Abstract
The aim of this study is to elucidate whether and how miR-107 participates in the modulation of paclitaxel sensitivity in non small cell lung cancer (NSCLC). By qRT-PCR, we found that miR-107 is significantly down-regulated in paclitaxel-resistant A549/Taxol cells compared with corresponding paclitaxel-sensitive counterparts. Overexpression of miR-107 suppresses paclitaxel resistance of A549/Taxol cells through directly inhibiting Bcl-w. Overexpression of miR-107 promotes apoptosis and inhibits proliferation and mobility of A549/Taxol cells under treatment with paclitaxel in vitro. Moreover, miR-107 inhibits in vivo paclitaxel resistance in xenograft model. MiR-107/Bcl-w axis regulates paclitaxel chemoresistance through PI3K-Akt pathway. Our results suggest that up-regulation of miR-107 resensitizes paclitaxel-resistant NSCLC cells by targeting Bcl-w, which reveals a potential mechanism of miR-107 in reversing drug resistance.
Keywords: Non small cell lung cancer, chemoresistance, microRNA-107, Bcl-w, PI3K-Akt pathway
Introduction
Non small cell lung cancer (NSCLC), which represents approximately 80% of all primary lung cancer, is regarded as a leading reason of cancer-associated mortalities around the world [1]. Although a great number of therapeutic methods were extensively explored in clinical application, it has been verified that systemic chemotherapy can provide promising improvement in both survival rate and life quality for NSCLC patients [2]. Currently, paclitaxel has become an integral part of several commonly used chemotherapy regimens in NSCLC.
Paclitaxel (Taxol), a microtubule-stabilizing agent applied as a first-line chemotherapy drug in clinical oncology, was isolated and extracted from Taxusbrevifolia and originally reported as an anti-cancer agent in 1971 [3]. Paclitaxel could regulate cell cycle via blocking cells in late G2 or M phases [4] and directly induce DNA damage and cell apoptosis [5,6]. However, the therapeutic efficiency of paclitaxel is increasingly getting worse for cancers due to chemotherapy drugs resistance. Therefore, in order to improve chemotherapeutic efficacy of paclitaxel, it is urgent for us to explore the underlying mechanisms of its function and develop effective chemotherapeutic strategies to overcome the paclitaxel resistance of NSCLC.
MicroRNAs (miRNAs), 18-22 nucleotides in length, are a series of small, single stranded, highly conserved, non-coding RNAs. Through binding with 3’-untranslated region (3’-UTR) of their target mRNAs, miRNAs can modulate expression of downstream genes [7,8]. In addition to participation in several biological processes, such as cell-cycle regulation [9] and cell differentiation [10], miRNAs were confirmed to be associated with a number of human cancers [11-13]. Increasing evidence also indicated that several miRNAs might be involved in regulating the sensitivity of cancer cells to chemotherapeutic compounds [14,15]. For example, Cai et al. revealed that overexpression of miR-205 could inhibit cell proliferation and enhance the sensitivity of breast cancer cells to docetaxel [16]. However, the miR-107 expression and its biological functions in paclitaxel resistance of NSCLC have been rarely reported.
BCL2-like 2 (Bcl-w), a member of BCL2 family, serves as an anti-apoptotic factor by promoting cell proliferation and suppressing cell apoptosis [17,18]. Recent articles have suggested that dysregulated expression of Bcl-w is significantly correlated to various types of cancer, such as breast cancer [19], colorectal cancer [20] and lung cancer [21].
In this study, we first aimed to explore the expression of miR-107 and its biological functions in NSCLC. The further investigations were performed to verify whether Bcl-w was a direct target of miR-107 and miR-107 could regulate sensitivity of NSCLC cell lines to paclitaxel. Finally, we hypothesized that miR-107 might participate in regulating NSCLC sensitivity to paclitaxel by regulating Bcl-w.
Materials and methods
Cell lines and animals
The human NSCLC cell line (A549) and HEK 293T cell line were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), and maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 U/ml of penicillin G sodium, and 100 µg/ml streptomycin sulfate (Sigma-Aldrich, Shanghai, China) at 37°C in a humidified air atmosphere containing 5% CO2.
Male BALB/C nude mice of 2-4 weeks old, 19 ± 1 g in weight, were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China) and housed five per cage under specific pathogen-free conditions. All animal experiments were carried out following the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised in 1985) and were approved by the Animal Care and Use Committee of Changhai Hospital.
Establishment of paclitaxel-resistant cell lines
For the establishment of paclitaxel-resistant cell subline A549/Taxol, parental cells A549 in the logarithmic growth phase were collected, digested with 0.25% trypsin and centrifuged, and then a cell suspension (1 × 109 cells/L) was obtained. Then, 10 mL cell suspension was seeded to a culture bottle and cultivated for 24 h in culture medium with a low concentration of 2 nM paclitaxel (Sigma-Aldrich). Then, cells were collected and re-inoculated in a 10-ml culture flask in culture medium containing an increased concentration. This protocol was repeated until the cells showed stable proliferation in a culture medium with 24 nM paclitaxel. Cells were further cultured in drug-free medium for one week before follow-up experiments. The level of paclitaxel resistance was investigated using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay.
Paclitaxel sensitivity MTT assay
Briefly, cells were seeded to the wells of a 96-well plate (1 × 104 cells per well) and allowed to adhere. After 24 h incubation, the media was replaced with fresh media and the cells were treated with various doses (0, 2, 4, 8, 12, 24, 36, 48 nM) of paclitaxel for another 24 h. 20 μl of MTT (Sigma Chemicals, St. Louis, MO, USA; 5 mg/ml in PBS) was added to each well, and the cells were cultured for an additional 4 h. Then, liquid was removed from the plate and 150 μL of DMSO was added to each well and incubated in darkness at room temperature for 2 h. The optical density (OD) value was measured at 490 nm using an ELISA reader (MultiskanEX, Lab systems, Helsinki, Finland). Paclitaxel sensitivity was determined using the IC50 value (paclitaxel concentration causing 50% decrease in absorbance compared with the control).
Lentivirus construction and transfection
To generate miR-107 and Bcl-w overexpression stable transfectants, A549/Taxol cells were transfected with lentiviral expressing vectors. The plasmid construction and lentivirus package were performed commercially (Genechem Co. Ltd., Shanghai, China). Viruses packaging was performed in HEK293T cells after the co-transfection of pGC-GFP-miR-107 vector or pGC-GFP-Bcl-w vector with the packaging plasmid pHelper 1.0 Vector and the envelope plasmid pHelper 2.0 vector through Lipofectamine 2000 (Invitrogen, Camarillo, CA, USA). Cells were transfected with lentivirus at 50% confluency. After 7 days of transfection, stable clones were selected with GFP by flow cytometry and cultured for further analysis. An empty pGC-GFP-NC-LV vector was used as a negative reference.
Dual-luciferase reporter assay
The putative binding sites for miR-107 within the 3’-UTR of Bcl-w were identified through using the Targetscan algorithm (http:www.targetscan.org) [22]. The miR-107 binding sites from 3’UTR Bcl-w or mutant 3’UTR was cloned into the pGL3-report luciferase vector (Sigma-Aldrich). 100 nM miR-107 mimics or control miRNA was co-transfected with 0.1 μg of the pGL3-3’UTR wildtype or mutant plasmid DNAs into HEK 293T cells in 96-well plates using Lipofectamine 2000. Cells were collected 24 h after transfection and analyzed using the Dual Luciferase Reporter Assay System (Promega, Madison, WI), and the relative luciferase activity was normalized to the activity of Renilla luciferase.
Quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cultured cell lines through TRIzol® (Life Technologies, Carlsbad, CA, USA) according to manufacturer’s instructions. The cDNA was synthesized using PrimeScript RT reagent Kit (Epicentre, Madison, WI, USA) or the miScript Reverse Transcription Kit (Qiagen) and was subsequently amplified by PCR using a SYBR Green mix (Takara, Tokyo, Japan) on an ABI7500 Real-Time PCR System (Applied Biosystems, CA, USA). U6 small RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for normalization and quantification of miR-107 and Bcl-w mRNA expression, respectively. The relative gene expression level (amount of target normalized to the endogenous control gene) was calculated using the 2-ΔΔCt method. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence passed the fixed threshold. The primers used in this study were listed in Table 1.
Table 1.
The sequences of primers
| Gene name | Primer sequences | |
|---|---|---|
| miR-107 | RT | 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGATAG-3’ |
| Forward | 5’-AGCAGCATTGTACAGGG-3’ | |
| Reverse | 5’-GTGCAGGGTCCGAGGT-3’ | |
| Bcl-w | Forward | 5’-CACCCAGGTCTCCGATGAAC-3’ |
| Reverse | 5’-TTGTTGACACTCTCAGCACAC-3’ | |
| U6 | RT | 5’-AACGCTTCACGAATTTGCGT-3’ |
| Forward | 5’-CTCGCTTCGGCAGCACA-3’ | |
| Reverse | 5’-AACGCTTCACGAATTTGCGT-3’ | |
| GAPDH | Forward | 5’-CGAGATCCCTCCAAAATCAA-3’ |
| Reverse | 5’-TTCACACCCATGACGAACAT-3’ | |
Western blot analysis
Cell lysates were collected according to routine procedures. The protein amount was investigated using the bovine serum albumin (BSA) kit (Beyotime, Shanghai, China). Proteins samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene difluoride (PVDF) membranes (Roche, Switzerland). The membranes were blocked overnight in 5% skimmed milk in Tris-buffered saline with Tween®-20 (TBST). After incubation with specific primary antibodies overnight at 4°C, membranes were further incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies. After applying electrochemiluminescent (ECL)-Plus detection reagents (Santa Cruz, CA, USA), the protein bands were quantified by densitometry using the Image J software (http://rsb.info.nih.gov/ij). GAPDH was applied as an internal control for relative quantification.
Flow cytometry-based apoptosis analysis
Apoptosis analysis was conducted using a BioVision Annexin V-FITC reagent kit (Sigma-Aldrich; St. Louis, MO, USA) and flow cytometry. Cells were seeded onto 6-well plates, and then 400 μL 1 × binding buffer, 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) were added. Cells were treated with 8 nM paclitaxel for 48 h to induce apoptosis. Cells were incubated for 5-15 min after mixing, and flow cytometry carried out within an hour.
Caspase-3 activity assay
The activity of caspase-3, a key executor of cell apoptosis, was investigated through a caspase-3 colorimetric protease assay kit (Thermo Fisher Scientific, Waltham, MA, USA). After treatment with 8 nM paclitaxel for 48 h, 1 × 105 cells were lysed, and the supernatant was collected after centrifugation. 40 μL protein of cell lysate per sample was added to 50 μL of reaction buffer with 5 μL of N-acetyl-Asp-Glu-Val-Asp-pNA substrate and incubated at 37°C for 2 h. The reaction was measured at 405 nm for absorbance.
Wound-healing assay
Upon reaching confluency, cell monolayers were wounded by dragging a 1 ml pipette tip across the cells and then cells were washed to remove cell debris and allowed to migrate for 24 h after treatment with 8 nM paclitaxel. Images were taken at 0 and 24 h after wounding using a DMI 6000 inverted microscope.
Tumor xenograft model
Cells were suspended in serum-free medium, mixed with Matrigel (BD Biosciences) and injected subcutaneously into a single side of the posterior flank of each anesthetized nude mouse. When the mice developed palpable tumors, they were randomly divided into the following four treatment groups (n=5 animals per group): (1) A549; (2) A549/Taxol; (3) A549/Taxol + lenti_miR-107; and (4) A549/Taxol + lenti_miR-107 + lenti_Bcl-w. 8 nM paclitaxel was intraperitoneally injected once a week for 4 weeks. Tumor volumes, measured by a single individual with a digital caliper every three days, were calculated using the formula: 1/2 × length × width2. Four weeks after implantation, all of the mice were sacrificed and the tumors were extracted and weighted.
Statistical analysis
Continuous data were presented with the mean ± standard deviation (SD) and a two-tail, unpaired Student’s t-test was used to compare the statistical difference between two groups. The value of P<0.05 were considered statistically significant. SPSS 17.0 software (SPSS, Chicago, IL, USA) and GraphPad Prism (GraphPad Software Inc., San Diego, USA) was employed to analyze all data.
Results
MiR-107 is down-regulated in paclitaxel-resistant NSCLC cells
As first step of the study, we conducted qRT-PCR analysis to investigate the expression of miR-107 in A549/Taxol and A549 cell lines. As shown in Figure 1, miR-107 expression were significantly suppressed in A549/Taxol cells in comparison to A549 cells (P<0.05), which suggested that the down-regulation of miR-107 might serve an important role in suppressing the sensitivity of NSCLC cells to treatment with paclitaxel.
Figure 1.
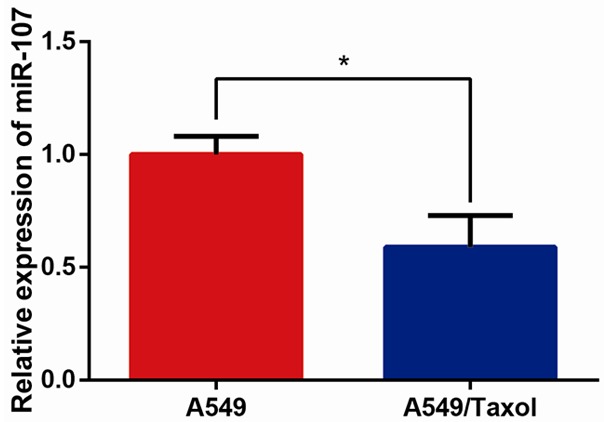
MiR-107 is down-regulated in paclitaxel-resistant NSCLC cells. Analysis of miR-107 expression levels was performed by qRT-PCR. U6 was used as the endogenous control. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
Bcl-w is a target directly inhibited by miR-107 in paclitaxel-resistant NSCLC cells
MiRNAs modulates various biological functions through down-regulating expression of downstream targets. We predicted the putative target genes of miR-107 in human cells using the tool TargetScan database (www.targetscan.org). Among hundreds of the predicted candidates, Bcl-w was of interest in the present study due to its complementary structure with miRNA-107 (Figure 2A). Through performing dual-luciferase reporter assay, as expected, we uncovered that the relative luciferase activities were remarkably reduced in HEK 293T cells transfected with Bcl-w WT-3’UTR/miR-107 mimics compared with those transfected with Bcl-w WT-3’UTR/control miRNA (P<0.05, Figure 2B).
Figure 2.
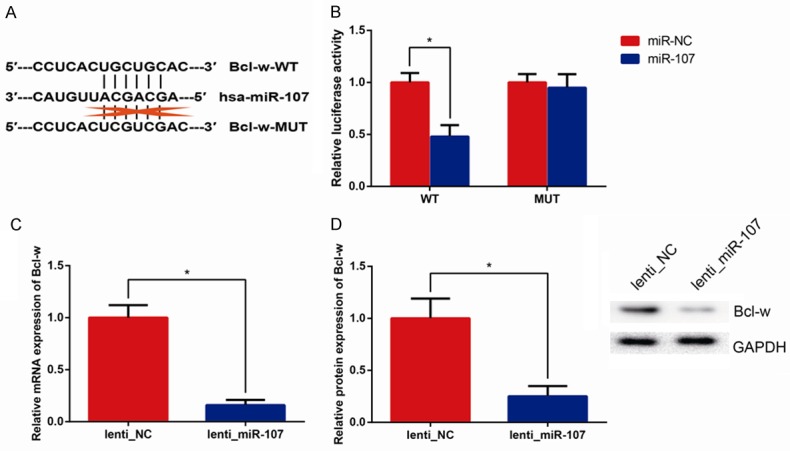
Bcl-w is a target of miR-107. (A) The miR-107 binding sites in the Bcl-w 3’UTR predicted by bioinformatics analysis. (B) Luciferase activities in HEK293T cells were measured by Dual-luciferase reporter assay. qRT-PCR (C) and western blot (D) showing Bcl-w mRNA and protein expression levels in A549/Taxol cells with miR-107 overexpression. GAPDH was used as the endogenous control. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
Furthermore, in order to up-regulate expression of miR-107 in A549/Taxol cells, which exhibited low levels of miR-107, we transfected lenti_miR-107, which remarkably increased miR-107 level (Data not shown). We observed that transfection of lenti_miR-107 could significantly inhibit Bcl-w expression at both the mRNA and protein levels in A549/Taxol cells (all P<0.05, Figure 2C, 2D).
MiR-107 promotes paclitaxel sensitivity of paclitaxel-resistant NSCLC cells
The levels of paclitaxel resistance of A549 and A549/Taxol cells to various concentrations of paclitaxel (0, 2, 4, 8, 16, 32, 64 nM) were investigated by MTT assay (Figure 3A), and IC50 values were subsequently calculated. As shown in Figure 3B, the IC50 value of paclitaxel in A549/Taxol cells (42.5 nM) was greatly higher compared with that of their parental A549 cells (10 nM) (P<0.05), and overexpression of miR-107 significantly reduced the IC50 value of paclitaxel in A549/Taxol cells (9 nM) (P<0.05). However, co-transfection of lenti_Bcl-w could partly reverse the downregulated IC50 value of paclitaxel in A549/Taxol cells transfected with lenti_miR-107 (25.1 nM). These data indicated that up-regulation of miR-107 effectively enhances the sensitivity of A549/Taxol cells to paclitaxel through inhibiting Bcl-w.
Figure 3.
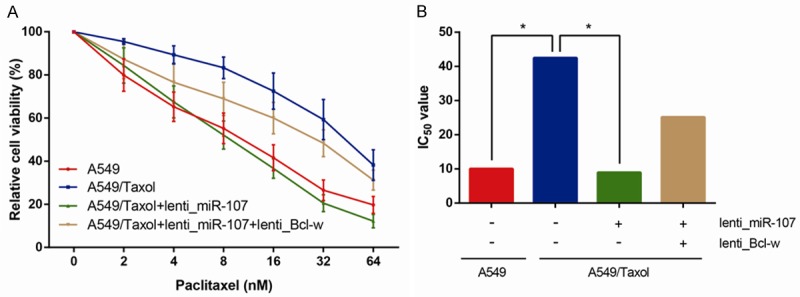
MiR-107 overexpression promotes paclitaxel sensitivity of A549/Taxol cells. The cell viability (A) was determined by MTT assay at 0, 2, 4, 8, 16, 32, 64 nM paclitaxel, and IC50 values (B) were subsequently calculated. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
MiR-107 promotes apoptosis and inhibits mobility of paclitaxel-resistant NSCLC cells in treatment with paclitaxel in vitro
On the basis of the MTT assay, the apoptotic rate to paclitaxel of A549/Taxol and A549 cells were determined through flow cytometer. As shown in Figure 4A, the apoptotic rate to paclitaxel of A549/Taxol cells was significantly inhibited than that of their parental A549 cells (P<0.05), and overexpression of miR-107 obviously promoted the apoptosis of A549/Taxol cells to paclitaxel (P<0.05). In addition, co-transfection of lenti_Bcl-w could reverse the relatively high apoptotic rate to paclitaxel of A549/Taxol cells transfected with lenti_miR-107. Remarkably increased caspase-3 activity to paclitaxel was also observed in A549/Taxol cells transfected with lenti_miR-107 (Figure 4B).
Figure 4.
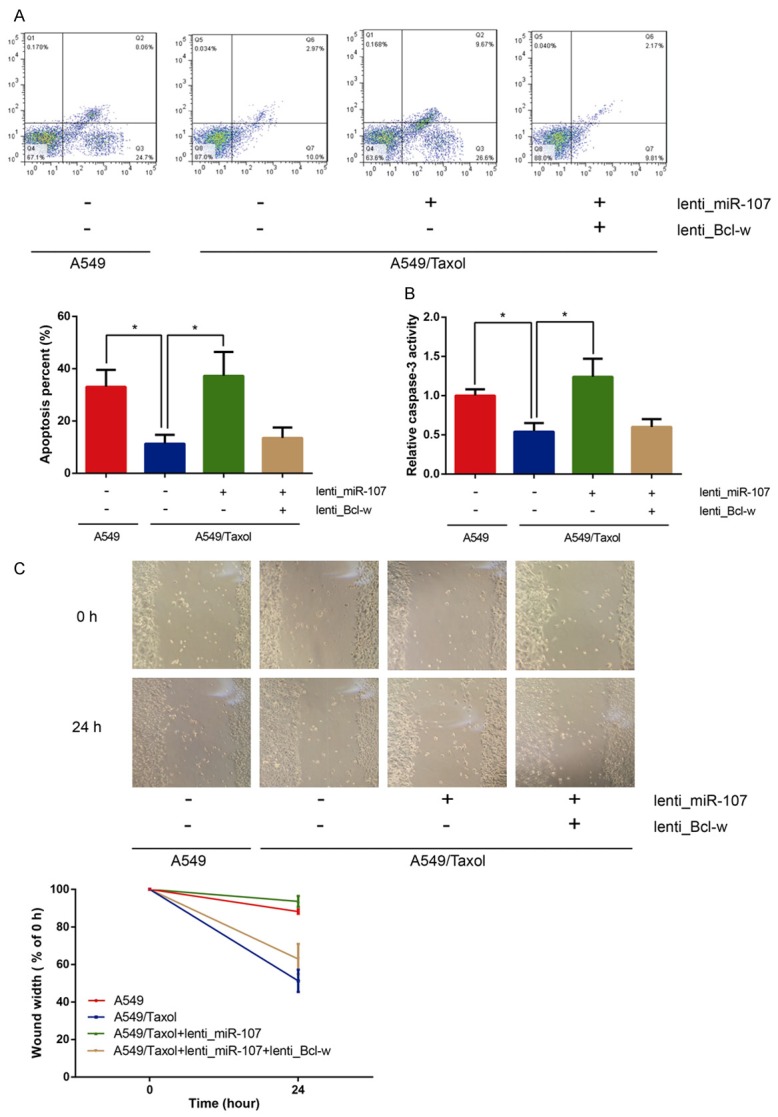
MiR-107 overexpression promotes apoptosis and inhibits mobility of A549/Taxol cells in treatment with paclitaxel in vitro. A. The percentages of apoptotic cells analyzed by flow cytometry. B. Cell apoptosis was determined by caspase-3 activity assay. C. Wound-healing assay was performed to determine cell mobility. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
Wound healing assay was conducted to assess the cell mobility to paclitaxel of A549/Taxol and A549 cells. As illustrated in Figure 4C, the cell mobility to paclitaxel of A549/Taxol cells was not remarkably suppressed than that of their parental A549 cells (P<0.05), and overexpression of miR-107 significantly inhibited the cell mobility to paclitaxel of A549/Taxol cells (P<0.05). Besides, co-transfection of lenti_Bcl-w could reverse the restrained cell mobility to paclitaxel of A549/Taxol cells transfected with lenti_miR-107.
MiR-107 inhibits paclitaxel resistance in vivo
On the basis of suppressive role of miR-107 to paclitaxel resistance, in order to further verify our findings in vivo, we investigated the effects of miR-107 on tumor growth received paclitaxel in nude mice. The combination of miR-107 overexpression and paclitaxel treatment resulted in a significant decrease of tumor growth. As shown in Figure 5A-C, the average tumor volume and weight were remarkably down-regulated in the group of miR-107 overexpression received paclitaxel compared to other groups (all P<0.05). Accordingly, these findings revealed that miR-107 could inhibit paclitaxel resistance in vivo.
Figure 5.
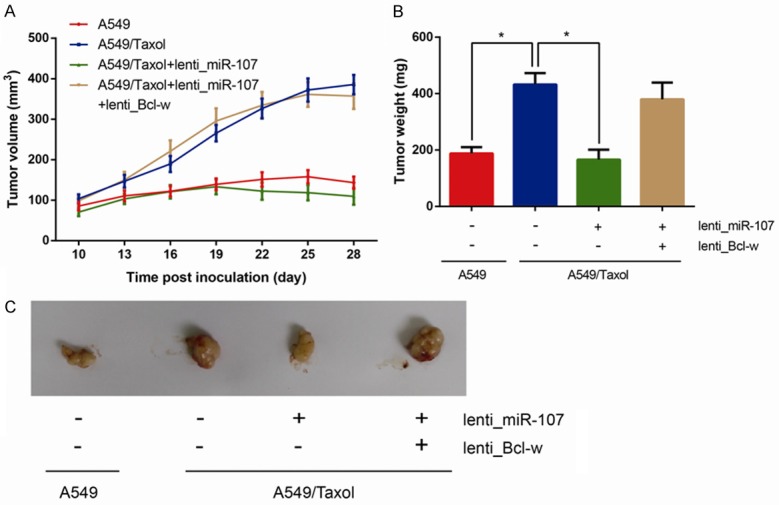
MiR-107 overexpression inhibits paclitaxel resistance in vivo. A. Tumor volume was measured and calculated every three days. B. Tumor weight was measured after four weeks of implantation. C. Representative pictures of xenograft tumors in four groups. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
MiR-107/Bcl-w axis regulates paclitaxel chemoresistance through PI3K-Akt pathway
We further explored the molecular mechanisms underlying miR-107/Bcl-w axis-regulated paclitaxel chemoresistance. Aberrant activation of PI3K-Akt pathway and genetic alterations of its components lead to tumorigenesis. We found that the levels of p-Akt and p-GSK-3β were significantly decreased in miR-107-overexpressing A549/Taxol cells, whereas overexpression of Bcl-w obviously restored these effects (all P<0.05, Figure 6). Based on these data, we suggested that miR-107/Bcl-w axis regulates paclitaxel chemoresistance through PI3K-Akt pathway.
Figure 6.
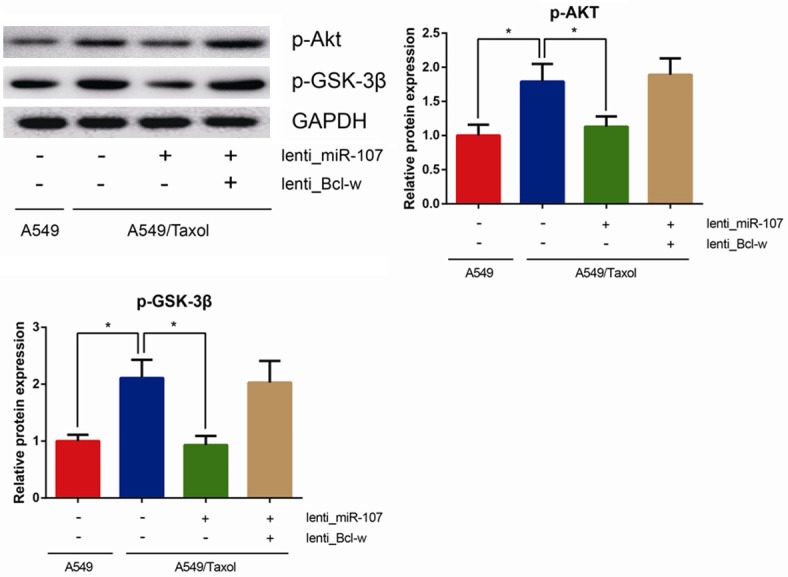
MiR-107/Bcl-w axis regulates paclitaxel chemoresistance through PI3K-Akt pathway. Levels of p-Akt and p-GSK3β in cell lysates were analyzed by western blotting using GAPDH as a loading control. Data are presented as means ± SDs, and *P<0.05 was considered to be statistically significant.
Discussion
NSCLC has become an increasingly prevalent malignancy worldwide. The combination of local radiotherapy/chemotherapy and surgery is regarded as one of the effective treatment approaches for NSCLC patients to make the unresectable tumors can be removed, and over the past decade, a wide variety of new chemotherapeutic agents have become available for the treatment of NSCLC, including paclitaxel and cisplatin [23]. However, chemoresistance still remains as one of the most leading obstacles to the successful therapeutic outcomes [24]. A large body of evidence revealed that the underlying mechanisms responsible for chemoresistance are likely to be multifaceted and extremely intricate, such as up-regulated DNA damage repair, inhibition of apoptosis and dysregulated metabolism of drugs.
Current studies suggest that miRNAs play a critical role in regulating the sensitivity of cancer cells to a great number of chemotherapeutic agents, including 5-FU (5-fluorouracil) [25,26], doxorubicin [27], and temozolomide [28] through post-transcriptionally regulating the expression of target proteins, such as drug transporters, drug targets, or apoptosis-associated components. Articles also revealed the role of multiple miRNAs in regulating sensitivities of NSCLC cells to the treatment of paclitaxel, including miR-135a [29], miR-337-3p [30] and miR-7 [31]. Abhisek Chatterjee et al. suggested revealed up-regulation of miR-16 prompted paclitaxel-induced apoptotic cell death via suppressing anti-apoptotic protein Bcl-2 [32].
MiR-107, a member of the miR-15/107 superfamily located on 10th chromosome, is aberrantly expressed in multiple solid tumors, including NSCLC, and its potential function has also been partly investigated. For example, the article of Yukari Takahashi et al. revealed that miR-107 induces cell cycle G1 arrest and suppresses invasion via directly modulating cyclin dependent kinase 6 (CDK6), thus suppressing tumor progression in NSCLC cells [33]. However, there is limited functional evidence of miR-107 in chemoresistance of NSCLC. In this study, by evaluating the miRNA expression patterns in paclitaxel-resistant cell subline A549/Taxol and parental cells A549, we uncovered that down-regulated miR-107 expression is significantly associated to paclitaxel-resistance in NSCLC, consistent with previous illustrated chemoresistance in gastric cancer because of reduced expression of miR-107 [34]. Besides, the sensitivity of A549/Taxol cells transfected with lenti_miR-107 to paclitaxel was significantly increased in comparison to negative control. Accordingly, we considered that down-regulation of miR-107 might contribute to the development and regulation of paclitaxel resistance in NSCLC cells.
Identification of miRNA gene targets helps us to further understand the function of miRNA in chemoresistance. According to the TargetScan database, miR-107 contained a length of specific converse binding sequence of Bcl-w. As we known, apoptosis is a fundamental process to both physiological and pathological development. Recently, alterations in the Bcl-2 family of anti-apoptotic proteins, including Bcl-2, Bcl-X, Bcl-w and Mcl-1, have been demonstrated to have a critical role in determining the effectiveness of chemotherapeutic agents for cancer treatment [35,36]. Xin Zhang et al. revealed overexpression of miR-203 could facilitate cisplatin sensitization through promoting apoptosis via directly targeting Bcl-w and Survivin [37]. As a member of the Bcl-2 family, Bcl-w has been indicated to promote tumorigenesis via suppressing the intrinsic pathway of apoptosis [38]. The mechanism by which Bcl-w inhibits apoptosis is that Bcl-w directly interacts with pro-apoptotic members (Bax and Bak) to suppress their apoptotic activities [39,40]. Lee et al. revealed that Bcl-w could restrain tumor cell apoptosis through blocking the SAPK/JNK pathway activation in gastric cancer cells [41]. Our experimental data also demonstrated that miR-107 could directly bind the 3’-UTR of Bcl-w, resulting in down-regulated expression of Bcl-w at post-transcriptional level, which might further contribute to increased paclitaxel-sensitivity in NSCLC cells.
In the present study, we originally indicated that down-regulation of miR-107 could sensitize NSCLC cells to paclitaxel treatment in a mouse model. Tumor volume was greatly decreased after miR-107 overexpression and paclitaxel treatment than in the group received paclitaxel only, indicating the potential role of miR-107 in regulating in vivo sensitivity of NSCLC cells to paclitaxel in the clinical application.
Over-activation of PI3K/AKT pathway has been found to be closely related to chemoresistance in NSCLC cells. MiR-107 overexpression inhibits, whereas Bcl-w overexpression promotes the activation of PI3K/AKT pathway in NSCLC cells and glioblastoma multiforme cells, respectively [42,43]. As expected, our results demonstrated that the levels of p-Akt and p-GSK-3β were reduced in miR-107-overexpressing A549/Taxol cells, whereas overexpression of Bcl-w obviously restored these effects, indicating that miR-107/Bcl-w axis might regulate paclitaxel chemoresistance through PI3K-Akt pathway.
In conclusion, we revealed that miR-107/Bcl-w signaling axis exerts a critical function in the development of paclitaxel-resistance in NSCLC cells, and that miR-107 overexpression could resensitize paclitaxel-resistant NSCLC cells through regulating Bcl-w expression and PI3K-Akt pathway. Our results demonstrated that chemotherapy combined with the modulation of miR-107 might be characterized as a promising therapeutic approach to overcome paclitaxel-resistance in NSCLC in the future.
Disclosure of conflict of interest
None.
References
- 1.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 2.Jalal SI, Ademuyiwa FO, Hanna NH. The role of maintenance chemotherapy in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2009;21:110–115. doi: 10.1097/CCO.0b013e328322cf49. [DOI] [PubMed] [Google Scholar]
- 3.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 4.De Furia MD. Paclitaxel (Taxol(R)): a new natural product with major anticancer activity. Phytomedicine. 1997;4:273–282. doi: 10.1016/S0944-7113(97)80081-5. [DOI] [PubMed] [Google Scholar]
- 5.Reeder A, Attar M, Nazario L, Bathula C, Zhang A, Hochbaum D, Roy E, Cooper KL, Oesterreich S, Davidson NE, Neumann CA, Flint MS. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer. 2015;112:1461–1470. doi: 10.1038/bjc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia SM, Harisa GI, Abd-Allah AR, Ahmad SF, Bakheet SA. The influence of lentinan on the capacity of repair of DNA damage and apoptosis induced by paclitaxel in mouse bone marrow cells. J Biochem Mol Toxicol. 2013;27:370–377. doi: 10.1002/jbt.21499. [DOI] [PubMed] [Google Scholar]
- 7.Diniz GP, Wang DZ. Regulation of Skeletal Muscle by microRNAs. Compr Physiol. 2016;6:1279–1294. doi: 10.1002/cphy.c150041. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Liang C. MicroRNAs: an emerging player in autophagy. ScienceOpen Res. 2015:2015. doi: 10.14293/S2199-1006.1.SOR-LIFE.A181CU.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Zhao L, Zhao F, Yang G, Wang J. MicroRNA-26b regulates cancer proliferation migration and cell cycle transition by suppressing TRAF5 in esophageal squamous cell carcinoma. Am J Transl Res. 2016;8:1957–1970. [PMC free article] [PubMed] [Google Scholar]
- 10.Undi RB, Kandi R, Gutti RK. MicroRNAs as Haematopoiesis Regulators. Adv Hematol. 2013;2013:695754. doi: 10.1155/2013/695754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto K, Oue N, Shinmei S, Sentani K, Sakamoto N, Naito Y, Hayashi T, Teishima J, Matsubara A, Yasui W. Expression of miR-486 is a potential prognostic factor after nephrectomy in advanced renal cell carcinoma. Mol Clin Oncol. 2013;1:235–240. doi: 10.3892/mco.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N, Li Y, Zhu LJ, Zhou RM, Jin W, Guo XQ, Wang CM, Chen ZF, Liu W. A functional polymorphism rs11614913 in microRNA-196a2 is associated with an increased risk of colorectal cancer although not with tumor stage and grade. Biomed Rep. 2013;1:737–742. doi: 10.3892/br.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, Wang H, Li Y, Li B, Li C, Ding C. A microRNA-related single nucleotide polymorphism of the XPO5 gene is associated with survival of small cell lung cancer patients. Biomed Rep. 2013;1:545–548. doi: 10.3892/br.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matuszcak C, Haier J, Hummel R, Lindner K. MicroRNAs: promising chemoresistance biomarkers in gastric cancer with diagnostic and therapeutic potential. World J Gastroenterol. 2014;20:13658–13666. doi: 10.3748/wjg.v20.i38.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolfo C, Fanale D, Hong DS, Tsimberidou AM, Piha-Paul SA, Pauwels P, Van Meerbeeck JP, Caruso S, Bazan V, Cicero G, Russo A, Giovannetti E. Impact of microRNAs in resistance to chemotherapy and novel targeted agents in non-small cell lung cancer. Curr Pharm Biotechnol. 2014;15:475–485. doi: 10.2174/1389201015666140519123219. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Yan X, Zhang G, Zhao W, Jiao S. MicroRNA-205 increases the sensitivity of docetaxel in breast cancer. Oncol Lett. 2016;11:1105–1109. doi: 10.3892/ol.2015.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Tang J, Liu X, Zeng L, Cheng C, Luo Y, Li L, Qin SL, Sang Y, Deng LM, Lv XB. Downregulation of miR-129-2 by promoter hypermethylation regulates breast cancer cell proliferation and apoptosis. Oncol Rep. 2016;35:2963–2969. doi: 10.3892/or.2016.4647. [DOI] [PubMed] [Google Scholar]
- 18.Datta S, Ray A, Singh R, Mondal P, Basu A, De Sarkar N, Majumder M, Maiti G, Baral A, Jha GN, Mukhopadhyay I, Panda C, Chowdhury S, Ghosh S, Roychoudhury S, Roy B. Sequence and expression variations in 23 genes involved in mitochondrial and non-mitochondrial apoptotic pathways and risk of oral leukoplakia and cancer. Mitochondrion. 2015;25:28–33. doi: 10.1016/j.mito.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Zubor P, Hatok J, Moricova P, Kajo K, Kapustova I, Mendelova A, Racay P, Danko J. Gene expression abnormalities in histologically normal breast epithelium from patients with luminal type of breast cancer. Mol Biol Rep. 2015;42:977–988. doi: 10.1007/s11033-014-3834-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Yu J, Liu H, Ma W, Yan L, Wang J, Li G. Novel Epigenetic CREB-miR-630 signaling axis regulates radiosensitivity in colorectal cancer. PLoS One. 2015;10:e0133870. doi: 10.1371/journal.pone.0133870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh M. Cardio-miRNAs and onco-miRNAs: circulating miRNA-based diagnostics for noncancerous and cancerous diseases. Front Cell Dev Biol. 2014;2:61. doi: 10.3389/fcell.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronnello C, Benos PV. ComiR: combinatorial microRNA target prediction tool. Nucleic Acids Res. 2013;41:W159–164. doi: 10.1093/nar/gkt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Govindan R, Grannis FW Jr, Grant SC, Horn L, Jahan TM, Komaki R, Kong FM, Kris MG, Krug LM, Lackner RP, Lennes IT, Loo BW Jr, Martins R, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Shapiro TA, Swanson SJ, Tauer K, Wood DE, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. quiz 653. [DOI] [PubMed] [Google Scholar]
- 24.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Jiang CF, Li DM, Ge X, Shi ZM, Li CY, Liu X, Yin Y, Zhen L, Liu LZ, Jiang BH. MicroRNA-497 inhibits tumor growth and increases chemosensitivity to 5-fluorouracil treatment by targeting KSR1. Oncotarget. 2016;7:2660–2671. doi: 10.18632/oncotarget.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Zhao L, Wei X, Wang L, Liu S, Yang Y, Wang F, Sun G, Zhang J, Ma Y, Zhao Y, Yu J. MicroRNA-320a promotes 5-FU resistance in human pancreatic cancer cells. Sci Rep. 2016;6:27641. doi: 10.1038/srep27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques SC, Ranjbar B, Laursen MB, Falgreen S, Bilgrau AE, Bodker JS, Jorgensen LK, Primo MN, Schmitz A, Ettrup MS, Johnsen HE, Bogsted M, Mikkelsen JG, Dybkaer K. High miR-34a expression improves response to doxorubicin in diffuse large B-cell lymphoma. Exp Hematol. 2016;44:238–246. e232. doi: 10.1016/j.exphem.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, You YP, Liu N, Jiang BH. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5:5416–5427. doi: 10.18632/oncotarget.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holleman A, Chung I, Olsen RR, Kwak B, Mizokami A, Saijo N, Parissenti A, Duan Z, Voest EE, Zetter BR. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene. 2011;30:4386–4398. doi: 10.1038/onc.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du L, Subauste MC, DeSevo C, Zhao Z, Baker M, Borkowski R, Schageman JJ, Greer R, Yang CR, Suraokar M, Wistuba II, Gazdar AF, Minna JD, Pertsemlidis A. miR-337-3p and its targets STAT3 and RAP1A modulate taxane sensitivity in non-small cell lung cancers. PLoS One. 2012;7:e39167. doi: 10.1371/journal.pone.0039167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Liu X, Zheng Y, Gu J, Xiong S, Jiang P, Jiang X, Huang E, Yang Y, Ge D, Chu Y. MicroRNA-7 sensitizes non-small cell lung cancer cells to paclitaxel. Oncol Lett. 2014;8:2193–2200. doi: 10.3892/ol.2014.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee A, Chattopadhyay D, Chakrabarti G. MiR-16 targets Bcl-2 in paclitaxel-resistant lung cancer cells and overexpression of miR-16 along with miR-17 causes unprecedented sensitivity by simultaneously modulating autophagy and apoptosis. Cell Signal. 2015;27:189–203. doi: 10.1016/j.cellsig.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Forrest AR, Maeno E, Hashimoto T, Daub CO, Yasuda J. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS One. 2009;4:e6677. doi: 10.1371/journal.pone.0006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Zhang L, Yin ZY, Fan XL, Hu B, Wang LQ, Zhang D. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int J Clin Exp Pathol. 2014;7:7236–7241. [PMC free article] [PubMed] [Google Scholar]
- 35.Kontos CK, Christodoulou MI, Scorilas A. Apoptosis-related BCL2-family members: key players in chemotherapy. Anticancer Agents Med Chem. 2014;14:353–374. doi: 10.2174/18715206113139990091. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Zhao Z, Wu K, Xu Z, Liu K. MCL-1 is the key target of adjuvant chemotherapy to reverse the cisplatin-resistance in NSCLC. Gene. 2016;587:147–154. doi: 10.1016/j.gene.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zhang Y, Liu X, Fang A, Li P, Li Z, Liu T, Yang Y, Du L, Wang C. MicroRNA-203 Is a prognostic indicator in bladder cancer and enhances chemosensitivity to cisplatin via apoptosis by targeting Bcl-w and survivin. PLoS One. 2015;10:e0143441. doi: 10.1371/journal.pone.0143441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 39.Bae IH, Yoon SH, Lee SB, Park JK, Ho JN, Um HD. Signaling components involved in Bclw-induced migration of gastric cancer cells. Cancer Lett. 2009;277:22–28. doi: 10.1016/j.canlet.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Um HD. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 2016;7:5193–5203. doi: 10.18632/oncotarget.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HW, Lee SS, Lee SJ, Um HD. Bcl-w is expressed in a majority of infiltrative gastric adenocarcinomas and suppresses the cancer cell death by blocking stress-activated protein kinase/c-Jun NH2-terminal kinase activation. Cancer Res. 2003;63:1093–1100. [PubMed] [Google Scholar]
- 42.Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol. 2016;49:1325–1333. doi: 10.3892/ijo.2016.3628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Lee WS, Woo EY, Kwon J, Park MJ, Lee JS, Han YH, Bae IH. Bcl-w enhances mesenchymal changes and invasiveness of glioblastoma cells by inducing nuclear accumulation of beta-Catenin. PLoS One. 2013;8:e68030. doi: 10.1371/journal.pone.0068030. [DOI] [PMC free article] [PubMed] [Google Scholar]


