Abstract
Background: We conducted a phase II study by combining FOLFOX4 plus bevacizumab (BV) with erlotinib (ER) as second-line chemotherapy for patients with metastatic colorectal cancer (mCRC). Methods: Patients were divided into two groups in randomized double-blind manner. One group was given FOLFOX4 plus 5 mg/kg BV on day 1 of 2-week cycle. The other group was given 2-week-cycle of BV + FOLFOX4, and 100 mg ER every day. The primary endpoint was progression-free survival (PFS). The secondary endpoints were overall survival (OS), clinical response rates and adverse events (AEs). Results: 66 patients received 2nd-line treatment of ER + BV+ FOLFOX4, and 65 received BV + FOLFOX4. Median PFS was 9.6 months of ER + BV + FOLFOX4 group, significantly better than 6.9 months of BV + FOLFOX4 group (P = 0.021, HR = 1.15, 95% CI = 0.88-1.39). Medium OS for ER + BV + FOLFOX4 group was 12.5 months, not statistically different than 12.1 months for BV + FOLFOX4 group (P = 00.146, HR = 0.63, 95% CI = 0.34-1.02). Combined partial response and stable disease rate was 48.5% for ER + BV + FOLFOX4 group, significantly higher than 32.2% for BV + FOLFOX4 group (P = 0.015). Patients in ER + BV + FOLFOX4 group had higher incidence rates of AEs. Conclusion: In second-line chemotherapy for patients with mCRC, combining erlotinib with FOLFOX4 plus bevacizumab may improve PFS, clinical response rates, but not OS. AEs, though with high incidence rates, were generally tolerable among patients receiving multiple reagents.
Keywords: Metastatic colorectal cancer, bevacizumab, erlotinib, FOLFOX4, second-line chemotherapy
Introduction
Colorectal cancer (CRC) is one of the most malign forms of carcinoma among both males and females. In United States along, CRC is one of the four major cancers among male patients, accounting for 44% of all new cancer cases every year [1]. Most of the patients with colorectal cancer would develop recurrent or metastatic colorectal cancer (mCRC), and the standard 1st-line and 2nd-line treatments include fluorouracil, leucovorin, and irinotecan (FOLFIRI), infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX), or capecitabine plus oxaliplatin (XELOX) [2-4]. In the setting of 2nd-line chemotherapy for patients with mCRC, emerging evidence showed that while standard chemotherapy was combined with targeted reagents, such as bevacizumab (BV), a human monoclonal vascular endothelial growth factor (VEGF) antibody, mCRC patients’ prognosis survivals were significantly improved [5-9]. Specifically, in clinical trial of ECOG-E3200, Giantonio and colleagues compared overall survivals (OS) and progression-free survivals (PFS) between mCRC patients who were treated with 2nd-line chemotherapy of FOLFOX4 only, and those treated with FOLFOX4 plus bevacizumab (BV + FOLFOX4) [9]. They discovered that patients’ OS and PFS were both significantly improved, from 10.8 months (FOLFOX4) to 12.9 months (BV + FOLFOX4) for OS, and 4.7 months (FOLFOX4) to 7.3 months (BV + FOLFOX4) for PFS [9]. Ultimately, the exciting clinical outcome of ECOG-E3200 trial leaded to FDA approval of using BV plus FOLFOX4 as standard second-line chemotherapy for patients with mCRC [7].
During past decade, in additional to using VEGF-antibody alone as cancer chemotherapy reagent, more and more studies had demonstrated that combining VEGF-antibody with human monoclonal antibody against epidermal growth factor receptor (EGFR), such as erlotinib (ER), could also be more beneficial for cancer patients, such as those suffer with melanoma or lung cancer [10-12]. In a recent phase III Nordic ACT trial, ER plus BV was used as maintenance setting for mCRC patients who were treated with BV-included 1st-line chemotherapy and experienced stable disease [13]. Although the investigators of Nordic ACT trial did not find significant improvement on patients’ survival by combining BV with ER, the study was encouraging as it showed moderate tolerance among patients for multiple monoclonal antibody chemo-reagents, and pointed out that strategy of combining VEGF and EGFR antibodies may potentially benefit patients with mCRC [13].
In this study, in light of the success of ECOG-E3200 trial [9], we performed a phase II clinical study of combining ER with BV + FOLFOX4 as 2nd-line chemotherapy for mCRC patients who failed standard platinum-based 1st-line chemotherapy. As comparison, patients in control group received BV + FOLFOX4 as 2nd-line chemotherapy. We thus present the efficacy and toxicity of this new scheme of 2nd-line chemotherapy for patients with mCRC.
Patients and methods
Ethic statement
In this study, all procedures were reviewed and approved by the Clinical Research & Ethic Committee at Liaocheng People’s Hospital in Liaocheng, Shandong Province, China. Participating patients all signed consent forms. In addition, this study was performed in accordance with Declaration of Helsinki and national regulation on good medical practices in China.
Patients
One hundred and thirty-two eligible patients participated in this 2nd-line phase II chemotherapy between June 2011 and June 2015. Patients were eligible if, their ages were between 18 and 75 years, they were diagnosed with metastatic colon or rectum cancer with visible lesion based on the guideline of Response Evaluation Criteria in Solid Tumors [14], they had Eastern Cooperative Oncology Group Performance Score (ECOG PS) between 0~2, they received 1st-line xaliplatin-based or irinotecan- based chemotherapy but still experienced tumor progression, they had not received any previous treatment involving bevacizumab or erlotinib. Patients were ineligible if, they had major cardiac, kidney or gastrointestinal malfunctions, they had HIV, hepatitis B or C viruses, their carcinoma metastatic sites include brain, they had received major surgeries 6 months prior to the study, they had uncontrollable hematopoietic conditions or bleeding.
2nd-line treatment plan
In this phase II, randomized double-blind clinical study, one hundred and thirty-two eligible patients were divided into two groups. In one group, patients were given bevacizumab (BV) + FOLFOX4 on 2-week cycles. Briefly, Patients were given intravenous (I.V.) administration of 2-hour 85 mg/m2 oxaliplatin on day 1, 2-hour 200 mg/m2 leucovorin on day 1 & 2, 400 mg/m2 I.V. bolus of fluorouracil on day 1, followed by 40-hour administration of 600 mg/m2 fluorouracil on days 1 & 2, plus I.V. administration of 5 mg/kg BV on day 1. In the other group, in addition to BV + FOLFOX4, patients received oral administration of 100 mg erlotinib (ER) every day. Patients were kept on study, until disease progression or death. If experiencing intolerable adverse events, patients may also withdraw from the study upon primary physician’ concur.
2nd-line study plan
The primary endpoint of our study is progression free survival (PFS). The secondary endpoint were overall survival (OS) and clinical response rates, including complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), which were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0) [15]. Adverse events (AEs) were evaluated according to the guideline of National Cancer Institute Common Toxicity Criteria, Common Terminology Criteria for Adverse Events version 3.0 [16].
Statistical analysis
PFS and OS were evaluated using the Kaplan-Meier model, along with unstratified log-rank test. Clinical response rates were estimated using two-sides χ2 test. Hazard ratios were estimated using cox proportional regression model with 95% confidence intervals (CI). Statistical difference was declared if P < 0.05.
Results
Patients
One hundred and thirty-two eligible patients participated in this phase II chemotherapy between June 2011 and June 2015 in the departments of General Surgery & Gastroenterology at Liaocheng People’s Hospital. One group of patients (n = 66) received 2nd-line treatment of ER + BV + FOLFOX4. The other group of patients (n = 65) received 2nd-line treatment of BV + FOLFOX4. Patients’ baseline properties were listed for two treatment groups (Table 1). The averaged ages were 62.5 years for ER + BV + FOLFOX4 group, and 61.8 years for BV + FOLFOX4 group. The number of male patients in ER + BV + FOLFOX4 group was 47, accounting for 71.2% of total patients in that group. In BV + FOLFOX4 group, there were 42 male patients, accounting for 64.6% of total patients in that group. In both groups, majority of the patients had ECOG PS of 0 (48.5% for ER + BV + FOLFOX4 group, 53.8% for BV + FOLFOX4 group), major carcinoma site be their colons (65.2% for ER + BV + FOLFOX4 group, 61.5% for BV + FOLFOX4 group), and had only one metastatic site (75.8% for ER + BV + FOLFOX4 group, 8.15% for BV + FOLFOX4 group). In addition, three chemotherapy regimens, 5-FU, FOLFIRI and XELOX were used during patients’ 1st-line chemotherapy. Thus, the baseline properties were well balanced between two groups of patients.
Table 1.
Baseline properties for participating patients with metastatic colorectal cancer
| ER + BV + FOLFOX4 (n = 66) | BV + FOLFOX4 (n = 65) | |
|---|---|---|
|
|
||
| Baseline properties | Patients, n (%) | Patients, n (%) |
| Age (years) | ||
| Average (range) | 62.5 (41-75) | 61.8 (38-72) |
| < 60 | 37 (56.1%) | 41 (63.1%) |
| ≥ 60 | 29 (43.9%) | 24 (36.9%) |
| Sex | ||
| Male | 47 (71.2%) | 42 (64.6%) |
| Female | 19 (28.8%) | 23 (35.4%) |
| ECOG PS | ||
| 0 | 32 (48.5%) | 35 (53.8%) |
| 1 | 26 (39.4%) | 22 (33.8%) |
| 2 | 8 (12.1%) | 8 (12.3%) |
| Carcinoma site | ||
| Colon | 43 (65.2%) | 41 (61.5%) |
| Rectum | 20 (30.3%) | 22 (33.8%) |
| Both | 3 (4.5%) | 2 (30.8%) |
| Metastatic sites | ||
| Liver | 27 (40.9%) | 24 (36.9%) |
| Lung | 21 (31.8%) | 25 (38.5%) |
| Lymph | 13 (19.7%) | 12 (18.5%) |
| Others | 5 (7.6%) | 4 (6.2%) |
| Number of metastatic sites | ||
| 1 | 50 (75.8%) | 53 (81.5%) |
| ≥ 2 | 16 (24.2%) | 12 (18.5%) |
| First-line chemotherapy | ||
| 5-FU | 25 (37.9%) | 20 (30.8%) |
| FOLFIRI | 23 (34.8%) | 32 (49.2%) |
| XELOX | 18 (27.3%) | 13 (20.0%) |
Abbreviations: ER, erlotinib; BV, bevacizumab; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Comparison of 2nd-line chemotherapy efficacy
The primary endpoint, PFS, was followed-up using Kaplan-Meier model. For BV + FOLFOX4 group, the median PFS was 6.9 months. For ER + BV + FOLFOX4 group, the medium PFS was 9.6 months. While compared PFS between two groups using the unstratified log-rank test, it demonstrated that the difference was significant (Figure 1A, P = 0.021, HR = 1.15, 95% CI = 0.88-1.39). It is worth noting that, in both groups, the majority of the patients were male. In order to eliminate the sex bias in our study, we thus conducted further subgroup analysis (male vs. female patients) on patient’s PFS. The result demonstrated that, both male and female patients had favorable PFS while they were treated with ER + BV + FOLFOX4, rather than BV + FOLFOX4 (Figure 2).
Figure 1.
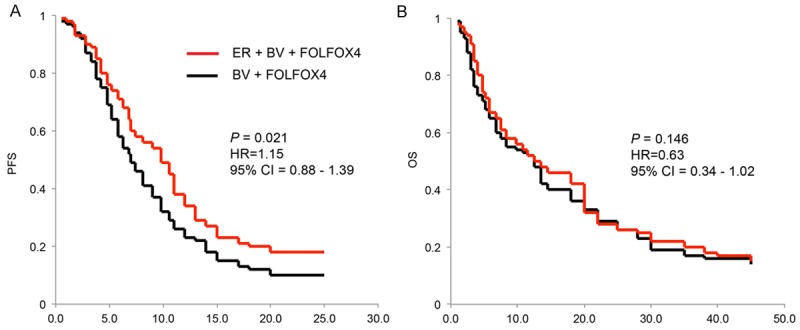
Progression-free survival (PFS) (A) and Overall survivals (OS) (B) were measured using Kaplan-Meier models, and compared between ER + BV + FOLFOX4 group and BV + FOLFOX4 group (log-rank test, 95% CI).
Figure 2.
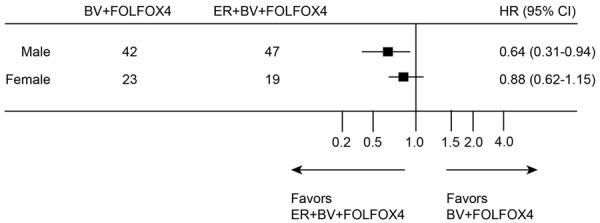
Progression-free survivals for treatment of ER + BV + FOLFOX4 and treatment of BV + FOLFOX4 were compared in subgroups of male and female patients.
Secondary endpoint of OS was also followed-up using Kaplan-Meier model. For BV + FOLFOX4 group, the median OS was 12.1 months. For ER + BV + FOLFOX4 group, the medium PFS was 12.5 months. Statistical analysis showed that OS were not significant different between two groups (Figure 1B, P = 00.146, HR = 0.63, 95% CI = 0.34-1.02).
Second endpoint of response rates was also followed up (Table 2). Complete response was not achieved in either of the group. However, in ER + BV + FOLFOX4 group, 48.5% (32 of 66) patients achieved PR or SD. This was much better than the response rate of 32.3% (21 of 65) in BV + FOLFOX4 group for patients achieved PR or SD (P = 0.015). Conceivably, response rates of PD were also significantly different between two groups. In ER + BV + FOLFOX4 group, 51.5% (34 of 66) patients had PD. This was markedly lower than PD rate of 67.7% (44 of 65) in BV + FOLFOX4 group (P = 0.028).
Table 2.
Response rates for participating patients with metastatic colorectal cancer
| ER + BV + FOLFOX4 (n = 66) | BV + FOLFOX4 (n = 65) | P-value (*, < 0.05) | |
|---|---|---|---|
|
|
|||
| Response rate | Patients, n (%) | Patients, n (%) | |
| CR | 0 (0%) | 0 (0%) | |
| PR + SD | 32 (48.5%) | 21 (32.3%) | 0.015* |
| PD | 34 (51.5%) | 44 (67.7%) | 0.028* |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DC, disease control.
Comparison of 2nd-line chemotherapy adverse events
The adverse events (AEs) of participating patients were also measured (Table 3). As compared to the patients in BV + FOLFOX4 group, those in ER + BV + FOLFOX4 group had higher incidence rates of almost all measured AEs, even including those of grade 3 & 4 AEs. In ER + BV + FOLFOX4 group, the top three overall AEs with highest incidence rates were vomiting (40.9%, 27 of 66), neuropathy (39.4%, 26 of 66) and rash (38.5%, 25 of 66). In BV + FOLFOX4 group, the top three overall AEs with highest incidence rates were also rash (30.1%, 20 of 65), neuropathy (26.2%, 17 of 65) and vomiting (24.6%, 16 of 65). In addition, the top three grade 3 & 4 AEs in ER + BV + FOLFOX4 group were rash (30.3%, 20 of 66), neuropathy (27.2%, 18 of 66) and vomiting (19.7%, 13 of 66). And the top three grade 3 & 4 AEs in BV + FOLFOX4 group were neuropathy (20.0%, 13 of 65), rash (18.5%, 12 of 65), and vomiting (10.8%, 7 of 65).
Table 3.
Adverse events for participating patients with metastatic colorectal cancer
| ER + BV + FOLFOX4 (n = 66) | BV + FOLFOX4 (n = 65) | |||
|---|---|---|---|---|
|
|
||||
| AE | All events | Grade 3 & 4 events | All events | Grade 3 & 4 events |
|
|
||||
| Patients, n (%) | Patients, n (%) | |||
| Bleeding | 23 (34.8%) | 10 (15.2%) | 11 (16.9%) | 3 (4.6%) |
| Vomiting | 27 (40.9%) | 13 (19.7%) | 16 (24.6%) | 7 (10.8%) |
| Rash | 25 (38.5%) | 20 (30.3%) | 20 (30.1%) | 12 (18.5%) |
| Neuropathy | 26 (39.4%) | 18 (27.2%) | 17 (26.2%) | 13 (20.0%) |
| Diarrhea | 13 (19.7%) | 8 (12.1%) | 6 (9.2%) | 3 (4.6%) |
| Fatigue | 24 (36.4%) | 11 (16.7%) | 12 (18.5%) | 5 (7.7%) |
| Proteinuria | 9 (13.6%) | 3 (4.5%) | 4 (6.2%) | 1 (1.5%) |
| Thrombocytopenia | 15 (23.1%) | 7 (10.6%) | 8 (12.3%) | 3 (4.6%) |
| Heart Failure | 10 (15.2%) | 4 (6.1%) | 5 (7.7%) | 2 (3.1%) |
| Asthenia | 5 (7.6%) | 3 (4.5%) | 7 (10.8%) | 1 (1.5%) |
| Anemia | 6 (9.1%) | 2 (3.0%) | 3 (4.6%) | 1 (1.5%) |
Abbreviations: AE, adverse events.
Discussions
In this phase II study, we combined FOLFOX4 plus bevacizumab with erlotinib as 2nd-line chemotherapy to treat mCRC patients who had unsuccessful 1st-line platinum-based chemotherapy. The major endpoint is PFS. The result of our study showed that, for mCRC patients treated with ER + BV + FOLFOX4, the median PFS was 9.6 months. This data is significantly better than the medium PFS in BV + FOLFOX4 group, which was measured as 6.9 months (P = 0.021, HR = 1.15, 95% CI = 0.88-1.39).
In ECOG-E3200 trial, measured medium PFS was 7.3 months [9], which was slightly better than the result of 6.9 months of BV + FOLFOX4 group in our study. This disparity may be attributed to the difference in bevacizumab dosage between two studies. In our study, patients were given 5 mg/kg bevacizumab on day 1 of 2-week cycle, only the half amount of 10 mg/kg bevacizumab in ECOG-E3200 trial [9]. However, the dosage of bevacizumab used in our study, or similar ones (such as 7.5 mg/kg bevacizumab on day 1 of 3-week cycle), was also commonly used in other combinational chemotherapy for treating mCRC patients in 2nd-line setting [5,6,8]. Most importantly, with only the half dosage of bevacizumab, we achieved almost same PFS as compared to ECOG-E3200 trial, suggesting that optimized chemotherapy scheme with reduced amount of bevacizumab may also be effective in prolonging patients’ PFS.
On the other hand, while comparing primary endpoints between two groups of tested patients in our study, the effect of erlotinib in improving patients’ PFS is obvious and without doubt. In a previous phase III maintenance study of Nordic ACT Trial, Johnsson and colleagues also tried combining erlotinib with bevacizumab, but failed to observe statistically significant improvement on patients’ clinical outcomes [13]. It is worth noting that, the chemotherapy settings between Nordic ACT Trial and our study are quite different. First, Nordic ACT Trial was a maintenance trial with mCRC patients previously received 1st-line chemotherapy and had stable conditions. Contrast to that, the patients enrolled in our study also received 1st-line chemotherapy but failed to establish conditions of partial response or stable disease. Second, Nordic ACT Trial did not include any platinum reagents in the study but we used FOLFOX4 as baseline chemotherapy. Third and most importantly, patients in Nordic ACT Trial all received 1st-line treatment of bevacizumab. But in our study, those patients receive any previous treatments involving bevacizumab or erlotinib were all excluded from the study. Therefore, it is possible that drug resistance to duplicate application of bevacizumab may contribute to the differences in patients’ PFS outcome between Nordic ACT Trial and our study.
Also in our study, we demonstrated that patients’ OSs were similar between two groups. Medium OS for ER + BV + FOLFOX4 group was 12.5 months, not statistically different than 12.1 months for BV + FOLFOX4 group (P = 00.146, HR = 0.63, 95% CI = 0.34-1.02). This result showed that it was still pre-mature to combine erlotinib with FOLFOX4 plus bevacizumab as standard 2nd-line chemotherapy for patients with mCRC. In a previous phase II 1st-line study, Meyerhardt and colleagues combined erlotinib with bevacizumab plus FOLFOX, but failed to improve patients’ survival [17]. It was suggested that combined reagents-induced toxicity was the major reason of not seeing treatment efficacy [17]. However, in our study, though incidence rates of adverse events were mostly higher in ER + BV + FOLFOX4 group, combination treatment of ER + BV + FOLFOX4 was generally tolerable among mCRC patients. Therefore, more clinical studies with bigger sampling-pool of patient size would undoubtedly help to optimize the combinational chemotherapy in 2nd-line setting to improve patients’ OS.
Conclusion
Overall, this phase II clinical study showed that combining erlotinib with FOLFOX4 plus bevacizumab is potentially beneficial to treating mCRC patients in 2nd-line chemotherapy, as it improved PFS and clinical response. More studies are needed to optimize the combination scheme to further improve OS.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Fakih MG. Metastatic Colorectal Cancer: Current State and Future Directions. J. Clin. Oncol. 2015;33:1809–24. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 3.Board RE, Valle JW. Metastatic colorectal cancer: current systemic treatment options. Drugs. 2007;67:1851–1867. doi: 10.2165/00003495-200767130-00004. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 5.Bennouna J, Borg C, Delord JP, Husseini F, Trillet-Lenoir V, Faroux R, Francois E, Ychou M, Goldwasser F, Bouche O, Senellart H, Kraemer S, Douillard JY. Bevacizumab combined with chemotherapy in the second-line treatment of metastatic colorectal cancer: results from the phase II BEVACOLOR study. Clin Colorectal Cancer. 2012;11:38–44. doi: 10.1016/j.clcc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Ciombor KK, Berlin J. Targeting metastatic colorectal cancer - present and emerging treatment options. Pharmgenomics Pers Med. 2014;7:137–144. doi: 10.2147/PGPM.S47582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 8.Suenaga M, Matsusaka S, Ueno M, Yamamoto N, Shinozaki E, Mizunuma N, Yamaguchi T, Hatake K. Predictors of the efficacy of FOLFIRI plus bevacizumab as second-line treatment in metastatic colorectal cancer patients. Surg Today. 2011;41:1067–1074. doi: 10.1007/s00595-010-4432-8. [DOI] [PubMed] [Google Scholar]
- 9.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 10.Schicher N, Paulitschke V, Swoboda A, Kunstfeld R, Loewe R, Pilarski P, Pehamberger H, Hoeller C. Erlotinib and bevacizumab have synergistic activity against melanoma. Clin Cancer Res. 2009;15:3495–3502. doi: 10.1158/1078-0432.CCR-08-2407. [DOI] [PubMed] [Google Scholar]
- 11.Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, Janne P, Kobayashi S, Halmos B, Tenen D, Tang XM, Engelman J, Yeap B, Folkman J, Johnson BE, Heymach JV. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franzini A, Baty F, Macovei II, Durr O, Droege C, Betticher D, Grigoriu BD, Klingbiel D, Zappa F, Brutsche M. Gene expression signatures predictive of bevacizumab/erlotinib therapeutic benefit in advanced non-squamous nonsmall cell lung cancer patients (SAKK 19/05 trial) Clin Cancer Res. 2015;21:5253–63. doi: 10.1158/1078-0432.CCR-14-3135. [DOI] [PubMed] [Google Scholar]
- 13.Johnsson A, Hagman H, Frodin JE, Berglund A, Keldsen N, Fernebro E, Sundberg J, De Pont Christensen R, Garm Spindler KL, Bergstrom D, Jakobsen A. A randomized phase III trial on maintenance treatment with bevacizumab alone or in combination with erlotinib after chemotherapy and bevacizumab in metastatic colorectal cancer: the Nordic ACT Trial. Ann Oncol. 2013;24:2335–2341. doi: 10.1093/annonc/mdt236. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Meyerhardt JA, Stuart K, Fuchs CS, Zhu AX, Earle CC, Bhargava P, Blaszkowsky L, Enzinger P, Mayer RJ, Battu S, Lawrence C, Ryan DP. Phase II study of FOLFOX, bevacizumab and erlotinib as first-line therapy for patients with metastatic colorectal cancer. Ann Oncol. 2007;18:1185–1189. doi: 10.1093/annonc/mdm124. [DOI] [PubMed] [Google Scholar]


