Abstract
Ovarian cancer accounts for the highest mortality among all gynecologic cancers. Cytoreductive surgery followed by chemotherapy with a platinum-based agent (cisplatin or carboplatin) plus paclitaxel is the first-line option for treatment of epithelial ovarian cancer. However, primary or acquired resistance to platinum-based agents is a major clinical challenge. MicroRNAs are a group of small non-coding RNAs that regulate gene expression post-transcriptionally and may function as oncogenes or tumor-suppressor genes through extensive crosstalk with intracellular signaling pathways. Importantly, their dysregulation has been implicated in ovarian tumorigenesis. Pertinent to chemotherapy, increasing evidence has revealed that miRNAs can be directly linked to chemosensitivity to platinum-based agents in ovarian cancer. In this review, we summarize current evidence concerning the role of miRNAs in prediction and modulation of cellular responses to cisplatin and carboplatin in ovarian cancer.
Keywords: Cisplatin, carboplatin, chemoresistance, microRNA, ovarian cancer, melanoma
Introduction
Ovarian cancer accounts for approximately 3% of all cancers in the Western world and has the highest mortality rate of all gynecologic cancers with the 5-year survival rate of approximately 30% [1-3]. Platinum-based chemotherapeutics, including cisplatin and carboplatin, are used in the treatment of a variety of cancers, including ovarian, bladder, lung, head and neck, and gastirc cancers [4-9]. Platinum compounds mediate their cytotoxic effects through binding to DNA molecules, thereby interfering with DNA repair mechanisms in cancer cells [10-12]. Standard treatment for epithelial ovarian cancer is primarily cytoreductive surgery followed by treatment with platinum-containing compound (i.e. cisplatin or carboplatin) in combination with paclitaxel [13-15]. However, primary or acquired drug resistance is a major challenge and decreases the treatment efficiency. Patients with platinum resistance experienced progression during chemotherapy or recurrence within six months of completed chemotherapy [16-18]. Nonetheless, the exact underlying mechanisms of chemoresistance to platinum agents are still unknown. Recently, growing number of studies have investigated the mechanisms underlying chemoresistance in ovarian cancer [4,19-21]. Understanding the molecular basis of chemoresistance is crucial to improving the effectiveness of ovarian cancer treatment.
MicroRNAs (miRNAs) are small (19-25 nucleotides in length), non-coding and evolutionarily conserved RNAs that regulate gene expression post-transcriptionally through induction of translation blockage or mRNA degradation via binding to their target mRNAs [22-25]. miRNAs play important roles in various biological and pathological processes, including development, immune response, inflammation and tumorigenesis [26-29]. Most importantly, aberrant expression of miRNAs has been documented in virtually all types of malignancies, including ovarian cancer [30-33]. Differentially expressed miRNAs exert their oncogenic or tumour-suppressive effects through regulating many cancer-pertinent cellular processes, such as proliferation, migration, apoptosis, stemness and chemoresistance [34-36]. miRNAs may also be used as biomarkers for predicting chemoresponsiveness to maximize therapeutic effect and minimize treatment toxicity [37-39].
In this review, we summarize the literature concerning the role of miRNAs in predicting and modifying response to platinum-based chemotherapy in ovarian cancer. We also discuss the associated molecular targets and intracellular pathways involved in these processes.
Cisplatin
Cisplatin is a major landmark in the history of successful anticancer drugs since its introduction to clinical trials in 1971 [40,41]. It is widely used in treatment of solid tumors, such as ovarian, lung, head and neck, and breast tumors [42-45]. Cisplatin is the backbone drug used in combination with other chemotherapeutic agents in the management of ovarian cancer in the clinical setting [46-48]. It is noteworthy that patients resistant to cisplatin very often exhibit cross-resistance to carboplatin [47,49].
Drug effects on miRNA expression
Boren et al. identified a total of 7 miRNAs that were significantly associated with OVCA cell responsiveness to cisplatin [50]. These miRNAs included miR-23b, miR-381, miR-340, miR-520, miR-331, miR-185 and miR-106a. They also investigated the molecular mechanisms underlying chemoresistance in which miRNA expression levels were measured on paired mother/daughter and cisplatin-sensitive/resistant ovarian cancer cell lines. Three of 7 differentially expressed miRNAs (i.e. miR-340, miR-381, and miR-520f) in inherent cisplatin-resistant cell lines also showed significantly differential expression in paired sensitive/resistant and mother/daughter cell lines. These results indicate common molecular mechanisms in inherent and acquired cisplatin resistance.
Another study compared the differentially expressed miRNAs in platinum-sensitive and -resistant ovarian tumor cell lines using miRNA array [51]. A total of 4 downregulated miRNAs and 13 upregulated miRNAs were identified. Among them, miR-141-3p was the most deregulated miRNA. Li et al. also screened for differentially expressed miRNAs in the cisplatin-resistant human ovarian cancer cell line A2780/DDP using microarray [52]. A total of 32 miRNAs were found to be differentially expressed in A2780/DDP cells compared with its parental A2780 cells. Four abnormally expressed miRNAs (i.e. miR-146a, miR-130a, miR-374a and miR-182) were further verified by quantitative reverse transcription-PCR. miR-146a was upregulated whereas miR-130a, miR-374a, and miR-182 were downregulated in A2780/DDP cells when compared with A2780 cells.
Yang et al. found that a total of 89 miRNAs differentially expressed in cisplatin-resistant cell line SKOV3/CIS as compared with SKOV3 cells. [53]. Among them, 35 miRNAs were downregulated and 54 miRNAs were upregulated. These results suggested that differential miRNA expression might contribute to acquisition of cisplatin resistance in ovarian cancer. In particular, the expression of miR-130a was increased in cisplatin-resistant SKOV3/CIS cells as compared with the parental SKOV3 cells. PTEN was found to be the potential target of miR-130a.
Jaarsveld et al. showed that 27 miRNAs were differentially expressed in an isogenic cisplatin-sensitive cell line as compared with a cisplatin-resistant ovarian cancer cell line using miRNA expression profiles [54]. Five of these deregulated miRNAs, including the family members of miR-141/200c, were correlated with cisplatin sensitivity (Table 1).
Table 1.
Drug effects on miRNA expression
| Num | Method | Drug | Deregulated | Upregulated | Downregulated | Reference |
|---|---|---|---|---|---|---|
| 1 | qRT-PCR | Cisplatin | miR-23b, miR-381, miR-340, miR-520, miR-331, miR-185, miR-106a | [50] | ||
| 2 | Microarray qRT-PCR | Cisplatin | miR-141-3p | 13 miRNAs | 4 miRNAs | [51] |
| 3 | Microarray qRT-PCR | Cisplatin | 32 miRNAs | miR-146a | miR-130a, miR-374a, miR-182 | [52] |
| 4 | Microarray qRT-PCR | Cisplatin | 54 miRNAs, miR-130a | 35 miRNAs | [53] | |
| 5 | Microarray qRT-PCR | Cisplatin | 27 miRNAs | miR-141, miR-200c, miR-215, miR-421 | miR-493-5p | [54] |
| 6 | qRT-PCR | Cisplatin | miR-21 | [57] | ||
| 7 | Microarray qRT-PCR | carboplatin | miR-21, miR-214 | [81] |
Modulation of chemosensitivity
miR-21 is a well-known oncogene promoting cell proliferation, migration and invasion in various types of tumor [8,55,56]. Importantly, miR-21 overexpression is associated with drug resistance. miR-21 expression was higher in cisplatin-resistant than -sensitive ovarian cancer cells [57]. Enforced expression of miR-21 promoted cell proliferation in cisplatin-sensitive cells. Furthermore, downregulating miR-21 significantly reduced cell proliferation and invasion in cisplatin-resistant ovarian cancer cells. In addition, the tumor suppressor gene programmed cell death 4 (PDCD4) was identified as a target of miR-21 and c-Jun N-terminal kinase (JNK)-1/c-Jun/miR-21 pathway was involved in miR-21-mediated regulation of cisplatin resistance. Generally, the passenger strand, or the non-incorporated strand, is considered non-functional. However, miR-21-3p, the passenger strand of miR-21, was also increased in cisplatin-resistant ovarian cancer cells [58]. Moreover, miR-21-3p conferred cisplatin resistance to many ovarian cell lines while miR-21-5p increased cisplatin sensitivity. NAV3 was identified to be a potential target of miR-21-3p. In summary, miR-21-3p could induce cisplatin resistance in ovarian cancer through targeting the NAV3 gene.
miR-103/107 overexpression sensitized ovarian cancer cells to cisplatin and reduced the percentage of RAD51 foci-positive cells in response to chemotherapy [59]. Expression of miR-130a was increased in cisplatin-resistant cell lines [60], in which inhibition of miR-130a could overcome the cisplatin resistance and inhibit MDR1 mRNA and P-glycoprotein (P-gp) expression [53]. miR-130a played a role in both MDR1/P-gp- and phosphoinositide 3-kinase (PI3K)/Akt/PTEN/mammalian target of rapamycin (mTOR)-mediated drug-resistance pathways in SKOV3/CIS cells, indicating a key role of miR-130a in the modulation of platinum-based chemotherapy [53]. miR-130b decreased sensitivity to cisplatin in ovarian cancer line compared with mock-transfected and negative control cancer cells [61]. In addition, the expression of MDR1, GST-π, P-gp and GST-π were decreased following miR-130b transfection. Expression levels of miR-141 were higher in non-serous ovarian tumors resistant to therapy and KEAP1 was identified to be its direct target [54]. Overexpression of KEAP1 increased cisplatin sensitivity through regulating the nuclear factor (NF)-kB pathway. These findings suggested that miR-141-mediated modulation of KEAP1 played a significant role in the response of ovarian cancer to cisplatin [54]. Let-7i was downregulated in chemotherapy-resistant ovarian cancer in which suppressing let-7i expression enhanced the resistance to cisplatin [62].
miR-130a and miR-374a overexpression decreased the cisplatin sensitivity of A2780 cells while their inhibitors re-sensitized A2780/DDP cells [52]. Ziliak et al. found that miR-193b increased cisplatin resistance in 7 ovarian cancer cell lines [63]. Akt is a crucial cell survival pathway and its activation plays a key role in cisplatin resistance. miR-214 was elevated in ovarian cancer and enhanced ovarian cancer cell survival and cisplatin resistance through targeting PTEN to activate the PTEN/Akt pathway. Moreover, inhibition of miR-214 expression sensitized ovarian cancer cells to cisplatin-induced apoptosis. miR-214 serves as an anti-apoptotic factor to mediate cisplatin resistance [64].
miR-506 increased ovarian cancer cells sensitivity to DNA damage through directly targeting DNA damage repair gene RAD51. Systemic delivery of miR-506 in nude mice significantly increased the cisplatin response [65]. Lysophosphatidic acid, epidermal growth factor and platelet-derived growth factor enhanced cell proliferation and increased miR-30c-2 expression in ovarian cancer. Ovarian cancer cells transfected with miR-30c-2 nearly eliminated cisplatin-induced cytotoxicity [66].
Dicer belongs to the RNase III family and controls maturation of miRNAs. Dicer downregulation results in a global decrease in miRNA expression and plays a significant role in cellular transformation and tumorigenesis. Kuang et al. demonstrated that Dicer downregulation increased cell proliferation, migration and cell cycle progression in ovarian cancer cells [67]. In addition, Dicer expression was lower in cisplatin-resistant ovarian cancer cells than parental cells. Knockdown of Dicer inhibited the sensitivity of ovarian cancer cells to cisplatin. These findings suggest that Dicer is involved in cisplatin resistance in ovarian cancer. DGCR8 binds to Drosha, an RNase III enzyme, to form the Microprocessor complex that cleaves a primary transcript of miRNA. The expression of DGCR8 was higher in ovarian cancer. Knockdown of DGCR8 sensitized ovarian cancer cells to cisplatin-induced apoptosis. In addition, deregulation of miRNA expression was observed in DGCR8-knockdown ovarian cancer cells, where miR-27b was the most highly downregulated miRNAs [68].
Aside from host miRNAs, viral miRNA also influenced cisplatin resistance. miR-BART7, a herpetic viral miRNAs from Epstein-Barr virus, induced cisplatin-resistance directly [69] (Figure 1, Table 2).
Figure 1.
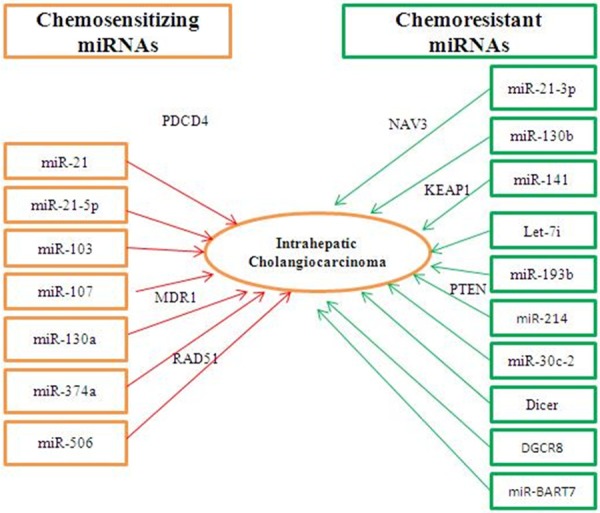
Modulation of cisplatin chemosensitivity by chemosensitizing and chemoresistant miRNAs in ovarian cancer.
Table 2.
Modulation of chemosensitivity
| miRNAs | Drug | Effect | Target gene | References |
|---|---|---|---|---|
| miR-21 | Cisplatin | Sensitivity | PDCD4 | [57] |
| miR-21-3p | Cisplatin | Resistance | NAV3 | [58] |
| miR-21-5p | Cisplatin | Sensitivity | [58] | |
| miR-103/107 | Cisplatin | Sensitivity | [59] | |
| miR-130a | Cisplatin | Sensitivity | MDR1 | [60] |
| miR-130b | Cisplatin | Resistance | [61] | |
| miR-141 | Cisplatin | Resistance | KEAP1 | [54] |
| Let-7i | Cisplatin | Resistance | [62] | |
| miR-130a | Cisplatin | Sensitivity | [52] | |
| miR-374a | Cisplatin | Sensitivity | [52] | |
| miR-193b | Cisplatin | Resistance | [63] | |
| miR-214 | Cisplatin | Resistance | PTEN | [64] |
| miR-506 | Cisplatin | Sensitivity | RAD51 | [65] |
| miR-30c-2 | Cisplatin | Resistance | [66] | |
| Dicer | Cisplatin | Resistance | [67] | |
| DGCR8 | Cisplatin | Resistance | [68] | |
| miR-BART7 | Cisplatin | Resistance | [69] | |
| miR-200c | Paclitaxel carboplatin | Sensitivity | [82-84] | |
| miR-141 | Paclitaxel carboplatin | Sensitivity | [82-84] | |
| miR-193b | Paclitaxel carboplatin | Resistance | CRIM1 | [84] |
| miR-200 | Paclitaxel carboplatin | Sensitivity | [82] | |
| chrXq27.3 miRNAs | Paclitaxel carboplatin | Sensitivity | [85] |
Response prediction
Expression levels of miR-141 were higher in patients with non-serous ovarian tumors that are resistant to platinum-based chemotherapy (platinum-free interval < 6 months) [54]. Elevated miR-506 expression was associated with better response to platinum chemotherapy, longer progression-free and overall survival in epithelial ovarian cancer patients [65]. Reduced let-7i expression was associated with the shorter progression-free survival in ovarian cancer [62]. Therefore, miR-141, miR-506 and let-7i might be used to predict chemotherapy response in patients with ovarian cancer.
Gu et al. showed that deregulation of miRNAs played a role in the favorable prognosis of patients with wild-type BRCA1/2 [70]. Ovarian cancer patients with alterations of BRCA1/2 have a better prognosis than non-carriers. Three miRNAs (i.e. miR-146a, miR-148a and miR-545) that target BRCA1/2 were associated with better survival outcomes in patients with wild-type BRCA1/2 treated with platinum-based chemotherapy. Therefore, patients who could benefit from platinum-based chemotherapy could be predicted from BRCA1/2-directed miRNA signature (Table 3).
Table 3.
Response prediction
| miRNAs | Target | Drug | Effect | Prediction | References |
|---|---|---|---|---|---|
| miR-141 | Platinum | Resistant | Shorter | [54] | |
| miR-506 | Platinum | Sensitivity | Longer | [65] | |
| let-7i | Platinum | Sensitivity | Longer | [62] | |
| miR-146a | BRCA1/2 | Platinum | Sensitivity | Longer | [70] |
| miR-148a | BRCA1/2 | Platinum | Sensitivity | Longer | [70] |
| miR-545 | BRCA1/2 | Platinum | Sensitivity | Longer | [70] |
Carboplatin
Since its introduction to clinical usage in 1992, carboplatin has become a commonly preferred agent over cisplatin because of its distinct toxicity profile. The advantage of carboplatin is its simple pharmacokinetics and a predictable toxicity profile [71-73]. The comparative therapeutic efficacy of cisplatin and carboplatin remains controversial [74,75]. However, carboplatin is one of the most effective chemotherapeutic drugs for the treatment of ovarian cancer [76,77]. Recently, it was proved that carboplatin plus paclitaxel is not inferior, when compared with cisplatin plus paclitaxel in patients with advanced ovarian cancer [78]. Carboplatin plus paclitaxel has less toxicity and is easier to administer [79,80].
Drug effects on miRNA expression
miRNA profiling was evaluated among ovarian cancer cells in ascites and matched omental metastasis in patients with epithelial ovarian cancer. After being treated with carboplatin, malignant ovarian cancer cells in ascites demonstrated higher cell viability compared to omental metastasis. In addition, the expression levels of miR-21 and miR-214 were significantly higher in malignant cells of ascites [81]. This finding implicated that miR-21 and miR-214 might contribute to intrinsic carboplatin resistance in ovarian cancer (Table 1).
Modulation of chemosensitivity
Inhibition of miR-200c or miR-141 conferred ovarian cancer cells with resistance to paclitaxel and carboplatin [82]. The miR-200 family plays crucial roles in modulating chemosensitivity to carboplatin and paclitaxel in ovarian cancer. Prislei et al. demonstrated that expression of miR-200c was higher in the cisplatin-sensitive isogenic cells [83]. In addition, enforced expression of miR-200c increased cisplatin activity in ovarian cancer cells. A decrease of the total colony area was also observed in the miR-200c-overexpressing cells treated with cisplatin. Cittelly et al. showed that restoration of miR-200c in ovarian cancer cells, alone or in combination with paclitaxel, significantly decreased tumor burden in established tumors [84]. miR-193b, which is located on the opposite arm of miR-193b, contributed to resistance to both carboplatin and cisplatin in ovarian cancer cell lines through decreasing CRIM1 expression (Table 2).
Response prediction
Leskela et al. found that miR-200 was correlated with treatment response to the paclitaxel-carboplatin regimen [82]. Patients with higher miR-200c levels demonstrated lower relapse/progression rates. Bagnoli et al. demonstrated that low expression of chrXq27.3 miRNAs was associated with early relapse in ovarian cancer patients [85] (Figure 1, Table 2).
Concluding remarks and future perspectives
miRNAs are a class of small, non-coding RNA which regulate gene expression at post-transcriptional levels. Recent studies have demonstrated that miRNAs could be used to predict clinical outcomes of chemotherapy in various cancers. More importantly, miRNAs can modulate efficacy of chemotherapy. In ovarian cancer, cancer cells resistant to platinum-based agents often showed altered miRNA expression profiles. Furthermore, many of these deregulated miRNAs were found to modulate cellular sensitivity to cisplatin and carboplatin. It is therefore hopeful that targeted delivery of chemosensitizing miRNAs might help to maximize therapeutic effect of platinum-based agents, thereby improving clinical outcomes in patients with metastatic ovarian cancer. However, the mechanisms underlying miRNA regulation of chemosensitivity in ovarian cancer remain largely uninvestigated. In addition, studies in mouse xenograft models are limited. More importantly, evidence on safety and efficacy of miRNA-based treatment are lacking in humans. Further investigations with systematic identification and functional characterization of miRNAs are thus required. Future studies should also reveal the targets and signaling pathways in the regulation of chemosensitivity.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant Numbers: 81401847).
Disclosure of conflict of interest
None.
References
- 1.Creighton CJ, Hernandez-Herrera A, Jacobsen A, Levine DA, Mankoo P, Schultz N, Du Y, Zhang Y, Larsson E, Sheridan R, Xiao W, Spellman PT, Getz G, Wheeler DA, Perou CM, Gibbs RA, Sander C, Hayes DN, Gunaratne PH. Integrated analyses of microRNAs demonstrate their widespread influence on gene expression in high-grade serous ovarian carcinoma. PLoS One. 2012;7:e34546. doi: 10.1371/journal.pone.0034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Liu J, Segura MF, Shao C, Lee P, Gong Y, Hernando E, Wei JJ. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012;228:204–215. doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 3.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Bi T, Qu Z, Jiang J, Cui S, Wang Y. Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol Rep. 2014;32:1003–1012. doi: 10.3892/or.2014.3311. [DOI] [PubMed] [Google Scholar]
- 5.Vinall RL, Ripoll AZ, Wang S, Pan CX, deVere White RW. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer. 2012;130:2526–2538. doi: 10.1002/ijc.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drayton RM, Dudziec E, Peter S, Bertz S, Hartmann A, Bryant HE, Catto JW. Reduced Expression of miRNA-27a Modulates Cisplatin Resistance in Bladder Cancer by Targeting the Cystine/Glutamate Exchanger SLC7A11. Clin Cancer Res. 2014;20:1990–2000. doi: 10.1158/1078-0432.CCR-13-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Li X, Cheng S, Wei W, Li Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol Med Rep. 2015;11:625–632. doi: 10.3892/mmr.2014.2688. [DOI] [PubMed] [Google Scholar]
- 8.Ren W, Wang X, Gao L, Li S, Yan X, Zhang J, Huang C, Zhang Y, Zhi K. MiR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol Cell Biochem. 2014;390:253–262. doi: 10.1007/s11010-014-1976-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl) 2014;127:2357–2362. [PubMed] [Google Scholar]
- 10.Montopoli M, Ragazzi E, Froldi G, Caparrotta L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009;42:195–206. doi: 10.1111/j.1365-2184.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, Zheng X, Xu X, Zhou Q, Yan H, Zhang X, Lu B, Wu C, Ju J. Prognostic significance of miR-181b and miR-21 in gastric cancer patients treated with S-1/Oxaliplatin or Doxifluridine/Oxaliplatin. PLoS One. 2011;6:e23271. doi: 10.1371/journal.pone.0023271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava AK, Han CH, Zhao R, Cui TT, Dai YT, Mao C, Zhao WQ, Zhang XL, Yu JH, Wang QE. Enhanced expression of DNA polymerase eta contributes to cisplatin resistance of ovarian cancer stem cells. Proc Natl Acad Sci U S A. 2015;112:4411–4416. doi: 10.1073/pnas.1421365112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita Y. Ovarian cancer: new developments in clear cell carcinoma and hopes for targeted therapy. Jpn J Clin Oncol. 2015;45:405–407. doi: 10.1093/jjco/hyu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung SW, Marrach S, Cummins S, Bhutia YD, Mody H, Hooks SB, Dhar S, Govindarajan R. Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Lett. 2015;359:233–240. doi: 10.1016/j.canlet.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang-Hartwich Y, Soteras MG, Lin ZP, Holmberg J, Sumi N, Craveiro V, Liang M, Romanoff E, Bingham J, Garofalo F, Alvero A, Mor G. p53 protein aggregation promotes platinum resistance in ovarian cancer. Oncogene. 2015;34:3605–3616. doi: 10.1038/onc.2014.296. [DOI] [PubMed] [Google Scholar]
- 16.Fu AQ, Yu Z, Song YB, Zhang EN. Silencing of glutaminase 1 resensitizes Taxol-resistant breast cancer cells to Taxol. Mol Med Rep. 2015;11:4727–4733. doi: 10.3892/mmr.2015.3261. [DOI] [PubMed] [Google Scholar]
- 17.McNeil EM, Astell KR, Ritchie AM, Shave S, Houston DR, Bakrania P, Jones HM, Khurana P, Wallace C, Chapman T, Wear MA, Walkinshaw MD, Saxty B, Melton DW. Inhibition of the ERCC1-XPF structure-specific endonuclease to overcome cancer chemoresistance. DNA Repair. 2015;31:19–28. doi: 10.1016/j.dnarep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Ryner L, Guan YH, Firestein R, Xiao YY, Choi YJ, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, Fu L, Amler LC, Bais C, Wang YL. Upregulation of Periostin and Reactive Stroma Is Associated with Primary Chemoresistance and Predicts Clinical Outcomes in Epithelial Ovarian Cancer. Clinical Cancer Research. 2015;21:2941–2951. doi: 10.1158/1078-0432.CCR-14-3111. [DOI] [PubMed] [Google Scholar]
- 19.Dai F, Zhang Y, Chen Y. Involvement of miR-29b signaling in the sensitivity to chemotherapy in patients with ovarian carcinoma. Hum Pathol. 2014;45:1285–1293. doi: 10.1016/j.humpath.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Liu N, Zhou C, Zhao J, Chen Y. Reversal of paclitaxel resistance in epithelial ovarian carcinoma cells by a MUC1 aptamer-let-7i chimera. Cancer Invest. 2012;30:577–582. doi: 10.3109/07357907.2012.707265. [DOI] [PubMed] [Google Scholar]
- 21.Wang YL, Ryner L, Guan YH, Firestein R, Xiao YY, Choi YJ, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, Fu L, Amler LC, Bais C. Upregulation of periostin and reactive stroma is associated with primary chemoresistance and predicts clinical outcomes in epithelial ovarian cancer. J. Clin. Oncol. 2015:33. doi: 10.1158/1078-0432.CCR-14-3111. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–13924. doi: 10.18632/oncotarget.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Li Z. The role of MicroRNAs expression in laryngeal cancer. Oncotarget. 2015;6:23297–305. doi: 10.18632/oncotarget.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6:4562–8. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Finniss S, Cazacu S, Bucris E, Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, Mikkelsen T, Slavin S, Brodie C. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu LL, Yang Y, Xu HL, Cheng Y, Wen X, Ouyang L, Bao JK, Wei YQ, Liu B. Identification of novel caspase/autophagy-related gene switch to cell fate decisions in breast cancers. Cell Prolif. 2013;46:67–75. doi: 10.1111/cpr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif. 2013;46:436–446. doi: 10.1111/cpr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Zhang SY, Gao YM, Liu YF, Liu YB, Zhao ZG, Yang K. MicroRNAs as oncogenes or tumour suppressors in oesophageal cancer: potential biomarkers and therapeutic targets. Cell Prolif. 2014;47:277–286. doi: 10.1111/cpr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei X, Chen D, Lv T, Li G, Qu S. Serum MicroRNA-125b as a Potential Biomarker for Glioma Diagnosis. Mol Neurobiol. 2016;53:163–70. doi: 10.1007/s12035-014-8993-1. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 35.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disc Degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ, Tang CH. CTGF increases matrix metalloproteinases expression and subsequently promotes tumor metastasis in human osteosarcoma through down-regulating miR-519d. Oncotarget. 2014;5:3800–3812. doi: 10.18632/oncotarget.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie T, Zhang J, Peng C, Lin Y, Chen J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget. 2014;5:7013–7026. doi: 10.18632/oncotarget.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Liao X, Lu N, Liu W, Wong CW. Chromatin-modifying drugs induce miRNA-153 expression to suppress Irs-2 in glioblastoma cell lines. Int J Cancer. 2011;129:2527–2531. doi: 10.1002/ijc.25917. [DOI] [PubMed] [Google Scholar]
- 39.Ujifuku K, Mitsutake N, Takakura S, Matsuse M, Saenko V, Suzuki K, Hayashi K, Matsuo T, Kamada K, Nagata I, Yamashita S. miR-195, miR-455-3p and miR-10a(*) are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010;296:241–248. doi: 10.1016/j.canlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Cho H, Nishiike S, Yamamoto Y, Takenaka Y, Nakahara S, Yasui T, Hanamoto A, Inohara H. Docetaxel, cisplatin, and fluorouracil for patients with inoperable recurrent or metastatic head and neck squamous cell carcinoma. Auris Nasus Larynx. 2015;42:396–400. doi: 10.1016/j.anl.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Lamarca A, Benafif S, Ross P, Bridgewater J, Valle JW. Cisplatin and gemcitabine in patients with advanced biliary tract cancer (ABC) and persistent jaundice despite optimal stenting: Effective intervention in patients with luminal disease. Eur J Cancer. 2015;51:1694–1703. doi: 10.1016/j.ejca.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen JH, Vogelius IR, Fischer BM, Friborg J, Aznar MC, Persson GF, Hakansson K, Kristensen CA, Bentzen SM, Specht L. Prognostic value of 18F-fludeoxyglucose uptake in 287 patients with head and neck squamous cell carcinoma. Head Neck. 2015;37:1274–1281. doi: 10.1002/hed.23745. [DOI] [PubMed] [Google Scholar]
- 43.Devery AM, Wadekar R, Bokobza SM, Weber AM, Jiang YY, Ryan AJ. Vascular endothelial growth factor directly stimulates tumour cell proliferation in non-small cell lung cancer. Int J Oncol. 2015;47:849–856. doi: 10.3892/ijo.2015.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habbel P, Kurreck A, Schulz CO, Regierer AC, Kaul D, Scholz CW, Neumann C, Possinger K, Eucker J. Cisplatin Plus Ifosfamide with/without Etoposide as Salvage Treatment in Heavily-pre-treated Patients with Metastatic Breast Cancer. Anticancer Res. 2015;35:5091–5095. [PubMed] [Google Scholar]
- 45.Tsutsui M, Kawakubo H, Hayashida T, Fukuda K, Nakamura R, Taicahashi T, Wada N, Saikawa Y, Omori T, Takeuchi H, Kitagawa Y. Comprehensive screening of genes resistant to an anticancer drug in esophageal squamous cell carcinoma. Int J Oncol. 2015;47:867–874. doi: 10.3892/ijo.2015.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novohradsky V, Zerzankova L, Stepankova J, Vrana O, Raveendran R, Gibson D, Kasparkova J, Brabec V. New insights into the molecular and epigenetic effects of antitumor Pt(IV)-valproic acid conjugates in human ovarian cancer cells. Biochem Pharmacol. 2015;95:133–144. doi: 10.1016/j.bcp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Chen JL, Solomides C, Parekh H, Simpkins F, Simpkins H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother Pharmacol. 2015;75:1217–1227. doi: 10.1007/s00280-015-2739-2. [DOI] [PubMed] [Google Scholar]
- 48.Maurmann L, Belkacemi L, Adams NR, Majmudar PM, Moghaddas S, Bose RN. A novel cisplatin mediated apoptosis pathway is associated with acid sphingomyelinase and FAS proapoptotic protein activation in ovarian cancer. Apoptosis. 2015;20:960–974. doi: 10.1007/s10495-015-1124-2. [DOI] [PubMed] [Google Scholar]
- 49.Paul BT, Blanchard Z, Ridgway M, ElShamy WM. BRCA1-IRIS inactivation sensitizes ovarian tumors to cisplatin. Oncogene. 2015;34:3036–3052. doi: 10.1038/onc.2014.237. [DOI] [PubMed] [Google Scholar]
- 50.Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Chan G, Kamath SG, Chen DT, Dressman H, Lancaster JM. MicroRNAs and their target messenger RNAs associated with ovarian cancer response to chemotherapy. Gynecol Oncol. 2009;113:249–255. doi: 10.1016/j.ygyno.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Ying HC, Xu HY, Lv J, Ying TS, Yang Q. MicroRNA signatures of platinum-resistance in ovarian cancer. Eur J Gynaecol Oncol. 2015;36:16–20. [PubMed] [Google Scholar]
- 52.Li N, Yang L, Wang H, Yi T, Jia X, Chen C, Xu P. MiR-130a and MiR-374a Function as Novel Regulators of Cisplatin Resistance in Human Ovarian Cancer A2780 Cells. PLoS One. 2015;10:e0128886. doi: 10.1371/journal.pone.0128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, Li N, Wang H, Jia X, Wang X, Luo J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep. 2012;28:592–600. doi: 10.3892/or.2012.1823. [DOI] [PubMed] [Google Scholar]
- 54.van Jaarsveld MT, Helleman J, Boersma AW, van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH, Berns EM, Verweij J, Pothof J, Wiemer EA. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 55.Xia B, Yang S, Liu T, Lou G. miR-211 suppresses epithelial ovarian cancer proliferation and cell-cycle progression by targeting Cyclin D1 and CDK6. Mol Cancer. 2015;14:322. doi: 10.1186/s12943-015-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 57.Echevarria-Vargas IM, Valiyeva F, Vivas-Mejia PE. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 2014;9:e97094. doi: 10.1371/journal.pone.0097094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DR. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol Oncol. 2015;137:143–151. doi: 10.1016/j.ygyno.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 59.Huang JW, Wang Y, Dhillon KK, Calses P, Villegas E, Mitchell PS, Tewari M, Kemp CJ, Taniguchi T. Systematic Screen Identifies miRNAs That Target RAD51 and RAD51D to Enhance Chemosensitivity. Mol Cancer Res. 2013;11:1564–1573. doi: 10.1158/1541-7786.MCR-13-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang LY, Wang HJ, Jia XB, Wang X, Luo J, Zhang XY. [Expression of miR-130a in cisplatin resistant cell lines of ovarian cancer] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:60–64. [PubMed] [Google Scholar]
- 61.Zong C, Wang J, Shi TM. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumour Biol. 2014;35:12151–12156. doi: 10.1007/s13277-014-2520-x. [DOI] [PubMed] [Google Scholar]
- 62.Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, Johnstone CN, Chen XM, Liu CG, Huang Q, Katsaros D, Calin GA, Weber BL, Butzow R, Croce CM, Coukos G, Zhang L. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ziliak D, Gamazon ER, Lacroix B, Kyung Im H, Wen Y, Huang RS. Genetic variation that predicts platinum sensitivity reveals the role of miR-193b* in chemotherapeutic susceptibility. Mol Cancer Ther. 2012;11:2054–2061. doi: 10.1158/1535-7163.MCT-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Yang D, Rupaimoole R, Pecot CV, Sun Y, Mangala LS, Li X, Ji P, Cogdell D, Hu L, Wang Y, Rodriguez-Aguayo C, Lopez-Berestein G, Shmulevich I, De Cecco L, Chen K, Mezzanzanica D, Xue F, Sood AK, Zhang W. Augmentation of response to chemotherapy by microRNA-506 through regulation of RAD51 in serous ovarian cancers. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/djv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia W, Eneh JO, Ratnaparkhe S, Altman MK, Murph MM. MicroRNA-30c-2* expressed in ovarian cancer cells suppresses growth factor-induced cellular proliferation and downregulates the oncogene BCL9. Mol Cancer Res. 2011;9:1732–1745. doi: 10.1158/1541-7786.MCR-11-0245. [DOI] [PubMed] [Google Scholar]
- 67.Kuang Y, Cai J, Li D, Han Q, Cao J, Wang Z. Repression of Dicer is associated with invasive phenotype and chemoresistance in ovarian cancer. Oncol Lett. 2013;5:1149–1154. doi: 10.3892/ol.2013.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Y, Tian P, Yang C, Liang Z, Li M, Sims M, Lu L, Zhang Z, Li H, Pfeffer LM, Yue J. Silencing the double-stranded RNA binding protein DGCR8 inhibits ovarian cancer cell proliferation, migration, and invasion. Pharm Res. 2015;32:769–778. doi: 10.1007/s11095-013-1219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandya D, Mariani M, McHugh M, Andreoli M, Sieber S, He S, Dowell-Martino C, Fiedler P, Scambia G, Ferlini C. Herpes virus microRNA expression and significance in serous ovarian cancer. PLoS One. 2014;9:e114750. doi: 10.1371/journal.pone.0114750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu Y, Zhang M, Peng F, Fang L, Zhang Y, Liang H, Zhou W, Ao L, Guo Z. The BRCA1/2-directed miRNA signature predicts a good prognosis in ovarian cancer patients with wildtype BRCA1/2. Oncotarget. 2015;6:2397–2406. doi: 10.18632/oncotarget.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baratti D, Kusamura S, Deraco M. Carboplatin plus paclitaxel scheduling for advanced ovarian cancer. Lancet Oncology. 2014;15:E249–E249. doi: 10.1016/S1470-2045(14)70197-4. [DOI] [PubMed] [Google Scholar]
- 72.Wei H, She Y, Lu T. Identification of novel carboplatin resistance gene in ovarian cancer. Cancer Res. 2014:74. [Google Scholar]
- 73.Liu EL, Liu Z, Zhou YX. Carboplatin-docetaxel-induced activity against ovarian cancer is dependent on up-regulated lncRNA PVT1. Int J Clin Exp Pathol. 2015;8:3803–3810. [PMC free article] [PubMed] [Google Scholar]
- 74.Mahner S, Meier W, du Bois A, Brown C, Lorusso D, Dell’Anna T, Cretin J, Havsteen H, Bessette P, Zeimet AG, Vergote I, Vasey P, Pujade-Lauraine E, Gladieff L, Ferrero A. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum-sensitive ovarian cancer patients: Results from a subset analysis of the CALYPSO phase III trial. Eur J Cancer. 2015;51:352–358. doi: 10.1016/j.ejca.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto K, Sasaki K, Horibe Y, Fukagawa F, Akizawa Y, Ishitani K, Hirai Y, Matsui H. Tolerance and Outcome of Primary Chemotherapy with Carboplatin and Ocetaxel in Elderly Ovarian Cancer Patients. Int J Gynecol Cancer. 2014;24:254–254. [Google Scholar]
- 76.Basen-Engquist K, Huang HQ, Herzog TJ, Armstrong DK, Sabbatini P, Walker JL, Kim BG, Fujiwara K, Tewari KS, O’Malley DM, Coleman RL. Randomized phase III trial of carboplatin/paclitaxel alone (CP) or in combination with bevacizumab followed by bevacizumab (CPB) and secondary cytoreduction surgery in platinum-sensitive recurrent ovarian cancer: GOG0213, an NRG Oncology/GOG Study-Analysis of patient reported outcomes (PRO) on chemotherapy randomization. J. Clin. Oncol. 2015:33. [Google Scholar]
- 77.Alcarraz C, Olivera M, Muniz J, Morante Z, Ruiz R, Valdiviezo N, Lopez LAM. Carboplatin and paclitaxel dose dense as neoadjuvant chemotherapy follow by interval cytoreduction in advanced ovarian cancer. J. Clin. Oncol. 2015:33. [Google Scholar]
- 78.Pignata S, Scambia G, Lauria R, Raspagliesi F, Panici PB, Cormio G, Katsaros D, Sorio R, Cavazzini G, Ferrandina G, Breda E, Murgia V, Sacco C, Sierra NMA, Pisano C, Salutari V, Weber BE, Pujade-Lauraine E, Gallo C, Perrone F. A randomized multicenter phase III study comparing weekly versus every 3 week carboplatin (C) plus paclitaxel (P) in patients with advanced ovarian cancer (AOC): Multicentre Italian Trials in Ovarian Cancer (MITO-7)-European Network of Gynaecological Oncological Trial Groups (ENGOT-ov-10)-Gynecologic Cancer Intergroup (GCIG) trial. J. Clin. Oncol. 2013:31. [Google Scholar]
- 79.Shimokata T, Ando Y. Carboplatin plus paclitaxel scheduling for advanced ovarian cancer. Lancet Oncology. 2014;15:E249–E250. doi: 10.1016/S1470-2045(14)70214-1. [DOI] [PubMed] [Google Scholar]
- 80.Brana I, Moore KN, Shapira-Frommer R, Welch S, Jou YM, Marinucci M, Freshwater T, Rose S, Oza AM. Targeting p53 mutant ovarian cancer: Phase I results of the WEE1 inhibitor MK-1775 with carboplatin plus paclitaxel in patients (pts) with platinum-sensitive, p53-mutant ovarian cancer (OC) J. Clin. Oncol. 2013:31. [Google Scholar]
- 81.Frederick PJ, Green HN, Huang JS, Egger ME, Frieboes HB, Grizzle WE, McNally LR. Chemoresistance in ovarian cancer linked to expression of microRNAs. Biotech Histochem. 2013;88:403–409. doi: 10.3109/10520295.2013.788736. [DOI] [PubMed] [Google Scholar]
- 82.Brozovic A, Duran GE, Wang YC, Francisco EB, Sikic BI. The miR-200 family differentially regulates sensitivity to paclitaxel and carboplatin in human ovarian carcinoma OVCAR-3 and MES-OV cells. Mol Oncol. 2015;9:1678–93. doi: 10.1016/j.molonc.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prislei S, Martinelli E, Mariani M, Raspaglio G, Sieber S, Ferrandina G, Shahabi S, Scambia G, Ferlini C. MiR-200c and HuR in ovarian cancer. BMC Cancer. 2013;13:72. doi: 10.1186/1471-2407-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cittelly DM, Dimitrova I, Howe EN, Cochrane DR, Jean A, Spoelstra NS, Post MD, Lu X, Broaddus RR, Spillman MA, Richer JK. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol Cancer Ther. 2012;11:2556–2565. doi: 10.1158/1535-7163.MCT-12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bagnoli M, De Cecco L, Granata A, Nicoletti R, Marchesi E, Alberti P, Valeri B, Libra M, Barbareschi M, Raspagliesi F, Mezzanzanica D, Canevari S. Identification of a chrXq27.3 microRNA cluster associated with early relapse in advanced stage ovarian cancer patients. Oncotarget. 2011;2:1265–1278. doi: 10.18632/oncotarget.401. [DOI] [PMC free article] [PubMed] [Google Scholar]


