Abstract
Dysregulated expression of rab31, a member of the large Rab protein family of the Ras superfamily of small GTPases, has been observed in several types of cancer, including breast cancer. Rab31, depending on its expression level, may regulate the switch between an invasive versus proliferative phenotype of breast cancer cells in vitro. Moreover, gene expression of rab31 is induced by the C-terminal subunit of mucin-1 (MUC1-C) and estrogen receptors (ER). To gain further insights into the clinical relevance of rab31 and mucin-1 expression in breast cancer, we analyzed the relation between rab31 and mucin-1 (CA15-3) antigen levels in detergent tissue extracts of ER-positive (ER+) tumors and clinicopathological parameters as well as patients’ prognosis. No significant correlation was observed between rab31 and CA15-3 antigen levels. Elevated rab31 antigen levels in tumor tissue extracts were significantly associated with higher tumor grade (P = 0.021). Strikingly, an inverse significant association was observed for CA15-3 with tumor grade (P = 0.032). Furthermore, high rab31 antigen levels were significantly associated with a high S-phase fraction (SPF, P = 0.047), whereas a trend for lower CA15-3 antigen levels in tumor tissue displaying higher SPF was observed. High rab31 antigen levels were significantly associated with poor 5-year disease-free survival (DFS) of ER+ breast cancer patients in univariate Cox regression analysis (HR = 1.91, 95% CI = 1.14-3.17, P = 0.013). In contrast, high levels of CA15-3 antigen levels were associated with better patients’ prognosis (HR = 0.56, 95% CI = 0.33-0.95, P = 0.031). In multivariable analysis, rab31 antigen levels contributed independent prognostic information for DFS when adjusted for prognostically relevant clinicopathological parameters with a HR for high versus low values of 1.97 (95% CI = 1.09-3.54, P = 0.024), whereas CA15-3 antigen levels were not significant. Our results strongly suggest that rab31 antigen levels in tumor tissue are associated with the proliferative status, and rab31 represents an independent biomarker for prognosis in ER+ breast cancer patients. Total mucin-1 (CA 15-3) levels are rather inversely associated with tumor grade and SPF, and elevated levels even indicate prolonged DFS in ER+ breast cancer patients.
Keywords: Rab31, mucin-1, CA15-3, antigen levels, tumour tissue extracts, breast cancer, prognosis
Introduction
Due to the complexity of compartmentalization of eukaryotic cells the need arises for a cellular machinery that enables an actively, highly dynamic transport of lipids and proteins between distinct membrane-bound organelles. This process, involving continuous recycling of regulatory proteins between the cytosol and membranes and the fusion and transport of membrane-bounded carriers between organelles, requires different classes of molecules for regulation. One key set of proteins for membrane traffic regulation consists of Rab GTPases which belong to the Ras superfamily of small GTP-binding proteins [1]. Up to now, more than 60 different human Rab proteins have been identified, and a growing number of Rab and Rab-related proteins have been functionally characterized (for review see [2-4]). The importance of Rabs for intra-/intercellular processes is underlined by an increasing number of diseases, including cancer, attributed to Rab protein dysfunction [1,2,5-8].
Rab31 (also known as rab22B) is a 194 amino acid protein (Mr ≈ 22,000) which shares highest homology with rab22A (71% sequence identity), and together with rab22A and rab21 belongs to the Rab5-subfamily [9]. Rab31 is expressed fairly ubiquitously in normal human tissue and is mainly localized to the trans-Golgi, the trans-Golgi network (TGN) and to endosomes [10,11].
Generally, dysregulation of Rab pathways can lead to immunodeficiencies and neurological disorders, and has been shown to affect cancer progression [12-16]. Rab31 plays a role in benign skin and kidney disease, and is involved in multiple aspects of tumor progression in various types of cancer such as liver, ovarian, cervical, or breast cancer as well as glioblastoma (for review see [17]). In breast cancer, rab31 was identified as one out of 11 genes that are overexpressed in estrogen receptor-positive (ER+) breast cancer patients [18]. Furthermore, elevated rab31 mRNA levels are reported to be significantly associated with shorter distant metastasis-free and overall survival of untreated, lymph node-negative breast cancer patients [19]. Recently, the important role of rab31 in the modulation tumor biological-relevant processes in breast cancer in vitro and in vivo was reported [20]. Increased rab31 protein levels were associated with enhanced proliferation of breast cancer cells, led to reduced adhesion of cells towards extracellular matrix proteins and a decreased invasive capacity in vitro and in vivo [20]. These results suggest that rab31 overexpression may lead to a switch from an invasive to a proliferative phenotype.
Recently, the C-terminal subunit of mucin-1 (MUC1-C), in concert with estrogen receptor α (ERα), was identified to activate rab31 gene expression in breast cancer [21]. Mucin-1 is overexpressed in various human epithelial cancer types including breast cancer [22]. Moreover, mucin-1-derived antigen is mainly known as serum biomarker CA15-3 for monitoring metastatic disease of breast cancer [23,24]. However, the prognostic information provided by mRNA and protein expression levels of mucin-1 detected by cDNA arrays and by immunohistochemistry in breast cancer remains controversial [25-27].
Mucin-1 is a heterodimeric transmembrane glycoprotein. The large (> 1,000 amino acids [aa]) extracellular N-terminal chain (MUC1-N) harbors variable numbers of 20-aa tandem repeats that are extensively modified by O-linked glycans and forms a non-covalent complex with the C-terminal subunit MUC1-C, comprising an extracellular (58 aa), transmembrane (28 aa), and cytoplasmic domain (72 aa; [28]). In breast cancer, MUC-1N - in contrast to MUC-1C - is shed from the surface of the cancer cells and is detectable (as CA15-3) at increased levels in the serum. The cytoplasmic tail of MUC1-C interacts with various receptor tyrosine kinases such as EGFR and HER2 and, by this, may modulate downstream signaling pathways [28,29]. MUC1-C, which is internalized by clathrin-mediated endocytosis, has been shown to interact with ERα and stimulate ERα-mediated transcription of several genes including RAB31 [28-31]. In turn, rab31 protein is proposed to stimulate upregulation of MUC1-C in breast cancer cells likely by attenuating the degradation of MUC1-C in lysosomes forming an auto-inductive loop [21]. Furthermore, rab31 and MUC1-C were reported to be significantly co-expressed in tumor tissue of ER+ breast cancer patients [21]. Recently, MUC1-C was described to block inhibitory effects of tamoxifen on ERα-mediated rab31 promoter activity in breast cancer cells [32].
In the present study, we aimed at further evaluating the clinical relevance of both rab31 and mucin-1 expression in breast cancer. For this, we analyzed the relation between rab31 and mucin-1 (CA15-3) antigen levels in detergent ER+ breast cancer tissue extracts and clinicopathological parameters as well as patients’ prognosis.
Material and methods
Patients and tissues
Tumor tissue samples from a total of 284 patients with primary, estrogen receptor-positive (ER+) breast carcinoma who underwent breast cancer surgery at the Dresden University Medical Center and in regional hospitals during 1992-2000, and 2005-2006, were used in this retrospective study for the generation of tumor tissue extracts as well as fine needle aspiration. The study adhered to national regulations on ethical issues and was approved by the local ethical committee. Histopathological grade was determined according to Bloom and Richardson as modified by Elston and Ellis [33]. Tumor staging was performed according to the TNM classification system of the UICC (6th Edition 2002). Estrogen receptors were determined by immunohistochemistry and assessed using the Remmele score system which is based on a final immunoreactivity score created by multiplication of the intensity score (classified on a scale of 0 to three) with the positivity score (scale of one to four) values [34]. Cases with a Remmele score greater 1 were regarded as estrogen receptor-positive. Histological sections of all patients included in the study were re-evaluated by one of us (G.B.) with regard to tumor grading and histological type of the tumors. All other histological features were taken from the original histological reports. Locoregional treatment of patients consisted of modified radical mastectomy or breast conserving lumpectomy with axillary lymph node dissection. Postoperative locoregional radiotherapy was given to the breast after an incomplete resection or after breast conserving treatment. Adjuvant treatment was administered according to respective consensus recommendations at the time, complete clinical details on adjuvant systemic therapy, however, were not available for this patient cohort.
Patients who had a previous diagnosis of cancer or had a carcinoma in situ as well as patients with recurrent disease or distant metastasis within two months after surgery or with incomplete clinical parameters were excluded from the study. The patients’ age at time of diagnosis ranged from 26 to 91 years (median 60 years). The median follow-up time of the patients was 29 months (range 3 to 141 months). During that time, 64 patients experienced a disease recurrence (locoregional and/or distant metastasis), 28 of the patients have died.
Tumor tissue extracts
After surgery, tumor samples were snap frozen and stored in liquid nitrogen. Tumor tissues were extracted in ice-cold extraction buffer (20 mM Tris/HCl, 125 mM NaCl, 1% [v/v] Triton X-100, pH 8.5) as previously described [35,36]. After centrifugation, the supernatants were aliquoted and stored frozen in liquid nitrogen until use. Protein content was determined by the Micro BCA protein assay reagent kit (Pierce, Rockford, IL). Antigen levels were calculated as ng (rab31) or units (CA15-3) per mg of total protein.
Generation of polyclonal antibodies directed against rab31
Recombinant rab31, harboring an N-terminal histidine (His)6-tag, was expressed in Escherichia coli and purified by nickel-nitrilotriacetic acid agarose affinity chromatography (Qiagen, Hilden, Germany) under denaturing/slightly reducing conditions as described previously [37]. The purified recombinant rab31-His protein was used as antigen for immunization of rabbits. The generated polyclonal antibodies (pAb) directed to rab31 were purified and subsequently tested for specificity as described [20]. Purified antibody (IgG fraction) from rabbit #3 (pAb RT3-IgG) was found to be the most suitable antibody as it showed a strong reaction with its immunogen rab31-His as well as with recombinant GST-rab31, and did not cross-react with other members of the Rab protein family such as rab5 and rab22A that are closely related/highly homologous to rab31 [20].
ELISA format for rab31
For detection of rab31, a sandwich ELISA format was developed using a commercially available monoclonal antibody (mAb M01, Novus Biologicals, Wiesbaden, Germany) as the catcher, and pAb RT3-IgG as the detecting antibody as described previously [38].
Briefly, ninety-six well microplates (MaxiSorpTM; Nunc, Wiesbaden, Germany) were coated with 100 µl per well of capture antibody (mAb M01, 0.5 mg/ml) diluted 1:2,500 in coating buffer (15 mM Na2CO3, 33 mM NaHCO3, pH 9.6) overnight at 4°C. After washing the plates twice with washing buffer (0.14 M NaCl, 20 mM Na2HPO4, 20 mM KH2PO4 (PBS), containing 0.5% [v/v] Tween 20, pH 7.6), unspecific protein binding sites were blocked by adding 200 µl per well of blocking buffer (washing buffer containing 2% neonatal calf serum [v/v]; Gibco BRL, Eggenstein, Germany) for 30 min at 37°C. Thereafter, plates were incubated with 100 µl/well of test samples diluted in sample buffer (50 mM Tris/HCl, 100 mM NaCl, 0.2% [v/v] Triton X-100, 1% [w/v] BSA, pH 7.6) for 90 min at 37°C. Two-fold serial dilutions in sample buffer (0.15; 0.31; 0.62; 1.25; 2.5; 5.0; 10 ng/ml) of a stock solution of purified recombinant GST-rab31 (25 mg/ml), consisting of human rab31 fused C-terminally to the bacterial glutathione S-transferase (Grismayer, 2012) were used as the standard antigen. Following three wash steps, 100 µl per well of polyclonal anti-rab31detection antibody (pAb RT3-IgG) diluted 1:200 in blocking buffer were added to each well and incubated for 90 min at 37°C. After washing plates three times, 100 µl per well of peroxidase-labeled goat anti-rabbit IgG antibody (Novus; 0.5 mg/ml) diluted 1:1,000 in blocking buffer were added to each well for 60 min at 37°C. Following washing, a total of 100 µl of 3,3’,5,5’-tetramethylbenzidine (TMB; K & P Laboratories, Gaithersburg, MD) was added to each well for 20 min at room temperature in the dark. The reaction was stopped by addition of 200 µl per well of 0.5 M H2SO4, and the optical density was measured at 450 nm (reference wavelength 620 nm) using a multichannel microplate reader (SLT Spectra, Salzburg, Austria). Absorbance values were converted into ng/ml of rab31 by reference to the standard curve. Finally, the rab31 concentration is expressed as ng rab31 per mg of total protein content (ng/mg) of tumor tissue extracts.
Determination of CA15-3
The CA15-3 content was determined in the same lot of breast cancer tissue extracts as for rab31 applying the commercially available LIAISON® CA15-3 sandwich chemiluminescence immunoassay (Diasorin, Dietzenbach, Germany) according to the manufacturer’s instructions. Since tumor tissue extracts (instead of serum samples) were used for CA15-3 determination, we analyzed test precision/reproducibility of the commercial Diasorin CA15-3 assay using tissue extracts. All tissue extracts were diluted 10-fold in the same sample buffer (50 mM Tris/HCl, 100 mM NaCl, 0.2% [v/v] Triton X-100, 1% [w/v] BSA, pH 7.6) in an identical manner as for rab31 antigen determination. The intra-assay precision was determined by assaying 12 replicates of three different tumor tissue extracts (diluted tenfold in sample buffer) at a concentration of 6.1, 32.2, and 49.9 U/ml resulting in a coefficient of variation (CV) of 1.9%, 3.4%, and 3.8%, respectively. The inter-assay CV was 4.9% and 3.9%, respectively, as determined by assaying tissue extracts with a CA15-3 content of 6.6 and 11.8 U/ml in duplicate in 12 separate assays over four weeks. These data are within the range of intra- and inter-assay CV values reported by the manufacturer (Diasorin) for serum samples. For the assessment of assay linearity, two-fold serial dilutions of three tumor tissue extract (281.0, 715.0, and 858.0 U/ml) were tested. The CA15-3 assay displayed a linear regression with zero intercept for all three samples (simple regression, regression coefficients of r = 1.000, r = 0.999, and r = 0.999, respectively). For further analyses the CA15-3 antigen concentration is expressed in units CA15-3 per mg of total protein content (U/mg) of tumor tissue extracts.
Determination of S-phase fraction
DNA analysis was performed on suspensions of cell nuclei derived from fine-needle aspirates of fresh tumor tissue biopsies. The aspirated cells were resuspended in 1 ml CycleTest buffer solution containing DMSO (BD Biosciences, Heidelberg, Germany), shock-frozen using a mixture of dry ice and 99% ethanol, and stored at -20°C until further use. After thawing the cell suspension was centrifuged (400 g for 5 min at room temperature), and the supernatant was decanted. For DNA analysis, the CycleTest PLUS DNA Reagent Kit (BD Biosciences) was used according to the manufacturer’s instructions. The analysis was performed on a FACScan flow cytometer (BD Biosciences) equipped with automated doublett discrimination modul (DDM) and CellQuest software (BD Biosciences) for data aquisition. For each histogram at least 10,000 nuclei were analyzed, which were recorded unconditionally without any previous electronic gating. DNA histograms were evaluated with the ModFit LT 2.0 software (Verity Software House, Topsham, ME) using the rectangle fit model, with automatic background substraction, for the calculation of the percentage of cells in S-phase fraction (SPF). In non-diploid cases, the SPF from the non-diploid cell population was calculated, in case of (few) multiploid cases, the SPF for the cell population with the greatest number of events was calculated. Generally we adhered to the criteria set forth by the DNA Cytometry consensus conference, retaining only histograms with a CV < 8% and > 2,000 cells and/or 15% of events in the aneuploid cycle studied [39]. Cases with > 20% debris and/or aggregated cells were excluded. The percentage of SPF values ranged from 1 to 31% (median 5%), and were dichotomized into groups with low and high SPF by the median.
Statistical analyses
The relation between tumor biological marker values and clinicopathological parameters was determined using the nonparametric Chi square test. For survival analysis, patients’ 5-year disease-free survival (DFS) was used as the follow-up end point as described [40]. The association of tumor biological marker values as well as of clinicopathological factors with DFS was analyzed using Cox’s univariate and multivariable proportional hazards regression models. The multivariable model was adjusted for clinicopathological factors that may affect survival: age, lymph node status, tumor size, and SPF. Survival curves were generated by univariate Kaplan-Meier estimation using the log-rank regression model. The statistical analyses were two-sided, and only P-values < 0.05 were considered to be statistically significant. Calculations were performed using the StatView 5.0 statistical package (SAS Institute, Cary, NC).
Results
Quantification of rab31 and mucin-1 (CA15-3) antigen levels in tumor tissue extracts of estrogen receptor-positive (ER+) breast cancer patients
Rab31 and CA15-3 levels were determined by ELISA in tumor tissue extracts of a patient cohort encompassing 284 patients with primary ER+ breast cancer. The rab31 and CA15-3 antigen concentration ranged from 0.026 to 2.23 ng/mg (median 0.41 ng/mg) and from 1.4 to 779.9 U/mg (median 49.5 U/mg), respectively. Applying both Spearman rank correlation and nonparametric Mann-Whitney analysis, no significant correlation was observed between rab31 and CA15-3 antigen levels.
To analyze, whether expression levels of both tumor biological markers differ between ER+ and estrogen receptor-negative (ER-) patients, we also determined antigen levels in a cohort of ER- breast cancer patients (n = 110; data not shown). Here, the rab31 and CA15-3 antigen concentration ranged from 0.005 to 3.17 ng/mg (median 0.36 ng/mg) and from 1.00 to 292.7 U/mg (median 7.50 U/mg), respectively. Whereas rab31 antigen levels did not significantly differ in tumor tissue between ER+ and ER- patients, significantly higher CA15-3 antigen levels were measured in the ER+ versus ER- subgroups. Similar to ER+ patients, no significant correlation was observed between rab31 and CA15-3 antigen levels in ER- patients as well.
Rab31 and mucin-1 (CA15-3) and their association with clinical and histomorphological parameters of ER+ breast cancer patients
The relationship between rab31 and CA15-3, dichotomized into groups with low and high levels by the median, and relevant clinicopathological characteristics, including the S-phase fraction (SPF), in the cohort of 284 ER+ breast cancer patients is summarized in Table 1. A strong association was observed between rab31 antigen levels and tumor grade with G3 tumors displaying the highest antigen levels (P = 0.021; Kruskall-Wallis test; Figure 1A). Strikingly, an inverse significant association was observed for CA15-3 with tumor grade (P = 0.032; Kruskall-Wallis-test; Figure 1B). Furthermore, high rab31 antigen levels were significantly associated with a high SPF (P = 0.047; Mann Whitney-test; Figure 1C), whereas a trend for lower CA15-3 antigen levels in tumor tissue displaying higher SPF was observed (Figure 1D). There were no significant associations of rab31 and CA15-3 with age, regional lymph node metastasis, or tumor size (Table 1).
Table 1.
Association of rab31 and mucin-1 (CA15-3) antigen levels in tumor tissue extracts with clinical and histomorphological characteristics of estrogen receptor-positive (ER+) breast cancer patients
| Clinicopathological parameters | No. patients | rab31b low/high | CA15-31b low/high |
|---|---|---|---|
| Totala | 284 | 143/141 | 140/138 |
| Age (years) | P = 0.720 | P = 0.629 | |
| ≤ 60 | 140 | 72/68 | 72/65 |
| > 60 | 138 | 68/70 | 67/68 |
| Lymph node status | P = 0.050 | P = 0.844 | |
| Negative | 150 | 85/65 | 72/72 |
| Positive | 125 | 56/69 | 64/61 |
| Tumor stage | P = 0.103 | P = 0.852 | |
| < 2 cm | 149 | 81/68 | 72/73 |
| > 2 cm | 128 | 57/71 | 64/62 |
| Tumor gradec | P = 0.021 | P = 0.032 | |
| Grade 1 | 48 | 30/18 | 19/29 |
| Grade 2 | 177 | 95/82 | 87/84 |
| Grade 3 | 57 | 17/40 | 34/23 |
| Proliferative status (SPF)d | P = 0.039 | P = 0.563 | |
| SPF low | 112 | 42/56 | 51/45 |
| SPF high | 98 | 64/48 | 53/55 |
Total n = 284; due to missing values, numbers do not always add up to 284.
P for Chi-square test (cut-off point = median);
P for Kruskal-Wallis test.
SPF: S-phase fraction. SPF values were dichotomized into groups with low and high SPF by the median (5%).
Figure 1.
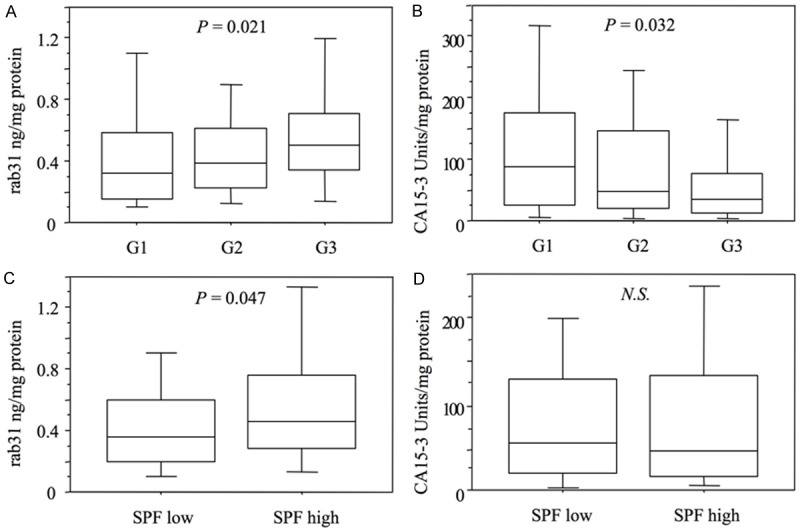
Correlation of rab31 and mucin-1 (CA15-3) antigen levels with tumor grade and S-phase fraction (SPF) in tumor tissues of ER+ breast cancer patients. A: Rab31 antigen levels in tumor tissue extracts are directly correlated with tumor grading (P = 0.021). B: Indirect correlation of CA15-3 antigen levels with tumor grading (P = 0.032). C and D: Correlation of rab31 (P = 0.047) and CA15-3 (n.s.) with the proliferation marker SPF. SPF values were dichotomized into groups with low and high SPF by the median (5%). Tumor grading was categorized in well-differentiated (G1), moderately differentiated (G2) and poorly differentiated (G3).
Association of rab31 and mucin-1 (CA15-3) with disease-free survival of ER+ breast cancer patients
The strength of association between clinicopathological and tumor biological markers with patients’ 5-year survival (DFS) is presented in Table 2. In univariate Cox regression analysis of DFS, the clinicopathological parameters age, nodal status, tumor size, and S-phase fraction reached statistical significance. In case of tumor grade, only patients with poorly differentiated G3 tumors, but not with moderately differentiated G2 tumors, displayed a statistically significant, about 4-fold increased risk for relapse as compared to the patients with well-differentiated G1 tumors. On one hand, high rab31 antigen levels were significantly related with poor DFS of ER+ breast cancer patients with hazard ratios (HR) of 1.91 (95% CI = 1.14-3.17, P = 0.013) as compared to those patients with low values. On the other hand, ER+ breast cancer patients displaying high CA15-3 levels had a significantly lower risk of relapse or death (HR = 0.56, 95% CI = 0.33-0.95, P = 0.031) as compared to patients with low values.
Table 2.
Univariate Cox regression analysis for disease-free survival of estrogen receptor-positive (ER+) breast cancer patients
| Factor | No. cases | Disease-free survival HR (95% CI)b | P |
|---|---|---|---|
| Totala | 284 | ||
| Age (years) | |||
| ≤ 60 | 140 | 1 | |
| > 60 | 138 | 0.60 (0.36-1.00) | 0.049 |
| Lymph node status | |||
| Negative | 150 | 1 | |
| Positive | 125 | 3.89 (2.22-6.84) | < 0.001 |
| Tumor stage | |||
| < 2 cm | 149 | 1 | |
| > 2 cm | 128 | 2.21 (1.31-3.72) | 0.003 |
| Tumor grade | |||
| Grade 1 | 48 | 1 | |
| Grade 2 | 177 | 2.05 (0.73-5.76) | 0.172 |
| Grade 3 | 57 | 3.96 (1.37-11.5) | 0.011 |
| Proliferative status (SPF)c | |||
| Low | 112 | 1 | |
| High | 98 | 3.42 (1.88-6.21) | < 0.001 |
| rab31d | |||
| Low | 143 | 1 | |
| High | 141 | 1.91 (1.14-3.17) | 0.013 |
| CA15-3d | |||
| Low | 140 | 1 | |
| High | 138 | 0.56 (0.33-0.95) | 0.031 |
Total n = 284; due to missing values, numbers do not always add up to 284.
HR: hazard ratio; 95% CI: 95% confidence interval of univariate Cox’s regression analysis.
SPF: S-phase fraction. SPF values were dichotomized into groups with low and high SPF by the median (5%).
Dichotomized into groups with high and low levels by the median values.
These findings were also confirmed by Kaplan-Meier estimation. The associations of rab31 and CA15-3 levels as well as tumor grading and SPF values with DFS are visualized by the respective survival curves: here, high rab31 antigen levels (P = 0.012; Figure 2A), G3 tumors (P = 0.005; Figure 2C), and high SPF values (P < 0.001; Figure 2D) are significantly associated with shorter DFS, whereas high CA15-3 antigen levels are related with better DFS (P = 0.028; Figure 2B).
Figure 2.
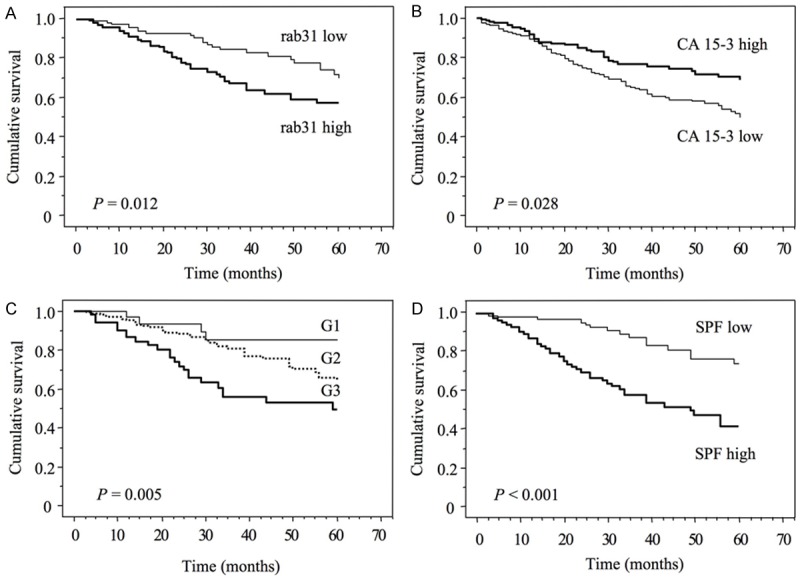
Probability of patients’ 5-year disease-free survival with regard to rab31 (A) and CA15-3 (B) antigen levels in tumor tissue extracts, and the clinicopathological parameters tumor grading (C) and S-phase fraction (SPF, D). The median values of rab31 and CA15-3 antigen levels, and of SPF values (0.41 ng/mg, 49.5 U/mg, and 5%, respectively) were used as cut-off points. Tumor grading was categorized in well-differentiated (G1), moderately differentiated (G2) and poorly differentiated (G3).
Rab31 but not mucin-1 (CA15-3) is an independent prognostic marker for disease-free survival (DFS) of ER+ breast cancer patients
The independent relationship of rab31 and CA15-3 with DFS was studied with Cox’s multivariable regression analysis. In the analyzed patient cohort of 193 ER+ breast cancer patients, high rab31 antigen levels contributed independent prognostic information when adjusted for prognostically relevant clinicopathological parameters including age, lymph node status, tumor size, and S-phase fraction with a HR of 1.97 (95% CI = 1.09-3.54, P = 0.024) for high versus low values (Table 3). The CA15-3 antigen levels were not significantly associated with prognosis when added to the base model of clinical prognostic factors for DFS in multivariable analysis (Table 3).
Table 3.
Multivariate Cox regression analysis for disease-free survival of estrogen receptor-positive (ER+) breast cancer patients
| Factor | No. cases | Disease-free survival HR (95% CI)a | P |
|---|---|---|---|
| Total | 193 | ||
| Age (years) | |||
| ≤ 60 | 95 | 1 | |
| > 60 | 98 | 0.78 (0.78-1.39) | 0.403 |
| Lymph node status | |||
| Negative | 104 | 1 | |
| Positive | 89 | 5.02 (2.49-10.1) | < 0.001 |
| Tumor size | |||
| < 2 cm | 105 | 1 | |
| > 2 cm | 88 | 1.82 (0.95-3.47) | 0.070 |
| Proliferative status (SPF)b | |||
| Low | 102 | 1 | |
| High | 91 | 2.98 (1.58-5.60) | < 0.001 |
| rab31c | |||
| Low | 100 | 1 | |
| High | 93 | 1.97 (1.09-3.54) | 0.024 |
| CA15-3c | |||
| Low | 97 | 1 | |
| High | 90 | 0.77 (0.43-1.41) | 0.402 |
HR: hazard ratio; 95% CI: 95% confidence interval of multivariate Cox’s regression analysis.
SPF: S-phase fraction. SPF values were dichotomized into groups with low and high SPF by the median (5%).
Dichotomized into groups with high and low levels by the median values.
Tumor biological factors were separately added to the base model consisting of age, nodal status, tumor size, and proliferative status (SPF).
Discussion
Dysreglated expression of both rab31 and mucin-1 has been observed in several types of human epithelial cancer, including breast cancer [17,22]. Transcription of the mucin-1 oncogene (MUC1) is known to be distinctly upregulated in breast cancer and is aberrantly expressed in > 90% of human breast carcinomas [41,42]. Overexpression of rab31 mRNA was observed in ER+ breast cancer tissue and found to be associated with poor prognosis of lymph node-negative breast cancer patients [18,19]. In accordance with this finding, in the present paper, we observed that high rab31 protein levels in ER+ tumor tissue were significantly associated with poor prognosis of breast cancer patients.
Concerning overexpression of rab31 in breast cancer, it has been reported that rab31 transcripts are targets of the mRNA-binding and stabilizing protein HuR, a member of the ELAV-Hu family [43,44]. High cytoplasmic HuR expression is associated with poor outcome in breast cancer [45]. Binding of HuR to rab31 transcripts may significantly increase their half-life resulting in elevated rab31 mRNA levels. Another explanation for increased rab31 levels in ER+ breast cancer is related to the observation that the rab31 promoter region harbors an ER responsive element [21] and gene transcription is, in fact, activated in an estrogen-dependent manner via binding of a complex of ERα and the C-terminal domain of mucin-1 (MUC-1C) as co-factor to the rab31 promoter.
Although the prognostic information provided by mRNA and protein expression levels of mucin-1 remains controversial [26], there is accumulating evidence that elevated mucin-1 antigen levels play a fundamental role in the progression of different human epithelial cancer types including breast cancer (for review see [22,46]). Using immunohistochemistry, there are detailed studies on distribution of mucin-1 antigen in breast cancer tissue [27,47,48]. Interestingly, the subcellular localization of mucin-1, but not the staining intensity, has been reported to display prognostic significance. On the other hand, CA15-3, which corresponds to the shed N-terminal domain of mucin-1, MUC-1N, is the most widely used serum marker in breast cancer patients, whereas MUC-1C is undetectable in serum [28]. Several commercial assays have been developed for the measurement of CA15-3, which generally use two monoclonal antibodies, DF3 and 115D8, both directed to epitopes of the MUC-1N subunit. Elevated serum levels of CA15-3 are found in the majority of breast cancer patients with distant metastases and thus, are highly associated with poor prognosis (reviewed in [23]). In addition, a prognostic significance of CA15-3 serum levels in lymph node-negative, node-positive and ER+ breast cancer patients was described [49].
Using such a CA15-3 sandwich immunoassay, we detected soluble mucin-1 antigen levels in detergent extracts of tumor tissue with excellent test precision and reproducibility. Therefore, we were able to quantify CA15-3 levels in breast cancer tissue and to compare these results with rab31 antigens levels obtained with our newly developed sandwich ELISA from the very same tissue extracts. It is important to note, however, that the measured CA15-3 values represent total MUC1-N concentrations in tissue extracts, which are most probably composed of shed subunit MUC1-N plus intact MUC1-N/MUC1-C-heterodimer. Therefore, these values should not be equivalent to either shed MUC-1N in sera or intracellular MUC1-C levels.
ER and mucin-1 ER cis-elements have been described to be involved in regulation of the mucin-1 gene transcription [31]. Thus, as expected also from results obtained with both mRNA analysis [21] as well as immunohistochemistry [27,50], we observed significantly higher tumor tissue CA15-3 antigen levels in ER+ versus ER- patients. In ER+ breast cancer cells, the C-terminal subunit of mucin-1 induces rab31 expression in vitro [21]. In tumor tissue extracts of both ER+ and ER- breast cancer, however, we did not find a significant correlation between rab31 and soluble mucin-1 (CA15-3) antigen levels. This suggests that in vivo subcellular localization of mucin-1 [26], the presence of mucin-1 splice variant isoforms [31], and/or the availability of free MUC-1C [28] may be more crucial in the regulatory interaction between the mucin-1 oncoprotein and the rab31 GTPase in breast cancer as compared to the total antigen level of mucin-1.
In addition to lymph node status, tumor size, and tumor grade, proliferation markers such immunohistochemical Ki-67 staining and cytometric SPF are useful clinicopathological parameters for assessing prognosis of breast cancer patients [51-53]. Especially, estimation of the SPF obtained by fine-needle sampling has been shown to be a reproducible method to quantitatively measure the proliferative status [51,54]. In a representative subgroup of ER+ patients, in which SPF was reliably determined, we observed that high rab31 antigen levels in breast cancer tissue were significantly associated with a high SPF. This finding confirms our previous in vitro data that overexpression of rab31 in breast cancer cells leads to enhanced proliferation of the tumor cells [20]. High SPF values were found to be significantly associated with high tumor grading (not shown, P < 0.001), which may, at least in part, be due to increased mitotic counts, e.g. in G3 versus G2 or G1 tumors. In consideration of this association, it is not surprising that a strong association was observed between rab31 antigen levels and tumor grade as well, with G3 tumors displaying the highest antigen levels. Interestingly, inverse significant association for CA15-3 with tumor grade as well as a trend for lower CA15-3 antigen levels in tumor tissue displaying higher SPF was observed. This is again in line with the results obtained by immunohistochemistry, demonstrating an inverse association of mucin-1 overexpression with tumor grade [27]. The presence of apical mucin-1 staining in the majority of breast cancer cells generally correlates with increased functional differentiation and better prognosis [47], which is in accordance with our finding that elevated CA15-3 antigen levels in tumor tissue extracts indicate prolonged DFS in ER+ breast cancer patients. Interestingly, however, it was shown that intense cytoplasmic staining, even by the use of MUC-1N specific mAbs (B27.29 and BC2), was associated with a worse prognosis [25]. Thus, CA15-3 or total MUC1-N values do not fully reflect the biological significance of mucin-1 overexpression in poorly differentiated tumors. It is tempting to speculate that the generation and the amount of tumor-supporting mucin-1-derived molecules, such as internalized, cytosolic MUC-1C acting as co-factor of ERα, is independent from the mucin-1 overall expression level. Notably, inhibitors of the MUC1-C subunit have been developed that directly block its oncogenic function and induce death of breast cancer cells in vitro and in xenograft models. Based on these findings, MUC1-C inhibitors are presently tested as potential agents for the treatment of patients with breast cancers [28]. With regard to the MUC-1C-mediated induction of rab31 overexpression, rab31 antigen levels in tumor tissue extracts may not only represent an independent biomarker for prognosis in ER+ breast cancer patients, but could also be helpful in selecting patients which may benefit from a MUC-1C-targeted therapeutic approach.
Acknowledgements
We thank S. Creutzburg, K. Barthel and J. Zönnchen for excellent technical assistance. The present work is dedicated to our former colleague S. Zotter.
Disclosure of conflict of interest
None.
References
- 1.Singan VR, Handzic K, Simpson JC. Quantitative image analysis approaches for probing Rab GTPase localization and function in mammalian cells. Biochem Soc Trans. 2012;40:1389–93. doi: 10.1042/BST20120145. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 3.Goud B, Gleeson PA. TGN golgins, Rabs and cytoskeleton: regulating the Golgi trafficking highways. Trends Cell Biol. 2010;20:329–36. doi: 10.1016/j.tcb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Porther N, Barbieri MA. The role of endocytic Rab GTPases in regulation of growth factor signaling and the migration and invasion of tumor cells. Small GTPases. 2015;6:135–44. doi: 10.1080/21541248.2015.1050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbeel L, Freson K. Rab proteins and Rabassociated proteins: major actors in the mechanism of protein-trafficking disorders. Eur J Pediatrics. 2008;167:723–9. doi: 10.1007/s00431-008-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramani D, Alahari SK. Integrin-mediated function of Rab GTPases in cancer progression. Mol Cancer. 2010;9:312. doi: 10.1186/1476-4598-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra S, Cheng KW, Mills GB. Rab GTPases implicated in inherited and acquired disorders. Semin Cell Dev Biol. 2011;22:57–68. doi: 10.1016/j.semcdb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzeng HT, Wang YC. Rab-mediated vesicle trafficking in cancer. J Biomed Sci. 2016;23:70. doi: 10.1186/s12929-016-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klöpper TH, Kienle N, Fasshauer D, Munro S. Untangling the evolution of Rab G proteins: implications of a comprehensive genomic analysis. BMC Biol. 2012;10:71. doi: 10.1186/1741-7007-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Gabin AG, Cammer M, Almazan G, Charron M, Larocca JN. Role of rRAB22b, an oligodendrocyte protein, in regulation of transport of vesicles from trans Golgi to endocytic compartments. J Neurosci Res. 2001;66:1149–60. doi: 10.1002/jnr.1253. [DOI] [PubMed] [Google Scholar]
- 11.Ng EL, Wang Y, Tang BL. Rab22B’s role in trans-Golgi network membrane dynamics. Biochem Biophys Res Commun. 2007;361:751–7. doi: 10.1016/j.bbrc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 12.Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–9. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 13.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–6. doi: 10.1016/j.bbcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Zhang Y, Chen L, Liu Y, Feng Y, Yan J. The critical role of Rab31 in cell proliferation and apoptosis in cancer progression. Mol Neurobiol. 2016;53:4431–7. doi: 10.1007/s12035-015-9378-9. [DOI] [PubMed] [Google Scholar]
- 16.Qin X, Wang J, Wang X, Liu F, Jiang B, Zhang Y. Targeting Rabs as a novel therapeutic strategy for cancer therapy. Drug Discov Today. 2017;22:1139–1147. doi: 10.1016/j.drudis.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Kotzsch M, Goettig P, Soelch S, Magdolen V. Rab31 (Ras-related protein in brain 31) Atlas Gen Cytogen Oncol Haematol. 2016 http://atlasgeneticsoncology.org/Genes/RAB31ID41978ch18p11.html. [Google Scholar]
- 18.Abba MC, Hu Y, Sun H, Drake JA, Gaddis S, Baggerly K, Sahin A, Aldaz CM. Gene expression signature of estrogen receptor alpha status in breast cancer. BMC Genomics. 2005;6:37. doi: 10.1186/1471-2164-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotzsch M, Sieuwerts AM, Grosser M, Meye A, Fuessel S, Meijer-van Gelder ME, Smid M, Schmitt M, Baretton G, Luther T, Magdolen V, Foekens JA. Urokinase receptor splice variant uPAR-del4/5-associated gene expression in breast cancer: identification of rab31 as an independent prognostic factor. Breast Cancer Res Treat. 2008;111:229–40. doi: 10.1007/s10549-007-9782-6. [DOI] [PubMed] [Google Scholar]
- 20.Grismayer B, Sölch S, Seubert B, Kirchner T, Schäfer S, Baretton G, Schmitt M, Luther T, Krüger A, Kotzsch M, Magdolen V. Rab31 expression levels modulate tumor-relevant characteristics of breast cancer cells. Mol Cancer. 2012;11:62. doi: 10.1186/1476-4598-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin C, Rajabi H, Pitroda S, Li A, Kharbanda A, Weichselbaum R, Kufe D. Cooperative interaction between the MUC1-C oncoprotein and the Rab31 GTPase in estrogen receptor-positive breast cancer cells. PLoS One. 2012;7:e39432. doi: 10.1371/journal.pone.0039432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu F, Liu F, Zhao H, An G, Feng G. Prognostic significance of mucin antigen MUC1 in various human epithelial cancers: a meta analysis. Medicine. 2015;94:1–10. doi: 10.1097/MD.0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–74. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–40. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol. 1995;26:432–9. doi: 10.1016/0046-8177(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 26.Ginestier C, Charafe-Jauffret E, Bertucci F, Eisinger F, Geneix J, Bechlian D, Conte N, Adélaïde J, Toiron Y, Nguyen C, Viens P, Mozziconacci MJ, Houlgatte R, Birnbaum D, Jacquemier J. Distinct and complementary information provided by use of tissue and DNA microarrays in the study of breast tumor markers. Am J Pathol. 2002;161:1223–33. doi: 10.1016/S0002-9440(10)64399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 28.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–81. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters betacatenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–32. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 31.Zaretsky JZ, Barnea I, Aylon Y, Gorivodsky M, Wreschner DH, Keydar I. MUC1 gene overexpressed in breast cancer: structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor alpha (ERalpha) in regulation of the MUC1 gene expression. Mol Cancer. 2006;5:57. doi: 10.1186/1476-4598-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharbanda A, Rajabi H, Jin C, Raina D, Kufe D. Oncogenic MUC1-C promotes tamoxifen resistance in human breast cancer. Mol Cancer Res. 2013;11:714–23. doi: 10.1158/1541-7786.MCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 34.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] . Pathologe. 1987;8:138–40. [PubMed] [Google Scholar]
- 35.Jänicke F, Pache L, Schmitt M, Ulm K, Thomssen C, Prechtl A, Graeff H. Both the cytosols and detergent extracts of breast cancer tissues are suited to evaluate the prognostic impact of the urokinase-type plasminogen activator and its inhibitor, plasminogen activator inhibitor type 1. Cancer Res. 1994;54:2527–30. [PubMed] [Google Scholar]
- 36.Luther T, Flössel C, Albrecht S, Kotzsch M, Müller M. Tissue factor expression in normal and abnormal mammary gland. Nat Med. 1996;2:491–2. doi: 10.1038/nm0596-491a. [DOI] [PubMed] [Google Scholar]
- 37.Debela M, Magdolen V, Schechter N, Valachova M, Lottspeich F, Craik CS, Choe Y, Bode W, Goettig P. Specificity profiling of seven human tissue kallikreins reveals individual subsite preferences. J Biol Chem. 2006;281:25678–88. doi: 10.1074/jbc.M602372200. [DOI] [PubMed] [Google Scholar]
- 38.Sölch S. TU München: PhD Thesis. 2014. Effects of the GTPase Rab31 on breast cancer cell proliferation, adhesion, and expression of other tumor-associated genes. [Google Scholar]
- 39.Hedley DW, Clark GM, Cornelisse CJ, Killander D, Kute T, Merkel D. Consensus review of the clinical utility of DNA cytometry in carcinoma of the breast. Report of the DNA cytometry consensus conference. Cytometry. 1993;14:482–5. doi: 10.1002/cyto.990140505. [DOI] [PubMed] [Google Scholar]
- 40.Kotzsch M, Farthmann J, Meye A, Fuessel S, Baretton G, Tjan-Heinen V, Schmitt M, Luther T, Sweep F, Magdolen V, Span P. Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression levels in breast cancer. Eur J Cancer. 2005;41:2760–68. doi: 10.1016/j.ejca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Zotter S, Hageman PC, Lossnitzer A, Mooi WJ, Hilgers J. Tissue and tumour distribution of human polymorphic epithelial mucin. Cancer Rev. 1988;11-12:55–101. [Google Scholar]
- 42.Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989;43:1072–6. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 43.Heinonen M, Hemmes A, Salmenkivi K, Abdelmohsen K, Vilen ST, Laakso M, Leidenius M, Salo T, Hautaniemi S, Gorospe M, Heikkila P, Haglund C, Ristimaki A. Role of RNA binding protein HuR in ductal carcinoma in situ of the breast. J Pathol. 2011;224:529–39. doi: 10.1002/path.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simone LE, Keene JD. Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev. 2013;23:35–43. doi: 10.1016/j.gde.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, Furneaux H, Hla T, Haglund C, Ristimäki A. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65:2157–61. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 46.Horm TM, Schroeder JA. MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr. 2013;7:187–98. doi: 10.4161/cam.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001;91:1973–82. doi: 10.1002/1097-0142(20010601)91:11<1973::aid-cncr1222>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 48.Iizuka M, Nakanishi Y, Fuchinoue F, Maeda T, Murakami E, Obana Y, Enomoto K, Tani M, Sakurai K, Amano S, Masuda S. Altered intracellular region of MUC1 and disrupted correlation of polarity-related molecules in breast cancer subtypes. Cancer Sci. 2015;106:307–14. doi: 10.1111/cas.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nisman B, Maimon O, Allweis T, Kadouri L, Maly B, Hamburger T, Peretz T. The prognostic significance of LIAISON(R) CA15-3 assay in primary breast cancer. Anticancer Res. 2013;33:293–9. [PubMed] [Google Scholar]
- 50.Do SI, Kim K, Kim DH, Chae SW, Park YL, Park CH, Sohn JH. Associations between the expression of mucins (MUC1, MUC2, MUC5AC, and MUC6) and clinicopathologic parameters of human breast ductal carcinomas. J Breast Cancer. 2013;16:152–8. doi: 10.4048/jbc.2013.16.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chassevent A, Jourdan ML, Romain S, Descotes F, Colonna M, Martin PM, Bolla M, Spyratos F. S-phase fraction and DNA ploidy in 633 T1T2 breast cancers: a standardized flow cytometric study. Clin Cancer Res. 2001;7:909–17. [PubMed] [Google Scholar]
- 52.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–83. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 53.Dayal JH, Sales MJ, Corver WE, Purdie CA, Jordan LB, Quinlan PR, Baker L, ter Haar NT, Pratt NR, Thompson AM. Multiparameter DNA content analysis identifies distinct groups in primary breast cancer. Br J Cancer. 2013;108:873–80. doi: 10.1038/bjc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Remvikos Y, Vielh P, Padoy E, Benyahia B, Voillemot N, Magdelénat H. Breast cancer proliferation measured on cytological samples: a study by flow cytometry of S-phase fractions and BrdU incorporation. Br J Cancer. 1991;64:501–7. doi: 10.1038/bjc.1991.338. [DOI] [PMC free article] [PubMed] [Google Scholar]


