Abstract
Poor prognosis of gastric cancer is related to not only malignancy of gastric cancer cells, but also the tumor microenvironment. Thus drugs, which can inhibit both of them, are urgently needed to be explored. Studies on effect of Proton-pump inhibitors (PPIs) in anti-neoplasms are increasing, but is rare in gastric in gastric cancer. Here we investigated how the gastric cancer microenvironment is regulated by PPIs. The objective response rate of gastric cancer patients in our hospital treated by PPIs is investigated. PPIs’ effects were further explored by observing the change of microRNAs, cytokines, cellular apoptosis. Bioinformatic pathway analysis of microarray was used to discover the pathway involved in PPIs’ regulation of gastric cancer microenvironments. Immunoblotting assays and qRT-PCR were used to define molecular events with PPIs treatment. We report here that PPIs can improve the prognosis of advanced gastric cancer patients; and inhibit the progress of gastric cancer both in vivo and in vitro. Moreover, high dose of PPIs can regulate the pathway associated with tumor malignancy and microenvironment via inhibiting the release of exosomes, which packed microRNAs. PPIs can inhibit the transformation of CAFs (cancer associated fibroblasts) and cytokines released from CAFs. In addition, PPIs inhibit the malignancy of gastric cancer through regulating HIF-1α-FOXO1 axis. High dose of PPIs can inhibit malignancy of gastric cancer and regulate its surrounding tumor microenvironment. This finding suggests that PPIs maybe of potential value as a therapeutic tool for treatment of gastric cancer.
Keywords: Proton-pump inhibitor, exosome, microRNA, gastric cancer
Introduction
Gastric cancer is the fourth most common cancer and the second most common cause of cancer-related mortality globally [1]. It is often diagnosed at an advanced stage [2] associated with poor survival and efficacy of systemic treatment, which is ascribed to the significant poor biological behavior [3-5]. Mounting evidences indicated tumor biologic behaviors were not only correlated to tumor cells but also to the tumor microenvironment [6]. Tumor microenvironment provides a shelter and supportive soil for tumor cells [7]. Hence, well-tolerated and effective anti-tumor agents, which can not only inhibit the progress of gastric cancer but also interfere with the complicated tumor microenvironment, are eagerly needed.
Gastric cancer cells survive in hypoxic and acid microenvironment [8]. The acid-outside pH gradient of cancer cells originates as a response to the metabolic adaptation to hypoxic tumor milieu. HIF-1α, which is known to regulate proton extrusion and PH homeostasis by enhancing the expression of plasma membrane ion pumps and transporters under hypoxic conditions [9], is activated. Moreover, extracellular pH affects the amount and characteristic of exosomes. Recent researches on cancer exosomes revealed that acidic microenvironment promotes exosomes traffic and uptake of cancer cells, contributing tumor favorable environment [10]. Previous studies have elucidated that exosomes carrying miRNAs secreted by cells was a new way of cell-cell interaction, which is potentially important in cell microenvironment regulation [11-13].
Clinically, Proton-pump inhibitors (PPIs) are safely used to treat a wide range of gastro-intestinal disorders like peptic ulcer, gastritis, etc. [14]. Recently a few studies found PPIs could improve chemosensitivity of gastric cancer cells [15-17] and change the acidity of the tumor microenvironment [18]. A pilot, prospective, randomized, phase II clinical study showed intermittent high dose of PPIs improved the efficacy of chemotherapy in breast cancer patients without obvious toxicity [19]. However, the effects and mechanism of PPIs in the treatment of gastric cancer remain unclear.
In this study, we explored the clinical results of PPIs in treating advanced gastric cancer patients from our hospital, and investigated the mechanism.
Material and methods
Patients in TCGA database and our hospital
RNA expression from TCGA stomach adenocarcinoma were downloaded from the website of THE CANCER GENOME ATLAS (https://cancergenome.nih.gov).
Seven cases of metastatic gastric adenocarcinoma treated in our hospital and enrolled in a single arm pilot study were analyzed. All patients received high dose of esomeprazole (120 mg, qd, for 2 days prior to chemotherapy; or 60 mg, qd, for 6 days, started 3 days before chemotherapy) combined with salvage chemotherapy. The study was approved by Ethical Committee of Tianjin Medical University Cancer Institute and Hospital, and performed in accordance with the Declaration of Helsinki of the World Medical Association. All patients had given written informed consent to the work.
Cell culture and transfection
The gastric cancer cell line SGC7901 was purchased from the Cell Resource Center, Peking Union Medical College (Beijing, China). Human skin fibroblast cell line HFF-1 were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The details of cell culture and transfection was performed as described in Supplementary Materials and Methods.
Ethics, consent and permissions
The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local regulatory requirements and laws. Study procedures were approved by institutional ethical board of Tianjin Medical University Cancer Institute and Hospital. Written informed consent was obtained from all patients.
Animal studies
Female 4-6-week-old BALB/c Nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd.) were maintained in a barrier facility on HEPA-filtered racks. All animal studies were conducted under an approved protocol in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals and was approved by Animal Care. Mice (approval 2016080) were approved by the Animal Care and Use Committee of Tianjin Medical University Cancer Institute and Hospital, China. Cells were harvested by trypsinization, washed in PBS, resuspended at 2 × 107 cells/ml in PBS, and then injected subcutaneously into the right flank of BALB/c Nude mice. Primary tumors were measured in 2 dimensions (a, b), and volume (mm3) was calculated as a (mm) × b2 (mm2)/2. Primary tumors were harvested from the flank of mice.
Statistical analysis
All of the data were representative of at least 3 independent experiments. The data were expressed as the mean ± S.E. of at least three separate experiments. Statistical significance was considered at P < 0.05 using Student’s t-test. In this study, GraphPad Software was used to conduct the analysis.
Other Supplementary Material and Methods were conducted as described in the Supplementary Materials and Methods.
Results
PPIs promoted the prognosis of patients with advanced gastric cancer and inhibited proliferation and metastasis in vivo
Table 1 showed the baseline characteristics of the seven patients in our hospital, including 6 male and 1 female. All patients were previously treated with at least one combination chemotherapy regimen but failed. The company regimens with high dose of PPIs are mainly iriontecan or docetaxel. The addition of esomeprazole to chemotherapy was well tolerated without obvious toxicity. Consequently, two patients had partial response (PR), three achieved stable disease (SD), and two presented with progressive disease (PD) (Table 1). The disease control rate was 71.4%, while the response rate was 28.6%. Results of the survival are not matured.
Table 1.
The baseline characteristics of the seven patients in our hospital
| Patient | Gender | Previous Regimen No | Salvage Chemotherapy | Esomeprazole | Cycles | Objective Response |
|---|---|---|---|---|---|---|
| Case 1 | Male | 1 | Irinotecan | 120 mg, for 2 days | 3 | PD |
| Case 2 | Male | 2 | Docetaxel | 120 mg, for 2 days | 2 | SD |
| Case 3 | Male | 1 | Irinotecan | 120 mg, for 2 days | 3 | PD |
| Case 4 | Male | 1 | Docetaxel | 120 mg, for 2 days | 9 | SD |
| Case 5 | Female | 1 | Docetaxel | 120 mg, for 2 days | 6 | SD |
| Case 6 | Male | 2 | Irinotecan | 60 mg, for 6 days | 5 | PR |
| Case 7 | Male | 1 | 5FU/LV+Oxaliplatin | 60 mg, for 6 days | 5 | PR |
After injecting 5.8 mg PPIs (Omeprazole) or Saline intraperitoneal into the paired tumor-bearing BALB/c Nude mice for 21 days. We found PPIs could limit the tumor volume of mice (Figure 1A-C).
Figure 1.
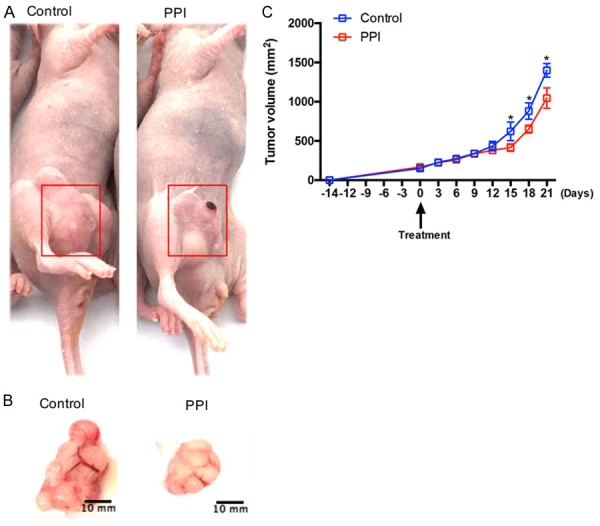
PPIs inhibites SGC-7901 tumor proliferation and metastasis in vivo xenograft model. A, B. Typical images of tumor that formed in nude mice treated with (and without) PPIs. SGC-7901 (4 × 106 cells per mouse) were injected subcutaneously into the right flank of BALB/c Nude mice and induced tumors. C. Effect of PPIs (5.8 mg/day) on the volume of tumor in vivo xenograft model during 21 days (n=5 per group). Asterisk indicates a significant difference determined by unpaired two-tailed t test (* indicates P < 0.05).
PPIs promote the effects of anti-tumor drugs and apoptosis and inhibits proliferation, cell migration and invasion of SGC-7901 gastric cancer cells
To explore whether PPIs (omeprazole) could suppress gastric cancer cell proliferation, we selected SGC-7901 cells, which were treated with different concentrations of PPIs and for various time points, then cell proliferation was evaluated by CCK8 assays. It was observed that the inhibitory role of PPIs on SGC-7901 cells gradually increased with time and change in concentration (Figure 2A). We then explored whether PPIs could enhance the chemosensitivity. The relative sensitivity to cisplatin (DDP), paclitaxel (TAX) and 5-FU of gastric cancer cells was determined by the CCK8 assay, with or without pretreatment with PPIs (80 µg/ml). The results of repeated experiments indicated that pretreatment with omeprazole induced the susceptibility of gastric cancer cells to the cytotoxic effect of cisplatin, paclitaxel and 5-FU (Figure 2B). To further verify whether PPIs could induce apoptosis in gastric cancer. We analyzed cell apoptosis using the annexin-V-FITC and propidium iodide (PI) staining assays. It wasfound that PPIs could enhance apoptosis SGC-7901 cells (Figure 2C) at high dose of PPIs. In order to determine whether omeprazole could affect cell motility, transwell assays were performed after incubating with different dose of omeprazole for 24 h. Transwell assays showed that gastric cancer cells treated at higher dose of PPIs showed a lower ratio in migration and invasion (Figure 2D).
Figure 2.
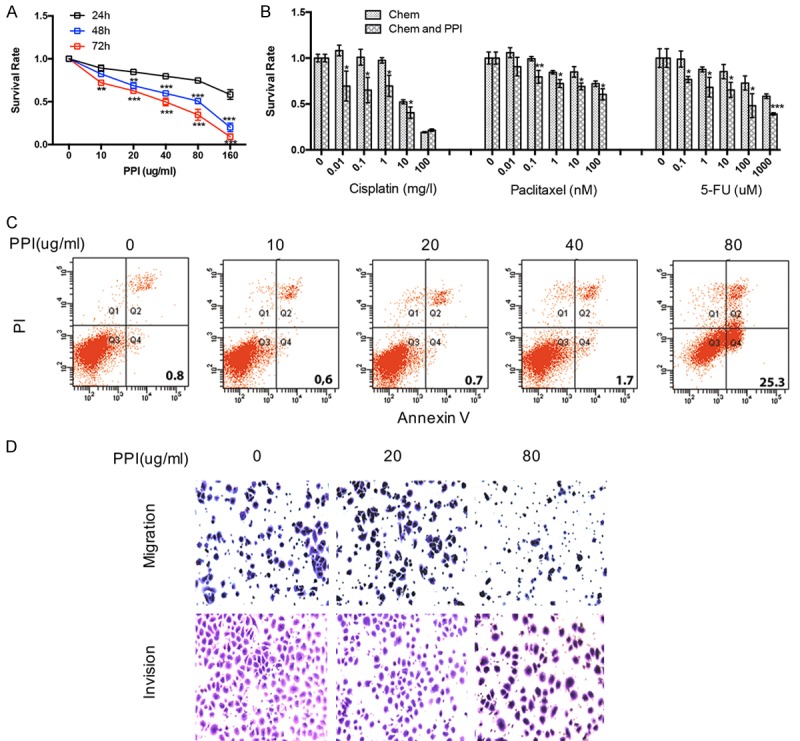
PPIs facilitate the effects of anti-tumor drugs, autophagy and apoptosis and inhibits proliferation, cell migration and invasion of SGC-7901 gastric cancer cells. A. CCK8 assay analysis showing cell viability following PPIs (80 ug/ml) treatment at various concentrations as indicated for 24, 48, and 72 h. Percentages of cell viability is presented as mean ± S.E.M. (n=5). B. SGC-7901 cells were treated with cisplatin, paclitaxel, and 5-FU at indicated concentration combined with (or without) PPIs (80 ug/ml) for 24 h. Percentages of cell viability is presented as mean ± S.E.M. (n=5). C. PPIs effect on apoptosis of SGC-7901 cells at indicated concentration for 24 h (n=3). D. Typical images of migrated and invasive SGC-7901 cells in transwell assays (n=3) following treatment with PPIs at indicated concentration for 24 h. All photographs were taken at a magnification of ×200. Asterisk indicates a significant difference determined by unpaired two-tailed t test (*** indicates P < 0.001; ** indicates P < 0.01; * indicates P < 0.05).
PPI regulates FOXO1 in SGC-7901 gastric cancer cells
TCGA database showed mRNA of FOXO1 had appositive correlation with AJCC tumor pathologic in gastric cancer patients (Figure 3A). Nature, 2014 database also proved this (Supplementary Figure 1A). These data showed FOXO1 had correlation with the progression of gastric cancer, and PPIs might regulate FOXO1 in gastric cancer.
Figure 3.
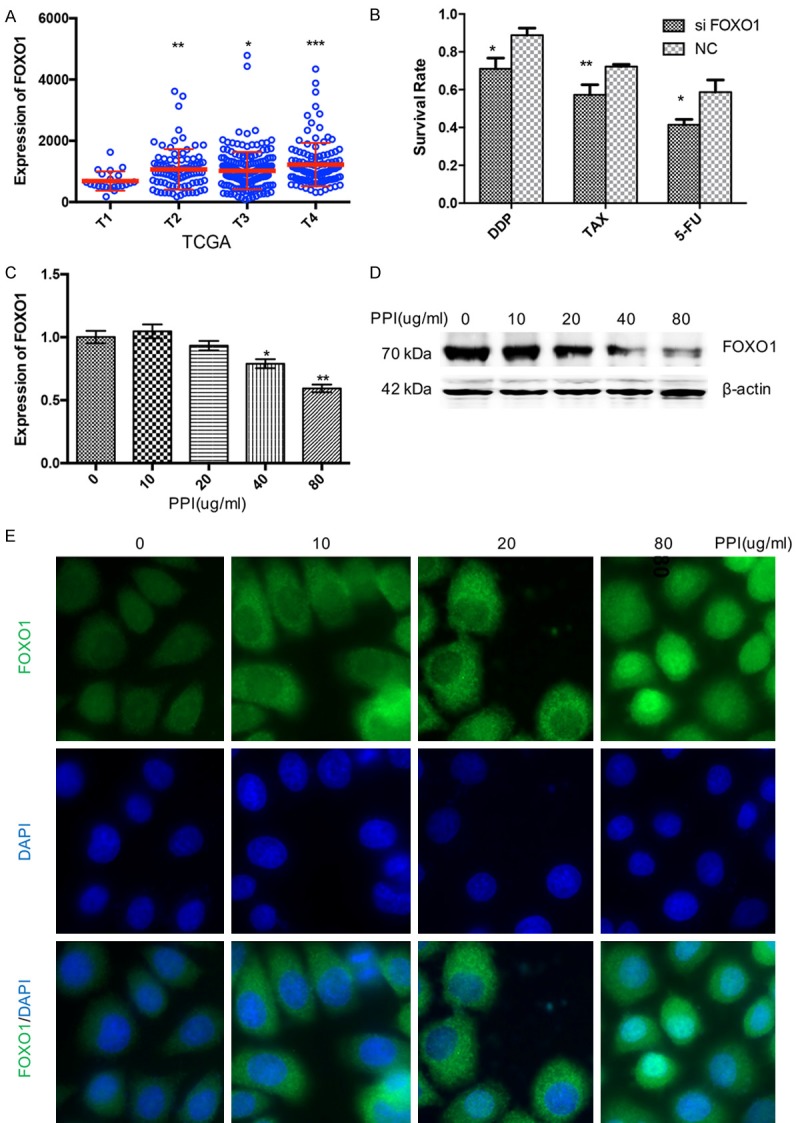
PPIs upregulates FOXO1 in SGC-7901 gastric cancer cells. A. TCGA databases show mRNA of FOXO1 have positive correlation with AJCC tumor pathologic in gastric cancer patients. B. SGC-7901 cells were treated with cisplatin (DDP, 10 mg/l), paclitaxel (TAX, 100 nM), and 5-FU (1 mM) combined with scrambled siRNA or FOXO1 siRNA for 24 h. Percentages of cell viability is presented as mean ± S.E.M. (n=5). C. PPIs effect on the mRNA expression of FOXO1 of SGC7901 cells at indicated concentration for 24 h (n=5). D. PPIs effect on the expression of FOXO1 and β-actin of SGC7901 cells at indicated concentration for 24 h (n=3). E. SGC7901 cells were treated with PPIs at indicated concentration for 24 h, and the location of FOXO1 were tested by immunofluorescence. And the nucleus of SGC7901 cells was stained by DAPI (n=3). All photographs were taken at a magnification of ×200. Asterisk indicates a significant difference determined by unpaired two-tailed t test (*** indicates P < 0.001; ** indicates P < 0.01; * indicates P < 0.05).
To explored whether FOXO1 was associated with drug resistance in gastric cancer, siRNA was used to knock down the expression of FOXO1. Following transfection, cells were treated with cisplatin, paclitaxel, 5-FU or PBS. Cell proliferation was evaluated by CCK8 assays. Transfection of siRNA of FOXO1 could enhance the cytotoxicity of the anti-tumor drugs cisplatin, paclitaxel and 5-FU in 24 h (Figure 3B). Furthermore, to verify whether PPI could regulate the expression of FOXO1 in gastric cancer, qRT-PCR was conducted and demonstrated omeprazole could inhibit expression of FOXO1 mRNA in high dose (Figure 3C). Western blotting showed that the expression of FOXO1 decreased at high dose of PPIs (Figure 3D). These data suggesting that high dose of PPIs could inhibit the expression of FOXO1 and enhance the effect of anti-tumor drugs though FOXO1.
Researches have proved that FOXO1 could promote apoptosis signaling through the activation or repression of apoptosis-related genes in the nucleus [45], and cytosolic FOXO1 is essential for the induction of autophagy [44]. We then explored whether PPIs affects the cellular distribution of FOXO1 in gastric cancer cells by immunofluorescence. FOXO1 was primarily localized to the cytoplasm. When we treated SGC-7901 cells with relative low dose of PPI, FOXO1 stayed the same. However, FOXO1 was shuttled into the nucleus when we treated gastric cancer cells with PPIs at 80 ug/ml (Figure 3E). It was revealed that high dose of PPIs induced apoptosis though regulating the cellular distribution of FOXO1.
PPIs regulates FOXO1 though HIF-1α in SGC-7901 gastric cancer cells
TCGA database showed mRNA of HIF-1α had positive correlation with AJCC metastasis pathologic in gastric cancer patients (Figure 4A). Nature, 2014 database also proved this (Supplementary Figure 1B). Then we explore whether FOXO1 had a relation with HIF-1α in gastric cancer. TCGA databases and Nature, 2014 database reveal the positive correlation of mRNA between FOXO1 and HIF-1α (Figure 4B and Supplementary Figure 1C). And these data showed HIF-1α had a correction with the malignancy of gastric cancer and PPIs might inhibit the progress of gastric cancer by affecting HIF-1α-FOXO1 axis.
Figure 4.
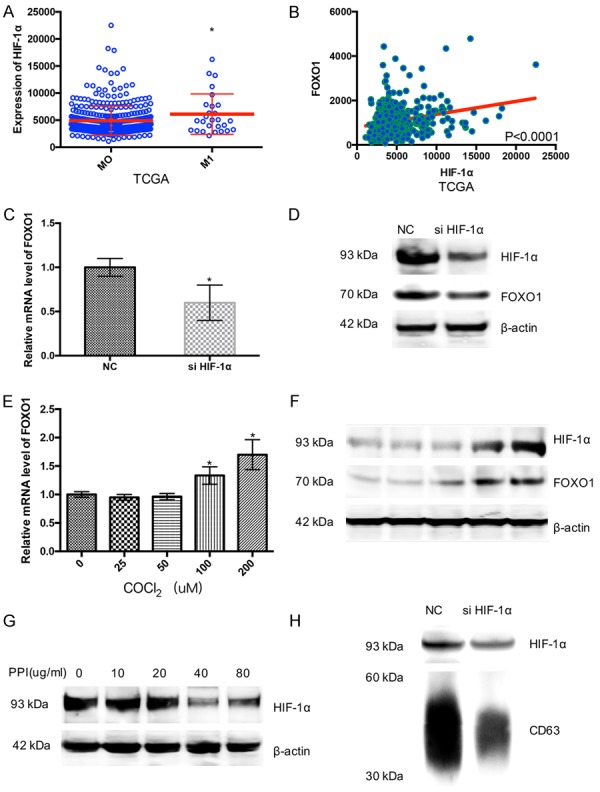
PPIs upregulates FOXO1 though HIF-1α in SGC-7901 gastric cancer cells. A. TCGA databases show mRNA of HIF-1α have positive correlation with AJCC metastasis pathologic in gastric cancer patients. B. TCGA database reveal the positive correlation of mRNA between FOXO1 and HIF-1α. C. Scrambled siRNA or HIF-1α siRNA effect on the expression of FOXO1 of SGC7901 cells for 24 h (n=3). D. Scrambled siRNA or HIF-1α siRNA effect on the mRNA expression of FOXO1 of SGC7901 cells for 24 h (n=3). E. COCl2 effect on the Mrna expression of FOXO1 of SGC7901 cells at indicated concentration for 24 h (n=3). F. COCl2 effect on the expression of HIF-1, FOXO1 and β-actin of SGC7901 cells at indicated concentration for 24 h (n=3). G. PPIs effect on the expression of HIF-1α and β-actin of SGC7901 cells at indicated concentration for 24 h (n=3). H. Scrambled siRNA or HIF-1α siRNA effect on the expression CD63 and HIF-1α in exosome of SGC7901 cells for 24 h (n=3). Asterisk indicates a significant difference determined by unpaired two-tailed t test (*** indicates P < 0.001; ** indicates P < 0.01; * indicates P < 0.05).
siRNA was used to knock down the expression of HIF-1α, and qRT-PCR showed the expression of FOXO1 decreased in SGC7901 cells with the transfection of si-HIF-1α (Figure 4C). Western blotting suggested that both of HIF-1α and FOXO1 decreased in SGC7901 cells (Figure 4D). SGC7901 cells were treated with different dose of CoCl2 which could maintain a high level of HIF-1α in cancer cells. qRT-PCR showed the RNA expression of FOXO1 increasedin hypoxia (Figure 4E). Western blotting showed that the expression of FOXO1 was markedly highas the expression of HIF-1α increased in SGC7901 cells (Figure 4F). These results showed HIF-1α could promote FOXO1 transcription and expression and highdose of omeprazole could decrease FOXO1 through inhibiting the expression of HIF-1α to restrainthe progress of gastric cancer.
It was revealed that the expression of HIF-1α experienced an opposite tendency with the concentration of PPIs enrichment (Figure 4G). siRNA was used to knock down the expression of HIF-1α, Western blotting showed siRNA could inhibit the HIF-1α expression in exosomes, and could also suppress exosomes release from gastric cancer cells (Figure 4H).
PPIs regulated tumor microenvironment by inhibiting the release of exosomes and exosomes carrying miRNAs
We explored whether PPIs could inhibit the exosomes release. Firstly, we confirmed the exosome by electronic speculum (Supplementary Figure 1D). SGC-7901 cells were treated with 80 ug/ml PPIs for 24 h, and then exosomes were isolated and resuspended in the PBS. The expression of CD63, a well-known marker of exosomes, was confirmed by means of Western blotting, which showed a lower level in the PPIs treated group (Figure 5A). And BCA protein assay proved that omeprazole could inhibit exosomes release (Figure 5B). What’s more, to explore whether PPIs could affect tumor microenvironment through exosomes, we added exosomes derived from SCG-7901 cells treated with or without PPIs into medium of HFF-1 cells, respectively (Figure 5C). Western blotting showed exosomes could induce CAFs transformation, however less CAFs transformation was induced by exosomes treated with PPIs. Furthermore, PKH67 was used to label exosomes. 30 ul exosomes was added in the medium of HFF-1 cells for 3 h, and fluorescence microscope showed exosomes could effectively enter into HFF-1 cells. However, less exosomes from SGC-7901 cells treated with PPIs could enter into HFF-1 cells (Supplementary Figure 1E).
Figure 5.
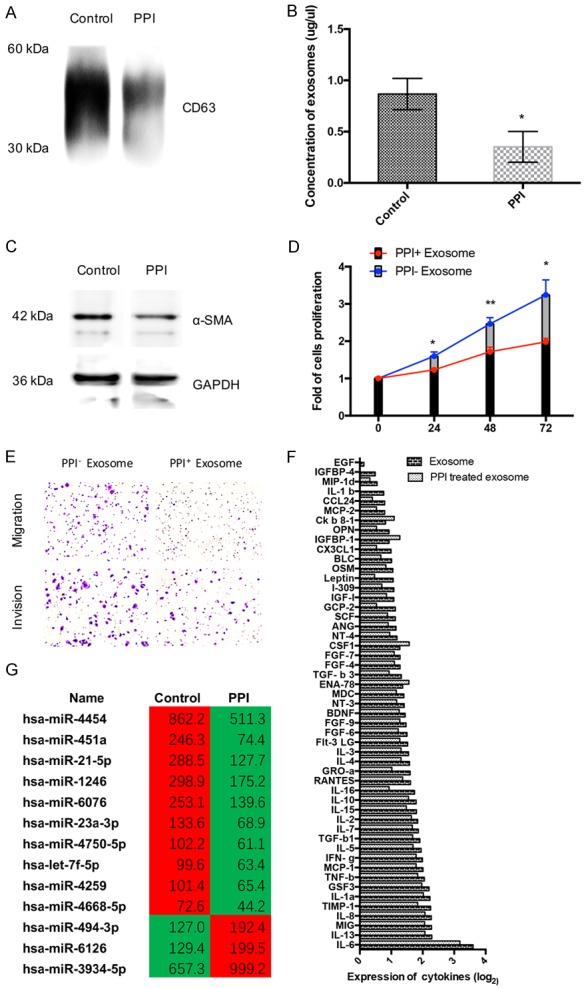
PPIs inhibited the release of exosomes and exosomes related miRNA and regulated tumor microenvironment though regulating exosomes. A. PPIs (80 ug/ml, 24 h) effect on the expression CD63 in the exosome of SGC7901 cells (n=3). B. PPI (80 ug/ml, 24 h) effect on the concentration of exosomes were measured by BCA protein assay (n=5). C. HFF-1 cell were treated with exosome of SGC7901 cells (80 ug/ml of PPI or PBS (control) treated for 24 h), and the expression of α-SMA and β-actin of HFF-1 cells were tested by Western Bloting (n=3). D. SGC7901 cells were cultured with the medium of HFF-1 cell, which were treated with exosome of SGC7901 cells (80 ug/ml of PPI or PBS (control) treated for 24 h). CCK8 assay was performed to analyze cell proliferation of SGC-7901 cells (n=5). E. SGC7901 cells were cultured with the medium of HFF-1 cell, which were treated with exosome of SGC7901 cells (80 ug/ml of PPI or PBS (control) treated for 24 h). Migration and invasion of SGC-7901 cells were tested by transwell assays (n=3). F. HFF-1 cell were treated with exosome of SGC7901 cells (80 ug/ml of PPI or PBS (control) treated for 24 h), and secreted cytokine were tested by Human Cytokine Array (n=3). G. The unsupervised hierarchical clustering analysis (microarray) of de-regulated miRNAs of exosome. The left is control group and right is the PPIs group. (|Fold change| ≥ 1.5 and P < 0.05) (n=3). All photographs were taken at a magnification of ×200. Asterisk indicates a significant difference determined by unpaired two-tailed t test (*** indicates P < 0.001; ** indicates P < 0.01; * indicates P < 0.05).
After cultured with exosome derived from SGC7901 cells (80 ug/ml of PPIs or PBS (control) treated for 24 h), the media of HFF-1 cells were collected and then replaced the normal media of SGC7901 cells for 24 h. To determine the impact of cytokines secreted from CAFs on gastric cancer cells, CCK8 and transwell assays were conducted. It showed that PPIs could also inhibit the proliferation (Figure 5D), migration and invasion (Figure 5E) of SGC7901 cells through the transportation of exosomes in tumor microenvironment.
We added exosomes, which were released from PPIs-treated (or untreated) SCG-7901 cells into medium of HFF-1 for 24 h. We measured cytokines via the Human Cytokine Array G5. The markedly changes of respectable cytokines were found in medium (Figure 5F), which could also promote the progression of gastric cancer. Our results inferred that PPIs could affect the CAFs transformation by regulating the exosomes derived from gastric cancer cells.
We performed microarray analysis to compare the signature difference of microRNA within normal SGC-7901 secreted exosomes and PPIs-treated exosomes (Figure 5G). Overall, we detected 13 miRNAs out 4774 arrayed miRNAs. The screening criteria was |Fold change| ≥ 1.5 and P < 0.05. Where in 3 miRNAs showed a significant upregulation and 10 miRNAs showed a significant down-regulation. In order to determine the biological processes regulated by up-regulated and down-regulated miRNAs, we performed Bioinformatics pathways analysis using DIANA-mirPath program. Our data demonstrated 30 significantly enriched KEGG pathways (P < 0.01, FDR corrected), which are probably under the control of aforementioned miRNAs (Table 2). Among those, tumor invasion and metastasis related pathway, adherence junction and focal adhesion pathway, malignancy of tumor related pathway, FoxO and HIF-1 pathway and TGF-beta pathway (tumor microenvironment related pathway) were involved in the pathway regulated by the exosomes contained miRNAs.
Table 2.
Top 30 cellular pathways influenced by dysregulated exosomes miRNAs
| KEGG pathway | p-value | Genes | miRNAs |
|---|---|---|---|
| MicroRNAs in cancer | 4.70E-42 | 94 | 11 |
| Proteoglycans in cancer | 5.98E-12 | 110 | 11 |
| Renal cell carcinoma | 2.70E-09 | 45 | 11 |
| Hepatitis B | 4.18E-08 | 74 | 11 |
| Pancreatic cancer | 3.70E-07 | 46 | 11 |
| Prion diseases | 8.29E-07 | 13 | 8 |
| Adherents junction | 8.29E-07 | 43 | 9 |
| Pathways in cancer | 8.29E-07 | 189 | 11 |
| Thyroid hormone signaling pathway | 1.18E-06 | 65 | 11 |
| Colorectal cancer | 2.01E-06 | 41 | 11 |
| Glioma | 2.01E-06 | 39 | 11 |
| Chronic myeloid leukemia | 6.23E-06 | 46 | 11 |
| Protein processing in endoplasmic reticulum | 1.20E-05 | 91 | 11 |
| Fatty acid biosynthesis | 1.27E-05 | 6 | 6 |
| FoxO signaling pathway | 2.28E-05 | 75 | 11 |
| Endometrial cancer | 2.28E-05 | 34 | 11 |
| Prostate cancer | 2.28E-05 | 54 | 11 |
| Non-small cell lung cancer | 2.45E-05 | 36 | 11 |
| Hippo signaling pathway | 3.24E-05 | 76 | 11 |
| ErbB signaling pathway | 6.07E-05 | 48 | 12 |
| Phosphatidylinositol signaling system | 6.10E-05 | 45 | 10 |
| Cell cycle | 9.15E-05 | 65 | 10 |
| Lysine degradation | 0.000108681 | 25 | 8 |
| TGF-beta signaling pathway | 0.000118299 | 40 | 10 |
| Viral carcinogenesis | 0.000428011 | 80 | 11 |
| Focal adhesion | 0.000587465 | 104 | 11 |
| p53 signaling pathway | 0.000610132 | 42 | 6 |
| Bacterial invasion of epithelial cells | 0.000740575 | 41 | 9 |
| HIF-1 signaling pathway | 0.001817139 | 56 | 10 |
| Neurotrophin signaling pathway | 0.002456577 | 63 | 11 |
Discussion
Several preclinical studies have elucidated that PPIs can modulate tumor acidification and restore chemotherapeutic sensitivity in drug-resistant cancer cells [20-24]. But only two recent small sample size clinical studies had reported the promising value of PPIs in treating osteosarcoma [25] and breast cancer patients [19] yet.
Based on our pilot study, high dose of PPIs (esomeprazole) had promising DCR and response rate, considering the extremely poor efficacy in advanced gastric cancer second or third line treatment [27,28]. It is interesting that all the patients with PR were given higher accumulated dose of esomeprazole (three days before chemotherapy, and three days concurrent with chemotherapy). No partial response was seen among the rest of patients who received esomeprazole only two days prior to chemotherapy. The dose of esomeprazole we used was lower than HU’s regimen [19]. Because our study was only a non-controlled pilot study, the optimal regimen of high dose esomeprazole still needed to be further verified. We didn’t observe obvious side reactions induced by esomeprazole. Previous studies had also shown PPIs had no significant side effects [26], even at high dosages (as in patients with Zollinger-Ellison syndrome) [29,30]. Furthermore, our study confirmed high dose of PPIs could inhibit the tumor size in tumor-bearing BALB/c Nude mice compared to placebo. All of these results indicated PPIs had great potential in treating gastric cancer patients. However, the biological and molecular changes PPIs treatment gastric cancer cells were not well studied.
Previous studies demonstrated tumor exosomes release was increased by acidic PH [31] and PPIs selectively accumulate in acidic spaces and target the H+, K+-ATPase of cell to regulate cellular PH gradient [14]. But whether PPIs could regulate the gastric cancer derived exosomes was rarely known. In this study, we found that PPIs could inhibit exosomes release at the concentration of 80 ug/ml. Exosomal miRN-As have emerged as micro-communicators of pathologic conditions including cancer. Cancers can educate the tumor microenvironment in favor of metastasis [32]. In this research, we purified exosomes from SCG-7901 cells which was treated with PPIs, and analyzed by microarray. We detected 13 significantly changed miRNAs out of 4774 arrayed miRNAs. According to bioinformatics pathway analysis, we found TGF-β signaling pathway was correlated with tumor microenvironment and the transformation of CAFs [33]. CAFs have recently received attention because of their pivotal roles in tumor growth, angiogenesis, invasion, and metastasis by interacting with tumor cells. The contribution of CAFs to tumor cell proliferation and motility include cytokines, chemokines, growth factors [34]. In this study, we demonstrated exosome from gastric cancer cells treated with PPIs induced less CAFs transformation and cytokines including IL-6, IL-8, TIMP-1 and TGF-β1 release than that in the control group. Studies have demonstrated that cytokines including IL-6 [35], IL-8 [36], TIMP-1 [37], and TGF-β1 [38] contribute to the progress of gastric cancer. These results implied that PPIs could play an effective role by the form of exosome in tumor microenvironment. PPIs could suppress the transformation into CAFs and the secretion of cytokines, consequently. Thereby, PPIs could restrain the progression of malignant behavior of gastric cancer cells by means of exosome to some extent. Western blotting showed siRNA of HIF-1α could inhibit the HIF-1α expression exosomes, and could also depress exosomes delivery from gastric cancer cells. These results indicated the inhibition of exosomes release by PPIs at least partially through HIF-1α.
Previous studies demonstrated HIF-1 [39-42] and FOXO1 [43-46] had a correlation with malignancy of cancer. In this study, we found HIF-1α and FOXO1 had positive correlation with AJCC metastasis pathologic in gastric cancer patients from TCGA database. We proved that high dose of PPIs could suppress cell proliferation and motility, enhance the effects of anti-carcinoma drugs, and induce apoptosis in gastric cancer cells. However, whether FoxO1 and HIF-1 pathway involved in these biological changes induced by PPIs remains unclear. We found PPIs could inhibit the expression of FOXO1 and induce cellular translocation of FOXO1. Studies have illuminated that FOXO1 could promote apoptosis signaling through the activation or repression of apoptosis-related genes in the nucleus [47,48], and cytosolic FOXO1 is essential for the induction of autophagy [46]. Consistent with previous studies, we discovered that high dose of PPIs induces nucleus translocation of FOXO1 to induce apoptosis. Meantime PPIs inhibit the tumor metastasis and enhance the effects of anti-cancer drugs by depressing the expression of FOXO1. Study has proved HIF-1α could lead to drug resistance in gastric cancer [49]. We found high-dose PPIs could also depress the expression of HIF-1α. We have proved HIF-1α could promote transcription of FOXO1 in gastric cancer. As a consequence, we demonstrated HIF-1α acted as upstream gene of FOXO1 and regulated the expression of FOXO1 with the administration of high-dose PPIs. These results indicate that PPIs can exert the most effect to inhibit gastric cancer at a high dose, and reasonable dose of PPIs should be formulated in clinical applications.
In this study, we found for the first time that high dose of PPIs could not only improve the prognosis of advanced gastric cancer patients, but also inhibit the progress of gastric cancer in vivo and in vitro. Furthermore, we showed that high-dose PPIs could not only inhibit the release of exosome and its packed microRNA to regulate gastric cancer and its microenvironment, but also enhance the effects of anti-tumor drugs, induce apoptosis, inhibit cell migration and invasion through regulating HIF-1α-FOXO1 axis in gastric cancer. Hence, high dose of PPIs will be used as a promising agent to relieve malignant progress of gastric cancer and regulate its surrounding tumor microenvironment in the future.
Data sharing statement
Microarray data deposited into the Gene Expression Omnibus (G.E.O.) with Accession NO. GSE87152 (https://www.ncbi.nlm.nih.gov/geo/). All the other supporting the findings of this study are available within the article and its Supplementary Information Files or from the corresponding author (D.H.) upon request.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (81572321), Nature Science Foundation of Tianjin City (15JCYBJC28200).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–44. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol. 2014;20:3905–15. doi: 10.3748/wjg.v20.i14.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 5.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for firstline treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 6.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XY, Hu SQ, Xiao L. The cancer-associated fibroblasts and drug resistance. Eur Rev Med Pharmacol Sci. 2015;19:2112–9. [PubMed] [Google Scholar]
- 8.Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM. Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer. 2005;41:2792–805. doi: 10.1016/j.ejca.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Taddei ML, Giannoni E, Comito G, Chiarugi P. Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett. 2013;341:80–96. doi: 10.1016/j.canlet.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Ban JJ, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461:76–9. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 11.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–74. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 13.Ren C, Chen H, Han C, Wang D, Fu D. Increased plasma microRNA and CD133/CK18-positive cancer cells in the pleural fluid of a pancreatic cancer patient with liver and pleural metastases and correlation with chemoresistance. Oncol Lett. 2012;4:691–4. doi: 10.3892/ol.2012.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn J. The proton-pump inhibitors: similarities and differences. Clin Ther. 2000;22:266–80. doi: 10.1016/S0149-2918(00)80032-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Yang Y, Shi X, Liao W, Chen M, Cheng AS, Yan H, Fang C, Zhang S, Xu G, Shen S, Huang S, Chen G, Lv Y, Ling T, Zhang X, Wang L, Zhuge Y, Zou X. Proton pump inhibitor pantoprazole abrogates adriamycin-resistant gastric cancer cell invasiveness via suppression of Akt/GSK-beta/beta-catenin signaling and epithelial-mesenchymal transition. Cancer Lett. 2015;356:704–12. doi: 10.1016/j.canlet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Gu M, Zhang Y, Zhou X, Ma H, Yao H, Ji F. Rabeprazole exhibits antiproliferative effects on human gastric cancer cell lines. Oncol Lett. 2014;8:1739–44. doi: 10.3892/ol.2014.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, Chen M, Ding X, Zhang X, Zou X. Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway. IntImmunopharmacol. 2013;17:585–92. doi: 10.1016/j.intimp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. 2013;2:e22058. doi: 10.4161/onci.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang BY, Zhang J, Wang JL, Sun S, Wang ZH, Wang LP, Zhang QL, Lv FF, Cao EY, Shao ZM, Fais S, Hu XC. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–86. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 21.Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho SW, Kim HC, Hahm KB. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin Cancer Res. 2004;10:8687–96. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 22.Ouar Z, Bens M, Vignes C, Paulais M, Pringel C, Fleury J, Cluzeaud F, Lacave R, Vandewalle A. Inhibitors of vacuolar H+-ATPase impair the preferential accumulation of daunomycin in lysosomes and reverse the resistance to anthracyclines in drug-resistant renal epithelial cells. Biochem J. 2003;370:185–93. doi: 10.1042/BJ20021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies RJ. Enhancement of chemotherapy by manipulation of tumourpH. Br J Cancer. 1999;80:1005–11. doi: 10.1038/sj.bjc.6690455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon S, Roy D, Schindler M. Intracellular pH and the control of multidrug resistance. Proc Natl Acad Sci U S A. 1994;91:1128–32. doi: 10.1073/pnas.91.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari S, Perut F, Fagioli F, Brach Del Prever A, Meazza C, Parafioriti A, Picci P, Gambarotti M, Avnet S, Baldini N, Fais S. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients’ bed. J Transl Med. 2013;11:268. doi: 10.1186/1479-5876-11-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 30:1513–8. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–25. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 28.Der G. An overview of proton pump inhibitors. Gastroenterol Nurs. 2003;26:182–90. doi: 10.1097/00001610-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Metz DC, Forsmark C, Lew EA, Starr JA, Soffer EF, Bochenek W, Pisegna JR. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison. Am J Gastroenterol. 2001;96:3274–80. doi: 10.1111/j.1572-0241.2001.05325.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramdani A, Mignon M, Samoyeau R. Effect of pantoprazole versus other protonpump inhibitors on 24-hour intragastric pH and basal acid output in Zollinger-Ellison syndrome. Gastroenterol Clin Biol. 2002;26:355–9. [PubMed] [Google Scholar]
- 31.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, Yan Y, Mao F, Zhao C, Shi Y, Xu W. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-β/Smad pathway. PLoS One. 2012;7:e52465. doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 35.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitadai Y, Haruma K, Mukaida N, Ohmoto Y, Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ, Tahara E. Regulation of disease-progression genesin human gastric carcinoma cells by interleukin 8. Clin Cancer Res. 2000;6:2735–40. [PubMed] [Google Scholar]
- 37.Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Yanoma S, Noguchi Y. Prognostic value of tissue inhibitor of matrix metalloproteinase-1 in plasma of patients with gastric cancer. Cancer Lett. 2000;151:81–6. doi: 10.1016/s0304-3835(99)00420-6. [DOI] [PubMed] [Google Scholar]
- 38.Tas F, Yasasever C, Karabulut S, Tastekin D, Duranyildiz D. Serum transforming growth factor-beta1 levels may have predictive and prognostic roles in patients with gastric cancer. Tumor Biol. 2015;36:2097–103. doi: 10.1007/s13277-014-2817-9. [DOI] [PubMed] [Google Scholar]
- 39.Nam SY, Ko YS, Jung J, Yoon J, Kim YH, Choi YJ, Park JW, Chang MS, Kim WH, Lee BL. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappaB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104:166–74. doi: 10.1038/sj.bjc.6606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q, Li Y, Tan BB, Fan LQ, Yang PG, Tian Y. HIF-1alpha induces multidrug resistance in gastric cancer cells by inducing MiR-27a. PLoS One. 2015;10:e132746. doi: 10.1371/journal.pone.0132746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka T, Kitajima Y, Miyake S, Yanagihara K, Hara H, Nishijima-Matsunobu A, Baba K, Shida M, Wakiyama K, Nakamura J, Noshiro H. The apoptotic effect of HIF-1alpha inhibition combined with glucose plus insulin treatment on gastric cancer under hypoxic conditions. PLoS One. 2015;10:e137257. doi: 10.1371/journal.pone.0137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohwer N, Lobitz S, Daskalow K, Jöns T, Vieth M, Schlag PM, Kemmner W, Wiedenmann B, Cramer T, Höcker M. HIF-1alpha determines the metastatic potential of gastric cancer cells. Br J Cancer. 2009;100:772–81. doi: 10.1038/sj.bjc.6604919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113:1186–96. doi: 10.1038/bjc.2015.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, Choi Y, Kim JH, Cheon Y, Lee BL. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17:423–30. doi: 10.1007/s10120-013-0314-2. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Yoon J, Ko YS, Chang MS, Park JW, Lee HE, Kim MA, Kim JH, Kim WH, Lee BL. Constitutive phosphorylation of the FOXO1 transcription factor in gastric cancer cells correlates with microvessel area and the expressions of angiogenesis-related molecules. BMC Cancer. 2011;11:264. doi: 10.1186/1471-2407-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–75. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–86. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32:221–31. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, Sun B, Zhang Q, Wang X, Jiang H, Krissansen GW, Qiao H, Sun X. Up-regulation of survivin by AKT andhypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer. FEBS J. 2014;281:115–28. doi: 10.1111/febs.12577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


