Abstract
Cataract is the most common cause of blindness worldwide. Multiple factors such as aging, eye injury, diabetes mellitus, ultraviolet exposure, drug use and other ocular diseases are etiologically linked to cataractogenesis. Due to a rapid increase in aging population, age-related cataract has become the leading cause of blindness. Therefore, it is urgent to understand the molecular mechanism underlying cataractogenesis. MicroRNAs (miRNAs) are a group of endogenous, small noncoding RNAs that regulate gene expression at the post-translational level through binding with the 3’-untranslated regions of target mRNAs. Studies have shown that miRNAs play important roles in multiple cellular functions, including apoptosis, cell proliferation, senescence and stress response. Deregulated expression of miRNAs is also linked to the pathogenesis of many diseases, including ocular diseases. In our review, we focus on miRNAs that are involved in cataract development and discuss their potential applications as novel diagnostic markers and therapeutic targets.
Keywords: Cataract, microRNAs, miRNAs
Introduction
Cataract, defined as the clouding of natural crystalline lens in the eye, is the leading contributing factor of blindness in the world, causing 47.8% of all cases of blindness [1-3]. It is estimated that the number of people suffering from cataract blindness will reach 40 million worldwide by 2025 [1,4-6]. Multiple factors, such as aging, eye injury, diabetes mellitus, ultraviolet exposure, drug use and other ocular diseases increase the risk for cataract [7-9]. Due to the global expansion of aging population, senile cataract has become the leading cause of blindness [10-12]. Surgery, including surgical extra-capsular lens fiber removal and synthetic lens implantation, is the major therapy for cataract [13-15]. However, a secondary lens opacification, also known as posterior capsular opacification (PCO), may result from cataract surgery [16-18]. Therefore, it is urgent to understand the molecular mechanism underlying cataractogenesis. There are three major forms of cataract, namely nuclear, cortical and subcapsular cataract, each of which is associated with different etiologies. Although our understanding of pathogenesis of cataract remains incomplete, at least three key mechanisms have been described: (1) oxidative stress and the associated loss of glutathione; (2) modifications and degradation of the major gene products of the crystallins; (3) aberrant signaling and cellular functions of lens epithelial cells [19,20].
miRNAs are a large group of endogenous, small non-coding RNAs of 20-25 nucleotides in length, which regulate gene expression post-transcriptionally by inducing mRNA degradation or translational repression through base-pairing with 3’-untranslated regions of their target mRNAs [21-30]. It is estimated that 40-90% of human protein-encoding genes are regulated by miRNA [31-33]. miRNAs play significant roles in many cellular processes, including cell differentiation, proliferation and apoptosis [34-36]. While most of the miRNA studies are related to cancers, accumulating evidence has demonstrated that miRNAs are also involved in the pathogenesis of ocular diseases, including cataract [37-40]. In particular, miRNAs are involved in the regulation lens epithelial cell functions [41]. In cataract research, several studies have embarked on miRNA profiling to identify deregulated miRNAs in diseased lenses [42,43]. Furthermore, several researchers have investigated the functional roles of deregulated miRNAs in the pathogenesis of cataract [44] (Table 1) (Figure 1).
Table 1.
Functional characterization of the deregulated miRNAs in cataract
| Name | Up or down regulation | Target gene | Reference |
|---|---|---|---|
| miR-34a | Up | Smad7 | 57 |
| miR-15a-5p | Up | bcl-2, mcl-1 | 44 |
| miR-15a-3p | Up | bcl-2, mcl-1 | 44 |
| miR-16-1-5p | Up | bcl-2, mcl-1 | 44 |
| Let-7 | Up | 37 | |
| MiR-125b | Down | p53 | 65 |
| miR-16-1-3p | Down | 44 |
Figure 1.
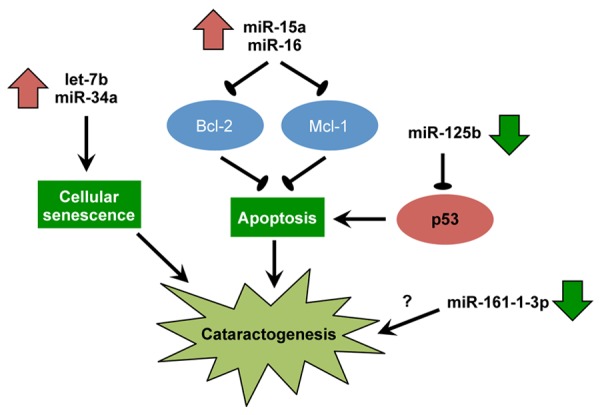
Key miRNAs dysregulated in human cataract and the proposed cataractogenic mechanisms.
In this review, we focus on miRNAs that are involved in cataract development. In addition, we will discuss their potential useas novel diagnostic tools and therapeutic strategies.
Deregulation of microRNAs in cataract
Many large-scale microarray analyses have been performed to investigate the differential expression of miRNAs in patients with cataract [43,45]. Most studies compared differential miRNA expression in lenses and aqueous humor between cataract patients and normal subjects.
Wu et al. compared miRNA expression in age-related cataractous human lenses with transparent lenses using microarrays and reverse transcription (RT)-PCR [46]. The investigators identified 20 (e.g. miR-933, miR-1308, miR-145, miR-143, miR-133a, miR-1207-5p) and 12 (e.g. miR-34a, miR768-3p, miR-486-5p, miR-378) miRNAs that were downregulated and upregulated by more than 2-fold, respectively, in the central epithelium of cataractous lenses compared with that of transparent lenses. Moreover, many predicted target genes of the identified miRNAs are involved in lens development or cataract formation. The significant differential expression of miRNAs in cataractous lenses indicates that miRNAs might play a crucial role in cataract formation.
Kubo et al. profiled miRNA expression in rat cataractous lens epithelial cells by a microarray-based approach [42]. miR-29a, miR-29c and miR-126 were significantly downregulated in lens epithelial cells of age-associated cataracts compared with non-cataractous controls. Moreover, the cytoskeleton-remodeling genes tropomyosin 1a and 2b were identified as the targets of miR-29c whose overexpression decreased the expression of these two genes. This study showed that miRNA expression was different in cataractous lens epithelial cells. However, whether these findings could be extrapolated to human remain unclear.
Previous studies demonstrated that several eye diseases, such as primary congenital glaucoma, myopia and Fuchs endothelial corneal dystrophy, are associated with changes of protein content in the aqueous humor [47-49]. A recent study analyzed miRNA in aqueous humor from patients undergoing cataract surgery using a real-time PCR array platform [45]. Among a total of 264 tested miRNAs, 110 were present in the aqueous humor. The most abundant miRNAs in the aqueous humor included miR-202, miR-193b, miR-135a, miR-365 and miR-376a. It has been postulated that these miRNAs in the aqueous humor were released from the cataractous lens and might play functional roles in regulating expression of target genes in tissues lining the anterior chamber. However, since obtaining aqueous humor from healthy eyes is invasive and unethical, it would be difficult to compare miRNA expression in aqueous humor from cataract patients with that from normal subjects. Nevertheless, these findings could still provide a basis for studying relative expression of miRNA in other ocular pathologies, such as glaucoma and anterior segment disease processes.
MicroRNAs upregulated in cataract
miR-34a
miR-34a, a p53-induced miRNA, is implicated in many diseases. For instance, miR-34a has been shown to reduce neointima formation through inhibiting smooth muscle cell proliferation and migration in vascular diseases [50]. Shikonin, a phytochemical, also inhibits adipogenic differentiation via regulation of miR-34a-FKBP1B pathway [51]. Moreover, miR-34a inhibits tumor invasion and metastasis in gastric cancer by targeting Tgif2 [52]. Interestingly, miR-34a also has a role in regulating senescence through interfering with cell cycle and apoptosis via the p53 pathway [53]. miR-34a was found to be upregulated in a cellular model of premature senescence induced by hydrogen peroxide [54]. Moreover, inhibition of miR-34a could delay the onset of replicative senescence [55]. The expression of miR-34a also increased with age in endothelial cells in many senescent human organs, including hearts and spleens of older mice [56]. To study the role of miR-34a during lens senescence, Chien et al. analyzed miR-34a expression levels in the lens epithelium of age-related cataracts in 110 patients [57]. Lens opacity was graded in accordance with a modified version of the Lens Opacities Classification System III with nuclear (N), cortical (C) and posterior subcapsular (P) cataract scores. Older patients showed higher N, C, and P scores. In addition, the expression levels of miR-34a were positively correlated with age of patients at the time of cataract surgery as well asN, C, and P cataract scores, indicating that high miR-34a was associated with high-grade lens opacity and serious lens senescence.
miR-15a-5p, miR-15a-3p, miR-16-1-5p
miR-15a and miR-16-1 could directly regulate up to 14% of genes in the human genome [58]. These two miRNAs could also induce apoptotic cell death through targeting some anti-apoptotic mediators, including bcl-2 and mcl-1 [44]. Li et al. compared the expression levels of miR-15a-5p, miR-15a-3p, miR-16-1-5p and miR-16-1-3p, and their targetsbcl-2and mcl-1 between normal and age-related cataract lens epithelial cells using real-time PCR [44]. The expression levels of miR-15a-5p, miR-15a-3p and miR-16-1-5p were significantly higher in lens epithelial cells of patients with cortical, nuclear, or posterior subcapsular cataracts than those from normal subjects. The expression levels of bcl-2 and mcl-1 were correspondingly lower in cataract patients than controls. These findings indicated that high expression of miR-15a-5p, miR-15a-3p and miR-16-1-5p in lens epithelial cells may contribute to the development of age-related cataract through inducing apoptosis.
Let-7
Let-7 family members play a role in regulating cell proliferation and differentiation through controlling many target genes [59]. Let-7-family miRNAs are frequently downregulated in tumors whereas enforced expression of let-7 suppresses tumor growth [60]. Let-7 is proved to regulate cellular ageing and tissue senescence [61], in which upregulation of let-7 has been documented in senescent fibroids and aged skeletal muscles [62]. Peng et al. evaluated the expression of let-7a/b/c in lens epithelia from 174 age-related cataracts [37]. Let-7b expression level was associated with patient age and severity of lens opacity as measured by N, C and P cataract scores in age-related cataracts. No significant correlation was identified between let-7a/c expression and either the severity of lens opacity or the patient age. These findings suggest that let-7b but not other let-7 family members might play a role in age-related cataracts.
MicroRNAs downregulated in cataract
miR-125b
Development of cataract is closely associated with abnormal apoptosis of lens epithelial cells. miR-125b plays important roles in various cellular processes, including cell proliferation, differentiation and apoptosis [63]. It has also been implicated in many diseases by targeting different transcription factors, growth factors, and matrixmetalloproteases [64]. Qin et al. investigated the role of miR-125b in age-related cataract. They showed that miR-125b was downregulated in the anterior lens capsules in age-related cataract compared with normal anterior lens capsule specimens [65]. miR-125b was also downregulated during ultraviolet irradiation-induced lens epithelial cell apoptosis. Furthermore, miR-125b levels were inversely correlated with p53 levels in age-related cataract tissues. It is therefore possible that miR-125b downregulation triggered human lens epithelial cell apoptosis by derepressing p53in the pathogenesis of age- and ultraviolet exposure-related cataract. Further investigations are needed to explore its potential therapeutic function in cataract.
miRNA-16-1-3p
The expression of miR-16-1-3p was not detected in lens epithelial cells from patients with cortical or nuclear cataract and was only slightly detected in corresponding cells from subcapsular cataract patients [44]. However, miR-16-1-3p was highly expressed in normal lens epithelial cells, suggesting that this miRNA might be crucial for maintaining the normal physiology of the lens. However, the detailed mechanism remains to be investigated.
MicroRNAs in posterior capsular opacification
PCO is a secondary lens opacification caused by of cataract surgery complication [66]. Following cataract surgery, residual lens epithelial cell proliferate rapidly under the anterior lens capsule and migrate onto the posterior capsule [67]. Such light scattering changes can lead to secondary visual loss [68]. In PCO, the remaining lens epithelial cells transform to mesenchymal cells, a process known as epithelial-to-mesenchymal transition (EMT), resulting in the formation of fibroblasts [69].
miRNA profiling of human PCO lens epithelial cells was performed using miRNA array. The results demonstrated that miR-204-5p was downregulated in human PCO tissues compared with normal attached lens epithelial cells [70]. Smad4, which is a mediator of transforming growth factor (TGF)-β/Smad signaling, was predicted to be a target of miR-204-5p. To this end, enforced expression of miR-204-5p upregulated E-cadherin expression and downregulated vimentin and α-smooth muscle actin expression in primary lens epithelial cells. Moreover, overexpression of miR-204-5p repressed EMT induced by TGF-β2. These data suggested that miR-204-5p could inhibit EMT through targeting Smad4 and consequently TGF-β signaling. The ability of miR-204-5p to repress EMT may provide a novel therapeutic avenue for PCO.
Quantitative RT-PCR showed that miR-181a was downregulated in both human PCO-attached and anterior polar cataract lens epithelial cells [71]. Restored expression of miR-181a significantly decreased proliferation and migration of lens epithelial cells. Furthermore, c-Met, Slug, and cyclooxygenase-2 (COX-2) were identified to be the direct targets of miR-181a. Restored expression of miR-181a not only decreased the expression of these targets, but also increased E-cadherin expression in lens epithelial cells. These data revealed that miR-181a is implicated in the proliferation, migration and EMT of lens epithelial cells while restoring miRNA-181a expression may be a potential novel therapeutic strategy for the prevention and treatment of PCO.
Another recent study profiled miRNA expression during mouse PCO formation using microarray to select miRNAs for therapeutic intervention. Within the first 3 weeks after cataract surgery, 55 miRNAs demonstrated expression changes and, among them, miR-184 and miR-204 were further investigated [72]. Transfection of miR-184 inhibitor (anti-miR-184) or the precursor miRNA for miR-204 (pre-miR-204) decreased the expansion and migration of lens epithelial cellsand markers of EMT. The different miRNA expression pattern in PCO and the attenuation of PCO by anti-miR-184 and pre-miR-204 indicated that miRNAs play a functional role in PCO formation. It is noteworthy that miRNAs, such as miR-204-5p and miR-181a, which are involved in PCO have not been shown to take part in cataract (Figure 2).
Figure 2.
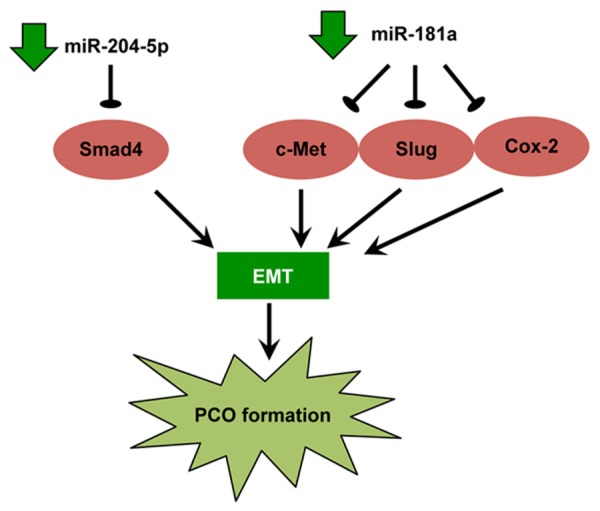
Key miRNAs dysregulated in human posterior capsular opacification (PCO) and the proposed cataractogenic mechanisms. EMT, epithelial-to-mesenchymal transition.
Mechanism of miRNA deregulation in cataract
Persistent high blood glucose levels are toxic to the eye, leading to the development of cataract [73]. Sugars are toxic to the lens by inducing protein glycation (non-enzymatic glycosylation) and production of reactive oxygen species [74]. Varma et al. investigated the role of miRNAs in oxidative stress-induced cataract [75]. They found that at least 24 and 6 apoptosis-relatedmiRNAs were upregulated and downregulated in galactosemic lenses, respectively, as compared with the normal lenses in mice. When added with sodium pyruvate, which could scavenge reactive oxygen species and inhibit their formation, the altered expression of 12 miRNAs could be completely prevented. In addition, the upregulation of 14 miRNAs was attenuated. These findings indicated that apoptotic miRNAs are differentially expressed between the galactose and the normal groups while pyruvate inhibits the expression of apoptotic miRNAs. Caffeine was previously reported to prevent high-galactose diet-induced cataracts. The same group also investigated the expression of apoptotic miRNAs in the protective effect of caffeine [76]. In this study, the elevation of 19 miRNAs in galactosemic lenses was inhibited by caffeine and the majority of these miRNAs are pro-apoptotic. Thus, the protective effect of caffeine against cataract might be attributed to its ability to prevent the induction of pro-apoptotic miRNAs.
Conclusions and future perspectives
It is now clear that miRNAs are crucial contributors to cataract. On one hand, accumulating miRNA profiling studies have demonstrated that miRNAs are deregulated in cataract. On the other hand, functional studies have shown that inhibition or enforced expression of specific miRNAs could affect lens epithelial cell migration and apoptosis in vitro, providing a mechanistic insight into the pathogenesis and progression of cataract. It is also reasonable to speculate that altered miRNA expression could be a crucial mechanistic link from environmental and genetic factors to cataractogenesis. It would also be interesting to investigate if altered levels of upregulated miRNAs (e.g. miR-34a and let-7) could be detected in plasma or tear in cataract patients for early and non-invasive diagnosis of this disease. Although miRNA-based therapies have been investigated largely in cancers, these approaches have not yet been applied to human ocular diseases, including cataract. More in-vivo experiments are therefore needed before the clinical application of miRNA-based therapeutics could be realized.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant Numbers: 81401847).
Disclosure of conflict of interest
None.
References
- 1.Ao M, Li X, Huang C, Hou Z, Qiu W, Wang W. Significant improvement in dynamic visual acuity after cataract surgery: a promising potential parameter for functional vision. PLoS One. 2014;9:e115812. doi: 10.1371/journal.pone.0115812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew MC, Lim LW, Tan CS. Multimedia interventions on the informed consent process for cataract surgery. Indian J Ophthalmol. 2014;62:1102–1103. doi: 10.4103/0301-4738.146732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zegarra M, Burga GH, Lansingh V, Samudio M, Duarte E, Ferreira R, Dorantes Y, Gines JC, Zepeda L. Late diagnosis and surgical treatment of patients diagnosed with unilateral congenital cataract at Fundacion Vision, in Asuncion, Paraguay. Arq Bras Oftalmol. 2014;77:297–299. doi: 10.5935/0004-2749.20140075. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ali N, Cheema RA, Abdelaziz MA, Khattak A. Randomized controlled trial to evaluate intraocular pressure following sub-Tenon’s local anesthesia for cataract surgery: With and without hyaluronidase added to anesthetic solution. Saudi J Anaesth. 2014;8:S63–66. doi: 10.4103/1658-354X.144080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aujla JS, Vincent SJ, White S, Panchapakesan J. Cataract Surgery in Eyes with Low Corneal Astigmatism: Implantation of the Acrysof IQ Toric SN6AT2 Intraocular Lens. J Ophthalmic Vis Res. 2014;9:324–328. doi: 10.4103/2008-322X.143369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Chen A, Wang Y, Fang X, Ye R, Lin J. Prevention and control of perioperative incision infection in patients undergoing day cataract surgery. Eye Sci. 2014;29:182–185. [PubMed] [Google Scholar]
- 7.Hall MD, Schultheiss TE, Smith DD, Nguyen KH, Wong JY. Dose response for radiation cataractogenesis: a meta-regression of hematopoietic stem cell transplantation regimens. Int J Radiat Oncol Biol Phys. 2015;91:22–29. doi: 10.1016/j.ijrobp.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TV, Pham VH, Abe K. Pathogenesis of Congenital Rubella Virus Infection in Human Fetuses: Viral Infection in the Ciliary Body Could Play an Important Role in Cataractogenesis. EBioMedicine. 2015;2:59–63. doi: 10.1016/j.ebiom.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiraphatthanavong P, Wattanathorn J, Muchimapura S, Thukham-mee W, Lertrat K, Suriharn B. The combined extract of purple waxy corn and ginger prevents cataractogenesis and retinopathy in streptozotocin-diabetic rats. Oxid Med Cell Longev. 2014;2014:789406. doi: 10.1155/2014/789406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye J, Lou LX, He JJ, Xu YF. Body mass index and risk of age-related cataract: a meta-analysis of prospective cohort studies. PLoS One. 2014;9:e89923. doi: 10.1371/journal.pone.0089923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh N, Nicholson BP, Agron E, Clemons TE, Bressler SB, Rosenfeld PJ, Chew EY. Visual acuity after cataract surgery in patients with age-related macular degeneration: age-related eye disease study 2 report number 5. Ophthalmology. 2014;121:1229–1236. doi: 10.1016/j.ophtha.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan CW, Lin Y. Overweight, obesity, and age-related cataract: a meta-analysis. Optom Vis Sci. 2014;91:478–483. doi: 10.1097/OPX.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 13.Limbu B, Jha HC. Intraoperative complications of high volume sutureless cataract surgery in Nepal: a prospective study. Kathmandu Univ Med J (KUMJ) 2014;12:194–197. doi: 10.3126/kumj.v12i3.13717. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Lin H, Qu B, Chen W. Exploration of management workflow of cataract surgery in an impoverished population in urban China. Eye Sci. 2014;29:116–120. [PubMed] [Google Scholar]
- 15.Duong HV, Westfield KC, Singleton IC. Treatment Paradigm After Uncomplicated Cataract Surgery: A Prospective Evaluation. Asia Pac J Ophthalmol (Phila) 2014;3:220–225. doi: 10.1097/APO.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 16.Li EY, Chan TC, Lam NM, Jhanji V. Cataract surgery outcomes in adult patients with Down’s syndrome. Br J Ophthalmol. 2014;98:1273–1276. doi: 10.1136/bjophthalmol-2013-304825. [DOI] [PubMed] [Google Scholar]
- 17.Alfawaz A, Alrashidi S, Kalantan H, Al-Mezaine H, Abu AM. Cataract surgery under systemic infliximab therapy in patients with refractory uveitis associated with Behcet disease. Ann Saudi Med. 2014;34:328–333. doi: 10.5144/0256-4947.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SK, Praveen MR, Vasavada AR, Vasavada VA, Carelli R, Trivedi RH, Rasoebala V. Long-term longitudinal assessment of postoperative outcomes after congenital cataract surgery in children with congenital rubella syndrome. J Cataract Refract Surg. 2014;40:2091–2098. doi: 10.1016/j.jcrs.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42:1945–1963. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, Feng F. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. doi: 10.18632/oncotarget.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Li Z, Liu J. MiRNAs in primary cutaneous lymphomas. Cell Prolif. 2015;48:271–277. doi: 10.1111/cpr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278–283. doi: 10.1111/cpr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–13924. doi: 10.18632/oncotarget.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Li Z. The role of MicroRNAs expression in laryngeal cancer. Oncotarget. 2015 doi: 10.18632/oncotarget.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: a new player enters the game. Oncotarget. 2015;6:23297–305. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing’s sarcoma. Cell Prolif. 2015;48:1–6. doi: 10.1111/cpr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Zhang SY, Gao YM, Liu YF, Liu YB, Zhao ZG, Yang K. MicroRNAs as oncogenes or tumour suppressors in oesophageal cancer: potential biomarkers and therapeutic targets. Cell Prolif. 2014;47:277–286. doi: 10.1111/cpr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Yu M, Liu C, Zhu H, He X, Peng S, Hua J. miR-34c works downstream of p53 leading to dairy goat male germline stem-cell (mGSCs) apoptosis. Cell Prolif. 2013;46:223–231. doi: 10.1111/cpr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif. 2013;46:436–446. doi: 10.1111/cpr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, You T, Jing J. MiR-125b inhibits cell biological progression of Ewing’s sarcoma by suppressing the PI3K/Akt signalling pathway. Cell Prolif. 2014;47:152–160. doi: 10.1111/cpr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, Qiu G. MicroRNA-10b Promotes Nucleus Pulposus Cell Proliferation through RhoC-Akt Pathway by Targeting HOXD10 in Intervetebral Disc Degeneration. PLoS One. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Lee HK, Finniss S, Cazacu S, Bucris E, Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, Mikkelsen T, Slavin S, Brodie C. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie T, Zhang J, Peng C, Lin Y, Chen J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget. 2014;5:7013–26. doi: 10.18632/oncotarget.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng CH, Liu JH, Woung LC, Lin TJ, Chiou SH, Tseng PC, Du WY, Cheng CK, Hu CC, Chien KH, Chen SJ. MicroRNAs and cataracts: correlation among let-7 expression, age and the severity of lens opacity. Br J Ophthalmol. 2012;96:747–751. doi: 10.1136/bjophthalmol-2011-300585. [DOI] [PubMed] [Google Scholar]
- 38.Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, Yang Q, Liu J, Wei JJ, Shao C, Liu Z, Kong B. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5:10816–10829. doi: 10.18632/oncotarget.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bier A, Giladi N, Kronfeld N, Lee HK, Cazacu S, Finniss S, Xiang C, Poisson L, deCarvalho AC, Slavin S, Jacoby E, Yalon M, Toren A, Mikkelsen T, Brodie C. MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1. Oncotarget. 2013;4:665–676. doi: 10.18632/oncotarget.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirmer U, Doberstein K, Rupp AK, Bretz NP, Wuttig D, Kiefel H, Breunig C, Fiegl H, Muller-Holzner E, Zeillinger R, Schuster E, Zeimet AG, Sultmann H, Altevogt P. Role of miR-34a as a suppressor of L1CAM in endometrial carcinoma. Oncotarget. 2014;5:462–472. doi: 10.18632/oncotarget.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf L, Gao CS, Gueta K, Xie Q, Chevallier T, Podduturi NR, Sun J, Conte I, Zelenka PS, Ashery-Padan R, Zavadil J, Cvekl A. Identification and characterization of FGF2-dependent mRNA: microRNA networks during lens fiber cell differentiation. G3 (Bethesda) 2013;3:2239–2255. doi: 10.1534/g3.113.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo E, Hasanova N, Sasaki H, Singh DP. Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens. J Cell Mol Med. 2013;17:1146–1159. doi: 10.1111/jcmm.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Lin H, Wang Q, Chen W, Luo H, Zhang H. Discrepant expression of microRNAs in transparent and cataractous human lenses. Invest Ophthalmol Vis Sci. 2012;53:3906–3912. doi: 10.1167/iovs.11-9178. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Liu S, Zhang F, Jiang P, Wu X, Liang Y. Expression of the microRNAs hsa-miR-15a and hsa-miR-16-1 in lens epithelial cells of patients with age-related cataract. Int J Clin Exp Med. 2015;8:2405–2410. [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka Y, Tsuda S, Kunikata H, Sato J, Kokubun T, Yasuda M, Nishiguchi KM, Inada T, Nakazawa T. Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci Rep. 2014;4:5089. doi: 10.1038/srep05089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C, Lin H, Wang Q, Chen W, Luo H, Chen W, Zhang H. Discrepant expression of microRNAs in transparent and cataractous human lenses. Invest Ophthalmol Vis Sci. 2012;53:3906–3912. doi: 10.1167/iovs.11-9178. [DOI] [PubMed] [Google Scholar]
- 47.Bouhenni RA, Al Shahwan S, Morales J, Wakim BT, Chomyk AM, Alkuraya FS, Edward DP. Identification of differentially expressed proteins in the aqueous humor of primary congenital glaucoma. Exp Eye Res. 2011;92:67–75. doi: 10.1016/j.exer.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Duan X, Lu Q, Xue P, Zhang H, Dong Z, Yang F, Wang N. Proteomic analysis of aqueous humor from patients with myopia. Mol Vis. 2008;14:370–377. [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson MR, Segu ZM, Price MO, Lai X, Witzmann FA, Mechref Y, Yoder MC, Price FW. Alterations in the aqueous humor proteome in patients with Fuchs endothelial corneal dystrophy. Mol Vis. 2010;16:2376–2383. [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Q, Yang F, Guo M, Wen G, Zhang C, Luong LA, Zhu J, Xiao Q, Zhang L. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J Mol Cell Cardiol. 2015;89:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Jang YJ, Jung CH, Ahn J, Gwon SY, Ha TY. Shikonin inhibits adipogenic differentiation via regulation of mir-34a-FKBP1B. Biochem Biophys Res Commun. 2015;467:941–947. doi: 10.1016/j.bbrc.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 52.Hu Y, Pu Q, Cui B, Lin J. MicroRNA-34a inhibits tumor invasion and metastasis in gastric cancer by targeting Tgif2. Int J Clin Exp Pathol. 2015;8:8921–8928. [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B, Li D, Kovalchuk O. p53 Ser15 phosphorylation and histone modifications contribute to IR-induced miR-34a transcription in mammary epithelial cells. Cell Cycle. 2013;12:2073–2083. doi: 10.4161/cc.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes OC, Sarojini H, Wang E. Stepwise upregulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol. 2009;221:109–119. doi: 10.1002/jcp.21834. [DOI] [PubMed] [Google Scholar]
- 55.Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, Huang Q, Chen S, Zhang Z, Xu Y, Lai L, Zheng Y. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015;36:1692–1701. doi: 10.1016/j.neurobiolaging.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 56.Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Chien KH, Chen SJ, Liu JH, Chang HM, Woung LC, Liang CM, Chen JT, Lin TJ, Chiou SH, Peng CH. Correlation between microRNA-34a levels and lens opacity severity in age-related cataracts. Eye (Lond) 2013;27:883–888. doi: 10.1038/eye.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Jiang L, Ji X, Yang B, Zhang Y, Fu XD. Hepatitis B viral RNA directly mediates down-regulation of the tumor suppressor microRNA miR-15a/miR-16-1 in hepatocytes. J Biol Chem. 2013;288:18484–18493. doi: 10.1074/jbc.M113.458158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SI, Jeon MH, Kim JS, Jeon IS, Byun SJ. The gga-let-7 family post-transcriptionally regulates TGFBR1 and LIN28B during the differentiation process in early chick development. Mol Reprod Dev. 2015;82:967–75. doi: 10.1002/mrd.22575. [DOI] [PubMed] [Google Scholar]
- 60.Liu K, Zhang C, Li T, Ding Y, Tu T, Zhou F, Qi W, Chen H, Sun X. Let-7a inhibits growth and migration of breast cancer cells by targeting HMGA1. Int J Oncol. 2015;46:2526–2534. doi: 10.3892/ijo.2015.2949. [DOI] [PubMed] [Google Scholar]
- 61.Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, Navarro VM, Sanchez-Garrido MA, Leon S, Dieguez C, Cordido F, Matagne V, Dissen GA, Ojeda SR, Pinilla L, Tena-Sempere M. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–955. doi: 10.1210/en.2012-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laser J, Lee P, Wei JJ. Cellular senescence in usual type uterine leiomyoma. Fertil Steril. 2010;93:2020–2026. doi: 10.1016/j.fertnstert.2008.12.116. [DOI] [PubMed] [Google Scholar]
- 63.Knackmuss U, Lindner SE, Aneichyk T, Kotkamp B, Knust Z, Villunger A, Herzog S. MAP3K11 is a tumor suppressor targeted by the oncomiR miR-125b in early B cells. Cell Death Differ. 2015;23:242–52. doi: 10.1038/cdd.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal. 2014;12:30. doi: 10.1186/1478-811X-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin Y, Zhao J, Min X, Wang M, Luo W, Wu D, Yan Q, Li J, Wu X, Zhang J. MicroRNA-125b inhibits lens epithelial cell apoptosis by targeting p53 in age-related cataract. Biochim Biophys Acta. 2014;1842:2439–2447. doi: 10.1016/j.bbadis.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 66.MacLean KD, Werner L, Kramer GD, Farukhi MA, Gardiner GL, Kahook MY, Mamalis N. Evaluation of stability and capsular bag opacification of a new foldable adjustable intraocular lens. Clin Experiment Ophthalmol. 2015;43:648–54. doi: 10.1111/ceo.12526. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Medina JJ, Del Rio-Vellosillo M, Zanon-Moreno V, Santos-Bueso E, Gallego-Pinazo R, Ferreras A, Pinazo-Duran MD. Does Posterior Capsule Opacification Affect the Results of Diagnostic Technologies to Evaluate the Retina and the Optic Disc? Biomed Res Int. 2015;2015:813242. doi: 10.1155/2015/813242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Bree MC, van der Meulen IJ, Franssen L, Coppens JE, Reus NJ, Zijlmans BL, van den Berg TJ. Imaging of forward light-scatter by opacified posterior capsules isolated from pseudophakic donor eyes. Invest Ophthalmol Vis Sci. 2011;52:5587–5597. doi: 10.1167/iovs.10-7073. [DOI] [PubMed] [Google Scholar]
- 69.Xiao W, Chen X, Li W, Ye S, Wang W, Luo L, Liu Y. Quantitative analysis of injury-induced anterior subcapsular cataract in the mouse: a model of lens epithelial cells proliferation and epithelial-mesenchymal transition. Sci Rep. 2015;5:8362. doi: 10.1038/srep08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Li W, Zang X, Chen N, Liu T, Tsonis PA, Huang Y. MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophthalmol Vis Sci. 2013;54:323–332. doi: 10.1167/iovs.12-10904. [DOI] [PubMed] [Google Scholar]
- 71.Dong N, Tang X, Xu B. miRNA-181a inhibits the proliferation, migration, and epithelialmesenchymal transition of lens epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:993–1001. doi: 10.1167/iovs.14-15860. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann A, Huang Y, Suetsugu-Maki R, Ringelberg CS, Tomlinson CR, Del Rio-Tsonis K, Tsonis PA. Implication of the miR-184 and miR-204 competitive RNA network in control of mouse secondary cataract. Mol Med. 2012;18:528–538. doi: 10.2119/molmed.2011.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman-Billard S, Sedira N, Boelle PY, Poisson F, Heron E. High prevalence of undiagnosed diabetes and high risk for diabetes using HbA1c criteria in middle-aged patients undergoing cataract surgery. Diabetes Metab. 2013;39:271–275. doi: 10.1016/j.diabet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Olafsdottir E, Andersson DK, Stefansson E. The prevalence of cataract in a population with and without type 2 diabetes mellitus. Acta Ophthalmol. 2012;90:334–340. doi: 10.1111/j.1755-3768.2011.02326.x. [DOI] [PubMed] [Google Scholar]
- 75.Varma SD, Kovtun S, Hegde K, Yin J, Ramnath J. Effect of high sugar levels on miRNA expression. Studies with galactosemic mice lenses. Mol Vis. 2012;18:1609–1618. [PMC free article] [PubMed] [Google Scholar]
- 76.Varma SD, Kovtun S. Protective effect of caffeine against high sugar-induced transcription of microRNAs and consequent gene silencing: a study using lenses of galactosemic mice. Mol Vis. 2013;19:493–500. [PMC free article] [PubMed] [Google Scholar]


