Abstract
Traumatic spinal cord injuries are major health problems and the underlying pathophysiological events and treatment strategies are currently under investigation. In this article, we critically reviewed the literature investigating the effects of estrogen, progesterone, and human chorionic gonadotropin on spinal cord damage or preservation following traumatic spinal cord injury. The National Library of Medicine database was searched through December 2016 using PubMed for articles addressing the clinical relevance of the hormones to improve neural structural integrity following traumatic spinal cord injury. It was found that each of these hormones, through varied mechanisms, could serve to reduce the harmful effects associated with spinal cord injury, and could aid in restoring some function to the injured spinal cord in the animal models. The most striking effects were seen in the reduction of inflammation commonly linked to injury of the central nervous system. The effects of human chorionic gonadotropin administration following spinal cord injury have received far less attention than those of either estrogen or progesterone, and additional inquiry could be of general benefit. In this article, we discussed the outstanding questions and suggested future directions for further investigation.
Keywords: Estrogen, progesterone, human chorionic gonadotropin, spinal cord injury, remyelination, inflammation
Introduction
Traumatic spinal cord injuries (SCI), both complete and incomplete can have devastating physiological consequences. Depending on the severity of injury, patients incur neurological deficits ranging from paralysis, loss of sensation, impaired bowel, bladder and sexual function, autonomic dysfunction and even death [1-4], and the sequelae of impairment carry with them systemic complications, including impaired wound healing, pneumonia, and ventilator dependence, to name a few. The irreversibility of SCI can be ascribed largely to the relative rarity with which axonal regeneration occurs in the adult spinal cord [5]. This lack of regeneration is attributable to glial scar formation, inflammation and cell death, dominance of inhibitory growth components, and the loss of substrates that support growth [2,6-8].
The incidence of SCI has been demonstrated, through systematic reviews to be more prevalent in developing countries than in developed nations [9,10], however, in the United States alone, the staggering incidence of SCI approximates to 40 injuries per million annually or about 12,400 injuries across the general population as reported in 2010 [11]. The occurrence of traumatic SCIs show a bimodal age distribution with one peak being between the ages of 15 and 29 years, and the second being above age 65 years [11,12]. Injuries occur more often in men. The leading cause of SCI is motor vehicle accidents, followed by falls, violence (particularly gunshot wounds), and sports accidents, in descending order [13,14]. The pronounced mobility of the cervical spine causes it to be the most commonly damaged spinal region followed by the thoracolumbar junction, which has greater mobility than that of the thoracic spine; the additional stability conferred by the ribs results in fewer traumatic thoracic injuries [10,12].
The underlying causes of SCI vary dramatically including crush injuries, piercing injuries, vertebral herniation, and gunshot wounds. Many injuries are associated with compression, flexion, extension, distraction, axial loading or rotation of the spinal cord or column.
In recent years, studies have been conducted on various substances and biomolecules that could be effective in enhancing repair of the spinal cord following trauma. As a result, many promising advances have come forth in understanding the pathophysiological and biological processes involved in SCI and developing potential treatments.
Cell transplantation has been explored as a useful technique in minimizing the damage associated with SCI, and in regenerating the injured spinal cord; astrocyte transplantation has been studied in particular detail [5]. All astrocyte transplantation attempts have not provided significant benefit [15,16]. However, recent work with embryonic glial-restricted precursor-derived astrocytes (GDAs) has proven to be effective in limiting lesion size, while also preserving white matter [17-24].
Furthermore, there have been promising advances in the delivery methods of cell and biomolecules that could improve tissue repair and regeneration in the central nervous system. Methods include encapsulated cell therapy, the use of implanted scaffolds, and biomolecule delivery in polymeric nano/microspheres and hydrogels [25]. Ji et al. observed that maintaining the integrity of the blood-spinal cord barrier following spinal cord ischemia reperfusion injury could lead to improved outcomes [26,27].
Potential effects of immune modulatory therapies on SCI have also been examined [28]. The steroid methylprednisolone has been shown to be neuroprotective; it provides functional benefit in SCI through its action on glucocorticoid receptors, its interaction with NF-κB, and its antioxidant activity [29-35]. Inhibiting neutrophil recruitment and adhesion through targeting of the ICAM-1 and/or P-selectin has been observed to improve results in some cases of SCI [36-39], however, one study involving anti-neutrophil serum did not offer corroboratively compelling results [40]. While macrophage depletion could modestly improve outcomes following SCI [41,42], there is a relative lack of specific methods for targeting macrophages which complicates the determination that macrophage impairment, and not another mechanism, is responsible. Despite some conflicting results [43-45] with anti-T cell methods, likewise therapies aimed at inhibiting T- and B-cells have been seen to offer some benefit [46-50]. Several anti-inflammatory therapies have been shown to provide benefit following SCI; these include the administration of anti-CD11d monoclonal antibody [51-53], the inhibition of monocytes and neutrophils together (as potential collaborators) [54], and the use of intravenous immunoglobulin [55,56]. The antibiotic, minocycline, provides neuroprotective benefit through its ability to attenuate inflammation and apoptosis [57-69]. Some plant-derived substances such as allicin and gastrodin have also been observed to mitigate the effects of SCI through anti-inflammatory mechanisms [70,71]. Current research is attempting to elucidate the potential benefits to be derived from mediators of inflammation.
Hormonal therapies such as those involving the administration of estrogen, progesterone, and human chorionic gonadotropin (HCG) have been shown to improve outcomes following SCI (Table 1). These hormones act through various mechanisms that will be reviewed in this paper. The use of endogenous hormones as SCI therapy is attractive because associated results and side effects may be limited or more readily anticipated as compared to the use of some exogenous therapies. These hormones are also relatively accessible and inexpensive which improve their potential for wide-spread use.
Table 1.
Effects of hormones in relation to spinal cord injury
| Hormone | Direct effect | Implications |
|---|---|---|
| Estrogen | Microglial activation, increased VEGF expression along with increased blood flow to site of injury, reduced calpain and caspase 3 expression, attenuated cellular calcium influx, decreased TNF-α and iNOS expression | Reduction in neuronal death, anti-apoptotic effect leading the increased cell survival, decreased inflammatory response; overall increase in preservation of function following injury |
| Progesterone | Downregulation of inflammatory cytokines including TNF-α and iNOS, NOS2, MCP-1, and IL-1β; downregulation of caspase 3 and GFAP | Neuroprotection due to attenuated inflammation and reduction of apoptosis; improved motor function and increased preservation of neuronal structural and functional integrity |
| Human chorionic gonadotropin | Reduced lesion volume in experimental stroke models | Potential improvement in functional and structural recovery following spinal cord injury |
Hormonal therapy
Historically, regeneration of peripheral nerves has been considered plausible in certain situations, however, the dogma persists that SCIs are permanently debilitating without chance of recovery. While the reality largely confirms this impression, there are areas of research in spinal cord regeneration that are demonstrating tremendous promise. Areas that have been recently explored include cell transplantation, steroid hormone administration, immune modulatory therapies, and the administration of inflammation suppressants and other biomolecules among others. This review will focus on the hormones, estrogen, progesterone, and HCG and their effects on the injured spinal cord.
Estrogen
Many pharmacological agents and their effects following traumatic spinal cord injury have been studied [72-75]. Among these, estrogen has been shown to exhibit a neuroprotective effect [76-79]. This effect is a result of anti-inflammatory processes and activation of varied serine proteases by estrogen [76]. Letaif et al. [80] note that this anti-inflammatory activity is perpetrated through microglial activation, increased blood flow to the site of injury, increased levels of anti-apoptotic proteins, attenuated cellular influx of calcium following injury and administration of estrogen [81-83]. In fact, increased estrogen levels may be partially responsible for improved outcomes in females relative to males following SCI [84].
The overexpression of cytokines has been observed following injury of the central nervous system [85,86]. Upregulation of genes expressing cytokines such as tumor necrosis factor-a (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) has been observed shortly following SCI [87-92] (Figure 1). TNF-α mediates inflammatory processes through its activation of NF-κB, which in turn upregulates other pro-inflammatory cytokines [93-97]. Pishva et al. [98], determined that administration of estrogen twice daily following SCI in rats significantly reduced the gene expression of TNF-α and its downstream cytokine iNOS and this likely accounts for at least some of the inhibitory effect estrogen has on inflammation following SCI (Figure 1).
Figure 1.

Schematic diagram showing underlying causes of SCI and immune response activation. Some of the major transcription factors involved in inflammatory injury following spinal cord trauma are shown. The complete signaling pathways are not depicted. Causes of SCI vary and include motor vehicle accidents, falls, violent encounters, and sports injuries. SCI is generally marked by an increase in cytokines such as TNF-α, IL-1β, and IL-6 that lead to upregulation of inflammatory and/or apoptotic agents including NF-κB, AP-1, JNK, p38 MAPK, and PGE2. The upregulation of these factors often contributes to secondary damage which worsens the outcome following the initial injury, typically presenting as increased lesion size or increased loss of cells.
While estrogen may not be effective in returning complete function and mobility to an individual following traumatic spinal cord injury, it has been shown to improve functional scores in studies performed in rats [80]. However, between weeks four and six following the induced injury, the group administered a single dose of 17b-estradiol intraperitoneally scored significantly higher than their control counterparts with mean BBB scores being reported as 15.1 for the estradiol group and 9.3 for the control by week 6, which was statistically significant [80]. Hubscher et al. [99] likewise reported improved scores through the sixth week of their experiment. Other studies reviewed cover a short period and only provide insight into the initial effects of estrogen following SCI [76]. While Ritz and Hausmann reported lower BBB scores after the fourth week [77], other groups have reported significant improvements in BBB scores in estrogen-treated rats earlier than the fourth week [76,78]. The conflicting findings underscores the importance of a potential benefit with additional long-term studies extending well beyond the fourth week to clarify our understanding of the effects of estrogen on functional nervous system scores, beyond the short-term. Moreover, some investigators have administered estradiol in multiple doses [76,78,83] and observed good cellular and physiological results. Olsen et al. [83] administered estrogen for the first 21 days following SCI. The studies employing multiple dosing schedules [76,78] reported earlier improvements in BBB scores compared to those that administered a single dose [80] (Figure 2).
Figure 2.
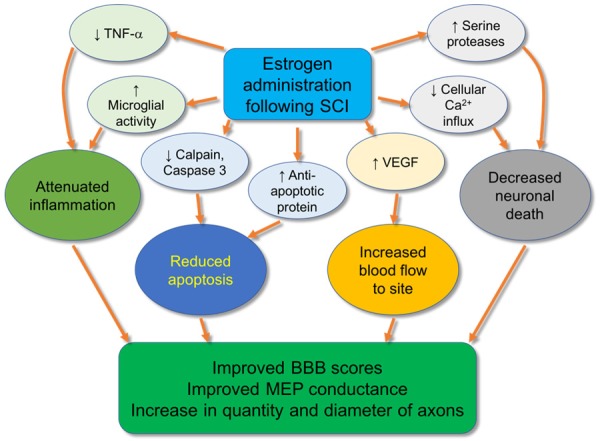
Compared to SCI controls, subjects receiving estrogen injections following injury exhibit reduction in TNF-α, increased microglial activity, reduced calpain and caspase 3 levels, increased levels of anti-apoptotic protein, decreased cellular calcium influx, increased serine protease activation, and increased VEGF expression. This, in turn, leads to attenuated inflammation, decreased apoptosis, and increased blood flow to the site of injury, resulting in improved scores on the Basso-Bresnahan-Beattie (BBB) scale for locomotion, improved conduction velocity on motor evoked potential (MEP) monitoring, and increased quantity and diameter of axons.
In addition, large differences between treatment and control animals have been seen using motor evoked potential monitoring (MEP) [80]. In this test, latency, or the time taken for an electrical impulse to travel from the head to the limbs, and the amplitude of the transmitted impulse were measured. Letaif et al. [80] reported a 17-fold increase in the speed of travel of the electrical impulse in estrogen-treated rats as compared to the control, and a 7-fold increase in the amplitude of same impulse. The strictly objective nature of this test makes it a valuable and evaluative tool for measuring neural function. Thus, MEP proves indispensable in future studies involving injuries of the nervous system in evaluating the effect of treatment (Figure 2).
No significant improvement has been observed from a histological perspective in rats treated with estrogen following SCI when analyzed for necrosis, hemorrhage, hyperemia, axon degeneration, and cellular infiltration. However, there has been a marked increase in quantity and diameter of axons observed in rats treated with estrogen compared to control [80]. The mean number of fibers for the estradiol group was reported as 92.6 and that of the control group was 56.9 which was statistically significant. Whereas the mean diameter of axons for the estradiol group was 92.4 compared to a mean of 55.1 for the control group, which was again, statistically significant [80].
So far, studies examining the effects of estrogen injection following SCI have administered the treatment directly following the injury. This provides useful insight into the results of early treatment with estrogen. However, it would be of benefit to directly compare the effects of early administration with later administration to ascertain any differences in benefit that exist. In fact, Sribnick et al. found that chronic cases of SCI are also amenable to estrogen therapy and exhibit improvements in motor function following treatment [78]. The effect on chronic cases could be further explored.
It has been shown that some molecules that bind the estrogen receptors such as G-1, tamoxifen, and other estrogen receptor agonists, can have neuroprotective functions similar or identical to those of estrogen following SCI [100-108]. High dose estrogen administration has not become a standard of care for SCI patients in large part due to the adverse effects associated with estrogen levels well above normal physiological levels. These side effects include increased rates of deep vein thrombosis and cancer [109,110], and the development of feminine physical traits in males. Samantaray et al. [1] reported that low dose estrogen administration attenuates gliosis and provides protection for neurons in the caudal penumbra following traumatic SCI in rats. It was observed that low dose estrogen administration (5-10 µg/kg) 48 hours following the injury resulted in the attenuation of inflammatory events, reduced calpain expression which induced an anti-apoptotic effect, reduced caspase-3 expression also inhibiting apoptosis, increased expression of the estrogen receptors ER-α and ER-β (suggesting effects relate to increased receptor signaling), attenuated neuronal death, and increased expression of VEGF which is a potent stimulator of vasculogenesis and angiogenesis. Apart from the increased expression of estrogen receptors, the observations failed to exhibit any significant difference between the 10 µg and 100 µg doses of estrogen.
Thus, the research findings to date suggest that low dose estrogen administration and estrogen receptor agonists exhibit potential to be further explored in animals, and in clinical trials in humans following additional knowledge on potentially adverse effects. Estrogen has been shown to ameliorate SCI, but the undesirable effects associated with high-dose estrogen administration limit its potential as a stand-alone therapy.
Progesterone
Progesterone (PROG) is a steroid hormone produced by the ovaries and placenta in females, and by the adrenal glands in both females and males. The nervous system has the capacity to locally synthesize PROG and convert PROG into its active metabolite, allopregnanolone [111]. As with estrogen, PROG has been shown to be promyelinating, anti-inflammatory, and neuroprotective in cases of nervous system injury [112-115]. When studied in relation to brain trauma, PROG was shown to prevent neuron loss and mitochondrial dysfunction, reduce edema and inhibit inflammatory cytokines, as well as, improve motor function on diagnostic scales [116-118]. Moreover, PROG has also been tested in two Phase II clinical trials which suggested its efficacy as a treatment option for traumatic brain injury patients [119-121]. With respect to SCI, PROG has been shown to prevent chromatolysis, preserve motor neuron structure, upregulate the expression of choline acetyltransferase and brain-derived neurotrophic factor (BDNF), which increase production of acetylcholine and help to support preservation, growth, and differentiation of neurons, respectively. PROG has also been shown to reduce the proliferation and activation of astrocytes and microglia, and increase the production of oligodendrocyte progenitor cells [113,122-124]. PROG could be a target for therapies aimed at improving neural function following injury by modulation of astrocytes and their pathogenesis [125]. As was observed with estrogen, 10 µg/kg/12 h PROG administration significantly reduced the expression of TNF-α and iNOS genes following SCI, which lead to production of inflammatory mediators and nitric oxide (NO) which can contribute to reactive radical damage [126]. Garcia-Ovejero et al. [127] observed comparable effects following SCI in rats administered PROG subcutaneously each day. After 60 days, there was a marked increase in spared white matter (SWM) preservation in PROG-treated rats compared to control with white matter measurements of 58.60 ± 4.06% and 22.99 ± 3.03% volume, respectively, as measured 2.5 mm rostrally and caudally from the epicenter of contusion. However, there was no significant difference in the volume of spared gray matter (SGM) observed between control rats and those treated with PROG. Increased oligodendrocytes, decreased myelin damage, improved axonal preservation, and improved locomotor function were all demonstrated following the administration of PROG after SCI.
While another study recapitulated the improved motor and histological outcome with PROG administration [128], the beneficial results are not universal: Fee et al. [129] found no improvement with PROG injection following SCI. Part of this discrepancy could be a result of differences in the duration of study, as reported observations in the study of Fee et al. [129] were limited to 5 days as opposed to 60 days and 6 weeks in the studies producing positive results.
Guennoun et al. [111] found the binding of progesterone to intracellular progesterone receptors (PR) via its classical pathway, and it may also bind to specific membrane sites (mPR/PGRMC1 complex) to activate intracellular signaling pathways (Figure 3). Moreover, allopregnanolone may act on GABAA receptors following its conversion from PROG [111,130-135]. They also determined that PROG acted on the effectors PR, mPRs, and PGRMC1. After conversion to 5a-dihydroprogesterone through a mechanism involving 5a-reductases, action occurred on the effectors PR and mPRa (Figure 3). Following conversion via 3a-HSOR to allopregnanolone (3a5a-THPROG), it can bind to GABAA receptors, PXR, and mPRd to induce neuroprotective effects [111] (Figure 3). Further understanding of the receptors upon which PROG and related molecules act could prove useful in developing treatments aimed at neuroprotection. Future work is required to ascertain potential side effects associated with synthetic progestins and allopregnanolone as many synthetic progestins may also bind androgen and glucocorticoid receptors producing undesirable effects [136,137], and allopregnanolone has been associated with some cognitive impairment and symptoms such as anxiety, irritability, aggressiveness, seizure, and increased pain [138-142]. Of note, it has been determined that PR reduces reactive gliosis and preserves oligodendrocyte precursor cells in the injured spinal cord in rats [143].
Figure 3.
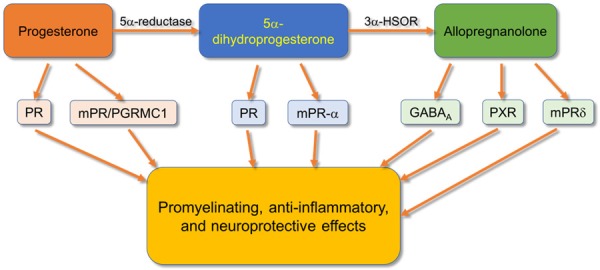
Compared to SCI controls, subjects receiving progesterone injections following injury exhibit reduced expression of TNF-α and subsequently iNOS, increased acetylcholine production and neuron growth through upregulation of choline acetyltransferase and brain-derived growth factor (BDNF), downregulation of NOS2, MCP-1, IL-1β, caspase-3, and GFAP. Observations have been noted of decreased chromatolysis, increased preservation of neuron structure, and a reduction of mitochondrial dysfunction in animals administered progesterone following SCI. It has been noted that progesterone and its metabolites 5a-dihydroprogesterone and allopregnanolone act on those receptors depicted to contribute to the mediation of the effects listed. In this schematic diagram, complete pathways are not depicted, but some of the major components are shown.
PROG shows potential as a modulator of neuropathic pain following SCI [144-146]. While there may be many mechanisms by which pain is transmitted following central nervous system injury [147,148], neuro-inflammation and reactive gliosis are primary causes of chronic pain following SCI [149-151]. Cytokines are involved in the modulation of neuronal function and pain transmission [151-156]. Coronel et al. [144] explored the effects of PROG on IL-1β and its receptors IL-1RI and IL-1RII, antagonist IL-1ra, IL-6, TNF-α, and NR1 subunit of N-methyl-D-aspartate receptor (NMDAR) following SCI. IL-1β, IL-6, and TNF-α mRNA (and protein) levels were significantly lower in rats receiving PROG than the placebo, on the first day status post injury; however, there was only a significant difference in mRNA levels observed on day 14 in IL-1β, and no significant difference on day 28 of the study. There was no significant difference in IL-1RI mRNA levels between the groups on days 1 or 14, but by day 28, the PROG-treated group had significantly lower levels than the placebo. IL-1RII mRNA levels were seen to be markedly higher in the PROG group on day one, with no significant difference thereafter. No differences were observed between the two groups in IL-1ra mRNA levels. These data as well as those showing fewer IL-1RI positive neurons in the spinal cords of PROG-treated rats compared to those receiving placebo suggest that PROG may provide benefit to those experiencing chronic pain following SCI, but may not have the same effect or mediate it in the same way in the immediate aftermath of the injury. Additional research on the effect of PROG following SCI, with a focus on IL-1RI, and its utility as a treatment option in cases of chronic pain following SCI, is warranted. There is indication that the effect of PROG could differ in acute and chronic settings.
Yang et al. [157] observed that PROG significantly reduces axonal dieback and neuronal death in mice following SCI when observed at intervals of 24 h, 48 h, and 72 h from the initial injury. This was mediated via down-regulation of inflammatory cytokines, including NOS2, MCP-1, and IL-1β as well as activated caspase-3 and GFAP. Interestingly, upregulation of myelin basic protein (MBP) was also noted. It was also suggested that PROG improved behavioral function following SCI. Further studies of a longer duration would help elucidate the effects of PROG on axon and neuron preservation beyond the acute stages.
PROG is effective as an anti-inflammatory agent that can improve motor function and histological outcomes following SCI. To date, the side-effects associated with PROG are not as immediately apprehensible as those of some others, and it continues to exhibit potential as an effective treatment following injury of the central nervous system.
HCG
Human chorionic gonadotropin (HCG) is a heterodimer consisting of an α and a β subunit, which are non-covalently linked. The hCGa subunit constitutes part of other hormones such as luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone. HCG is produced in the adrenal pituitary gland by gonadotropin cells [158,159].
HCG presents potential as a treatment in the case of central nervous system injury. It was observed in a preliminary study that HCG helped to increase the amount of adrenal medulla tissue survival following autologous transplant in the lateral ventricles of rat brains [160]. Proliferation of endogenous stem cells in the subgranular zone and the subventricular zone has been observed to increase with administration of HCG [161]. Meng et al. [162] observed that HCG induced neuronal differentiation of PC12 cells by activating the stably expressed lutropin/choriogonadotropin receptor in vitro. In a study of rats with experimental strokes, treatment with HCG + erythropoietin (EPO) significantly reduced the lesion volumes (by 82-89%) and significantly improved neurological scores compared to three other treatment groups including HCG + saline, saline + EPO, and saline + saline [163]. This would also suggest that EPO may play a role in the reduction of lesion size following stroke.
Extensive literature reviews illustrate a relative void in research performed using HCG in association with SCI as compared with studies using estrogen and progesterone. Patil and Nagaraj [164] found that 12 rats receiving HCG injections following SCI exhibited a significant improvement in functional recovery (assessed by measurement and grading of the return of bladder function and the ability to climb up an inclined plane) within 6 weeks as compared to 10 rats serving as control. In a later study, Patil et al. [165] transected the spinal cords of 21 rats at the midthoracic level; the 11 rats administered HCG exhibited significantly increased amplitudes of the cortical evoked motor action potentials after six weeks compared to the control group. These findings suggest that the administration of HCG may serve to improve spinal cord function following traumatic injury. However, the relatively small number of animals studied and the limited number of retrievable studies of the effects of HCG on individuals with SCI preclude any conclusive determinations of universal effectiveness or possible side effects until further research is undertaken.
HCG may act to improve motor function and minimize neuronal damage following spinal cord lesion. The minimal side effects associated with HCG, and the relative lack of research that has been performed on its effect on SCI mandate further investigation as a potential therapeutic option.
Conclusion
Estrogen, progesterone, and HCG are hormones with diverse functions that could serve to attenuate the harmful consequences of SCI through their abilities to interact with the GABA system, reduce excitotoxicity, free radicals, edema, and apoptosis, inhibit inflammatory cytokines, induce increased angiogenesis, mitochondrial recoupling, remyelination [166], and induce stem cell migration to the site of injury. These functions have potential to improve prognoses for individuals suffering after SCI with promise of increased motor function, preserved structure, and a reduction of neuropathic pain. Further investigation into each of these methods is of paramount importance.
Acknowledgements
This work was supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of conflict of interest
None.
References
- 1.Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL. Administration of low dose estrogen attenuates gliosis and protects neurons in acute spinal cord injury in rats. J Neurochem. 2016;136:1064–1073. doi: 10.1111/jnc.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao HQ, Dong ED. An update on spinal cord injury research. Neurosci Bull. 2013;29:94–102. doi: 10.1007/s12264-012-1277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donovan WH. Spinal cord injury-past, present, and future. J Spinal Cord Med. 2007;30:85–100. doi: 10.1080/10790268.2007.11753918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickelsimer E, Shiroma EJ, Wilson DA. Statewide investigation of medically attended adverse health conditions of persons with spinal cord injury. J Spinal Cord Med. 2010;33:221–231. doi: 10.1080/10790268.2010.11689699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu T, Zhou H, Li F, Wang T, Lu L, Feng S. Astrocyte transplantation for spinal cord injury: current status and perspective. Brain Res Bull. 2014;107:18–30. doi: 10.1016/j.brainresbull.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Maier IC, Schwab ME. Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1611–1634. doi: 10.1098/rstb.2006.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Warsz) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 8.Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- 9.Chiu WT, Lin HC, Lam C, Chu SF, Chiang YH, Tsai SH. Review paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health. 2010;22:9–18. doi: 10.1177/1010539509355470. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg MEL, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184–192. doi: 10.1159/000279335. discussion 192. [DOI] [PubMed] [Google Scholar]
- 11.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 12.Wolf M, Weber MA. Neuroimaging of the traumatic spine. Magn Reson Imaging Clin N Am. 2016;24:541–561. doi: 10.1016/j.mric.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 13.NSCISC National Spinal Cord Injury Statistical Center: Spinal Cord Injury Model Systems: 2014 Annual Report. 2015. [Google Scholar]
- 14.Jakoi A, Iorio J, Howell R, Zampini JM. Gunshot injuries of the spine. Spine J. 2015;15:2077–2085. doi: 10.1016/j.spinee.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Olby NJ, Blakemore WF. Reconstruction of the glial environment of a photochemically induced lesion in the rat spinal cord by transplantation of mixed glial cells. J Neurocytol. 1996;25:481–498. doi: 10.1007/BF02284817. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Hashimoto M, Koda M, Naito AT, Murata A, Okawa A, Takahashi K, Yamazaki M. Increase of sensitivity to mechanical stimulus after transplantation of murine induced pluripotent stem cell-derived astrocytes in a rat spinal cord injury model. J Neurosurg Spine. 2011;15:582–593. doi: 10.3171/2011.7.SPINE10775. [DOI] [PubMed] [Google Scholar]
- 17.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJ. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies JE, Pröschel C, Zhang N, Noble M, Mayer-Pröschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glialrestricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies SJ, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–732. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan C, Zheng Y, Cheng X, Qi X, Bu P, Luo X, Kim DH, Cao Q. Transplantation of D15A-expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int J Biol Sci. 2013;9:78–93. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Li J, Chen L, Zhang H, Yuan L, Davies SJ. Combined transplantation of GDAsBMP and hr-decorin in spinal cord contusion repair. Neural Regen Res. 2013;8:2236–2248. doi: 10.3969/j.issn.1673-5374.2013.24.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott Donaghue I, Tam R, Sefton MV, Shoichet MS. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J Control Release Off J Control Release Soc. 2014;190:219–227. doi: 10.1016/j.jconrel.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Yu Q, Xie L, Zhu H. Targeting the bloodspinal cord barrier: a therapeutic approach to spinal cord protection against ischemia-reperfusion injury. Life Sci. 2016;158:1–6. doi: 10.1016/j.lfs.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Yu Q, Huang J, Hu J, Zhu H. Advance in spinal cord ischemia reperfusion injury: Blood-spinal cord barrier and remote ischemic preconditioning. Life Sci. 2016;154:34–38. doi: 10.1016/j.lfs.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury--past, present and future. Exp Neurol. 2014;258:91–104. doi: 10.1016/j.expneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx J Am Soc Exp Neurother. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 32.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 33.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 34.Braughler JM, Hall ED. Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+ + K+)-ATPase, lipid peroxidation, and alpha motor neuron function. J Neurosurg. 1982;56:838–844. doi: 10.3171/jns.1982.56.6.0838. [DOI] [PubMed] [Google Scholar]
- 35.Hall ED, Braughler JM. Acute effects of intravenous glucocorticoid pretreatment on the in vitro peroxidation of cat spinal cord tissue. Exp Neurol. 1981;73:321–324. doi: 10.1016/0014-4886(81)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y, Fukuzawa K. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996;66:1525–1531. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- 37.Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 38.Farooque M, Isaksson J, Olsson Y. Improved recovery after spinal cord trauma in ICAM-1 and P-selectin knockout mice. Neuroreport. 1999;10:131–134. doi: 10.1097/00001756-199901180-00024. [DOI] [PubMed] [Google Scholar]
- 39.Farooque M, Isaksson J, Olsson Y. White matter preservation after spinal cord injury in ICAM-1/P-selectin-deficient mice. Acta Neuropathol (Berl) 2001;102:132–140. doi: 10.1007/s004010000307. [DOI] [PubMed] [Google Scholar]
- 40.Holtz A, Nyström B, Gerdin B. Spinal cord injury in rats: inability of nimodipine or anti-neutrophil serum to improve spinal cord blood flow or neurologic status. Acta Neurol Scand. 1989;79:460–467. doi: 10.1111/j.1600-0404.1989.tb03815.x. [DOI] [PubMed] [Google Scholar]
- 41.Iannotti CA, Clark M, Horn KP, van Rooijen N, Silver J, Steinmetz MP. A combination immunomodulatory treatment promotes neuroprotection and locomotor recovery after contusion SCI. Exp Neurol. 2011;230:3–15. doi: 10.1016/j.expneurol.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci Off J Soc Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauben E, Agranov E, Gothilf A, Nevo U, Cohen A, Smirnov I, Steinman L, Schwartz M. Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease. J Clin Invest. 2001;108:591–599. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci Off J Soc Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet Lond Engl. 2000;355:286–287. doi: 10.1016/s0140-6736(99)05140-5. [DOI] [PubMed] [Google Scholar]
- 46.Luchetti S, Beck KD, Galvan MD, Silva R, Cummings BJ, Anderson AJ. Comparison of immunopathology and locomotor recovery in C57BL/6, BUB/BnJ, and NOD-SCID mice after contusion spinal cord injury. J Neurotrauma. 2010;27:411–421. doi: 10.1089/neu.2009.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu B, Matic D, Djogo N, Szpotowicz E, Schachner M, Jakovcevski I. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp Neurol. 2012;237:274–285. doi: 10.1016/j.expneurol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 49.Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 51.Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD 11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5:343–352. doi: 10.3171/spi.2006.5.4.343. [DOI] [PubMed] [Google Scholar]
- 52.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. Anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate, and ventral horns of the injured spinal cord. J Neurosci. 2005;25:637–647. doi: 10.1523/JNEUROSCI.3960-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SM, Rosen S, Weinstein P, van Rooijen N, Noble-Haeusslein LJ. Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J Neurotrauma. 2011;28:1893–1907. doi: 10.1089/neu.2011.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gok B, Sciubba DM, Okutan O, Beskonakli E, Palaoglu S, Erdamar H, Sargon MF. Immunomodulation of acute experimental spinal cord injury with human immunoglobulin G. J Clin Neurosci. 2009;16:549–553. doi: 10.1016/j.jocn.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen DH, Cho N, Satkunendrarajah K, Austin JW, Wang J, Fehlings MG. Immunoglobulin G (IgG) attenuates neuroinflammation and improves neurobehavioral recovery after cervical spinal cord injury. J Neuroinflammation. 2012;9:224. doi: 10.1186/1742-2094-9-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- 58.Lee SM, Yune TY, Kim SJ, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem. 2004;91:568–578. doi: 10.1111/j.1471-4159.2004.02780.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–9. doi: 10.1097/00006123-200106000-00051. discussion 1399-401. [DOI] [PubMed] [Google Scholar]
- 61.Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SS, Kong PJ, Kim BS, Sheen DH, Nam SY, Chun W. Inhibitory action of minocycline on lipopolysaccharide-induced release of nitric oxide and prostaglandin E2 in BV2 microglial cells. Arch Pharm Res. 2004;27:314–318. doi: 10.1007/BF02980066. [DOI] [PubMed] [Google Scholar]
- 64.Kremlev SG, Roberts RL, Palmer C. Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. J Neuroimmunol. 2004;149:1–9. doi: 10.1016/j.jneuroim.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–171. doi: 10.1016/j.neulet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 67.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279:19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 70.Du F, Wang X, Shang B, Fang J, Xi Y, Li A, Diao Y. Gastrodin ameliorates spinal cord injury via antioxidant and anti-inflammatory effects. Acta Biochim Pol. 2016;63:589–593. doi: 10.18388/abp.2016_1272. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Ren D. Allicin protects traumatic spinal cord injury through regulating the HSP70/Akt/iNOS pathway in mice. Mol Med Rep. 2016;14:3086–3092. doi: 10.3892/mmr.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cristante AF, Barros Filho TE, Marcon RM, Letaif OB, da Rocha ID. Therapeutic approaches for spinal cord injury. Clinics. 2012;67:1219–1224. doi: 10.6061/clinics/2012(10)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cristante AF, Filho TE, Oliveira RP, Marcon RM, Ferreira R, Santos GB. Effects of antidepressant and treadmill gait training on recovery from spinal cord injury in rats. Spinal Cord. 2013;51:501–507. doi: 10.1038/sc.2013.18. [DOI] [PubMed] [Google Scholar]
- 74.Marcon RM, Cristante AF, de Barros Filho TE, de Oliveira RP, dos Santos GB. Potentializing the effects of GM1 by hyperbaric oxygen therapy in acute experimental spinal cord lesion in rats. Spinal Cord. 2010;48:808–813. doi: 10.1038/sc.2010.37. [DOI] [PubMed] [Google Scholar]
- 75.Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–82. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-7. [DOI] [PubMed] [Google Scholar]
- 76.Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- 77.Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 78.Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- 80.Letaif OB, Cristante AF, de Barros Filho TE, Ferreira R, dos Santos GB, da Rocha ID, Marcon RM. Effects of estrogen on functional and neurological recovery after spinal cord injury: an experimental study with rats. Clinics. 2015;70:700–705. doi: 10.6061/clinics/2015(10)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–33. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 159-60. [DOI] [PubMed] [Google Scholar]
- 82.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17β-oestradiol treatment. Brain. 2010;133:1013–1025. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Datto JP, Yang J, Dietrich WD, Pearse DD. Does being female provide a neuroprotective advantage following spinal cord injury? Neural Regen Res. 2015;10:1533–1536. doi: 10.4103/1673-5374.165213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu CY, Doster K, Hu ZY. Cell-mediated injury. In: Narayan K, Wilberger JE Jr, Polishock PT, editors. Neurotrauma: a comprehensive textbook of head and spinal cord injury. St. Louis, MO: McGraw-Hill; 1996. pp. 1433–44. In: no date. [Google Scholar]
- 86.Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- 87.Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 88.Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17:203–218. doi: 10.1089/neu.2000.17.203. [DOI] [PubMed] [Google Scholar]
- 89.Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 1997;759:190–196. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- 90.Wang CX, Nuttin B, Heremans H, Dom R, Gybels J. Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol. 1996;69:151–156. doi: 10.1016/0165-5728(96)00080-x. [DOI] [PubMed] [Google Scholar]
- 91.Yakovlev AG, Faden AI. Sequential expression of c-fos protooncogene, TNF-alpha, and dynorphin genes in spinal cord following experimental traumatic injury. Mol Chem Neuropathol. 1994;23:179–190. doi: 10.1007/BF02815410. [DOI] [PubMed] [Google Scholar]
- 92.Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- 93.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- 96.Grell M, Zimmermann G, Hülser D, Pfizenmaier K, Scheurich P. TNF receptors TR60 and TR80 can mediate apoptosis via induction of distinct signal pathways. J Immunol. 1994;153:1963–1972. [PubMed] [Google Scholar]
- 97.Caldenhoven E, Liden J, Wissink S, Van de Stolpe A, Raaijmakers J, Koenderman L, Okret S, Gustafsson JA, Van der Saag PT. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol Endocrinol Baltim Md. 1995;9:401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- 98.Amini Pishva A, Akbari M, Farahabadi A, Arabkheradmand A, Beyer C, Dashti N, Moradi F, Hassanzadeh G. Effect of estrogen therapy on TNF-α and iNOS Gene Expression in Spinal Cord Injury Model. Acta Med Iran. 2016;54:296–301. [PubMed] [Google Scholar]
- 99.Hubscher CH, Fell JD, Gupta DS. Sex and hormonal variations in the development of At-level Allodynia In a Rat chronic spinal cord injury model. Neurosci Lett. 2010;477:153–156. doi: 10.1016/j.neulet.2010.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng Q, Meng J, Wang X, Kang W, Tian Z, Zhang K, Liu G, Zhao J. G-1 exerts neuroprotective effects through G protein-coupled estrogen receptor 1 following spinal cord injury in mice. Biosci Rep. 2016:36. doi: 10.1042/BSR20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J, Hu R, Ge H, Duanmu W, Li Y, Xue X, Hu S, Feng H. G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol Med Rep. 2015;12:1733–1740. doi: 10.3892/mmr.2015.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colón JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11:1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ray SK, Samntaray S, Banik NL. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res. 2016;11:1418–1419. doi: 10.4103/1673-5374.191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res Bull. 2014;109:22–31. doi: 10.1016/j.brainresbull.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O. The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma. 2014;31:268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mosquera L, Colón JM, Santiago JM, Torrado AI, Meléndez M, Segarra AC, Rodríguez-Orengo JF, Miranda JD. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Colón JM, Torrado AI, Cajigas Á, Santiago JM, Salgado IK, Arroyo Y, Miranda JD. Tamoxifen administration immediately or 24 hours after spinal cord injury improves locomotor recovery and reduces secondary damage in female rats. J Neurotrauma. 2016;33:1696–1708. doi: 10.1089/neu.2015.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, Yang Q, Li X, Cheng Q, Zhang K, Han J, Zhao J, Liu G, Zhao M. Estrogen-like neuroprotection of isopsoralen against spinal cord injury through estrogen receptor ERα. Metab Brain Dis. 2017;32:259–265. doi: 10.1007/s11011-016-9913-z. [DOI] [PubMed] [Google Scholar]
- 109.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 111.Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol. 2015;146:48–61. doi: 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- 113.De Nicola AF, Labombarda F, Gonzalez Deniselle MC, Gonzalez SL, Garay L, Meyer M, Gargiulo G, Guennoun R, Schumacher M. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30:173–187. doi: 10.1016/j.yfrne.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Giatti S, Caruso D, Boraso M, Abbiati F, Ballarini E, Calabrese D, Pesaresi M, Rigolio R, Santos-Galindo M, Viviani B, Cavaletti G, Garcia-Segura LM, Melcangi RC. Neuroprotective effects of progesterone in chronic experimental autoimmune encephalomyelitis. J Neuroendocrinol. 2012;24:851–861. doi: 10.1111/j.1365-2826.2012.02284.x. [DOI] [PubMed] [Google Scholar]
- 115.Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 117.Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 118.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience. 2011;191:101–106. doi: 10.1016/j.neuroscience.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 120.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. 402.e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 121.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care Lond Engl. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.González SL, Labombarda F, González Deniselle MC, Guennoun R, Schumacher M, De Nicola AF. Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord. Neuroscience. 2004;125:605–614. doi: 10.1016/j.neuroscience.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 123.Labombarda F, González S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol. 2011;231:135–146. doi: 10.1016/j.expneurol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 124.Labombarda F, González SL, Lima A, Roig P, Guennoun R, Schumacher M, de Nicola AF. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2009;57:884–897. doi: 10.1002/glia.20814. [DOI] [PubMed] [Google Scholar]
- 125.Arbo BD, Bennetti F, Ribeiro MF. Astrocytes as a target for neuroprotection: modulation by progesterone and dehydroepiandrosterone. Prog Neurobiol. 2016;144:27–47. doi: 10.1016/j.pneurobio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 126.Farahabadi A, Akbari M, Amini Pishva A, Zendedel A, Arabkheradmand A, Beyer C, Dashti N, Hassanzadeh G. Effect of progesterone therapy on TNF-α and iNOS gene expression in spinal cord injury model. Acta Med Iran. 2016;54:345–351. [PubMed] [Google Scholar]
- 127.Garcia-Ovejero D, González S, Paniagua-Torija B, Lima A, Molina-Holgado E, De Nicola AF, Labombarda F. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J Neurotrauma. 2014;31:857–871. doi: 10.1089/neu.2013.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine. 1999;24:2134–2138. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- 129.Fee DB, Swartz KR, Joy KM, Roberts KN, Scheff NN, Scheff SW. Effects of progesterone on experimental spinal cord injury. Brain Res. 2007;1137:146–152. doi: 10.1016/j.brainres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 130.Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 131.Mani SK, O’Malley BW. Mechanism of Progesterone Receptor Action in the Brain. In: Pfaff DW, Arnold P, Etgen AM, editors. molecular mechanisms of hormone actions on behavior. Amsterdam: Academic Press; 2009. pp. 375–411. [Google Scholar]
- 132.Schumacher M, Zhu X, Guennoun R. hormones, brain and behavior. Elsevier; 2017. Progesterone: synthesis, metabolism, mechanism of action, and effects in the nervous system; pp. 215–244. [Google Scholar]
- 133.Li X, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–3773. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 135.Mani SK. Signaling mechanisms in progesterone-neurotransmitter interactions. Neuroscience. 2006;138:773–781. doi: 10.1016/j.neuroscience.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 136.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171–208. doi: 10.1210/er.2012-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moore NL, Hickey TE, Butler LM, Tilley WD. Multiple nuclear receptor signaling pathways mediate the actions of synthetic progestins in target cells. Mol Cell Endocrinol. 2012;357:60–70. doi: 10.1016/j.mce.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 138.Rabinowitz A, Cohen SJ, Finn DA, Stackman RW. The neurosteroid allopregnanolone impairs object memory and contextual fear memory in male C57BL/6J mice. Horm Behav. 2014;66:238–246. doi: 10.1016/j.yhbeh.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 139.Johansson IM, Birzniece V, Lindblad C, Olsson T, Bäckström T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- 140.Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26:1747–1751. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- 141.Turkmen S, Lundgren P, Birzniece V, Zingmark E, Backstrom T, Johansson IM. 3beta-20betadihydroxy-5alpha-pregnane (UC1011) antagonism of the GABA potentiation and the learning impairment induced in rats by allopregnanolone. Eur J Neurosci. 2004;20:1604–1612. doi: 10.1111/j.1460-9568.2004.03610.x. [DOI] [PubMed] [Google Scholar]
- 142.Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011;191:46–54. doi: 10.1016/j.neuroscience.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 143.Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol. 2015;154:274–284. doi: 10.1016/j.jsbmb.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 144.Coronel MF, Raggio MC, Adler NS, De Nicola AF, Labombarda F, González SL. Progesterone modulates pro-inflammatory cytokine expression profile after spinal cord injury: implications for neuropathic pain. J Neuroimmunol. 2016;292:85–92. doi: 10.1016/j.jneuroim.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 145.Coronel MF, Sánchez Granel ML, Raggio MC, Adler NS, De Nicola AF, Labombarda F, González SL. Temporal changes in the expression of the translocator protein TSPO and the steroidogenic enzyme 5α-reductase in the dorsal spinal cord of animals with neuropathic pain: effects of progesterone administration. Neurosci Lett. 2016;624:23–28. doi: 10.1016/j.neulet.2016.04.067. [DOI] [PubMed] [Google Scholar]
- 146.González SL, Coronel MF. Beyond reproduction: the role of progesterone in neuropathic pain after spinal cord injury. Neural Regen Res. 2016;11:1238–1240. doi: 10.4103/1673-5374.189177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yezierski RP. Spinal cord injury pain: spinal and supraspinal mechanisms. J Rehabil Res Dev. 2009;46:95–107. [PubMed] [Google Scholar]
- 149.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Exp Neurol. 2014;258:48–61. doi: 10.1016/j.expneurol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–372. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Viviani B, Boraso M, Marchetti N, Marinovich M. Perspectives on neuroinflammation and excitotoxicity: a neurotoxic conspiracy? Neurotoxicology. 2014;43:10–20. doi: 10.1016/j.neuro.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 153.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 154.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glialcytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci Off J Soc Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic Pain: promises and potential caveats. Neurosci Biobehav Rev. 2014;45:19–27. doi: 10.1016/j.neubiorev.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Z, Xie W, Ju F, Khan A, Zhang S. In vivo two-photon imaging reveals a role of progesterone in reducing axonal dieback after spinal cord injury in mice. Neuropharmacology. 2017;116:30–37. doi: 10.1016/j.neuropharm.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 158.Matsuura S, Ohashi M, Chen HC, Shownkeen RC, Hartree AS, Reichert LE, Stevens VC, Powell JE. Physicochemical and immunological characterization of an HCG-like substance from human pituitary glands. Nature. 1980;286:740–741. doi: 10.1038/286740a0. [DOI] [PubMed] [Google Scholar]
- 159.Cole LA, Sasaki Y, Muller CY. Normal Production of Human Chorionic Gonadotropin in Menopause. N Engl J Med. 2007;356:1184–1186. doi: 10.1056/NEJMc066500. [DOI] [PubMed] [Google Scholar]
- 160.Patil A, Fillmore K, Valentine J, Hill D. The study of the effect of human chorionic gonadotrophic (HCG) hormone on the survival of adrenal medulla transplant in brain. Preliminary study. Acta Neurochir (Wien) 1987;87:76–78. doi: 10.1007/BF02076021. [DOI] [PubMed] [Google Scholar]
- 161.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin Reprod Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 162.Meng XL, Rennert OM, Chan WY. Human chorionic gonadotropin induces neuronal differentiation of PC12 cells through activation of stably expressed lutropin/choriogonadotropin receptor. Endocrinology. 2007;148:5865–5873. doi: 10.1210/en.2007-0941. [DOI] [PubMed] [Google Scholar]
- 163.Belayev L, Khoutorova L, Zhao KL, Davidoff AW, Moore AF, Cramer SC. A novel neurotrophic therapeutic strategy for experimental stroke. Brain Res. 2009;1280:117–123. doi: 10.1016/j.brainres.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 164.Patil AA, Nagaraj MP. The effect of human chorionic gonadotropin (HCG) on functional recovery of spinal cord sectioned rats. Acta Neurochir (Wien) 1983;69:205–218. doi: 10.1007/BF01401807. [DOI] [PubMed] [Google Scholar]
- 165.Patil AA, Filmore K, Hill D. The effect of human chorionic gonadotropin (HCG) on restoration of physiological continuity of the spinal cord. A preliminary report. Int Surg. 1990;75:54–57. [PubMed] [Google Scholar]
- 166.Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr Neuropharmacol. 2016;14:641–653. doi: 10.2174/1570159X14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]


