression significantly inhibited cancer cell proliferation; colony formation; and migration, compared to controls. Zhao et al. [43] report that serum Dkk1 level was associated with various clinical features of papillary thyroid cancer, including tumor size, lymph node metastasis, and tumor-node-metastasis stage. Jiang et al. [47] reported significantly higher serum Dkk1 level in cervical cancer patients compared with healthy women. They also reported that the high serum Dkk1 was associated with lymphatic metastasis and tumor diameter in cervical carcinoma, and was associated with poor patient prognosis. However, Hou et al. [45] noted significantly lower Dkk1 expression in carcinoma tissue compared to normal cervical tissue, with the Dkk1 expression level being correlated with clinical stage, tumor differentiation, depth of invasion, and lymph node metastasis of the tumors. These contrasting reports evidence the need for further elucidation of the role of Dkk1 in cancer.
Figure 2.
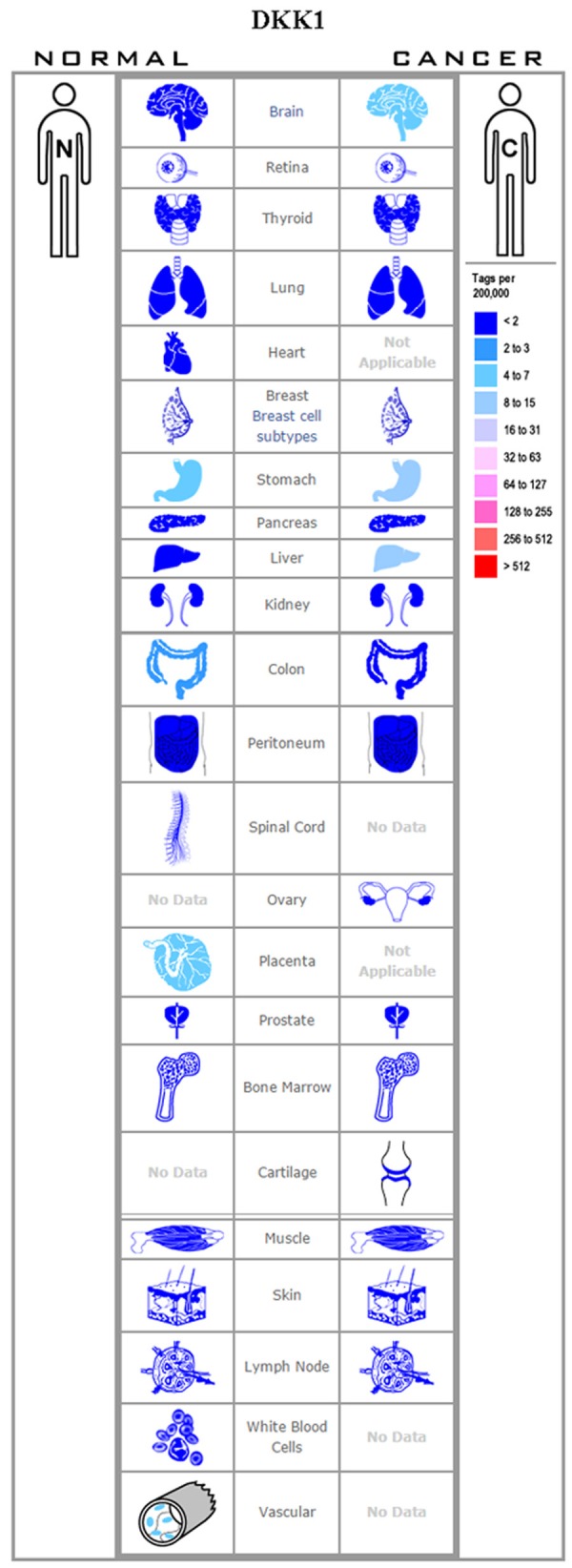
Expression profile for Dkk1 in human cancers.
Unlike Dkk1, Dkk2 can function as an LRP6 agonist or an antagonist, the particular role being dictated by the cellular context. The Krm2 serves as a co-factor that regulates Dkk2 activity in the Wnt/LRP6 signaling. Mao and Niehrs [48] have confirmed that Dkk2 can function as a Wnt/LRP6 signaling agonist or antagonist and that both contradictory functions are mediated via Krm2. Furthermore, as Dkk2 alone cannot activate the Wnt/β-catenin signaling pathway, the LRP6 level is vital to the Dkk2-mediated activation of the Wnt pathway. Like Dkk1, aberrant expression of Dkk2 has been detected in many tumor tissues. Several epigenetic alterations leading to a decline in the expression of Dkk2 have been reported in tumors (Figure 3). RNA interference-mediated silencing of Dkk2 has been observed in oral tongue squamous cell carcinoma [49]; esophageal adenocarcinoma [50]; and frequent epigenetic Dkk2 silencing has been found in ovarian carcinoma [51]; renal cancer [52]; and hepatocellular carcinoma [8]. In the abovementioned studies, Dkk2 played an inhibitory role in the development of the various tumors. However, increased Dkk2 expression can promote proliferation and invasion via the Wnt signaling in prostate cancer [53], Ewing sarcoma [54], and colorectal cancer [55]. These examples exhibit that even as the role of Dkk2 is closely related to the tumor environment, its function in the cellular context is incredibly complex.
Figure 3.
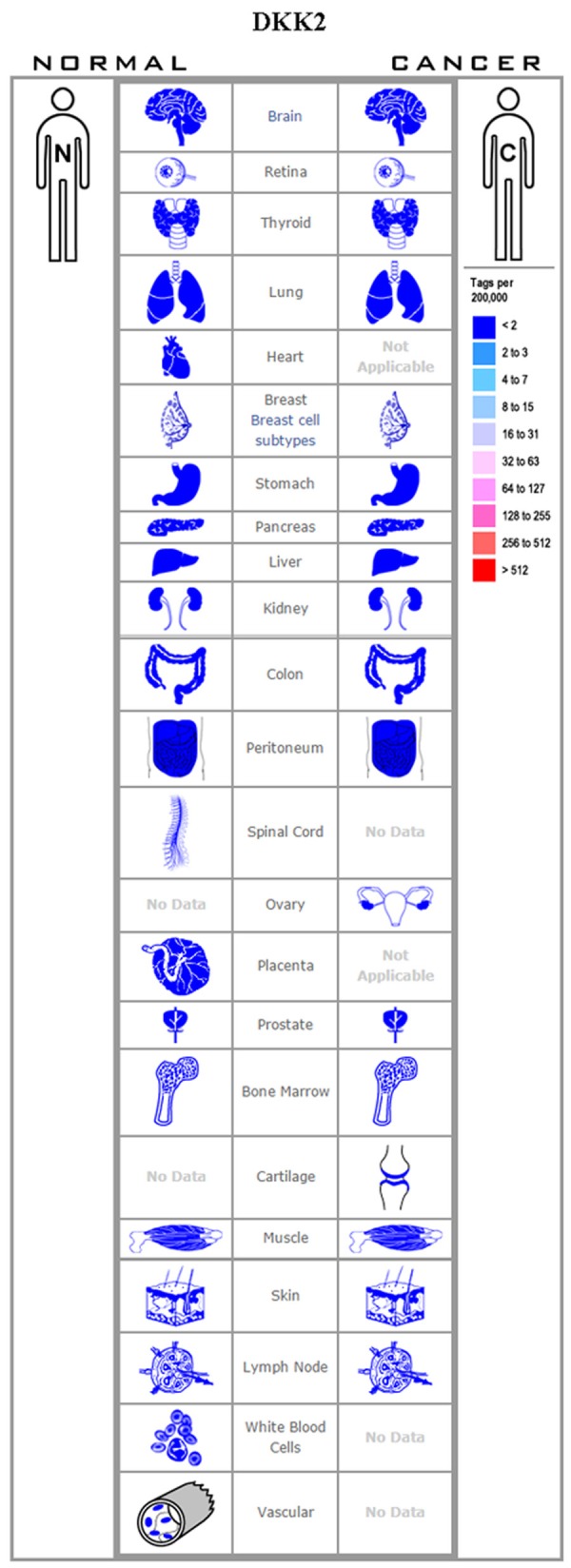
Expression profile for Dkk2 in human cancers.
The role of Dkk3 in Wnt/β-catenin signaling has remained unclear. Although previous studies have stated that Dkk3 does not influence Wnt signaling, recent studies indicate that Dkk3 is capable of interacting with Krm1/2 and LRP5/6 with comparable binding energies, thereby disrupting downstream signaling [56,57]. There are numerous pieces of evidence reflecting the inhibitory effect of Dkk3 on tumor growth and development via the Wnt pathway. However, the mechanism of this inhibitory effect is not clear, and only a few studies have reported a putative mechanism for the Dkk3-mediated tumor suppression. One such study reported that while Dkk3 could interact with Krm1/2, it could not interact with, or affect, the expression of LRP6 [57]. Dkk3 downregulation has been detected in melanoma [58]; basal breast cancer [59]; gastric cancer [60,61]; clear cell renal cell carcinoma [62]; and pancreatic cancer [63], while its overexpression has been reported in hepatoblastoma [64] and esophageal adenocarcinoma [65] (Figure 4).
Figure 4.
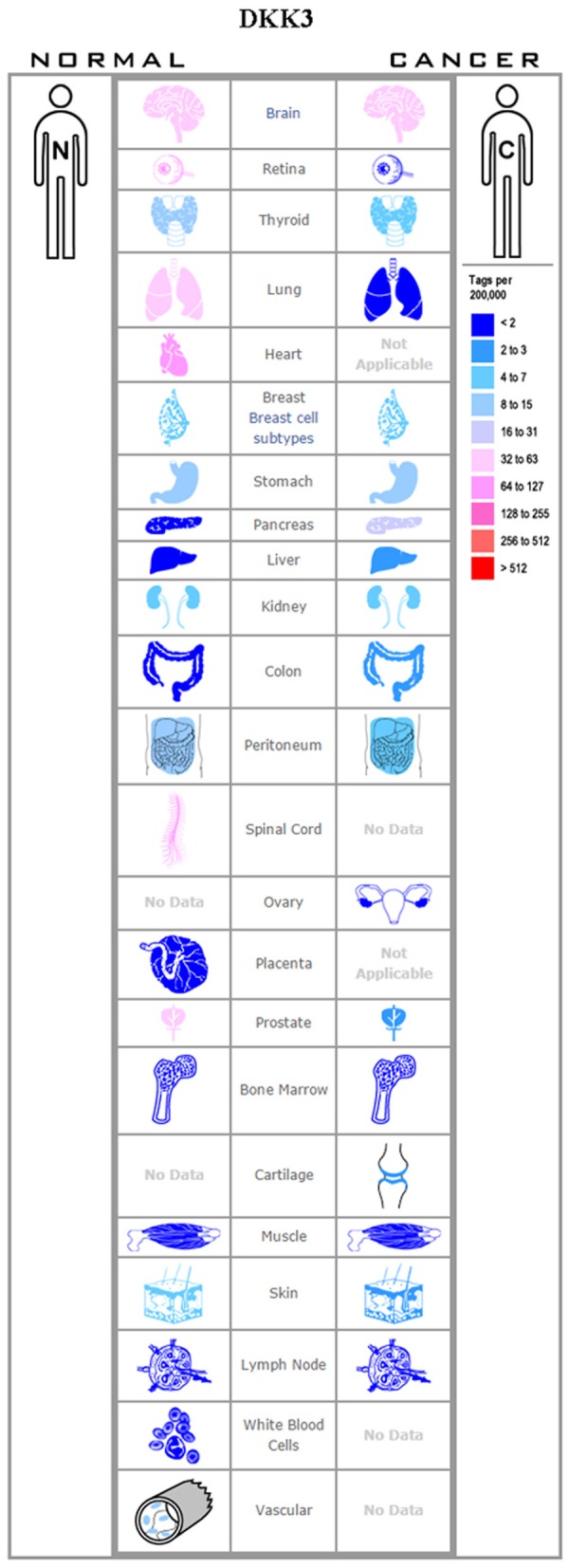
Expression profile for Dkk3 in human cancers.
The Dkk4 binds LRP5/LRP6 and Krm1/2 and demonstrates a Wnt/β-catenin antagonistic activity similar that of Dkk1. Thus, the downregulation of Dkk4 can promote tumor progression. However, its upregulation may also contribute to tumor progression. Pan et al. [66] observed that Dkk4 expression was high in pancreatic cancer tissues, and was associated with tumor and organ development, and with inflammation. Immunohistochemical and immunofluorescence studies indicated that Dkk4 was co-expressed with the mitogen-activated protein kinase 3 (MAPK3) and guanine nucleotide exchange factor (VAV3) in pancreatic cancer tissues, suggesting that in pancreatic cancer, Dkk4 may function through other signaling pathways such as the MAPK pathway. Hu et al. [67] reported that the Dkk4 mRNA and protein expression levels were significantly upregulated in clear cell renal cell carcinoma along with the downregulation of cyclin D1, c-myc, and β-catenin. Additionally, Hinoda et al. [52] reported that Dkk4 expression was increased in renal cancer tissues wherein it could activate the non-canonical c-Jun Nterminal kinase (JNK) signaling pathway while inhibiting the canonical Wnt pathway. Dkk4 expression was reportedly also upregulated in colorectal tumors and could promote tumor progression [55,68]. Contrary to these reports, however, a short hairpin (shRNA)-mediated knockdown of Dkk4 was noted to promote cell proliferation in hepatocellular carcinoma [69] (Figure 5).
Figure 5.
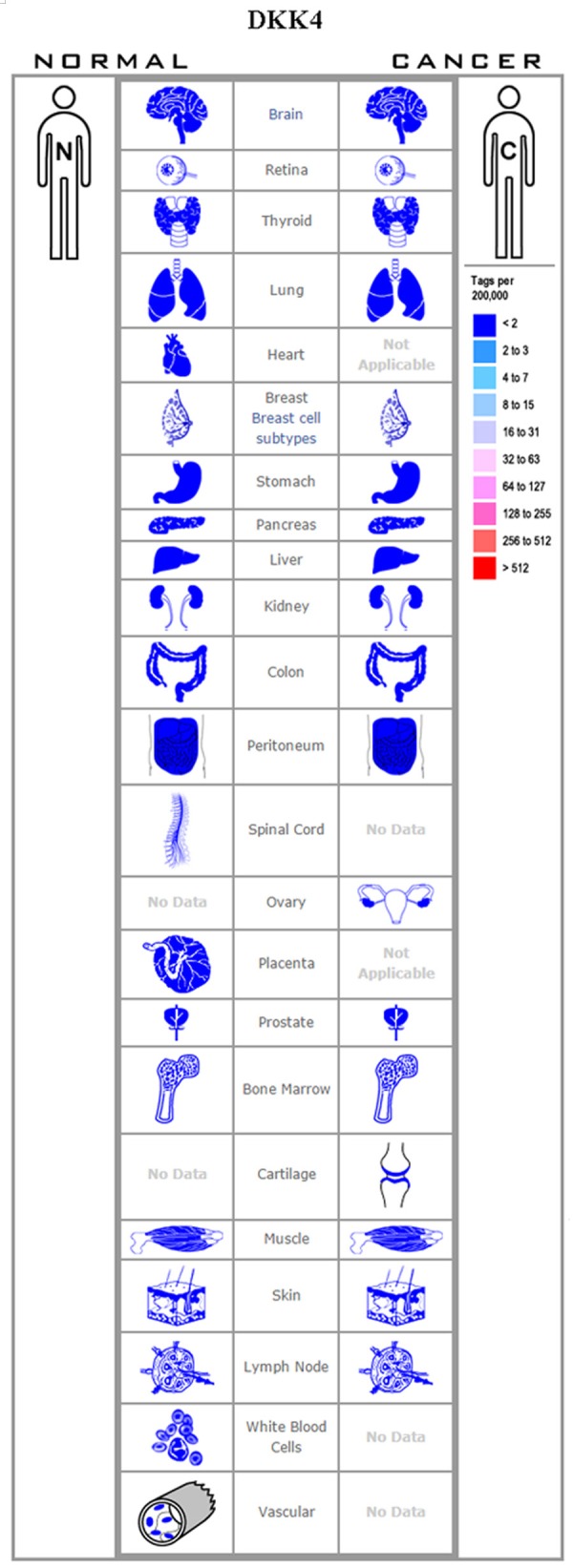
Expression profile for Dkk4 in human cancers.
Role of Dkks in the biological phenotype of cancer cells
Dkks and cancer cell proliferation
In normal tissue, cellular proliferation is strictly controlled to maintain homeostasis. Tumor cells, however, amass the ability to propagate indefinitely. Cellular proliferation is controlled through the regulation of cell cycle, growth factors, and their receptors, and oncogenes/tumor suppressor genes [70]. Dkk1 could inhibit the proliferation of MCF-7 breast cancer cell line, whereas LM-MCF-7 cells, where Dkk1 expression was knocked down via siRNAs, exhibited increased proliferation. The high Dkk1 level can downregulate the expression of β-catenin, c-Myc, cyclin D1, and Survivin by accelerating their phosphorylation-dependent degradation, thereby blocking the heightened proliferation of breast cancer cells [71]. Conversely, Dkk1 can promote cancer cell proliferation independent of the Wnt signaling pathway [14]. Cytoskeleton-associated protein 4 (CKAP4) is a receptor for Dkk1, and the expression of Dkk1 and CKAP4 are frequently dysregulated in human pancreatic and lung cancer lesions. Binding of Dkk1 to CKAP4 activates the PI3K/AKT pathway, resulting in cellular proliferation [72].
The Dkk2 expression is significantly upregulated in prostate cancer, and its knockdown can suppress cell proliferation and invasion. Downregulation of Dkk2 can decrease the expression of β-catenin, cyclin D1, and c-Myc in prostate cancer cells through Wnt signaling inhibition [53]. An in silico study has shown that Dkk3 could efficiently interact with LRP, Krm, and epidermal growth factor receptor (EGFR) with comparable binding energies. Dkk3 can inhibit cancer cell growth by blocking Wnt and EGFR downstream signaling [56]. Dkk4 is frequently downregulated in colorectal cancer cell lines and primary tumors, and the ectopic overexpression of Dkk4 or treatment with recombinant Dkk4 could both inhibit the growth of colorectal cancer cells. Cell cycle regulation, specifically a G0/G1 arrest, may partially explain this inhibitory effect of Dkk4 on tumor proliferation [73].
Dkks and cancer cell apoptosis
Controlled cellular proliferation and apoptosis ensure normal growth and development of the body and normal physiological functions. Apoptosis is regulated by a balance of anti-apoptotic and pro-apoptotic factors, and tumor cells have gained the ability to avoid apoptosis [70].
The role of Dkks in apoptosis is well described in renal cell carcinoma (RCC). The expression of Dkk1 is significantly lower in the RCC tissue compared with the adjacent normal tissue. However, Dkk1 overexpression in cells could induce apoptosis and inhibit proliferation. Interestingly, while T-cell factor/lymphoid enhancer factor (TCF/LEF) activity; the expression of nuclear β-catenin; cyclin D1; and c-Myc remained unchanged in Dkk1 transfectants, the expression levels of cleaved caspase 3; p53; p21; and p53 upregulated modulator of apoptosis (puma) were significantly upregulated. These results indicate that the pro-apoptotic effect of Dkk1 is independent of Wnt signaling [74].
Like Dkk1, the expression level of Dkk2 is decreased in RCC cell lines. However, unlike Dkk1, the downregulation of Dkk2 in RCC cell line A498 could inhibit RCC progression by inducing apoptosis and G1 phase cell cycle arrest [52]. The expression of Dkk-3 is downregulated by histone modification. RCC proliferation was significantly inhibited, and apoptosis promoted, in Dkk3-overexpressing RCC cell lines. Further, the expression levels of p21, MDM-2, and Puma genes were increased in the Dkk3-overexpressing cells. Thus, Dkk3 upregulation in renal cancer cells can induce apoptosis via the non-canonical JNK pathway [75]. Besides RCC, Dkk3 exhibits tumor-suppressive and pro-apoptotic effects, inducing apoptosis through mitochondrial and Fas death receptor pathways [76]. Pro-apoptotic effect of Dkk1 has also been reported in lung cancer; however, the mechanism remains unclear. Dkk3 gene knockout in the non-small cell lung cancer cell line H460 increased the expressions of cyclin-dependent kinases D1 and E, p53, p21, and Bax, thereby activating the apoptotic pathway [77]. In contrast, Dkk3 overexpression in lung cancer cell line resulted in significant upregulation of E-cadherin, while the expression levels of matrix metalloproteinase-7 (MMP7), survivin, c-myc, and cyclin D1 were downregulated. In cisplatin-resistant lung adenocarcinoma cell lines, Dkk3 overexpression induced cell cycle arrest and apoptosis [78]. Lastly, Terauchi et al. [79] have reported that the downregulation of Dkk4 expression could suppress apoptosis in osteoblasts via the Wnt signaling pathway.
Dkks and cancer angiogenesis
Angiogenesis is critical to provide the body with sufficient nutrients and oxygen and to eliminate metabolic wastes. Angiogenesis is strictly and precisely regulated to maintain homeostasis. Tumor angiogenesis or neovascularization, however, is a result of a loss of angiogenic control. Tumor angiogenesis is essential for invasive growth and metastasis and consists of two patterns: general angiogenesis and vasculogenic mimicry [70,80]. Emerging reports indicate that the Wnt signaling pathways and their antagonists modulate vessel neogenesis during both developmental and pathological angiogenesis [29].
Dkk2 can promote angiogenesis in murine and human endothelial cells, and the Dkk2-mediated angiogenesis is distinct from VEGF-mediated angiogenesis [81]. Compared to VEGF-induced blood vessels, Dkk2-induced vessel displays closer interconnections. Additionally, Dkk2-induced vessels consistently show higher coverage of endothelial cells (ECs) by pericytes and smooth muscle cells (SMCs), which in turn plays an important role in vessel maturity and stability. Dkk2-mediated angiogenesis involves a signaling cascade induced through LRP6-mediated APC/Asef2/Cdc42 activation.
Dkk1 reportedly suppresses angiogenesis, and its expression is downregulated upon induction of morphogenesis [81,82]. In non-small cell lung cancer, Dkk1 promotes vasculogenic mimicry by inducing the expression of epithelial-mesenchymal transition (EMT)-related and cancer stem cell (CSC)-related proteins [83].
Dkks and cancer invasion & metastasis
Invasion and metastasis are the most prominent biological characteristics of cancer and are also the most important causes of cancer-related deaths. Tumor metastasis is a continuous and dynamic process, wherein tumor cells translocate from their primary position via local invasion, blood vessels, and lymphatic tracts.
Recent studies have identified a role of Dkk1 in tumorigenesis and the invasiveness of several tumors. Dkk1 is upregulated in human HCCA tissues and increases tumor cell invasion and metastasis. The high Dkk1 level in HCCA correlates with metastasis to the hilar lymph nodes. Downregulation of Dkk1 in HCCA cells, however, significantly inhibits proliferation, colony formation, and migration. Dkk1 exerts its pro-invasive effect, at least in part, through the β-catenin/MMP-7 signaling pathway [33]. Similarly, β-catenin/MMP-7 signaling, a pathway independent of the canonical Wnt signaling pathway, is one of the pathways responsible for the pro-invasive effect of Dkk1 in hepatocellular carcinoma [84].
Expression of Dkk1 is elevated in an intrahepatic cholangiocarcinoma (ICC) cell line and promotes migration and invasion via elevated MMP-9 and VEGF-C [34]. Conversely, Dkk1 overexpression inhibits the proliferation and migration of human retinal pigment epithelial cells by suppressing the Wnt/β-catenin signaling pathway [85]. The expression of Dkk1 is markedly decreased in gastric cancer (GC) tissue and serum samples, and an upregulation of Dkk1 in chemo-resistant GC cells inhibits their proliferation and invasion [86].
Interestingly, there are several hormone response elements in the Dkks genes, which allow these hormones to regulate important physiological processes by modulating the expression of Dkks genes. Thyroid hormone receptor (TR) binds to nucleotides -1645 to -1629 of the Dkk4 gene promoter and induces its expression in HepG2-TR cells at the transcriptional level. Dkk4 overexpression suppresses hepatoma cell invasion in vitro and reduces metastasis in SCID mice via decreased MMP-2 expression [87]. The 1,25(OH)2D3 induces an early and transient binding of the vitamin D receptor (VDR) and the Silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) co-repressor to a region adjacent to the transcription start site of Dkk4. Ectopic Dkk4 expression increases the migration and invasion of colon cancer cells, which can be reversed by 1α,25-dihydroxyvitamin D3 [88].
Dkks and cancer diagnosis
Even as a growing body of literature confirms the essentiality of early diagnosis in improved patient prognosis, there is a dearth of early diagnostic markers for a vast number of cancers. In a previous study, Dkks were reported to be a highly sensitive and specific early detection markers for hepatocellular carcinoma (HCC) [89]. The role of Dkk1 in the diagnosis of HCC is relatively clear. In a large multicenter study conducted by Fan et al. [90], Dkk1 exhibited multiple advantages in the diagnosis of HCC: (1) Serum Dkk1 levels were significantly higher in HCC patients compared to unaffected controls; (2) Dkk1 level retained diagnostic accuracy in AFP-negative HCC patients, as well in patients with early-stage HCC; (3) Elevated serum Dkk1 concentration could differentiate between HCC, chronic HBV infection, and cirrhosis; and (4) A combination of Dkk1 and AFP level measurement greatly improved HCC diagnostic accuracy than when either test was used separately. Dkk1 can thus serve as a potential biomarker for HCC diagnosis, and subsequent studies have further confirmed the usefulness of a combined Dkk1-AFP measurement in improving early diagnostic efficacy [2].
In cervical cancer patients, serum levels of Dkk1 were associated with the histological type and lymphatic metastasis [45]. Serum levels of Dkk1 were also significantly higher in gastric adenocarcinoma patients. More importantly, a Dkk1 cutoff level of 25 U/mL could discriminate between gastric cancer patients and unaffected controls with 100% specificity and sensitivity. Thus, serum Dkk1 level may serve as a potent, novel serological marker for gastric cancers [91]. Similar to Dkk1, the serum level of Dkk3 was also significantly associated with lymphatic metastasis and tumor diameter in cervical cancer patients [92]. We also found that the serum Dkk3 level was significantly higher in healthy individuals than the gastric carcinoma patients and inversely linked to tumor size [61].
Dkks and the treatment of cancer
In assimilating the various roles played by the Dkks family in tumor development and metastasis, Dkks emerge as potential therapeutic targets for cancer treatment. In this regard, researchers have extensively studied Dkk3 as a genetic therapeutic target, and have demonstrated its tumor-suppressive effects. In one such study, Shiraha et al. [63], using intra-tumoral injections of an adenovirus vector carrying the human Dkk3 gene (Ad-REIC), showed that Dkk3 expression could be a potential therapeutic tool for pancreatic cancer. They further reported that Ad-REIC could induce apoptosis and inhibit the growth of pancreatic cancer cell lines, which were the underlying mechanisms for Dkk3-mediated tumor suppression. In another study, Kurozumi et al. [93] reported that in malignant glioma, Dkk3 expression could regulate cell growth through caspase-dependent apoptosis. Dkk3 expression was also reported to induce apoptosis in human prostate cancer cells through the activation of c-Jun-NH2-kinase [94].
Dkks and cancer molecular mechanism
With the rapid development of modern molecular biology, genetic and epigenetic alterations have been identified as key players in cancer initiation and progression. In the Dkks gene family, methylation is the most commonly identified epigenetic hallmark associated with tumorigenesis.
Dkks methylation in cancer
DNA methylation is a crucial epigenetic modification with key functions during development. Moreover, aberrant DNA methylation has been linked to many human diseases, particularly cancer. Sites of differential and aberrant DNA methylation include regulatory DNA sequences, such as CpG islands in promoters; and distal cis-regulatory elements, such as enhancers and promoters [95]. Recent studies have shown that Dkks gene methylation is involved in tumorigenesis and cancer progression. Furthermore, the Dkks family members exhibit different methylation status in different cancer types [96].
Dkks related miRNAs in cancer
MicroRNAs (miRNAs) are a subset of highly conserved, small noncoding RNAs that are approximately 18-22 nucleotides in length. miRNAs bind to the 3’-untranslated regions of mRNAs and bring about their degradation or translational repression, thereby acting as vital post-transcriptional regulators of gene expression [97]. miRNAs play important regulatory roles in many biological processes, including cellular differentiation, proliferation, and apoptosis of cancer cells [98]. Dysregulated miRNAs expression has been reported is a large number of human cancers.
Ren et al. [98] noted that miR-501-5p was markedly upregulated in gastric cancer cell lines and affected tissues and that its expression level was significantly correlated with a more aggressive phenotype in gastric cancer patients. They further demonstrated that miR-501-5p could directly target and suppress multiple repressors of the Wnt/β-catenin signaling cascade, including Dkk1. Yu et al. [99] demonstrated that miR-522 overexpression could promote cell proliferation, colony formation, and cell cycle progression, whereas a knockdown of miR-522 could attenuate these effects.
Dkks gene polymorphisms
In a recent study, Singh et al. [100] showed that Dkk3 rs7396187 exhibited a protective effect on lung cancer patients. Subjects with a heterozygous genotype of Dkk2 rs17037102 and rs419558 were at an increased risk. Subjects with the variant genotypic combination of Dkk3 rs3206824 and Dkk2 rs419558 showed a two-fold higher risk of developing lung cancer. Additionally, subjects with all three Dkk2 genotypic variants had a four-fold higher risk of developing lung cancer.
Other mechanisms
In addition to the regulatory mechanisms noted above, other mechanisms have been noted through which Dkks expression levels are modulated in certain cancer subtypes. In HBV-expressing liver cancer cell lines, HBV could bind to the Dkk1 promoter region and trigger its increasing expression at mRNA and protein level [101]. 1,25(OH)2D3 promotes the up-expression of Dkk1 mRNA and protein through an indirect transcriptional mechanism, which act as a tumor suppressor in colon cancer. In contrast, 1,25(OH)2D3 blocks Dkk4 transcription by targeting its promoter via VDR binding [102]. Additionally, thyroid hormone (T3) can induce Dkk4 mRNA and protein expression in HCC cells.
Perspective
The Dkk proteins have lately attracted a wide range of attention in cancer research as both a diagnostic biomarker and a potential therapeutic target. A large number of studies report the pleiotropic roles of Dkk proteins in physiological processes, while Dkks dysfunctions have been implicated in many diseases. In this review, we summarized the different functional pathways of Dkks gene family, in addition to the canonical and widely-studied Wnt pathway (Figure 6). Of note, even as the underlying mechanisms remain unclear, the growing body of literature provides irrefutable evidence of the important role played by Dkks in the diagnosis and treatment of tumors.
Figure 6.
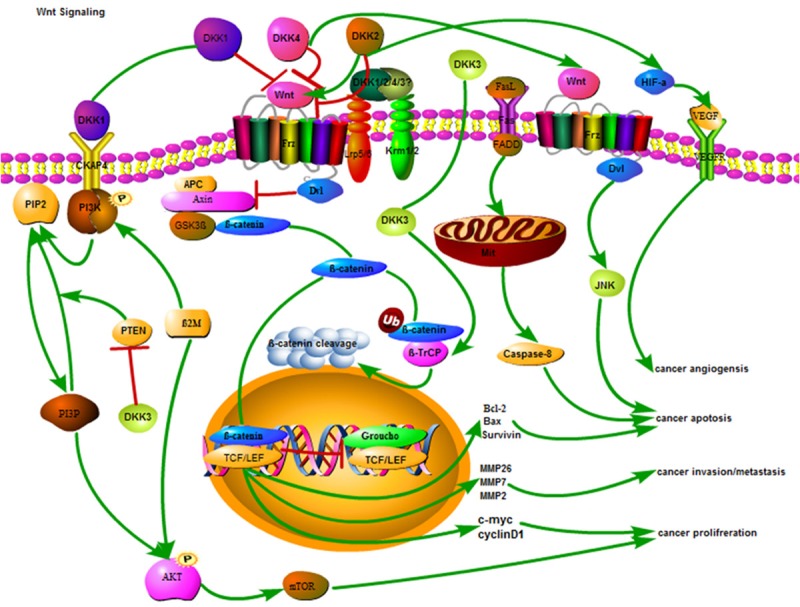
Schematic representation of the antitumor mechanisms of Dkks in cancer cell.
Acknowledgements
This study was supported by National Natural Scientific Foundation of China (No. 81572777). We thank Dr. Pu Xia for his valuable comments and excellent technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted lifeyears for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdal H, Gül Utku Ö, Karatay E, Çelik B, Elbeg Ş, Doğan İ. Combination of DKK1 and AFP improves diagnostic accuracy of hepatocellular carcinoma compared with either marker alone. Turk J Gastroenterol. 2016;27:375–81. doi: 10.5152/tjg.2016.15523. [DOI] [PubMed] [Google Scholar]
- 3.Raskin W, Harle I, Hopman WM, Booth CM. Prognosis, treatment benefit and goals of care: what do oncologists discuss with patients who have incurable cancer? Clin Oncol (R Coll Radiol) 2016;28:209–14. doi: 10.1016/j.clon.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Attard CL, Pepper AN, Brown ST, Thompson MF, Thuresson PO, Yunger S, Dent S, Paterson AH, Wells GA. Cost-effectiveness analysis of neoadjuvant pertuzumab and trastuzumab therapy for locally advanced, inflammatory, or early HER2-positive breast cancer in Canada. J Med Econ. 2015;18:173–88. doi: 10.3111/13696998.2014.979938. [DOI] [PubMed] [Google Scholar]
- 5.Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL, Chang DT, Gibbs IC, Goldhaber-Fiebert JD, Horst KC. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2016;34:902–9. doi: 10.1200/JCO.2015.62.9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–9. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawa M, Masuda M, Yamada T. Targeting the Wnt signaling pathway in colorectal cancer. Expert Opin Ther Targets. 2016;20:419–29. doi: 10.1517/14728222.2016.1098619. [DOI] [PubMed] [Google Scholar]
- 8.Fatima S, Luk JM, Poon RT, Lee NP. Dysregulated expression of dickkopfs for potential detection of hepatocellular carcinoma. Expert Rev Mol Diagn. 2014;14:535–48. doi: 10.1586/14737159.2014.915747. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Wei H, Zhang S. Dickkopf-4 is frequently overexpressed in epithelial ovarian carcinoma and promotes tumor invasion. BMC Cancer. 2017;17:455. doi: 10.1186/s12885-017-3407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariz K, Ingolf JB, Daniel H, Teresa NJ, Erich-Franz S. The Wnt inhibitor dickkopf-1: a link between breast cancer and bone metastases. Clin Exp Metastasis. 2015;32:857–66. doi: 10.1007/s10585-015-9750-1. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Salinas P. Introduction: Wnt signaling mechanisms in development and disease. Dev Neurobiol. 2014;74:757–8. doi: 10.1002/dneu.22192. [DOI] [PubMed] [Google Scholar]
- 13.Hermans KC, Blankesteijn WM. Wnt signaling in cardiac disease. Compr Physiol. 2015;5:1183–209. doi: 10.1002/cphy.c140060. [DOI] [PubMed] [Google Scholar]
- 14.Inestrosa NC, Montecinos-Oliva C, Fuenzalida M. Wnt signaling: role in Alzheimer disease and schizophrenia. J Neuroimmune Pharmacol. 2012;7:788–807. doi: 10.1007/s11481-012-9417-5. [DOI] [PubMed] [Google Scholar]
- 15.Maarouf OH, Ikeda Y, Humphreys BD. Wnt signaling in kidney tubulointerstitium during disease. Histol Histopathol. 2015;30:163–71. doi: 10.14670/HH-30.163. [DOI] [PubMed] [Google Scholar]
- 16.Arnes M, Casas Tinto S. Aberrant Wnt signaling: a special focus in CNS diseases. J Neurogenet. 2017:1–7. doi: 10.1080/01677063.2017.1338696. [DOI] [PubMed] [Google Scholar]
- 17.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishita M, Endo M, Minami Y. [Regulation of cellular responses by non-canonical Wnt signaling] . Clin Calcium. 2013;23:809–15. [PubMed] [Google Scholar]
- 19.Kikuchi A. [Canonical Wnt signaling pathway and cellular responses] . Clin Calcium. 2013;23:799–807. [PubMed] [Google Scholar]
- 20.Takada S, Fujimori S, Shinozuka T, Takada R, Mii Y. Differences in the secretion and transport of Wnt proteins. J Biochem. 2017;161:1–7. doi: 10.1093/jb/mvw071. [DOI] [PubMed] [Google Scholar]
- 21.Wang HY, Malbon CC. Wnt-frizzled signaling to G-protein-coupled effectors. Cell Mol Life Sci. 2004;61:69–75. doi: 10.1007/s00018-003-3165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–41. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 24.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 25.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita Y, Fukumoto S. [Antagonists of Wnt pathway] . Clin Calcium. 2013;23:817–23. [PubMed] [Google Scholar]
- 27.Levasseur R, Lacombe D, de Vernejoul MC. LRP5 mutations in osteoporosis-pseudoglioma syndrome and high-bone-mass disorders. Joint Bone Spine. 2005;72:207–14. doi: 10.1016/j.jbspin.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Liu JT, Guo WB, Sun JY. Serum Dickkopf-1 acts as a new biomarker in human breast cancer. Minerva Med. 2017;108:334–340. doi: 10.23736/S0026-4806.17.04807-8. [DOI] [PubMed] [Google Scholar]
- 29.Choi HJ, Park H, Lee HW, Kwon YG. The Wnt pathway and the roles for its antagonists, DKKS, in angiogenesis. IUBMB Life. 2012;64:724–31. doi: 10.1002/iub.1062. [DOI] [PubMed] [Google Scholar]
- 30.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–81. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 31.Bhavanasi D, Speer KF, Klein PS. CKAP4 is identified as a receptor for Dickkopf in cancer cells. J Clin Invest. 2016;126:2419–21. doi: 10.1172/JCI88620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/betacatenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 33.Shi XD, Yu XH, Wu WR, Xu XL, Wang JY, Xu LB, Zhang R, Liu C. Dickkopf-1 expression is associated with tumorigenity and lymphatic metastasis in human hilar cholangiocarcinoma. Oncotarget. 2016;7:70378–70387. doi: 10.18632/oncotarget.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y, Qiu SJ, Sun YF, Zhang X, Wang Z, Zhu K, Qin WX, Tang ZY, Fan J, Zhou J. High expression of Dickkopfrelated protein 1 is related to lymphatic metastasis and indicates poor prognosis in intrahepatic cholangiocarcinoma patients after surgery. Cancer. 2013;119:993–1003. doi: 10.1002/cncr.27788. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Zhou H, Zhang X, Ma X, Liu Z, Liu X. Elevated levels of Dickkopf-1 are associated with beta-catenin accumulation and poor prognosis in patients with chondrosarcoma. PloS One. 2014;9:e105414. doi: 10.1371/journal.pone.0105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watany M, Badawi R, Elkhalawany W, Abd-Elsalam S. Study of Dickkopf-1 (DKK-1) gene expression in hepatocellular carcinoma patients. J Clin Diagn Res. 2017;11:Oc32–Oc34. doi: 10.7860/JCDR/2017/23095.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun DK, Wang L, Wang JM, Zhang P. Serum Dickkopf-1 levels as a clinical and prognostic factor in patients with bladder cancer. Genet Mol Res. 2015;14:18181–7. doi: 10.4238/2015.December.23.5. [DOI] [PubMed] [Google Scholar]
- 38.Han SX, Zhou X, Sui X, He CC, Cai MJ, Ma JL, Zhang YY, Zhou CY, Ma CX, Varela-Ramirez A, Zhu Q. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015;6:19907–17. doi: 10.18632/oncotarget.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser M, Mieth M, Liebisch P, Oberländer R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, Stover D, Sezer O, Heider U. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–4. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 40.Rachner TD, Thiele S, Göbel A, Browne A, Fuessel S, Erdmann K, Wirth MP, Fröhner M, Todenhöfer T, Muders MH, Kieslinger M, Rauner M, Hofbauer LC. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer. 2014;14:649. doi: 10.1186/1471-2407-14-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begenik H, Kemik AS, Emre H, Dulger AC, Demirkiran D, Ebinc S, Kemik O. The association between serum Dickkopf-1 levels and esophageal squamous cell carcinoma. Hum Exp Toxicol. 2014;33:785–8. doi: 10.1177/0960327113510537. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Gong HL, Zhou L, Tian J, Wang Y. Dickkopf-1 is a novel prognostic biomarker for laryngeal squamous cell carcinoma. Acta Otolaryngol. 2014;134:753–9. doi: 10.3109/00016489.2014.894251. [DOI] [PubMed] [Google Scholar]
- 43.Zhao YP, Wang W, Wang XH, Xu Y, Wang Y, Dong ZF, Zhang JJ. Downregulation of serum DKK-1 predicts poor prognosis in patients with papillary thyroid cancer. Genet Mol Res. 2015;14:18886–94. doi: 10.4238/2015.December.28.38. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Sun B, Qi L, Li Y, Zhao X, Zhang D, Zhang Y. Dickkopf-1 expression is down-regulated during the colorectal adenoma-carcinoma sequence and correlates with reduced microvessel density and VEGF expression. Histopathology. 2015;67:158–66. doi: 10.1111/his.12474. [DOI] [PubMed] [Google Scholar]
- 45.Jiang T, Wang S, Huang L, Zhang S. Clinical significance of serum DKK-1 in patients with gynecological cancer. Int J Gynecol Cancer. 2009;19:1177–81. doi: 10.1111/IGC.0b013e31819d8b2d. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Wu J, Chen B, Li M, Tian Y, He M, Xue J, Wang J, Bai S, Sharma A, Liu H, Tang J, She JX. Serum Dickkopf-1 (DKK1) is significantly lower in patients with lung cancer but is rapidly normalized after treatment. Am J Transl Res. 2014;6:850–6. [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T, Huang L, Zhang S. DKK-1 in serum as a clinical and prognostic factor in patients with cervical cancer. Int J Biol Markers. 2013;28:221–5. doi: 10.5301/jbm.5000005. [DOI] [PubMed] [Google Scholar]
- 48.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–83. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 49.Kawakita A, Yanamoto S, Yamada S, Naruse T, Takahashi H, Kawasaki G, Umeda M. MicroRNA-21 promotes oral cancer invasion via the Wnt/beta-catenin pathway by targeting DKK2. Pathol Oncol Res. 2014;20:253–61. doi: 10.1007/s12253-013-9689-y. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Zhao Y, Herbst A, Kalinski T, Qin J, Wang X, Jiang Z, Benedix F, Franke S, Wartman T, Camaj P, Halangk W, Kolligs FT, Jauch KW, Nelson PJ, Bruns CJ. miR-221 mediates chemoresistance of esophageal adenocarcinoma by direct targeting of DKK2 expression. Ann Surg. 2016;264:804–814. doi: 10.1097/SLA.0000000000001928. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Zhang S, Gu L, Di W. Epigenetic silencing of DKK2 and Wnt signal pathway components in human ovarian carcinoma. Carcinogenesis. 2012;33:2334–43. doi: 10.1093/carcin/bgs278. [DOI] [PubMed] [Google Scholar]
- 52.Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, Ishii N, Dahiya R. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–87. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Pang K, Zhou ZG, Chen YF, Mo T, Li M, Liu CB. Dickkopf 2 promotes proliferation and invasion via Wnt signaling in prostate cancer. Mol Med Rep. 2016;14:2283–8. doi: 10.3892/mmr.2016.5502. [DOI] [PubMed] [Google Scholar]
- 54.Hauer K, Calzada-Wack J, Steiger K, Grunewald TG, Baumhoer D, Plehm S, Buch T, Prazeres da Costa O, Esposito I, Burdach S, Richter GH. DKK2 mediates osteolysis, invasiveness, and metastatic spread in Ewing sarcoma. Cancer Res. 2013;73:967–77. doi: 10.1158/0008-5472.CAN-12-1492. [DOI] [PubMed] [Google Scholar]
- 55.Matsui A, Yamaguchi T, Maekawa S, Miyazaki C, Takano S, Uetake T, Inoue T, Otaka M, Otsuka H, Sato T, Yamashita A, Takahashi Y, Enomoto N. DICKKOPF-4 and -2 genes are upregulated in human colorectal cancer. Cancer Sci. 2009;100:1923–30. doi: 10.1111/j.1349-7006.2009.01272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohammadpour H, Pourfathollah AA, Nikougoftar Zarif M, Khalili S. Key role of Dkk3 protein in inhibition of cancer cell proliferation: an in silico identification. J Theor Biol. 2016;393:98–104. doi: 10.1016/j.jtbi.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors (Chur, Switzerland) 2010;28:232–42. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huo J, Zhang Y, Li R, Wang Y, Wu J, Zhang D. Upregulated MicroRNA-25 mediates the migration of melanoma cells by targeting DKK3 through the WNT/beta-catenin pathway. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorsy E, Topuz AS, Geisler C, Stahl S, Garczyk S, von Stillfried S, Hoss M, Gluz O, Hartmann A, Knüchel R, Dahl E. Loss of Dickkopf 3 promotes the tumorigenesis of basal breast cancer. PloS One. 2016;11:e0160077. doi: 10.1371/journal.pone.0160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park JM, Kim MK, Chi KC, Kim JH, Lee SH, Lee EJ. Aberrant loss of dickkopf-3 in gastric cancer: can it predict lymph node metastasis preoperatively? World J Surg. 2015;39:1018–25. doi: 10.1007/s00268-014-2886-3. [DOI] [PubMed] [Google Scholar]
- 61.Xu XY, Xia P, Yu M, Nie XC, Yang X, Xing YN, Liu YP, Takano Y, Zheng HC. The roles of REIC gene and its encoding product in gastric carcinoma. Cell Cycle (Georgetown, Tex.) 2012;11:1414–31. doi: 10.4161/cc.19823. [DOI] [PubMed] [Google Scholar]
- 62.Guo CC, Zhang XL, Yang B, Geng J, Peng B, Zheng JH. Decreased expression of Dkk1 and Dkk3 in human clear cell renal cell carcinoma. Mol Med Rep. 2014;9:2367–73. doi: 10.3892/mmr.2014.2077. [DOI] [PubMed] [Google Scholar]
- 63.Uchida D, Shiraha H, Kato H, Nagahara T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K, Nasu Y, Yagi T, Kumon H, Yamamoto K. Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer. J Gastroenterol Hepatol. 2014;29:973–83. doi: 10.1111/jgh.12501. [DOI] [PubMed] [Google Scholar]
- 64.Pei Y, Yao Q, Yuan S, Xie B, Liu Y, Ye C, Zhuo H. GATA4 promotes hepatoblastoma cell proliferation by altering expression of miR125b and DKK3. Oncotarget. 2016;7:77890–77901. doi: 10.18632/oncotarget.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Lin L, Thomas DG, Nadal E, Chang AC, Beer DG, Lin J. The role of Dickkopf-3 overexpression in esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2015;150:377–385. e2. doi: 10.1016/j.jtcvs.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ouyang Y, Pan J, Tai Q, Ju J, Wang H. Transcriptomic changes associated with DKK4 overexpression in pancreatic cancer cells detected by RNA-Seq. Tumour Biol. 2016;37:10827–38. doi: 10.1007/s13277-015-4379-x. [DOI] [PubMed] [Google Scholar]
- 67.Zhai W, Hu GH, Zheng JH, Peng B, Liu M, Huang JH, Wang GC, Yao XD, Xu YF. High expression of the secreted protein dickkopf homolog 4: roles in invasion and metastasis of renal cell carcinoma and its association with Von Hippel-Lindau gene. Int J Mol Med. 2014;33:1319–26. doi: 10.3892/ijmm.2014.1673. [DOI] [PubMed] [Google Scholar]
- 68.Ebert MP, Tänzer M, Balluff B, Burgermeister E, Kretzschmar AK, Hughes DJ, Tetzner R, Lofton-Day C, Rosenberg R, Reinacher-Schick AC, Schulmann K, Tannapfel A, Hofheinz R, Röcken C, Keller G, Langer R, Specht K, Porschen R, Stöhlmacher-Williams J, Schuster T, Ströbel P, Schmid RM. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:44–53. doi: 10.1056/NEJMoa1009473. [DOI] [PubMed] [Google Scholar]
- 69.Chouhan S, Singh S, Athavale D, Ramteke P, Pandey V, Joseph J, Mohan R, Shetty PK, Bhat MK. Glucose induced activation of canonical Wnt signaling pathway in hepatocellular carcinoma is regulated by DKK4. Sci Rep. 2016;6:27558. doi: 10.1038/srep27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Zhou XL, Qin XR, Zhang XD, Ye LH. Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling. Acta Pharmacol Sin. 2010;31:202–10. doi: 10.1038/aps.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H, Eguchi H, Shintani Y, Endo H, Inoue M, Doki Y, Okumura M, Morii E, Kikuchi A. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest. 2016;126:2689–705. doi: 10.1172/JCI84658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baehs S, Herbst A, Thieme SE, Perschl C, Behrens A, Scheel S, Jung A, Brabletz T, Göke B, Blum H, Kolligs FT. Dickkopf-4 is frequently down-regulated and inhibits growth of colorectal cancer cells. Cancer Lett. 2009;276:152–9. doi: 10.1016/j.canlet.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y, Saini S, Majid S, Deng G, Ishii N, Dahiya R. Wnt antagonist DKK1 acts as a tumor suppressor gene that induces apoptosis and inhibits proliferation in human renal cell carcinoma. Int J Cancer. 2011;128:1793–803. doi: 10.1002/ijc.25507. [DOI] [PubMed] [Google Scholar]
- 75.Ueno K, Hirata H, Majid S, Chen Y, Zaman MS, Tabatabai ZL, Hinoda Y, Dahiya R. Wnt antagonist DICKKOPF-3 (Dkk-3) induces apoptosis in human renal cell carcinoma. Mol Carcinog. 2011;50:449–57. doi: 10.1002/mc.20729. [DOI] [PubMed] [Google Scholar]
- 76.Takata A, Terauchi M, Hiramitsu S, Uno M, Wakana K, Kubota T. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int J Gynecol Cancer. 2015;25:372–9. doi: 10.1097/IGC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 77.Jung IL, Kang HJ, Kim KC, Kim IG. Knockdown of the Dickkopf 3 gene induces apoptosis in a lung adenocarcinoma. Int J Mol Med. 2010;26:33–8. doi: 10.3892/ijmm_00000431. [DOI] [PubMed] [Google Scholar]
- 78.Wang Z, Ma LJ, Kang Y, Li X, Zhang XJ. Dickkopf-3 (Dkk3) induces apoptosis in cisplatinresistant lung adenocarcinoma cells via the Wnt/beta-catenin pathway. Oncol Rep. 2015;33:1097–106. doi: 10.3892/or.2014.3704. [DOI] [PubMed] [Google Scholar]
- 79.Hiramitsu S, Terauchi M, Kubota T. The effects of Dickkopf-4 on the proliferation, differentiation, and apoptosis of osteoblasts. Endocrinology. 2013;154:4618–26. doi: 10.1210/en.2013-1387. [DOI] [PubMed] [Google Scholar]
- 80.Williamson SC, Metcalf RL, Trapani F, Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N, Polanski R, Nonaka D, Priest L, Fusi A, Carlsson F, Carlsson A, Hendrix MJ, Seftor RE, Seftor EA, Rothwell DG, Hughes A, Hicks J, Miller C, Kuhn P, Brady G, Simpson KL, Blackhall FH, Dive C. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Min JK, Park H, Choi HJ, Kim Y, Pyun BJ, Agrawal V, Song BW, Jeon J, Maeng YS, Rho SS, Shim S, Chai JH, Koo BK, Hong HJ, Yun CO, Choi C, Kim YM, Hwang KC, Kwon YG. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882–93. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park H, Jung HY, Choi HJ, Kim DY, Yoo JY, Yun CO, Min JK, Kim YM, Kwon YG. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17:221–34. doi: 10.1007/s10456-013-9390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao L, Zhang D, Zhao X, Sun B, Liu Y, Gu Q, Zhang Y, Zhao X, Che N, Zheng Y, Liu F, Wang Y, Meng J. Dickkopf-1-promoted vasculogenic mimicry in non-small cell lung cancer is associated with EMT and development of a cancer stem-like cell phenotype. J Cell Mol Med. 2016;20:1673–85. doi: 10.1111/jcmm.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L, Li M, Li Q, Wang CJ, Xie SQ. DKK1 promotes hepatocellular carcinoma cell migration and invasion through beta-catenin/MMP7 signaling pathway. Mol Cancer. 2013;12:157. doi: 10.1186/1476-4598-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou J, Jiang J, Wang S, Xia X. DKK1 inhibits proliferation and migration in human retinal pigment epithelial cells via the Wnt/betacatenin signaling pathway. Exp Ther Med. 2016;12:859–863. doi: 10.3892/etm.2016.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia X, Li N, Peng C, Deng Y, Wang J, Deng M, Lu M, Yin J, Zheng G, Liu H, He Z. miR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer cells. Oncotarget. 2016;7:7044–54. doi: 10.18632/oncotarget.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chi HC, Liao CH, Huang YH, Wu SM, Tsai CY, Liao CJ, Tseng YH, Lin YH, Chen CY, Chung IH, Wu TI, Chen WJ, Lin KH. Thyroid hormone receptor inhibits hepatoma cell migration through transcriptional activation of Dickkopf 4. Biochem Biophys Res Commun. 2013;439:60–5. doi: 10.1016/j.bbrc.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 88.Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, González-Sancho JM. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–77. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 89.Yang H, Chen GD, Fang F, Liu Z, Lau SH, Zhang JF, Lau WY, Yang LY. Dickkopf-1: as a diagnostic and prognostic serum marker for early hepatocellular carcinoma. Int J Biol Markers. 2013;28:286–97. doi: 10.5301/jbm.5000015. [DOI] [PubMed] [Google Scholar]
- 90.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, Qiu SJ, Shi YH, Yu B, Tang N, Chu W, Wang M, Wu J, Zhang Z, Yang S, Gu J, Wang H, Qin W. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–26. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 91.Gomceli I, Bostanci EB, Ozer I, Kemik AS, Turhan N, Tez M, Kilic S, Demiriz B, Akoglu M. A novel screening biomarker in gastric cancer: serum Dickkopf-1. Hepatogastroenterology. 2012;59:1661–4. doi: 10.5754/hge11516. [DOI] [PubMed] [Google Scholar]
- 92.Jiang T, Huang L, Wang S, Zhang S. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J Obstet Gynaecol Res. 2010;36:769–73. doi: 10.1111/j.1447-0756.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 93.Shimazu Y, Kurozumi K, Ichikawa T, Fujii K, Onishi M, Ishida J, Oka T, Watanabe M, Nasu Y, Kumon H, Date I. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Ther. 2015;22:146–54. doi: 10.1038/gt.2014.100. [DOI] [PubMed] [Google Scholar]
- 94.Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–22. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 95.Meier K, Recillas-Targa F. New insights on the role of DNA methylation from a global view. Front Biosci (Landmark edition) 2017;22:644–668. doi: 10.2741/4508. [DOI] [PubMed] [Google Scholar]
- 96.Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N, Miyamoto C, Akutsu N, Yamaoka S, Itoh F. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol. 2008;14:2702–14. doi: 10.3748/wjg.14.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Izaurralde E. GENE REGULATION. Breakers and blockers-miRNAs at work. Science (New York, N.Y.) 2015;349:380–2. doi: 10.1126/science.1260969. [DOI] [PubMed] [Google Scholar]
- 98.Fan D, Ren B, Yang X, Liu J, Zhang Z. Upregulation of miR-501-5p activates the wnt/betacatenin signaling pathway and enhances stem cell-like phenotype in gastric cancer. J Exp Clin Cancer Res. 2016;35:177. doi: 10.1186/s13046-016-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Yu C, Chen M, Li Z, Tian S, Jiang J, Sun C. miR-522 contributes to cell proliferation of hepatocellular carcinoma by targeting DKK1 and SFRP2. Tumour Biol. 2016;37:11321–9. doi: 10.1007/s13277-016-4995-0. [DOI] [PubMed] [Google Scholar]
- 100.Bahl C, Singh N, Behera D, Sharma S. Association of polymorphisms in Dickopff (DKK) gene towards modulating risk for lung cancer in north Indians. Future Oncol (London, England) 2017;13:213–232. doi: 10.2217/fon-2016-0117. [DOI] [PubMed] [Google Scholar]
- 101.Peng H, Li Y, Liu Y, Zhang J, Chen K, Huang A, Tang H. HBx and SP1 upregulate DKK1 expression. Acta Biochim Pol. 2017;64:35–39. doi: 10.18388/abp.2016_1250. [DOI] [PubMed] [Google Scholar]
- 102.Pendás-Franco N, Aguilera O, Pereira F, González-Sancho JM, Muñoz A. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008;28:2613–23. [PubMed] [Google Scholar]


