Abstract
Long non-coding RNAs (lncRNAs) are a group of non-protein-coding RNAs with more than 200 nucleotides in length. lncRNAs are involved in diverse biological processes, including development, cell proliferation and differentiation. Emerging evidences also suggest that lncRNAs may participate in cancer development by functioning as tumor suppressors and oncogenes. BRAF-activated non-coding RNA (BANCR) was first identified as an oncogene in melanoma. Later studies demonstrated that BANCR was frequently deregulated in human cancers, including lung cancer, gastric cancer, colorectal cancer, thyroid cancer and osteosarcoma. Nevertheless, the direction of deregulation was tissue-specific in which BANCR could as an oncogene or tumor-suppressor gene. In this review, we compile current evidences concerning the functional roles and molecular mechanisms of BANCR in tumor development.
Keywords: Long non-coding RNAs, BANCR, cancer, oncogene
Introduction
Long noncoding RNAs (lncRNAs) are long RNAs (longer than 200 nucleotides) with limited protein coding potential [1-4] and play critical roles in transcriptional and post-transcriptional regulation of gene expression [2,5,6]. Recent studies have shown that deregulation of lncRNAs is involved in the initiation and progression of various types of cancer and correlated with cancer prognosis, including metastasis, recurrence and response to therapy [7-11]. Deregulation of lncRNAs contributes to the progression of cancer through modulating several cellular processes crucial to oncogenesis, including cell proliferation, migration and apoptosis [12-16].
BRAF-activated non-coding RNA (BANCR) was first identified in 2012 by Flockhart et al. in a RNA sequencing screen with an aim to identify transcripts affected by oncogenic BRAF V600E in melanoma [17,18]. They established that BANCR was a 693-bp lncRNA with its gene having four exons located on chromosome 9 [17,19]. Increasing number of studies later showed that BANCR was deregulated in various types of human cancer [17,20-22]. The expression and function of BANCR, however, are tissue-specific. BANCR contributes to tumorigenesis through modulating various mechanisms, including altering cell proliferation and migration.
In this review, we examine current evidences regarding the deregulation and roles of BANCR as one of the most important regulatory RNAs in human cancers. In addition, we review the potential molecular functions and mechanisms associated with BANCR deregulation in cancer. Moreover, we discuss the potential clinical applications of BANCR and its future perspectives.
Lung cancer
Lung cancer has become the second most common cancer and one of the leading causes of cancer-related deaths in the United States [23,24]. Lung cancer can be divided into two classes, namely small cell lung cancer and nonsmall cell lung cancer (NSCLC) [25-27]. Somatic mutations in BRAF have been found in ~3% of all NSCLC, in which ~50% is V600E [28]. The expression of BANCR was significantly lower in NSCLC tissues than normal tissues [19]. Lower BANCR expression was also associated with advanced clinical and pathological stages, as well as shorter overall survival of NSCLC patients independent of other clinicopathological parameters. Functionally, overexpression of BANCR inhibited cell viability, invasion and metastasis while knockdown of BANCR increased cell migration and invasion. In particular, enforced expression of BANCR suppressed epithelial-mesenchymal transition (EMT) through regulating E-cadherin, N-cadherin and vimentin. Further investigations revealed that histone deacetylation of BANCR promoter could mediate its downregulation in NSCLC cells. BANCR expression was higher in lung cancer-bearing C57BL/6 mice exposed to radiation therapy than in the control mice [29]. Consistent with previous studies, lower levels of BANCR were associated with larger lung tumor size in these mice and increased tolerance to radiation in vitro. These findings suggested that radiation therapy might exert its anti-tumor role in lung cancer at least in part through upregulating BANCR.
Gastric cancer
Gastric cancer is the third leading cause of cancer-related death worldwide [30-33]. Most patients with gastric cancer present with advanced stage of disease at the time of diagnosis, resulting in poor prognosis [34-37]. Therefore, molecular biomarkers are urgently needed to promote early diagnosis and prognostication in gastric cancer patients. While BRAF mutation is extremely rare in gastric cancer [38], BANCR was significantly upregulated in gastric cancer tissues compared with adjacent normal tissues [39]. Moreover, high expression of BANCR as an independent unfavorable prognostic factor was associated with advanced clinical and tumor-node-metastasis (TNM) staging in gastric cancer patients. An independent study also showed that BANCR was abnormally overexpressed in gastric cancer tissues and cell lines [40], in which knockdown of BANCR reduced cancer cell proliferation and induced apoptosis, accompanied by the inhibition of NF-κB1 expression. Importantly, overexpression of NF-κB1 reversed the tumor-suppressing effects of BANCR knockdown in gastric cancer cells. NF-κB1 was a target of microRNA-9, whose inhibitor could also reverse the effects of BANCR knockdown in gastric cancer cells. These results suggested that BANCR is overexpressed and exerts oncogenic function in gastric cancer through the microRNA-9/NF-κB1 cascade. Overexpression of BANCR is also an unfavorable prognostic marker in gastric cancer patients.
Colorectal cancer
Colorectal cancer is common worldwide, with most patients diagnosed at the late stage in China [41-45]. It is therefore urgent to identify novel molecular markers and therapeutic targets for early detection and treatment, respectively, to improve the clinical outcomes. BRAF mutations occur in ~10% of colorectal cancer with 3% in non-hypermutated tumors and 47% in and hypermutated tumors [46].
Two contrasting views on the role of BANCR in colorectal cancer exist in the literature. Guo et al. reported that BANCR expression was frequently upregulated in colorectal cancer as compared with the matched adjacent normal tissues and positively correlated with lymph node metastasis and tumor stage [47]. In addition, enforced expression of BANCR promoted the migration of colon cancer cells in vitro, while downregulation of BANCR exerted an opposite effect. Further investigation demonstrated that BANCR induced EMT in colorectal cancer whereas the MAP kinase-ERK kinase (MEK) inhibitor U0126 decreased migration and reversed EMT in BANCR-overexpressing colon cancer cells, indicating that BANCR-induced EMT was MEK/extracellular signal-regulated kinase (ERK)-dependent. However, another study reported contradictory evidence in which BANCR expression was found to be significantly lower in colorectal cancer tissues as compared with normal tissues [48]. In addition, ectopic expression of BANCR inhibited colon cancer cell proliferation in vitro and in vivo. p21 is a well-known cyclin-dependent kinase inhibitor that arrests the cell cycle to inhibit cell proliferation. In this regard, ectopic expression of BANCR upregulated p21 and induced G0/G1 cell-cycle arrest and apoptosis in colorectal cancer cells, suggesting that downregulation of BANCR might contribute to the proliferation of colorectal cancer cells, at least in part, through the regulation of p21. Consistent with the latter study, BANCR was found to be involved in the unexpected tumor-suppressing effect of fentanyl, which is an anesthetic analgesic drug widely used in cancer pain management. To this end, fentanyl downregulated the transcription factor Ets-1 to derepress BANCR expression via altering histone 3 acetylation in colon cancer cells [49]. Furthermore, fentanyl-induced inhibition of cell proliferation, migration and invasion was reversed by Ets-1 overexpression and such rescuing effects of Ets-1could be abrogated by BANCR co-overexpression. This study supports that BANCR could exert tumor-suppressing effects in colorectal cancer. The reason underlying these contradictory findings remain unclear but it is hopeful that larger sample size together with more information on BRAF V600E and microsatellite instability statuses of tumor tissues in future studies will minimize inconsistency arising from inter-individual or subtype-specific difference.
Melanoma
Melanoma is a leading cause of skin cancer deaths with a poor survival rate [50-53]. Methods for early melanoma detection and innovative therapies to control advanced melanomas are needed [54-56]. Activating mutations in the BRAF oncogene are present in >70% of melanomas, 90% of which produce the active mutant BRAF V600E protein [57]. Flockhart et al. identified 39 differentially regulated lncRNAs, including BANCR, in BRAF V600E-positive melanomas cells [17,18], in which BANCR was recurrently upregulated and associated with cell migration. BANCR knockdown reduced melanoma cell migration by downregulating several related genes, including the chemokine CXCL11, which is a mediator of cell migration. Consistently, Li et al. showed that BANCR was upregulated in human melanoma cell lines and tissues [20,58]. In addition, increased BANCR expression was associated with higher tumor stages and lower survival rates. Knockdown of BANCR significantly reduced melanoma cell proliferation through inhibiting the mitogen-activated protein kinase (MAPK) pathway, in which the reduced phosphorylation of ERK1/2 and JNK caused by pharmacological inhibition could be rescued by BANCR overexpression. Moreover, combination of BANCR knockdown with ERK1/2 or JNK suppression resulted in synergistic inhibitory effects on melanoma cell proliferation in vitro. BANCR knockdown could also inhibit melanoma growth in vivo in BALB/c nude mice. In summary, BANCR is abnormally upregulated in human malignant melanoma and promotes cell proliferation and migration. The oncogenic effect of BANCR is dependent at least in part on CXCL11 and the MAPK pathway, suggesting the existence of a novel molecular circuitry that regulates malignant phenotypes in melanoma.
Thyroid cancer
Papillary thyroid carcinoma (PTC) accounts for approximately 80% of all thyroid cancers [59-61]. Most PTCs exhibit excellent prognoses due to early diagnosis and effective treatment, including surgery [62,63]. Accumulating evidence has shown that epigenetic alteration plays a critical role in the development of thyroid cancer [60,64]. BRAF V600E is the most common somatic mutation in PTC and could be detected in ~45% of tumor tissues [65]. Concordantly, BANCR expression was higher in PTC tissues and cell lines than in the normal controls [66]. Overexpression of BANCR induced cell proliferation, inhibited apoptosis and activated autophagy in PTC cells in vitro whereas BANCR knockdown exerted opposite effects. These results suggested that BANCR is oncogenic in PTC. Consistently, an independent study showed that the expression level of BANCR was significantly higher in PTC than in normal tissue [67]. In addition, BANCR knockdown dramatically inhibited thyroid-stimulating hormone receptor and suppressed PTC cell proliferation through inducing cell cycle arrest. BANCR silencing in PTC cells also reduced chromatin recruitment of enhancer of zeste homolog 2 (EZH2), an oncogenic histone methyltransferase whose overexpression inhibits a repertoire of tumor suppressors in different types of cancer. Therefore, BANCR is a potential therapeutic target in PTC.
Osteosarcoma
Osteosarcoma is the most common primary and aggressive bone malignancy in adolescents. It is characterized by poor prognosis because of its high local aggressiveness and metastasizing potentials as well as its resistance to chemotherapy. However, no useful biomarker for osteosarcoma detection and prognostication has been identified. BRAF was found to be mutated in approximately one-tenth of osteosarcoma patients [68]. The expression level of the BANCR was lower in osteosarcoma MG-63 cells as compared with normal osteoblasts SV-HFO. Overexpression of BANCR significantly reduced the level of β-catenin and suppressed MG-63 cell viability. Its expression was also induced by a phytochemical that exerted potent anti-cancer effects in osteosarcoma cells [69]. These findings suggest that BANCR could function as a tumor suppressor in osteosarcoma, in which this lncRNA negatively regulates cell proliferation through targeting the oncogenic Wnt/β-catenin signaling.
Retinoblastoma
Retinoblastoma is the most common primary intraocular malignancy of childhood, seriously impairing patient’s vision [70,71]. Retinoblastoma has become largely curable thanks to the advancement in its diagnosis and treatment [72-74]. The identification of novel molecular markers may also help develop new prognostic and therapeutic strategies. BRAF mutations are rare in retinoblastoma [75] but BANCR expression was higher in retinoblastoma tissues and cell lines than in normal retina samples [22]. In addition, BANCR expression was associated with tumor size, choroidal and optic nerve invasion as well as poor survival. Functionally, downregulation of BANCR inhibited proliferation, migration, and invasion of retinoblastoma cells in vitro. Taken together, BANCR plays a critical role in retinoblastoma progression and may serve as a promising candidate for prognostication and therapeutic targeting in retinoblastoma patients.
Hepatocellular carcinoma
The epidemiology of hepatocellular carcinoma (HCC) is undergoing a dynamic change in which its incidence in many Asian countries is declining because of infant hepatitis B virus immunization but on the rise in Western countries owing to increasing chronic hepatitis C virus infection [76]. The usual outcome of HCC is poor as only 10%-20% of tumors could be surgically removed. Moreover, there is no effective therapeutic agent for HCC except sorafinib which could extend the median survival of advanced-stage patients for ~3 months [77]. It is therefore pivotal to better understand the molecular pathogenesis of HCC in order to identify novel therapeutic targets.
While BRAF mutation occurs in <1% of HCC [78], a recent study showed that BANCR expression was remarkably upregulated in HCC tissues and cell lines compared with adjacent noncancerous tissues and normal hepatocyte CL-48O cells, respectively. High BANCR expression was also associated with high tumor grade, large tumor size, venous infiltration, advanced TNM staging, and shorter overall survival. Multivariate analysis further revealed that BANCR was an independent unfavorable prognostic factor in HCC. Functional characterization in HCC cells showed that knockdown of BANCR impaired cell proliferation, promoted apoptosis and reduced cell invasion and migration. The latter might be attributed to EMT reversal, which was marked by upregulation of E-cadherin and downregulation of vimentin [79]. These findings suggested BANCR is not only an oncogenic lncRNA in HCC, but also a novel prognostic marker as well as a potential therapeutic target.
Conclusions and future perspectives
Emerging evidences have shown the crucial roles of BANCR in the initiation and progression of different cancers (Table 1), in which the expression and functions of BANCR are tissue-specific. BANCR is upregulated in gastric cancer, melanoma, thyroid cancer, retinoblastoma and HCC where it promotes multiple oncogenic signaling cascades (Figure 1). In contrast, BANCR is downregulated in lung cancer and osteosarcoma where suppression of EMT and inhibition of Wnt/β-catenin signaling mediate its tumor-suppressing action. In colorectal cancer, different studies yielded contradictory results. Although BANCR seems to be upregulated in cancers with high prevalence of BRAF mutations, such as melanoma and PTC, the relationship between BRAF mutations and BANCR dysregulation is less apparent in other cancer types. Moreover, the mechanism underlying the tissue-specific effect of BANCR remains elusive. To this end, many lncRNAs have been shown to function as a microRNA-sponge to mediate their biological actions [80-82]. Thus, it is highly possible that tissue-specific expression profiles of microRNAs [83] in different cancer types would dictate whether BANCR functions as an oncogene or tumor-suppressor gene. Understanding the deregulated expression and functional roles of BANCR in cancers together with its association with clinic pathological parameters will help develop BANCR-based diagnostic or prognostic methods. In particularly, expression analysis of BANCR can be readily achieved by standardized quantitative techniques, including RT-PCR.
Table 1.
Dysregulation and functions of BANCR in human cancers
| Cancer type | Dysregulation | Phenotypes affected | Related genes | Role | References |
|---|---|---|---|---|---|
| Lung cancer | Downregulated | Viability, invasion metastasis, EMT | Tumor suppressor gene | [19,29] | |
| Gastric cancer | Upregulated | Growth, apoptosis | NF-kB1 | Oncogene | [39,40] |
| miR-9 | |||||
| Colorectal cancer | Upregulated | Migration, EMT, cell cycle | MEK | Oncogene or tumor suppressor gene | [47-49] |
| Downregulated | p21 | ||||
| Ets-1 | |||||
| Melanoma | Upregulated | Growth, migration | CXCL11 | Oncogene | [17,18,20,58] |
| ERK1/2 | |||||
| JNK | |||||
| Thyroid cancer | Upregulated | Proliferation, apoptosis, autophagy | TSHR | Oncogene | [64,67] |
| EZH2 | |||||
| Osteosarcoma | Downregulated | Viability, Proliferation, apoptosis | JNK | Tumor suppressor gene | [67] |
| Retinoblastoma | Upregulated | Proliferation, migration, invasion | Oncogene | [22] | |
| Hepatocellular carcinoma | Upregulated | Proliferation, apoptosis, migration, invasion, EMT | Oncogene | [79] |
Figure 1.
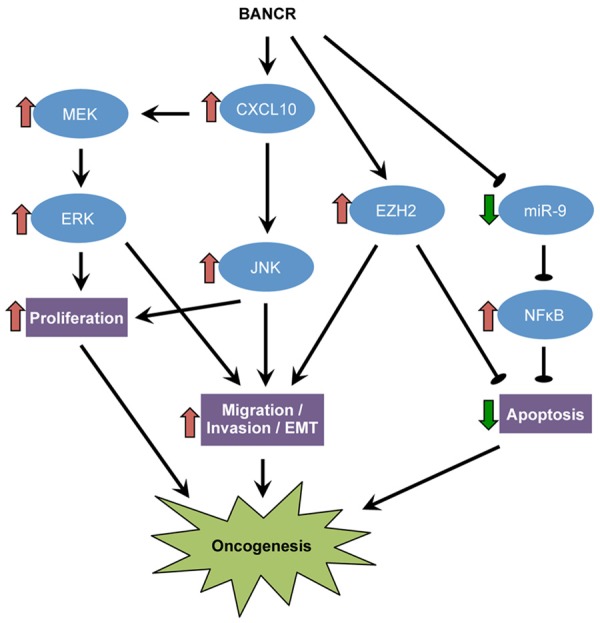
Proposed oncogenic mechanisms of BANCR.
Future investigation should analyze BANCR levels in urine, blood, and mucus for non-invasive detection or monitoring of cancer. A systematic analysis of BANCR expression using existing datasets, including The Cancer Genome Atlas (TCGA), may also help to identify cancers with deregulated BANCR expression levels. Moreover, although some upstream regulatory pathways mediating BANCR deregulation in cancers have been reported, the exact molecular mechanisms remain largely unknown. Further experiments are also required to delineate the downstream mechanism of BANCR, including elucidation of the molecular identities of its binding partners.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant Numbers: 81401847).
Disclosure of conflict of interest
None.
References
- 1.Yoshimoto R, Mayeda A, Yoshida M, Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochim Biophys Acta. 2016;1859:192–9. doi: 10.1016/j.bbagrm.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Geng PL, Yin P, Wang XL, Jia JP, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14:2311–2315. doi: 10.7314/apjcp.2013.14.4.2311. [DOI] [PubMed] [Google Scholar]
- 3.Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:555–559. doi: 10.1002/jso.23264. [DOI] [PubMed] [Google Scholar]
- 4.Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS, De W, Shu YQ, Wang ZX. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, Yin R. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36:1643–1651. doi: 10.1007/s13277-014-2763-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–55. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Li Z. Long non-coding RNA HOTAIR: A novel oncogene (Review) Mol Med Rep. 2015;12:5611–5618. doi: 10.3892/mmr.2015.4161. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Li Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953–1958. doi: 10.3892/ol.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng YJ, Xie SL, Li Q, Ma J, Wang GY. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39:2119–2128. doi: 10.1177/147323001103900608. [DOI] [PubMed] [Google Scholar]
- 13.Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219. doi: 10.3389/fgene.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104:458–464. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J, Liu M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012;22:1006–1014. doi: 10.1101/gr.140061.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy N. Epigenetics. Going places with BANCR. Nat Rev Cancer. 2012;12:451. doi: 10.1038/nrc3302. [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Liu XH, Wang KM, Nie FQ, Kong R, Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, Chen JF, Zhang EB, De W, Wang ZX. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68. doi: 10.1186/1476-4598-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L, Sha N. Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One. 2014;9:e100893. doi: 10.1371/journal.pone.0100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang L, Tian Y, Han X, Tian D. Long non-coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. Biomed Pharmacother. 2015;69:90–95. doi: 10.1016/j.biopha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015;36:7205–7211. doi: 10.1007/s13277-015-3413-3. [DOI] [PubMed] [Google Scholar]
- 23.Tejero R, Navarro A, Campayo M, Vinolas N, Marrades RM, Cordeiro A, Ruiz-Martinez M, Santasusagna S, Molins L, Ramirez J, Monzo M. miR-141 and miR-200c as markers of overall survival in early stage non-small cell lung cancer adenocarcinoma. PLoS One. 2014;9:e101899. doi: 10.1371/journal.pone.0101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Chi YL, Wang PY, Wang YQ, Zhang YX, Deng J, Lv CJ, Xie SY. miR-511 and miR-1297 inhibit human lung adenocarcinoma cell proliferation by targeting oncogene TRIB2. PLoS One. 2012;7:e46090. doi: 10.1371/journal.pone.0046090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZY, Fu SL, Xu SQ, Zhou X, Liu XS, Xu YJ, Zhao JP, Wei S. By downregulating Ku80, hsa-miR-526b suppresses non-small cell lung cancer. Oncotarget. 2015;6:1462–1477. doi: 10.18632/oncotarget.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HH, Pang M, Dong W, Xin JX, Li YJ, Zhang ZC, Yu L, Wang PY, Li BS, Xie SY. miR-511 induces the apoptosis of radioresistant lung adenocarcinoma cells by triggering BAX. Oncol Rep. 2014;31:1473–1479. doi: 10.3892/or.2014.2973. [DOI] [PubMed] [Google Scholar]
- 27.Gao F, Chang J, Wang H, Zhang G. Potential diagnostic value of miR-155 in serum from lung adenocarcinoma patients. Oncol Rep. 2014;31:351–357. doi: 10.3892/or.2013.2830. [DOI] [PubMed] [Google Scholar]
- 28.Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, Ladanyi M, Riely GJ. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J. Clin. Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JX, Chen M, Zheng YD, Wang SY, Shen ZP. Up-regulation of BRAF activated noncoding RNA is associated with radiation therapy for lung cancer. Biomed Pharmacother. 2015;71:79–83. doi: 10.1016/j.biopha.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Xiao YF, Zhang JW, Xie R, Hu CJ, Tang B, Wang SM, Wu YY, Hao NB, Yang SM. miR-1182 attenuates gastric cancer proliferation and metastasis by targeting the open reading frame of hTERT. Cancer Lett. 2015;360:151–159. doi: 10.1016/j.canlet.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Liu X, Xue H, Qiu B, Wei B, Sun K. MicroRNA-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-Myb. Cell Prolif. 2015;48:78–85. doi: 10.1111/cpr.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Q, Chen X, Zhang M, Fan Q, Luo S, Cao X. miR-137 is frequently down-regulated in gastric cancer and is a negative regulator of Cdc42. Dig Dis Sci. 2011;56:2009–2016. doi: 10.1007/s10620-010-1536-3. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka S, Olaru AV, An F, Luvsanjav D, Jin Z, Agarwal R, Tomuleasa C, Popescu I, Alexandrescu S, Dima S, Chivu-Economescu M, Montgomery EA, Torbenson M, Meltzer SJ, Selaru FM. MicroRNA-21 inhibits Serpini1, a gene with novel tumour suppressive effects in gastric cancer. Dig Liver Dis. 2012;44:589–596. doi: 10.1016/j.dld.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y, Mori M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–2733. doi: 10.1158/1078-0432.CCR-10-2132. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang X, Jiang L, Sun Z, Miao Z, Xu H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Grieken NC, Aoyama T, Chambers PA, Bottomley D, Ward LC, Inam I, Buffart TE, Das K, Lim T, Pang B, Zhang SL, Tan IB, Carvalho B, Heideman DA, Miyagi Y, Kameda Y, Arai T, Meijer GA, Tsuburaya A, Tan P, Yoshikawa T, Grabsch HI. KRAS and BRAF mutations are rare and related to DNA mismatch repair deficiency in gastric cancer from the East and the West: results from a large international multicentre study. Br J Cancer. 2013;108:1495–1501. doi: 10.1038/bjc.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Zhang L, Zhang Y, Zhou F. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer. Biomed Pharmacother. 2015;72:109–112. doi: 10.1016/j.biopha.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZX, Liu ZQ, Jiang B, Lu XY, Ning XF, Yuan CT, Wang AL. BRAF activated non-coding RNA (BANCR) promoting gastric cancer cells proliferation via regulation of NF-kappaB1. Biochem Biophys Res Commun. 2015;465:225–231. doi: 10.1016/j.bbrc.2015.07.158. [DOI] [PubMed] [Google Scholar]
- 41.Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C, Zhou XY, Du X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J Transl Med. 2013;11:122. doi: 10.1186/1479-5876-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji S, Ye G, Zhang J, Wang L, Wang T, Wang Z, Zhang T, Wang G, Guo Z, Luo Y, Cai J, Yang JY. miR-574-5p negatively regulates Qki6/7 to impact beta-catenin/Wnt signalling and the development of colorectal cancer. Gut. 2013;62:716–726. doi: 10.1136/gutjnl-2011-301083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu X, Li Z, Yu J, Chan MT, Wu WK. MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif. 2015;48:503–510. doi: 10.1111/cpr.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Z, Xu Y, Cai S. Down-regulated GAS1 expression correlates with recurrence in stage II and III colorectal cancer. Hum Pathol. 2011;42:361–368. doi: 10.1016/j.humpath.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Mansour MA, Hyodo T, Ito S, Kurita K, Kokuryo T, Uehara K, Nagino M, Takahashi M, Hamaguchi M, Senga T. SATB2 suppresses the progression of colorectal cancer cells via inactivation of MEK5/ERK5 signaling. FEBS J. 2015;282:1394–1405. doi: 10.1111/febs.13227. [DOI] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D, Sun Y. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol Lett. 2014;8:869–875. doi: 10.3892/ol.2014.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Y, Liu Y, Wang J, Jie D, Yun T, Li W, Yan L, Wang K, Feng J. Downregulated Long Noncoding RNA BANCR Promotes the Proliferation of Colorectal Cancer Cells via Downregualtion of p21 Expression. PLoS One. 2015;10:e0122679. doi: 10.1371/journal.pone.0122679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li AX, Xin WQ, Ma CG. Fentanyl inhibits the invasion and migration of colorectal cancer cells via inhibiting the negative regulation of Ets-1 on BANCR. Biochem Biophys Res Commun. 2015;465:594–600. doi: 10.1016/j.bbrc.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Howell PM, Riker AI. Up-Regulation of miR-182 Expression after Epigenetic Modulation of Human Melanoma Cells. Ann Surg Oncol. 2013;20:1745–52. doi: 10.1245/s10434-012-2467-3. [DOI] [PubMed] [Google Scholar]
- 51.Perera RJ, Ray A. Epigenetic regulation of miRNA genes and their role in human melanomas. Epigenomics. 2012;4:81–90. doi: 10.2217/epi.11.114. [DOI] [PubMed] [Google Scholar]
- 52.Tang L, Zhang W, Su B, Yu B. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed Res Int. 2013;2013:251098. doi: 10.1155/2013/251098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, Diederichs S, Eichmuller SB. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 54.Bennett PE, Bemis L, Norris DA, Shellman YG. miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol Genomics. 2013;45:1049–1059. doi: 10.1152/physiolgenomics.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell RE, Khaled M, Netanely D, Schubert S, Golan T, Buxbaum A, Janas MM, Postolsky B, Goldberg MS, Shamir R, Levy C. Transcription factor/microRNA axis blocks melanoma invasion program by miR-211 targeting NUAK1. J Invest Dermatol. 2014;134:441–451. doi: 10.1038/jid.2013.340. [DOI] [PubMed] [Google Scholar]
- 56.Levy C, Khaled M, Iliopoulos D, Janas MM, Schubert S, Pinner S, Chen PH, Li S, Fletcher AL, Yokoyama S, Scott KL, Garraway LA, Song JS, Granter SR, Turley SJ, Fisher DE, Novina CD. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol Cell. 2010;40:841–849. doi: 10.1016/j.molcel.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roller DG, Capaldo B, Bekiranov S, Mackey AJ, Conaway MR, Petricoin EF, Gioeli D, Weber MJ. Combinatorial drug screening and molecular profiling reveal diverse mechanisms of intrinsic and adaptive resistance to BRAF inhibition in V600E BRAF mutant melanomas. Oncotarget. 2016;7:2734–53. doi: 10.18632/oncotarget.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staff PO. Correction: Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One. 2015;10:e0118728. doi: 10.1371/journal.pone.0118728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossing M, Borup R, Henao R, Winther O, Vikesaa J, Niazi O, Godballe C, Krogdahl A, Glud M, Hjort-Sorensen C, Kiss K, Bennedbaek FN, Nielsen FC. Down-regulation of microRNAs controlling tumourigenic factors in follicular thyroid carcinoma. J Mol Endocrinol. 2012;48:11–23. doi: 10.1530/JME-11-0039. [DOI] [PubMed] [Google Scholar]
- 60.Esposito F, Tornincasa M, Pallante P, Federico A, Borbone E, Pierantoni GM, Fusco A. Down-regulation of the miR-25 and miR-30d contributes to the development of anaplastic thyroid carcinoma targeting the polycomb protein EZH2. J Clin Endocrinol Metab. 2012;97:E710–718. doi: 10.1210/jc.2011-3068. [DOI] [PubMed] [Google Scholar]
- 61.Zhu H, Fang J, Zhang J, Zhao Z, Liu L, Wang J, Xi Q, Gu M. miR-182 targets CHL1 and controls tumor growth and invasion in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2014;450:857–862. doi: 10.1016/j.bbrc.2014.06.073. [DOI] [PubMed] [Google Scholar]
- 62.Yang Z, Yuan Z, Fan Y, Deng X, Zheng Q. Integrated analyses of microRNA and mRNA expression profiles in aggressive papillary thyroid carcinoma. Mol Med Rep. 2013;8:1353–1358. doi: 10.3892/mmr.2013.1699. [DOI] [PubMed] [Google Scholar]
- 63.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif. 2007;40:921–935. doi: 10.1111/j.1365-2184.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma Y, Qin H, Cui Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013;441:958–963. doi: 10.1016/j.bbrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu J, Sun Y. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett. 2014;8:1947–1952. doi: 10.3892/ol.2014.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng H, Wang M, Jiang L, Chu H, Hu J, Ning J, Li B, Wang D, Xu J. BRAF-activated Long Non-coding RNA Modulates Papillary Thyroid Carcinoma Cell Proliferation through Regulating Thyroid Stimulating Hormone Receptor. Cancer Res Treat. 2016;48:698–707. doi: 10.4143/crt.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, Bottos A, Gammaitoni L, Migliardi G, Camussi G, Alberghini M, Torchio B, Ferrari S, Bussolino F, Fagioli F, Picci P, Aglietta M. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer. 2009;8:118. doi: 10.1186/1476-4598-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He N, Zhang Z. Baicalein suppresses the viability of MG-63 osteosarcoma cells through inhibiting c-MYC expression via Wnt signaling pathway. Mol Cell Biochem. 2015;405:187–196. doi: 10.1007/s11010-015-2410-6. [DOI] [PubMed] [Google Scholar]
- 70.Jo DH, Kim JH, Park WY, Kim KW, Yu YS. Differential profiles of microRNAs in retinoblastoma cell lines of different proliferation and adherence patterns. J Pediatr Hematol Oncol. 2011;33:529–533. doi: 10.1097/MPH.0b013e318228280a. [DOI] [PubMed] [Google Scholar]
- 71.Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, Xu J, Cheng JQ, Lin JY, Ma X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv Syst. 2009;25:13–20. doi: 10.1007/s00381-008-0701-x. [DOI] [PubMed] [Google Scholar]
- 72.Korah J, Canaff L, Lebrun JJ. The Retinoblastoma Tumor Suppressor Protein (pRb)/E2 Promoter Binding Factor 1 (E2F1) Pathway as a Novel Mediator of Transforming Growth Factor-beta (TGFbeta)-Induced Autophagy. J Biol Chem. 2016;291:2043–54. doi: 10.1074/jbc.M115.678557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seigel GM, Sharma S, Hackam AS, Shah DK. HER2/ERBB2 immunoreactivity in human retinoblastoma. Tumour Biol. 2016;37:6135–42. doi: 10.1007/s13277-015-4475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aldiri I, Ajioka I, Xu B, Zhang J, Chen X, Benavente C, Finkelstein D, Johnson D, Akiyama J, Pennacchio LA, Dyer MA. Brg1 coordinates multiple processes during retinogenesis and is a tumor suppressor in retinoblastoma. Development. 2015;142:4092–4106. doi: 10.1242/dev.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen Y, Merhavi-Shoham E, Avraham RB, Frenkel S, Pe’er J, Goldenberg-Cohen N. Hypermethylation of CpG island loci of multiple tumor suppressor genes in retinoblastoma. Exp Eye Res. 2008;86:201–206. doi: 10.1016/j.exer.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Wu WK, Sung JJ. Focus on gastrointestinal and liver cancers. Semin Cancer Biol. 2013;23:469–470. doi: 10.1016/j.semcancer.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 78.Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou T, Gao Y. Increased expression of LncRNA BANCR and its prognostic significance in human hepatocellular carcinoma. World J Surg Oncol. 2016;14:8. doi: 10.1186/s12957-015-0757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paci P, Colombo T, Farina L. Computational analysis identifies a sponge interaction network between long non-coding RNAs and messenger RNAs in human breast cancer. BMC Syst Biol. 2014;8:83. doi: 10.1186/1752-0509-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]


