Abstract
Spinal cord injury (SCI) is a severe trauma of central nervous system (CNS). Numerous stem cells have been applied for SCI therapy. Peripheral blood-derived mesenchymal stem cells (PBMSCs) have captured researchers’ attention by virtue of pluripotency and effectiveness. However, little work has been performed on whether PBMSCs play roles and what role, if any, in the lesion microenvironment. Through the investigation of the differentiation, neuroprotection and immunoloregulation of engrafted PBMSCs, we found that the expression of glial fibrillary acidic protein (GFAP) was inhibited. Meanwhile, myelin basic protein (MBP), neurofilament protein-200 (NF-200) and microtubule associated protein-2 (MAP-2) were promoted after PBMSC transplantation (PBMSCT) by immunohistochemistry. Though engrafted PKH26+PBMSCs could survive in vivo for at least 8 w, they could not respectively express GFAP, MBP and neuronal specific neucleoprotein (NeuN) by immunofluorescence. Additionally, Flow cytometry demonstrated that the number of CD4+IL17+Th17 cells decreased while CD4+CD25+Foxp3+Treg ones increased after PBMSCT (P < 0.01). Immunohistochemistry and Elisa both showed a lower expression of IL-6 and IL-17a while a higher expression of TGF-β after PBMSCT (P < 0.05). RT-PCR indicated that Th17-relevant genes including RORγT, IL-6 and IL-21 were inhibited and resulted in the decrease of IL-23a and IL-22 secretion (P < 0.05); Treg-relevant genes including FoxP3 and TGF-β and the secretion of IL-10 were improved (P < 0.05). Accordingly, we concluded that the PBMSCT-relevant therapy took effect not through the differentiation of PBMSCs into CNS cells, but through regulating Th17/Treg-relevant gene expression, inhibiting Th17-relevant gene expression and meanwhile promoting Treg-relevant gene expression, and eventually resulted in promotion of the functional recovery of SCI rats.
Keywords: Peripheral blood, mesenchymal stem cells (MSCs), spinal cord injury (SCI), cell transplantation, immunoloregulation, rats
Introduction
Spinal cord injury (SCI), a severe trauma often caused by car accidents or falls, usually induces loss of autonomic function and/or sensation below the level of injury [1]. It presents a high incidence, high morbidity, huge cost and lowering trend of age. SCI therapy conventionally includes methylprednisolone or/and surgery. However the situation has not been substantially improved yet. Additionally, methylprednisolone pulse therapy may increase rates of respiratory infection and hyperglycaemia etc., which usually lengthens hospital stay [2]. Though surgery can relieve spinal cord compression and restore the stability of spinal column, a secondary injury could not be relieved. It may eventually result in the loss of motor/sensory function.
With the capacity to self-renew and differentiate into various lineages, stem cells represent an important paradigm of cell-based therapy for SCI. Currently the various sources of engrafted cells mainly include neural stem cells, olfactory ensheathing cells, induced pluripotent stem cells, Schwann cells and mesenchymal stem cells (MSCs) [3-9]. Of those, Bone marrow-derived mesenchymal stem cells (BMMSCs) and Umbilical cord-derived mesenchymal stem cells (UCMSCs) possess convenient collection, low immunogenicity and easy expansion ex vivo. Thus, BMMSCs and UCMSCs have generated great interest among researchers for SCI therapy [3,4,10]. Nevertheless, there is a significant decline in the number, and proliferative and differential capacity of BMMSCs with ages [11]. In addition, the collection of bone marrow aspirate may cause infection and pain. As for UCMSCs, the ethical dispute and inconvenience for autograft have limited their widespread application to some extent. Recently, some studies have documented that peripheral blood-derived mesenchymal stem cells (PBMSCs) own biological characteristics which are similar to that of BMMSCs and UCMSCs, and also possess much more effective cost, less trauma and no anesthesia [12-16]. While, when originating from different sources, capacities of generation and differentiation of MSCs may differ [17]. It is also reported that PBMSCs, isolated from rabbit peripheral blood after mobilization with Granulocyte Colony-Stimulating Factor (G-CSF) and AMD3100 owes stronger differential capacities into adipocytes and chondroblasts than BMMSCs in vitro [18]. Many researchers have applied PBMSCs for dental implants, critical-sized calvarial bone defects, middle cerebral artery occlusion and traumatic brain injury etc. [19-23] and we have also transplanted PBMSCs for osteonecrosis of the femoral head in rabbits and SCI in rats [24,25], which all received good outcomes. In the present research, we engrafted PBMSCs into injury sites after SCI and study the survival, differentiation, protection and immunoregulation of the engrafted cells. It may provide useful experimental evidence of PBMSC-based therapy on SCI for further application.
Materials and methods
Animals
SD rats in a specific pathogen free (SPF) grade were purchased regardless of sex from the Animal Center of Third Military Medical University (production license: SCXK Yu 2012-0005). The animal studies were performed in accordance with the recommendations of the Weatherall report after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Zunyi Medical College.
Isolation, culture, identification and labeling of PBMSCs
Isolation and culture of PBMSCs were performed according to our previous method as it was illustrated in Figure 1 [24]. Briefly, SD rats weighing 80-120 g (n = 5) were subcutaneously injected with G-CSF (100 μg/kg/d) for 6 d. After the rat was anesthetized with 10% chloral hydrate (0.35 ml/100 g), 5-8 ml of peripheral blood was harvested from the left ventricle. Then the sample was diluted with a same volume phosphate buffer saline (PBS) and immediately overlaid on rat Ficoll lymphocyte separation liquid (1.083 g/L; Chuanye Biochemicals, Tianjin, China). After centrifugation at 400×g for 20 min, the intermediate layer was collected and rinsed twice with appropriate PBS. After centrifugation at 250×g for 10 min, the cells were cultured in 5 ml of α-Eagle’s Minimum Essential Medium (α-MEM, Gibco, New York, USA), supplemented with 15% fetal bovine serum (FBS, Gibco, New York, USA), 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mmol/L glutamine (Hyclone, Logan, USA). Half of the medium was replaced after 4 d and the full medium was replaced every 3 d. The cells were subcultured at 80%-90% confluence. Passage 2 (P2) cells were analyzed by flow cytometry. Briefly, P2 were collected, treated with 0.125% trypsin (Gibco, New York, USA), suspended by 0.1% BSA and adjusted to 2×106/ml. Afterwards, 100 µl of the suspension was placed into flow tubes, Alexa Fluor® 488 anti-rat CD29, Alexa Fluor® 488 anti-rat CD90, Alexa Fluor® 488 anti-rat CD45, Alexa Fluor® 488 anti-rat CD44, Alexa Fluor® 488 anti-rat CD34 and Alexa Fluor® 488 anti-rat G-CSFR (eBioscience, San Diego, USA) were added into each tube respectively. The samples were detected and analyzed using the FACS Calibur (BD, Franklin L, USA) and the analytic software was Cell Quest. Additionally, 0.125% trypsin-treated P2 cells (4×106) were labelled with PKH26 (PKH26 Fluorescent Cell Linker Kits, Sigma, USA) and then cultured till P3 for transplantation. Mesoderm multi-lineage differentiation capacities were analyzed according to our previous research [24].
Figure 1.
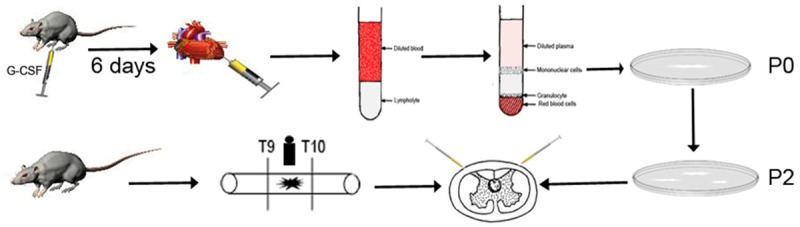
Process of isolation, culture transplantation of PBMSCs. SD rats weighing 80-120 g were subcutaneously injected with G-CSF for 6 d. About 6 ml of peripheral blood was harvested from the left ventricle after anesthetization. Then the sample was diluted with a same volume of PBS and immediately overlaid on rat Ficoll lymphocyte separation liquid. After centrifugation, the cells were cultured until at 80%-90% confluence and then subcultured. Adult rats underwent a contusion of spinal cord for model control after dorsal laminectomy. Following dorsal laminectomy at T9-T10 level, spinal cord was contused with a new type spinal cord impactor. Thirty minutes after SCI, 10 μl of PBS and PBMSC suspension (2×104 cells/μl) were respectively injected into each rat in both the PBS and PBMSCT groups at 2 μl/min and the injection sites were adjacent rostral and caudal spinal cord at a distance of 0.05 mm from the lesion.
Animal model of SCI and PBMSCs transplantation
Adult SD rats (weighting 200-250 g) were randomly selected according to coin tossing and divided into 4 groups: sham operation group (n = 13), model group (n = 15), PBS control group (n = 15) and PBMSCs transplantation (PBMSCT) group (n = 24, nine for immunofluorescence). Rats in the sham operation group only underwent dorsal laminectomy for blank control. Model group, underwent a contusion of spinal cord for model control after dorsal laminectomy. Rats were anesthetized with 10% chloral hydrate. Following dorsal laminectomy at T9-T10 level, spinal cord was contused with a new type spinal cord impactor (Chinese patent number: ZL201420557908.8) as previously described [24]. Thirty minutes after SCI, 10 μl of PBS and PBMSC suspension (2×104 cells/μl) with PBS were respectively injected into each rat in both the PBS and PBMSCT groups at 2 μl/min and the injection sites were adjacent rostral and caudal spinal cord at a distance of 0.05 mm from the lesion (Figure 1). The rats for immunofluorescence were injected with PKH26-labelled PBMSCs. Then, muscle and skin layers were sutured. The bladder was emptied by manual abdominal pressure every 8 h until the bladder function recovered. Penicillin was intraperitoneally injected at 16×104 IU/d for 5 d for infection prevention.
Behavioral analysis
Functional recovery of hindlimb motor was evaluated according to the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale [26]. Briefly, preoperative and postoperative testing session were conducted for 4 min when rats in each group walked continuously in the open field using the BBB locomotor rating scale. The standardized 21 point scale is mainly based on hindlimb movement and locomotor efficiency, 0 represents complete paralysis and 21 normal. Tilt table test was operated for detection of the degree of paralysis according to the reported method [27]. Briefly, rats in each group were placed onto a rubber-covered movable board, and the longitudinal axis of the tested rat was perpendicular to that of the inclined plate. Then the plate end which rat heads were directed towards was raised until the animal finally slid down. The maximum angle at which the rats could stay for at least 5 s was then recorded. Both the person producing the lesion and the tester assessing outcomes were masked to all group assignment. Additionally, the time of bladder functional recovery was recorded any time.
Histopathology analysis and Luxol Fast Blue staining
Histopathology and Luxol Fast Blue (LFB) were performed according to the reported methods to see the protection of PBMSCs on myelin sheathes and neurons with the previous methods [28]. Briefly, after anesthetized with 10% chloral hydrate, three rats in each group were transcardially perfused with 4% paraformaldehyde (PFA) at the corresponding time. Then a 6 mm-long spinal cord including the lesion was collected. After fixed with 4% PFA for 24 h, the tissue specimen underwent conventional dehydration and was embedded in paraffin wax and cut at 4 μm. Some were used for H&E staining, some for Luxol Fast Blue (LFB) staining, the left for immunohistochemistry. For LFB staining, after deparaffinization and clearance with 100% and 95% ethanol, staining with 60% LFB solution, rinse with 95% ethanol, 0.05% lithium chloride solution and 70% ethanol and distilled water, slides were sealed with Polyvinylpyrrolidone Mounting Medium (PVP, Beyotime, Shanghai, China).
Immunohistochemical staining
Immunohistochemical staining was performed according to instructions of manufacturer (GTVisionTM+ Detection system kit, Gene Tech, Shanghai, China). Briefly, paraffin sections were blocked with 10% goat serum for 30 min at room temperature. Primary antibodies were then respectively dropped onto the fixed sections overnight at 4°C. For primary antibodies, we used Anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (1:300; Abnova, Taiwan, China), Anti-myelin basic protein (MBP) antibody (1:200; Abcam, Massachusetts, USA), Anti- neurofilament protein-200 (NF-200) antibody (1:100; Abcam, Massachusetts, USA) and Anti-glial fibrillary acidic protein (MAP-2) antibody (1:200; Abcam, Massachusetts, USA), Anti-IL6 antibody (1:100, Sigma), Anti-IL17 antibody (1:100, Sigma) and Anti-TGF-β antibody (1:100, Sigma) and PBS was used as negative control. After the sections were washed with PBS the following day, HRP-conjugated secondary antibodies (Solution A of GTVisionTM+ Detection system kit) were added and then the sections were incubated for 30 min at room temperature. Thereafter, the sections were washed with PBS 3 times, covered with DAB working solution, counterstained with hematoxylin, dehydrated through graded alcohols, cleared with xylene and sealed with Polyvinylpyrrolidone Mounting Medium (Beyotime, Shanghai, China). Integral optical density (IOD) of the positive staining was then measured.
Immunofluorescence staining
After perfused with 4% PFA, dehydrated through graded sucrose and embedded in OCT, the injured spinal cord specimen (n = 3 at each time point) was cut at 8 μm by freezing microtome (CM 1850, Leica, Germany) for immunofluorescence staining. The collected frozen sections were dried at room temperature for 10 min and washed 3 times. After blocked with 10% goat serum and dropped with primary antibodies overnight according to the steps mentioned above, the sections were covered with Goat anti-rabbit/mouse IgG-FITC (Jackson, West Grove, PA) and incubated for 30 min at 37°C. For primary antibodies, we used Anti-GFAP polyclonal antibody (1:300; Abnova, Taiwan, China), Anti-MBP antibody (1:200; Abcam, Massachusetts, USA), and Anti-Neuronal specific neucleoprotein (NeuN) antibody (1:300; Abcam, Massachusetts, USA). Subsequently, these sections were washed with PBS, stained with DAPI, sealed with Antifade Polyvinylpyrrolidone Mounting Medium (Beyotime, Shanghai, China) for analysis under a fluorescence microscope (DMIR, Leica, Solms, GER).
Flow cytometric analysis
On days 14, 28, and 56 after transplantation we investigated the frequency of CD4+IL17+Th17/CD4+CD25+Foxp3+ regulatory T (Treg) cells according to the previous reports [29]. Individual cell suspensions from the PBMNCs and spleen were isolated and 2×105 cells in each tube were resuspended with RPMI 1640 medium modified (Hyclone; Logan, U-tah, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, New York, N.Y., USA) and pre-cultured with BFA/Monensin Mixture (250×, 0.75 mg/ml and 0.35 mg/ml, Multi Sciences), PMA/Ionomycin Mixture (250×, 12.5 mg/ml and 0.25 mg/ml, Multi Sciences) for 4 h before intracellular staining. After resuspended by Flow cytometry Staining buffer, Th17 cells were stained with Anti-Rat CD4 FITC (eBioscience, San Diego, Calif., USA), incubated for 1 h at 4°C avoiding light and then pretreated with Fix & PERM Kit (Multi Sciences). Anti-Mouse/Rat IL-17A PE (eBioscience) were then added and the cells were incubated in the dark for 1 h at room temperature. For CD4+CD25+Foxp3+Treg cells staining, Anti-Rat CD4 FITC and Anti-Rat CD25 PE (eBioscience) were added into the individual cell suspensions from the PBMNCs and spleen and those cells were incubated for 1 h at 4°C avoiding light. One ml of Foxp3 Fixtion/Permeabilization working solution were then added to each tube for incubation in the dark at room temperature for 1 h. Data analysis were performed with a flow cytometer (FACS Calibur; BD) using Cell Quest analytic software.
Cytokine assay
Serum was collected from rats 14 d, 28 d and 56 d postinjury. Cytokines TGF-β (Rat TGF-beta1 Platinum ELISA, eBioscience), IL-6 (Rat IL-6 Platinum ELISA, eBioscience) and IL-17a (Rat IL-17A Platinum ELISA, eBioscience) were measured using commercial ELISA kit according to the manufacturer’s instructions.
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was performed according to the previous study [25]. Total RNA was extracted from the freshly isolated spinal cords of every group at the end of the experiment (day 56) using Trizol (Invitrogen, Carlsbad, USA) reagent according to the manufacturer’s protocol, a reverse transcription reaction was performed using PrimeScript® RT Reagent Kit (TaKaRa, No.RR037A, Dalian, China); a reverse transcription reaction was performed by a PrimeScript® RT Reagent Kit (TaKaRa, No.RR037A, Dalian, China) in a 10-μl reaction volume and the reaction conditions was set to 37°C for 15 min and then 85°C for 5 s; the reaction system of fluorescent quantitative PCR was carried out with a SYBR® Premix Ex TaqTM Kit (TaKaRa, No.RR420A, Dalian, China). Primer sequences were listed as follows and β-actin gene was used as an internal control. RORγT: 5’-AGACACCACCGAACATCTCG-3’ (forward), 5’-AGGAGTAGGCCACATTGCAC-3’ (reverse); FoxP3: 5’-AGCTTGTTTGCTGTGCGGAG-3’ (forward), 5’-GTGGCATAGGTGAAAGGGGG-3’ (reverse); IL-17a: 5’-CAAACGCCGAGGCCAATAAC-3’ (forward), 5’-AGAGTCCAGGGTGAAGTGGA-3’ (reverse); IL-6: 5’-TTCACAGAGGATACCACCCACA-3’ (forward), 5’-AATCAGAATTGCCATTGCACAA-3’ (reverse); IL-22: 5’-AGTCACCTCAGCCCCTGTCAC-3’ (forward), 5’-CCCGATCGCTTTAATCTCTCC-3’ (reverse); IL-23a: 5’-GGTGATGGTTGTGATCCCCA-3’ (forward), 5’-ATGTCCGAGTCCAGCAGGTG-3’ (reverse); IL-21: 5’-GCACGAAGCTTTTGCCTGTT-3’ (forward), 5’-GGCATTTAGCCATGTGCCTC-3’ (reverse); IL-10: 5’-GAAAAATTGAACCACCCGGCA-3’ (forward), 5’-TTCCAAGGAGTTGCTCCCGT-3’ (reverse); TGF-β: 5’-CCGCAACAACGCAATCTATG-3’ (forward), 5’-AGCCCTGTATTCCGTCTCCTT-3’ (reverse).
Statistical analysis
Experiment data were presented as mean ± standard error (SEM). Statistical analyses were conducted using the SPSS 13.0 software package. One-way or two-way ANOVA was performed for comparison between groups. Statistical significance was set at P < 0.05.
Result
Morpholog and identification of PBMSCs
A few cultured cells could grow adherently 48 h after primary culture, obvious colony formed 9 d later, cell confluence got 70%-80% 16 d later and those cells could cover the bottom of the bottle 20 d later (Figure 2A). Passaged cells owned a uniform and long-spindle shape and arranged radially (Figure 2A). Flow cytometry showed positive expression of CD90, CD29, CD44, and G-CSFR, but negative expression of CD45 or CD34 (Figure 2B). Also, those cells owned multi-lineage differentiation potency into adipocytes, chondrocytes and osteocytes (Figure 2C).
Figure 2.
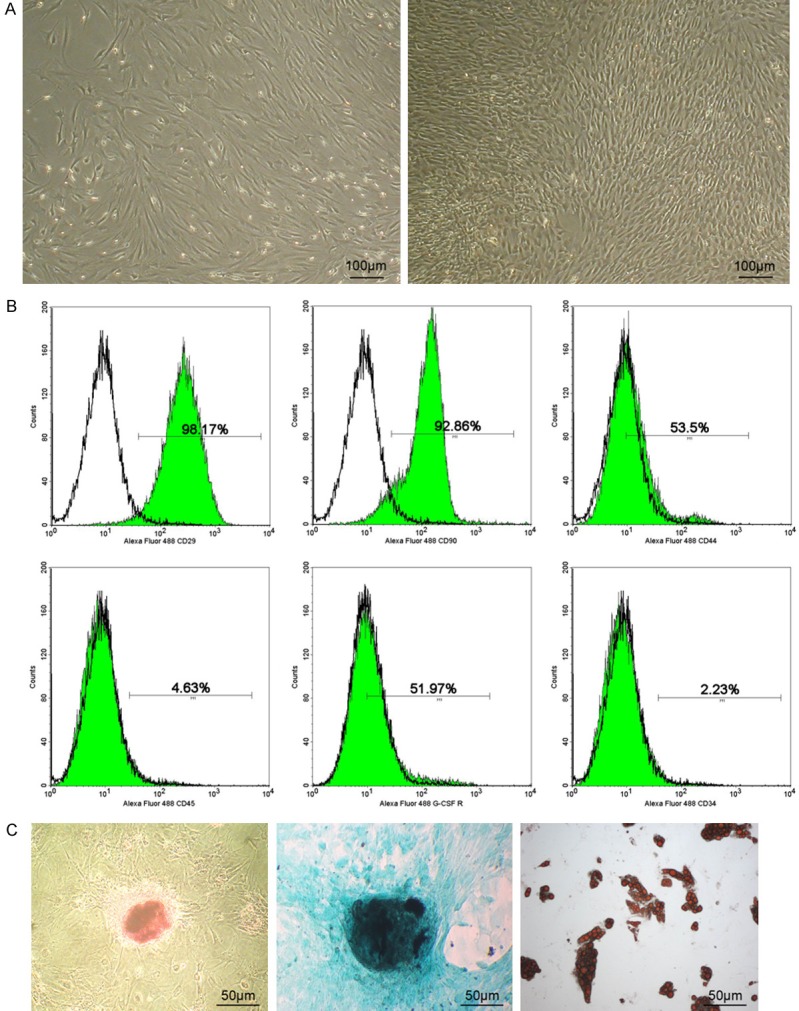
Morphology and identification of PBMSCs. A: Primary culturing PBMSCs owned a uniform and long-spindle shape and arranged radially at 16th day under light microscope and PBMSCs after subculture still kept that shape; B: P2 cells could express positively CD90, CD29, CD44, G-CSFR, but negatively CD34 and CD45. C: Osteogenesis, chondrogenesis, adipogenesis-committed differentiation of PBMSCs respectively by alizarin red, alcian blue and oil red.
Functional recovery
After labelled with PKH26, those cells, with an intact cell membrane, showed a well-delineated red fluorescence. After calculation, we got that the labeling rate > 99% (Figure 3A). BBB locomotor rating scales of all animals were 21 points 1 d before operation. BBB scoring of the rats in the sham operation group were also 21 points throughout the period of the experiment. On the day of operation, all models in the other 3 groups showed complete paralysis. Until 3rd week after operation, PBMSC-injected rats showed a significantly higher BBB rating scale than the PBS-injected and nothing-injected rats (P < 0.05). There was no significant difference observed between PBS-injected and nothing-injected rats after SCI (Figure 3B). Similar to the BBB data, tilt plate test signified a lager angle after PBMSCT (Figure 3C). In addition, rats after PBMSCT treatment owned a quicker micturation recovery (P < 0.05; Figure 3D).
Figure 3.
Labelling rate of PBMSCs, functional recovery after PBMSCT. A: PBMSCs after BrdU labelling in vitro under phase contrast microscope and under fluorescent microscope). B, C: BBB scoring and tilt table test of rats in each group. There were a lager angle and higher BBB scoring after PBMSCT (n = 22 as two died from apastia 7 d after surgery) than both in the Model group (n = 13 as one died 1 d after surgery and another died 5 d later) and PBS group (n = 14 as one died 4 d after surgery). D: Rats after PBMSCT showed a quicker micturition recovery time. E-G: Clear boundary still existed between gray and white matter, neat-arranged nerve fiber bundles, regular neuronal morphology in the sham operation group; boundary between gray and white matter disappeared and severe disintegration of neuron-like cells, cavity formation and gliosis were observed in both the model and PBS groups after SCI. While there was less cavities and more tissue preservation in the PBMSCT group at 8 w (n = 3). H, I: LFB results manifested that PBMSC-treated rats presented a relatively lower cavities and discontinuous myelin sheath (n = 3). Data represented Mean ± SEM, *P < 0.05, **P < 0.01, ****P < 0.00001, NS: no significance.
PBMSCT could promote the restoration of histopathological alteration
H&E results implied that the margins between gray and white matter was distinct after sham operation 56 d postinjury. Significant gliosis and abundant cavities occurred in the PBS and model group. By contrast, less gliosis and cavities could be observed after PBMSCT (P < 0.05; Figure 3E, 3F). Additionally, tissue could be preserved more (Figure 3G). Meanwhile, LFB staining manifested a regular arrangement of myelin sheath in rats only undergoing sham operation, while presented an irregular or completely disappear myelin sheath, flooded with abundant cavities after SCI. In contrast, PBMSC-treated rats presented a relatively lower cavities and discontinuous myelin sheath (P < 0.05; Figure 3H, 3I). These result inferred PBMSCT could promote the injury recovery, protect the neurons and myelin sheathes in the injured lesion.
Protection-relevant gene expression analysis by immunohistochemistry and immunofluorescence
GFAP, MBP, NF-200 and MAP-2 could express to some extend in all groups. At 2 w after SCI, GFAP, MBP, NF-200 and MAP-2 expression in the PBMSCT group were respectively higher than that in the model and PBS groups (P < 0.01); at 4 and 8 w, GFAP expression in the model and PBS groups increased abruptly and were higher than that in the PBMSCT group (P < 0.01) and glial scars formed significantly, and MBP, NF-200 and MAP-2 expression still kept the same tendency as that at 2 w (P < 0.01, Figure 4A, 4B). Immunofluorescence staining revealed engrafted PKH26+PBMSCs (the red color) could survived at the injury site for at least 8 w. However, there were little PKH26+GFAP+FITC astrocytes, PHK26+MBP+FITC oligodendrocytes, PKH26+NeuN+FITC neurons could be observed, which indicated the PBMSCT-relevant therapy took effect not through the differentiation of PBMSCs into CNS cells (Figure 4C).
Figure 4.
Immunohistochemistry and immunofluorescence analysis of the protective-relative proteins after PBMSCT. A, B: There was a lower IOD at 4 w and 8 w and there was no glial scar observed and a lower GFAP expression after PBMSCT (n = 3 in each group at each time point); higher expression of MAP-2, MBP and NF-200 could be observed obviously after PBMSCT corresponding time point. C-E: There were little implanted PKH26-labelled PBMSCs could co-express GFAP, MBP and NeuN. The number of survival PKH26+ cells reached as long as 8 w. Data represented Mean ± SEM, **P < 0.01.
Effect of PBMSCs on Th17/Treg cell balance
It was reported that suppression of Th17 lymphocyte differentiation could promote locomotor recovery in mice after SCI, which implied the impairment of Th17 lymphocytes in SCI [30]. CD4+T lymphocytes, could be differentiated from CD4+T cells in the presence of TGF-β and possessed a crucial role in controlling immune responses to self-antigens and preventing autoimmune diseases and [31]. The similar regulatory pathway (STAT3 signaling), makes it paramount to study Th17/Treg balance. The data implied that CD4+IL17+Th17 cells presented a high population in spleen and PBMNCs in the model and PBS group while a relatively low number after PBMSCT treatment 14 d postinjury (Figure 5A-C). Meanwhile there was an increased percentage of CD4+CD25+Foxp3+Treg cells after PBMSCT, higher notably than that without PBMSCT (Figure 5D-F). These results suggested that PBMSCT could prevent the differentiation of Th17 cells and promote the differentiation of Treg cells after SCI.
Figure 5.
Flow cytometry of Th17/Treg cells in peripheral blood serum and spleen. A-C: CD4+IL17+Th17 cells presented a lower population in PBMNCs and spleen after PBMSCT treatment 14 d post injury. D-F: There were an increased percentage of CD4+CD25+Foxp3+Treg cells after PBMSCT. n = 3 in each group at every time point, data represented Mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS: no significance.
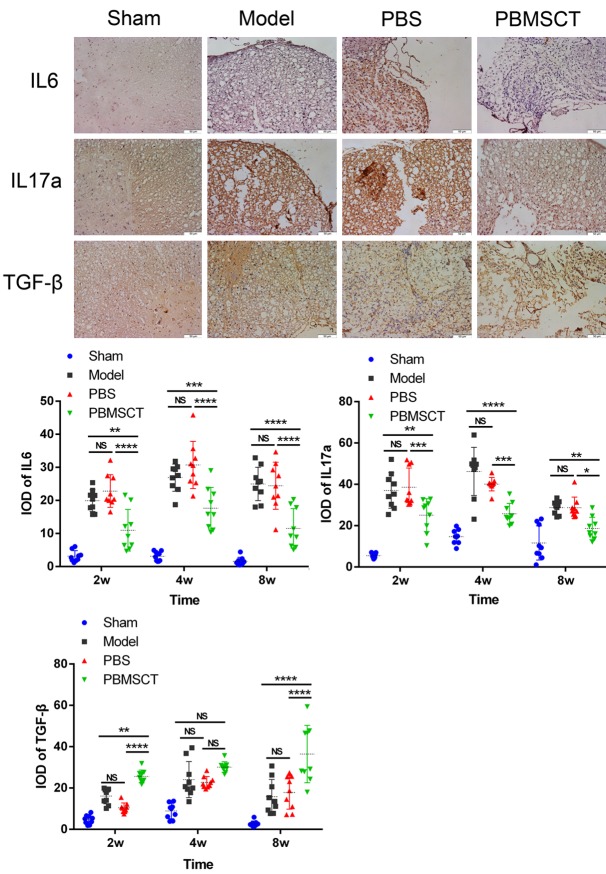
Analysis IL-6, IL-17a, and TGF-beta expression in the injured lesion
To further certify the immunoregulation function of PBMSCs on injured lesion, we analyzed IL-6, IL-17a and TGF-β expression by immunohistochemistry. As presented in the Figure 6, IL-6 and IL-17a expression were suppressed while TGF-β expression were promoted in the injured lesion after PBMSCT.
Figure 6.
Immunohistochemical analysis IL-6, IL-17a, and TGF-β expression in the injured lesion. Measured IOD data implied a higher expression of IL-6 and IL-17a while a lower TGF-β expression was promoted in the injured lesion 14 d after PBMSCT. The result indicated PBMSCT might block the IL-6 and IL-17a expression and promote TGF-β expression. (Mean ± SEM, n = 3) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.00001, NS: no significance.
Analysis cytokines IL-6, IL-17a and TGF-β levels in SCI rats
To study whether PBMSCs impacted on repair by immunoregulary function through blood circulation, we analyzed TGF-β, IL-6 and IL-17a level changes. As demonstrated in the Figure 7A, IL-6 and IL-17a were markedly suppressed (P < 0.05) while the production of TGF-β showed a distinct increase (P < 0.05) in the PBMSC-treated serum by Elisa from day 14 on. The data implied that PBMSCs might immunoregulate protein expression of Th17/Treg cell in SCI rats.
Figure 7.

IL-6, IL-17a and TGF-β expression in Peripheral serum and Relative gene expression analysis. A: Unanimous with the result above, IL-6 and IL-17a were markedly suppressed. The production of TGF-β showed a distinct increase in the PBMSC-treated serum by Elisa from day 14 on. The data implied that PBMSCs might immunoregulate protein expression of Th17/Treg cell in SCI rats. n = 3 at each time point in each group. B: Expression of RORγT, IL-6, IL-21, IL-23a, IL-22 were suppressed after PBMSCT and that of FoxP3, IL-10 and TGF-β were promoted to varying extents at 8 w. The data indicated that PBMSCs could change the Th17/Treg cell balance though factor secretion change. n = 4 in each group, data represented Mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 model group vs PBMSCT group; #P < 0.05, ##P < 0.01, ###P < 0.001 PBS group vs PBMSCT group; NS: no significance.
Expression analysis of genes relative to Th17/Treg balance
RT-PCR data indicated that a lower expression of Th17-relevant genes including RORγT, IL-6 and resulting in the secretion of IL-23a and IL-22; meanwhile and the expression of Treg-relevant genes including FoxP3 and TGF-β were improved, resulting in the overexpression of IL-10 and TGF-β (P < 0.05, Figure 7B). The data indicated that PBMSCs could change the Th17/Treg cell balance though factor secretion change.
Discussion
The differential potency into neural-like and glial-like cells and self-renew capacity makes MSCs suitable for therapy of CNS diseases, such as SCI [32,33]. To data, numerous studies have reported MSC transplantation for SCI improves tremendously the recovery of locomotor function after SCI [3,4,10,34-36]. Though PBMSCs were similar to BMMSCs in many characteristics, the differentiation of two seed cells into adipocytes had significant difference. There are little reports about PBMSCT for SCI therapy until now. In our previous studies, PBMSCs could differentiate into neurons under induced medium in vitro, which makes PBMSCT possible for SCI treatment [37]. Here, the data of tilt table test, BBB locomotor rating scales and bladder functional recovery suggested that rats after PBMSC therapy owned better motor performance. Additionally, there was not any abnormal behavior emerging in the whole progress. H&E as well as LFB staining revealed there were less necrotic cavities formed, more tissue preserved and no glial scar occurred in the species from PBMSC-injected rats at 8 w. It preliminarily confirmed PBMSCT was a useful strategy to enhance functional recovery, inhibit glial scar formation and protect myelin sheath.
How did PBMSCs take effects? Nowadays, the mechanisms of MSC therapy mainly focuses on the differentiation into the lost CNS cells, that is replacement therapy, and another is immunoregulation. Firstly, replacement therapy means the differentiation into the lost astrocytes, oligodendrocytes and neurons. Therefore, it’s necessary to explore the expression of the relevant characteristic proteins such as MBP and NeuN. As a major component of oligodendrocytes and Schwann cells, MBP plays important roles in the velocity of axonal impulse conduction and maintenance of correct structure of myelin [38,39]. The inhibition of glial scar formation mainly depends upon astrocyte cells, which can express numerous GFAP [40]. It plays an important role in mitosis and astrocyte-neuron interactions as well as cell-cell communication [41,42]. NF-200 is a maker of axon sprouting and regeneration [43]. MAP-2 mainly exists in the neuronal cell bodies and dendrites and is the maker of dendrite growth and plays an important role in neuronal development and synaptic plasticity [44]. Once the regeneration of neurons occurs, NF-200 and MAP-2 can be overexpressed. Therefore, we explored the relevant-protein expression combining immunohistochemistry with immunofluorescence to study the underlying mechanisms of replacement treatment. It showed GFAP, MBP, NF-200 and MAP-2 expression after PBMSCT were respectively higher 2 w post-injury. As time gone, GFAP decreased while other 3 factors were still a higher tendency. Astrocyte cells at different stage (naïve and mature) play different roles in the pathological and physiological process of SCI [45,46]. Naïve astrocytes, with active anabolic effects, form the cellular grid framework and induce the regeneration of nerve fibers while mature ones transform structural cells and proliferate forming scars which can block nerve regeneration. We hypothesized that engrafted PBMSCs could differentiate into naïve astrocytes or improve the number of naïve astrocytes and meanwhile inhibit the number of mature ones. Additionally, there were little PKH26+GFAP+FITC astrocytes, PHK26+MBP+FITC oligodendrocytes, PKH26+NeuN+FITC neurons could be observed, which indicated the PBMSCT-relevant therapy took effect not through the differentiation of PBMSCs into CNS cells. Even so, the engrafted PKH26+PBMSCs could survived at the injury site for at least 8 w, obtaining a longer survival time comparing with the previous reports [3,35,47,48].
Another key is inmunoregulatory. It was reported that the number of arginase1+CD206+ macrophage (M2) increased while that of iNOS+CD16/32+ macrophage (M1) decreased after human BMMSC transplantation for SCI in rats [49]. In our current study on the immunoregulation comparison between BMMSCs and PBMSCs, we found that PBMSCs own a stronger immunoregulation which also inhibited M1 macrophages and promoted M2 macrophages expression (unpublished data). It has been aninamously recognized traditionally that Th17/Treg balance possesses a crucial role in autoimmune diseases. We supposed that PBMSCs could also promote the differentiation of CD4+T lymphocytes into CD4+CD25+Foxp3+Treg ones and meanwhile inhibit into Th17 cells. The flow cytometry data confirmed our reference. In the normal process, Th17/Treg balance is regulated by TGF-β, which can induce gene expression of Foxp3 and RORγT; meanwhile, Foxp3 expression can in turn inhibit RORγT expression [50]. However, this inhibition can be abrogated when IL-6 is present and as a result Th17 differentiation can be improved [51]. Then Th17 cells secrete IL-17a and IL-6 to medicate tissue inflammation [52]. Therefore, we analyzed the IL-17a, IL-6 and TGF-β expression at the injury lesion. The results inferred a lower IL-17a and IL-6 while a higher TGF-β level. After the extra analysis of peripheral blood serum and spleen level of IL-6, IL-17a and TGF-β, we could further confirm the effect of PBMSCs on SCI rats. Additionally, in the process of Th17 differentiation, IL-23a and IL-21 might produce marked effects. IL-23a serves as a survival signal for Th17 cells and IL-21, similar to IL-6, may be an additional signal for full function of Th17 cells [51,53]. When IL-6 is absent, CD4+T cells can differentiate into Treg cells and express IL-10 and TGF-β for anti-inflammatory. PCR data demonstrated the coincidental results of those relative genes. The relative mechanism could be included as Figure 8. What’s more, blockade of IL-6 may be an effective therapy on SCI.
Figure 8.

The potential Th17/Treg-relative mechanism of PBMSCs therapy on SCI. Th17/Treg cell balance is mainly regulated by FoxP3 and RORγT; FoxP3 expression can be inhibited by RORγT expression. SCI insulted that M1 macrophage migrated to the lesion and secreted proinflammatory factors such as IL-6 and IL-21 which enabled CD4+T cells to differentiate into CD4+IL17a+Th17 cells which could aggravate the injury and tissue loss in the presence of TGF-β. Then Th17 cells could secret IL-17a, IL-6 and IL-22 etc. to promote inflammatory. Meanwhile CD4+T cells are forced into CD+IL17+Th17 cells, as a result the number of Th17 cells are sharply improved and the loss of injured CNS cells. After PBMSCT, IL-6 expression is inhibited obviously, and CD4+T cells are forced into CD4+CD25+FoxP3+Treg cells under the co-influence of TGF-β and FoxP3 could reduce the injury and promote the tissue preservation. Theoretically implanted PBMSCs could not only differentiate the lost oligodendrocytes and Neurons, but also inhibit the overexpression of GFAP. However, there is little differential cells could be observed in this study.
Conclusion
PBMSCT-relevant therapy took effect not through the differentiation of PBMSCs into CNS cells, but through regulating Th17/Treg-relevant genes expression, inhibiting Th17-relevant gene expression and meanwhile promoting Treg-relevant gene expression, and eventually resulted in promotion of the functional recovery of SCI rats.
Acknowledgements
We would like to thank Prof. Lin Xu for excellent technical assistance. This manuscript was reviewed by a professional science editor and by a native English-speaking copy editor to improve readability. This work was supported by grants from Guizhou Province Science and Technology Project funded projects, No.SY[2012]3124.
Disclosure of conflict of interest
None.
References
- 1.Bareyre FM. Neuronal repair and replacement in spinal cord injury. J Neurol Sci. 2008;265:63–72. doi: 10.1016/j.jns.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Suberviola B, Gonzalez-Castro A, Llorca J, Ortiz-Melon F, Minambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 2008;39:748–752. doi: 10.1016/j.injury.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Park SI, Lim JY, Jeong CH, Kim SM, Jun JA, Jeun SS, Oh WI. Human umbilical cord bloodderived mesenchymal stem cell therapy promotes functional recovery of contused rat spinal cord through enhancement of endogenous cell proliferation and oligogenesis. J Biomed Biotechnol. 2012;2012:362473. doi: 10.1155/2012/362473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrowderived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 6.Golden KL, Pearse DD, Blits B, Garg MS, Oudega M, Wood PM, Bunge MB. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol. 2007;207:203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui SP, Nag TC, Ghosh S. Characterization of proliferating neural progenitors after spinal cord injury in adult zebrafish. PLoS One. 2015;10:e0143595. doi: 10.1371/journal.pone.0143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menezes K, Nascimento MA, Goncalves JP, Cruz AS, Lopes DV, Curzio B, Bonamino M, de Menezes JR, Borojevic R, Rossi MI, Coelho-Sampaio T. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One. 2014;9:e96020. doi: 10.1371/journal.pone.0096020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.All AH, Gharibani P, Gupta S, Bazley FA, Pashai N, Chou BK, Shah S, Resar LM, Cheng L, Gearhart JD, Kerr CL. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One. 2015;10:e0116933. doi: 10.1371/journal.pone.0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S. Concise review: spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells. 2014;32:829–843. doi: 10.1002/stem.1579. [DOI] [PubMed] [Google Scholar]
- 11.Pekovic V, Hutchison CJ. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J Anat. 2008;213:5–25. doi: 10.1111/j.1469-7580.2008.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 13.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Shin JM, Jeon YJ, Chung HM, Chae JI. Proteomic validation of multifunctional molecules in mesenchymal stem cells derived from human bone marrow, umbilical cord blood and peripheral blood. PLoS One. 2012;7:e32350. doi: 10.1371/journal.pone.0032350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu CC, Lin SS, Yuan LJ, Chen LH, Pan TL, Yang CY, Lai PL, Chen WJ. Identification of mesenchymal stem cells and osteogenic factors in bone marrow aspirate and peripheral blood for spinal fusion by flow cytometry and proteomic analysis. J Orthop Surg Res. 2014;9:32. doi: 10.1186/1749-799X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 17.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu WL, Zhou CY, Yu JK. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med. 2014;42:592–601. doi: 10.1177/0363546513512778. [DOI] [PubMed] [Google Scholar]
- 19.Zheng RC, Park YK, Kim SK, Cho J, Heo SJ, Koak JY, Lee SJ, Park JM, Lee JH, Kim JH. Bone regeneration of blood-derived stem cells within dental implants. J Dent Res. 2015;94:1318–1325. doi: 10.1177/0022034515590368. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Huang KJ, Wu JC, Hu MS, Sanyal M, Hu M, Longaker MT, Lorenz HP. Peripheral bloodderived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med. 2015;4:359–368. doi: 10.5966/sctm.2014-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Chen D, Li F, Tan Z. Netrin-1 with stem cells promote angiogenesis in limb ischemic rats. J Surg Res. 2014;192:664–669. doi: 10.1016/j.jss.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Nichols JE, Niles JA, DeWitt D, Prough D, Parsley M, Vega S, Cantu A, Lee E, Cortiella J. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ABCG2+CXCR4+ mesenchymal stem cells: development of autologous cellbased therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4:3. doi: 10.1186/scrt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Q, Zhang Q, Jia LY, Fang N, Chen L, Yu LM, Liu JW, Zhang T. Isolation and characterization of rat mesenchymal stem cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood. Cells Tissues Organs. 2016 doi: 10.1159/000445855. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Fu Q, Tang NN, Zhang Q, Liu Y, Peng JC, Fang N, Yu LM, Liu JW, Zhang T. Preclinical study of cell therapy for osteonecrosis of the femoral head with allogenic peripheral blood-derived mesenchymal stem cells. Yonsei Med J. 2016;57:1006–1015. doi: 10.3349/ymj.2016.57.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 27.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 28.Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, Saunders NR. Pathological changes in the white matter after spinal contusion injury in the rat. PLoS One. 2012;7:e43484. doi: 10.1371/journal.pone.0043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang CB, Zhou DX, Zhan SX, He Y, Lin Z, Huang C, Li J. Amelioration of experimental autoimmune uveitis by leflunomide in Lewis rats. PLoS One. 2013;8:e62071. doi: 10.1371/journal.pone.0062071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi J, Wang D, Niu X, Hu J, Zhou Y, Li Z. MicroRNA-155 deficiency suppresses Th17 cell differentiation and improves locomotor recovery after spinal cord injury. Scand J Immunol. 2015;81:284–290. doi: 10.1111/sji.12276. [DOI] [PubMed] [Google Scholar]
- 31.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 32.Ma K, Fox L, Shi G, Shen J, Liu Q, Pappas JD, Cheng J, Qu T. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. 2011;33:1083–1093. doi: 10.1179/1743132811Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 35.Veeravalli KK, Dasari VR, Tsung AJ, Dinh DH, Gujrati M, Fassett D, Rao JS. Human umbilical cord blood stem cells upregulate matrix metalloproteinase-2 in rats after spinal cord injury. Neurobiol Dis. 2009;36:200–212. doi: 10.1016/j.nbd.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Nandoe Tewarie RD, Hurtado A, Levi AD, Grotenhuis JA, Oudega M. Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant. 2006;15:563–577. doi: 10.3727/000000006783981602. [DOI] [PubMed] [Google Scholar]
- 37.Liu XH, Zhang T, Zhang Q, Liu JW, Liu Zl, Tang NN. Colony froming capacity and neuron differentiation of rabbit peripheral blood-derived mesenchymal stem cells after G-CSF mobilization. Journal of Xi’an Jiaotong University (Medical Sciences) 2014;35:26–29. [Google Scholar]
- 38.Sakamoto Y, Kitamura K, Yoshimura K, Nishijima T, Uyemura K. Complete amino acid sequence of PO protein in bovine peripheral nerve myelin. J Biol Chem. 1987;262:4208–4214. [PubMed] [Google Scholar]
- 39.Deber CM, Reynolds SJ. Central nervous system myelin: structure, function, and pathology. Clin Biochem. 1991;24:113–134. doi: 10.1016/0009-9120(91)90421-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacque CM, Vinner C, Kujas M, Raoul M, Racadot J, Baumann NA. Determination of glial fibrillary acidic protein (GFAP) in human brain tumors. J Neurol Sci. 1978;35:147–155. doi: 10.1016/0022-510x(78)90107-7. [DOI] [PubMed] [Google Scholar]
- 41.Tardy M, Fages C, Le Prince G, Rolland B, Nunez J. Regulation of the glial fibrillary acidic protein (GFAP) and of its encoding mRNA in the developing brain and in cultured astrocytes. Adv Exp Med Biol. 1990;265:41–52. doi: 10.1007/978-1-4757-5876-4_4. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein DE, Shelanski ML, Liem RK. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J Cell Biol. 1991;112:1205–1213. doi: 10.1083/jcb.112.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lever IJ, Robinson M, Cibelli M, Paule C, Santha P, Yee L, Hunt SP, Cravatt BF, Elphick MR, Nagy I, Rice AS. Localization of the endocannabinoid-degrading enzyme fatty acid amide hydrolase in rat dorsal root ganglion cells and its regulation after peripheral nerve injury. J Neurosci. 2009;29:3766–3780. doi: 10.1523/JNEUROSCI.4071-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Ekmark-Lewen S, Lewen A, Israelsson C, Li GL, Farooque M, Olsson Y, Ebendal T, Hillered L. Vimentin and GFAP responses in astrocytes after contusion trauma to the murine brain. Restor Neurol Neurosci. 2010;28:311–321. doi: 10.3233/RNN-2010-0529. [DOI] [PubMed] [Google Scholar]
- 46.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, Rosmalen CF, Vissers JL. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75:1786–1793. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]
- 47.Abrams MB, Dominguez C, Pernold K, Reger R, Wiesenfeld-Hallin Z, Olson L, Prockop D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor Neurol Neurosci. 2009;27:307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 48.Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, Rao JS, Dinh DH. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 2007;24:391–410. doi: 10.1089/neu.2006.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-betainduced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 52.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 53.Janke M, Peine M, Nass A, Morawietz L, Hamann A, Scheffold A. In-vitro-induced Th17 cells fail to induce inflammation in vivo and show an impaired migration into inflamed sites. Eur J Immunol. 2010;40:1089–1098. doi: 10.1002/eji.200939487. [DOI] [PubMed] [Google Scholar]