Abstract
Rhizoma Anemarrhena, a widely used traditional Chinese medicine, has previously been shown to have neuroprotective effect. Sarsasapogenin-AA13 (AA13) is a novel synthetic derivative of Sarsasapogenin, which is extracted from Rhizoma Anemarrhena. The aim of this study is to investigate the nootropic and neurotrophic effects of AA13 and underlying mechanisms. In vitro, cell viability of rat primary astrocytes treated with AA13 and neurons cultured with conditioned medium of AA13-treated rat primary astrocytes was tested by MTT assays. In vivo, a pharmacological model of cognitive impairment induced by scopolamine was employed and spatial memory of the mice was assessed by Morris water maze. This study found that AA13 increased cell viability of primary astrocytes and AA13-treated astrocyte-conditioned medium enhanced the survival rate of primary neurons. Interestingly, AA13 markedly enhanced the level of BDNF in astrocytes. Furthermore, AA13 (6 mg/kg) improved the cognitive deficits in animal models (p<0.05) and BDNF and PSD95 levels were increased in brain. Therefore, we hypothesize that AA13 exerts nootropic and neurotrophic activities through astrocytes mediated upregulation of BDNF secretion. The results suggest that AA13 could be a potential compound for cognitive impairment after further research.
Keywords: Brain-derived neurotrophic factor (BDNF), astrocytes, neurons, Sarsasapogenin-AA13, nootropic, neurotrophic
Introduction
Cognitive impairment occurs as a transitional stage between normal aging and dementia. Memory loss is usually one of clinical signs of prodromal stage of Alzheimer’s disease (AD). AD is a complex progressive neurodegenerative brain disorder, which leads to cognitive deficits and even death [1]. The pathological hallmarks of the AD brain are Aβ plaques and neurofibrillary tangles [2]. Unfortunately, there are no efficacious and available medicines for AD.
Neuroglia is important for the proper functioning of the central nervous system (CNS). The fundamental function of neuroglia is to maintain the homeostasis, form myelin, provide support and protection for neurons, and defense infection [3-5]. Astrocytes, the most numerous and diverse neuroglial cells, play a crucial role in neurotrophy, neural repair and brain homeostasis. There are different types of astrocytes in CNS like fibrous astrocytes, protoplasmic astrocytes and redial astrocytes [6-8]. Astrocytes participate in the development of brain synapse during initial establishment and remodeling [9]. Astrocytes secreted neural active substances including neuromodulators, gliotransmitters and trophic factor [10,11], which are vital in information transfer and neural plasticity. So far, astrocytes have been proved to secret numerous synaptic related molecules, including interleukin [12], D-serine [13], neurotrophic factor [14], estrogen [15], and tumor necrosis factor alpha [16].
Neurotrophic factors are mainly produced by astrocytes and neurons, especially by astrocytes, which play important roles in learning and memory. And the reduction of neurotropic factors has been found in many neurodegenerative diseases [17,18]. Neuroglia is closely associated with neuronal damage, and the changes of astrocytes and microglia of the brain were considered to be important in the pathogenesis of AD [19]. In the brains of AD patients, both the activation of astrocytes and the damage of astrocytes were found, and those changes often appeared before the formation of neurofibrillary tangles and aggregation of β-amyloid (Aβ) [20]. In the studies of mice and patients, astrocytes can gather and phagocytose Aβ [21,22]. Aβ aggregation and plaque formation can promote activation of astrocytes in AD animals [23,24]. Similar astrocytes activation was also found in the brains of AD patients [19,25,26]. Using fluorescent tags to trace astrocytes, researchers found that the astrocytes transplanted from healthy mice could remove senile plaques and phagocytose them [27], indicating pathological changes of astrocytes play an important role in the process of AD, which makes astrocytes a promising therapeutic target.
Brain-derived neurotrophic factor (BDNF), a well-understood trophic factor, is important in many physiological courses in brains, such as neuron maturation, synapsis plasticity and neural restoration [28]. BDNF decreased in the brains of mild cognitive impairment patients [29]. Furthermore, BDNF is lower in the brains of AD patients than that of normal brains [30]. Researchers have found that BDNF could inhibit Aβ-induced neurotoxicity in vitro [31]. Since extrinsic BDNF cannot across blood brain barrier, using of compounds that promote the expression of BDNF can be a feasible way in the treatment of cognitive deficits or even AD.
Rhizoma Anemarrhena is a famous Chinese herb, which was often applied in the therapy of diabetic, thrombus and depression [32-34]. Recent studies have shown that Rhizoma Anemarrhenae has neuroprotective and anti-inflammatory effects [35,36]. Saponin mixture of Rhizoma Anemarrhenae ameliorated memory deficits induced by amyloid β-peptide [37]. Sarsasapogenin-AA13 (AA13), a novel synthetic derivative of sarsasapogenin, was found to have neuroprotective effect, but its underling mechanism has not been determined. AA13 is a new compound which was tested for neuroprotection from synthetic derivatives of sarsasapogenin. In our previous study, the anti-inflammatory effects of AA13 were demonstrated in RAW264.7 cells and peritoneal macrophages [38].
In this study, we found the potential neurotrophic activity of AA13 both in vivo and in vitro. Moreover, AA13 had the function of promoting the expression of BDNF, which could be a potential therapy in nerve damage for further research.
Materials and methods
Reagents
Ham’s/F12 (F12) medium, Dulbecco’s modified Eagle’s medium (DMEM) and Roswell Park Memorial Institute (RPMI)-1640 media were obtained from Hyclone (Logan, UT, USA). B27 and Neurobasal-A Medium were obtained from Gibco BRL (Carlsbad, CA, USA). Protease inhibitor cocktail, Phenylmethanesulfonyl fluoride (PMSF), Fetal bovine serum (FBS) and Poly-L-lysine were obtained from Sigma-Aldrich (St Louis, MO, USA). Thiazolyl blue (MTT), Phosphatase inhibitor complex III, and electrochemiluminescence (ECL) reagent kit were obtained from Sangon Biotech (Shanghai, China). Rabbit anti-BDNF polyclonal antibody, rabbit anti-Bcl2 polyclonal antibody, rabbit ant-Bax polyclonal antibody and rabbit anti-GAPDH polyclonal antibody were obtained from Proteintech Group, Inc (Rosemont, IL, USA). Rabbit anti-PSD95 monoclonal antibody was from Abcam (Shanghai) Company Ltd (Pudong, Shanghai, China). Secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). AA13 (Figure 1) was synthesized by Lei Ma’s lab from East China University of Science and Technology.
Figure 1.
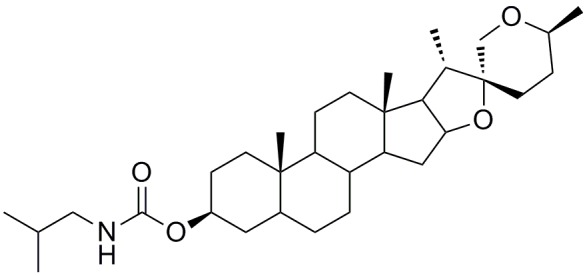
Chemical Structure of Sarsasapogenin-AA13.
Animals
Sprague Dawley rats and male ICR mice (30 g) were obtained from Shanghai Sippr-bk Laboratory Animal Co., LTD (Shanghai, China). All rats and mice were group-housed in clean and dry cages at 25 ± 1°C on a 12 h light 12 h dark automatic lightening with food and water ad libitum. All the experiments were complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals in Shanghai, China. All the experiments were performed with the permission of the Ethics Committee on Laboratory Animal Care of the East China University of Science and Technology.
CHO cells and Hela cells culture
CHO cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured with DMEM: Ham’s/F12 (D/F12) containing 10% FBS in incubator at 37°C. Cells were transfered to 96-well or 6-well plates at a concentration of 1×105/mL before experiments. Experiments were carried after cells attached the bottom of the culture dishes.
Hela cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured with RPMI-1640 media containing 10% FBS in incubator at 37°C. Cells were transfered to 96-well or 6-well plates at a concentration of 1×105/mL before experiments. Experiments were carried after cells attached the bottom of the culture dishes.
Primary astrocytes culture
Primary rat astrocytes were acquired from the cortex of 1-2 days’ neonatal rats according to previously description [39,40]. Rat pups were immersed in 75% alcohol for 5 min, and then washed with D-Hank’s. The head of neonatal rats were cut off quickly and cerebral cortex was isolated on ice. After removing meninx and large blood vessels neatly, cortex was minced mechanically in D/F12 medium containing 10% FBS and 100 U/mL antibiotics. After trituration and digestion with papain (2 mg/mL) at 37°C for 30 min, suspension liquid was filtered through a 100 μM sterile filter. Cell suspension liquid was centrifuged at 200 g for 5 min and deposits were mixed in a new culture medium. Cells were counted and density of cells was adjusted to 1×106 cells/mL. Cells were infused into dishes and incubated at 37°C and 5% CO2. When cells reached confluence, microglia was removed by shaking at 200 rpm for at least 24 h.
Primary neurons preparation
1-2 days’ neonatal Sprague Dawley rats were doused in 75% alcohol for 5 min, and then washed with D-Hank’s. Hippocampus was dissected after removing meninges and blood vessels in ice-cold neurobasal-A medium. The hippocampus was cut into a cubic millimeter pieces and digested by 2 mg/mL papain and 0.05 mg/mL DNAase for 30 min in serum-free medium. The tissue suspension was mixed with neurobasal-A medium with B27 (1%), penicillin (50 U/mL), streptomycin (50 U/mL) and glutamine (0.5 mmol/L) in sterile 50 mL centrifuge tube and gently pipetted to disperse the cells. After filtering through 100 μM sterile filter, density of cells was adjusted to 4×105 cells/mL. Then the cells were incubated in a poly-lysine-treated plate at 37°C and 5% CO2. After 4 hours, the medium was removed and new neurobasal-A medium with B27 (1%), glutamine (0.5 mmol/L), penicillin (50 U/mL) and streptomycin (50 U/mL) was added in the plate.
Astrocyte-conditioned medium preparation and treatments
Primary astrocytes were cultured in 6-well plate at a concentration of 2×105 cells/mL. Astrocytes were administered with AA13 at the concentration of 5 μM and 10 μM. After 24 h, the culture medium of astrocytes was collected in 50 mL centrifuge tube and centrifuged at 1000×g to discard floating cells and debris. Replace half the neurobasal-A medium of neuronal cells with conditioned medium from astrocytes. After 48 h, the activity of neuronal cells was evaluated by MTT assay.
MTT assay
MTT assay is a sensitive measurement of cell viability, depending on the activity of mitochondrial enzymes. Cells were cultured in 96-well plates. After treatments, MTT solution was added into the culture medium. The formazan crystals formed in cells was dissolved sufficiently with dimethylsulfoxide 4 hours later. The absorbance was quantified at 490 nm and 540 nm with a Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT).
Western blot analysis
After treatment, cells were washed by ice-cooled D-Hank’s for three times. Western blot lysing buffer containing multiple protease inhibitors were added into 6-well plate and lysed for 30 min on ice. Brian tissues were also washed with ice-cooled D-Hank’s, and broke down mechanically by glass homogenizer for 30 min on ice. After enough lysed, cells and tissue samples were centrifuged at 10000×g for 10 min. the supernatant was carefully collected in clean centrifuge tube and boiled at 100°C with loading buffer for 5 min. Samples were separated through SDS-PAGE and then the protein in gel were moved onto polyvinylidene difluoride (PVDF) membranes. After blocking in 5% Bull Serum Albumin (BSA), the target protein on membranes were probed with primary antibodies first and then horseradish peroxidase labeled secondary antibodies. Immunoreactivity was detected after using ECL reagent kit and the light was detected by cameras that capture image of medical X-ray film.
Drug treatment on mice
All the mice were divided into five groups: vehicle-treated group (saline), scopolamine-treated group, donepezil hydrochloride-treated group, AA13 (6 mg/kg)-treated group and AA13 (30 mg/kg)-treated group. Donepezil hydrochloride was used as a positive drug. Scopolamine and donepezil hydrochloride were dissolved in saline, while AA13 was dissolved in saline suspended 0.3% sodium carboxymethyl cellulose. Donepezil hydrochloride and AA13 were given intragastrically (i.g.) for 15 days. At day 15, all groups of mice except the vehicle-treated mice were administered intraperitoneally (i.p.) with scopolamine 25 min before probe test (see Figure 5A).
Figure 5.

Sarsasapogenin-AA13 attenuated scopolamine-induced cognitive impairment. A. Experimental timeline of Morris water maze test. Mice were administrated with AA13 (6 mg/kg, 30 mg/kg, i.g.) and donepezil (3 mg/kg, i.g.). Cognitive deficits were induced by injection of scopolamine (2 mg/kg, i.p.) at the last day of Morris water maze test (the 15th day). B. Swimming track of mice. C. Latency of mice in hidden platform trials. D. Time in the target quadrant. E. Swimming speed of mice in probe trials. Date are presented as mean ± SEM (n=10). p<0.05 is considered significant. *p<0.05 vs. Control; #p<0.05 vs. Scopolamine.
Morris water maze test
The effects of AA13 on memory impairment in mice were detected by Morris water maze test as previously described [40,41]. The experimental equipment (AniLab Software & Instruments Co., Ltd, China) includes a circular metal pool with a diameter of 150 cm and a depth of 50 cm (22 ± 1°C). The water was opaque with non-toxic black granules above. The pool was divided into four quadrants with fixed visual cues. A platform (10 cm in diameter) was placed at the midpoint of target quadrant under the surface of the maze. The equipment was placed in a behavior room maintained at 25°C. To test the swimming ability and the escape motivation of mice, each mouse received four visible platform tests before behavioral experiment: each mouse was given 60 s to swim freely and climb onto the platform that was 1 cm above the water surface. In day 9-14, hidden platform trials were performed four times per day. The mouse was put into the pool facing the edge of the pool at randomized positions and allowed to swim for 60 s to find the platform and stay for another 20 s. The mice that cannot find the platform within 60 s were leaded by experimenter to the platform and allowed to stay 20 s. Probe trial was in day 15 (See timeline in Figure 5A). The platform was taken away and all the mice swim individually in the pool for 60 s. Time spent in the platform quadrant for each mouse was collected by video analysis system. After the experiment, mice were sacrificed and the brains were isolated.
Statistical analysis
Dates were displayed as mean ± SEM. The statistical significance was evaluated by SPSS 15.0 version. The data was analyzed by One-way analysis of variance test, and multiple comparisons were assessed by a post hoc (LSD) test. Escape latency in Morris water maze was analyzed by two-way analysis of variance (ANOVA). P value of <0.05 was considered significant.
Results
Sarsasapogenin-AA13 increased cell viability of rat primary astrocytes
The effect of AA13 on rat primary astrocytes was examined. Primary astrocytes were cultured in 96-well plate at a concentration of 2×105 cells/mL. Cells were treated with different concentration of AA13 (0, 1, 5, 10 μM) for 24 h, and the viability of astrocytes was measured by MTT assay. As shown in Figure 2, AA13 increased the cell viability of rat primary astrocytes obviously at the concentration of 5 μM and 10 μM (Figure 2A). To confirm above mentioned effect was tissue-specific, we tested the effect of AA13 on CHO and Hela cells. The result indicated that AA13 cannot affect the viability of CHO cells (Figure 2B) and slightly inhibited Hela cell growth in the concentration of 5 μM and 10 μM (Figure 2C).
Figure 2.

Sarsasapogenin-AA13 increased the cell viability of rat primary astrocytes. A. Primary astrocytes; B. CHO cells; C. Hela cells were treated by AA13 for 24 h at the concentration of 1, 5 and 10 μM. The viability of cells was detected by MTT assays. The results are shown as a percentage of the control group. Date are presented as mean ± SEM (n=3). p<0.05 is considered significant; *p<0.05; **p<0.01; ***p<0.001 vs. AA13 (0 μM).
Besides, proteins related to proliferation like Bcl2/Bax were also detected. The results showed that the relative ratio of Bcl2/Bax was increased after AA13 treatment at the concentration of 5 μM and 10 μM (Figure 3).
Figure 3.

Sarsasapogenin-AA13 increased the relative ratio of Bcl2/Bax in rat primary astrocytes. A. Expression of Bcl2. B. Bax were measured by Western blot analysis. C. Relative ratio of Bcl2/Bax. D. Relative protein levels were analyzed by gray value. Date are presented as mean ± SEM (n=3). p<0.05 is considered significant; *p<0.05; **p<0.01; ***p<0.001 vs. AA13 (0 μM).
Sarsasapogenin-AA13 increased the survival of rat primary neurons mediated by astrocytes
To study the neurotrophic activity of AA13 on neurons, we tested the effects of astrocytes conditioned medium (with/without treatment of AA13) on primary neurons. The result demonstrated non-AA13 treated astrocytes conditioned medium (AA13 (0 μM)-CM) could markedly increase the survival rate of primary neurons compared with the control group (p<0.01). Furthermore, AA13 (10 μM)-treated astrocytes conditioned medium significantly raised the survival rate of primary neurons compared with AA13 (0 μM)-CM group (p<0.001). AA13 (10 μM) alone had no effect on the survival rate of primary neurons (Figure 4).
Figure 4.

Sarsasapogenin-AA13 increased the survival of rat primary neurons mediated by astrocytes. Rat primary astrocytes were administered with AA13 (10 μM) for 24 h, and the cultured medium of astrocytes were collected as conditioned medium (CM). Then rat primary neurons were treated with the conditioned medium. Date are presented as mean ± SEM (n=3). p<0.05 is considered significant. **p<0.01 vs. Control; ###p<0.001 vs. AA13 (0 μM)-CM.
Sarsasapogenin-AA13 reversed scopolamine induced amnesia
Morris water maze test was used to evaluate the nootropic activities of AA13 on scopolamine-induced cognitive impairment in mice model. In the training sessions, the latencies of AA13 (6 or 30 mg/kg) and donepezil treated groups are shorter than that of the control group (non-treated) without significant difference though (Figure 5C). In probe trial (the 7th day of training), the mice of scopolamine treatment spent shorter in the platform quadrant, and the mice of AA13 (6 mg/kg) and donepezil treatment stayed significantly longer in the platform quadrant compared with scopolamine-treated group (p<0.05). The time spent in AA13 (30 mg/kg) treated group was also increased in the target quadrant even though the difference was not significance (Figure 5B, 5D). Meanwhile, swimming speed of all the groups was of no difference (Figure 5E).
Sarsasapogenin-AA13 increased the expression of BDNF in vitro and in vivo
To determine whether BDNF involved in the effects of AA13, we first detected the expression of BDNF in vitro. Primary astrocytes were cultured in 6-well plate at a concentration of 2×105 cells/mL. After attached, cells were treated with different concentration of AA13 (0, 1, 5, 10 μM) for 24 h. The level of BDNF was detected by western blot analysis. As shown in Figure 6A, AA13 increased the level of BDNF at a concentration dependent manner with the statistical significance at 10 μM.
Figure 6.

Sarsasapogenin-AA13 increased the expression of BDNF in vitro and in vivo. A. Expression of BDNF in AA13-treated primary astrocytes were measured by Western blot analysis. After two weeks’ AA13 treatment, brain tissues of mice were separated. B, C. BDNF in cortex and hippocampus were detected by western blot analysis. Date are presented as mean ± SEM (n=3). p<0.05 is considered significant. *p<0.05, **p<0.01, ***p<0.001 vs. Control.
Next, the effect of AA13 on the expression of BDNF in mice brain was measured by Western blot analysis. After two weeks’ oral administration of AA13 (6 mg/kg, 30 mg/kg), the BDNF levels were significantly increased in cortex (Figure 6B) and hippocampus (Figure 6C), compared with the control group.
Sarsasapogenin-AA13 increased the expression of PSD95 in cortex and hippocampus
To further investigate the effect of AA13 on synaptogenesis which somewhat reflecting the function of neurons, the expression of PSD95 in cortex and hippocampus were detected. The result showed AA13 significantly increased the expression of PSD95 in cortex (Figure 7A, 7C) and hippocampus (Figure 7B, 7E). Meanwhile, AA13 did not increase the level of NeuN in cortex (Figure 7A, 7D) and hippocampus (Figure 7B, 7F).
Figure 7.
Sarsasapogenin-AA13 increased the expression of PSD95 in brains of mice. A, C, D. Levels of PSD95 and NeuN were detected in cortex. B, E, F. In hippocampus. Date are presented as mean ± SEM (n=3). p<0.05 is considered significant. **p<0.01, ***p<0.001 vs. Control.
Discussion
Development of potential nootropic and neurotrophic agents is a promising strategy for treatment of cerebral injury, neurodegenerative diseases and even encephalodysplasia [42]. Rhizoma anemarrhenae has been used in China for centuries. Based on the saponins isolated from Rhizoma anemarrhenae which are of multiple pharmacological activities, dozens of sarsasapogenin derivatives were synthetized and preliminarily screened. We have previously reported AA13, one of the sarsasapogenin derivatives, could attenuate H2O2- or Aβ-induced neurons and glia injuries, LPS-stimulated inflammation and cognitive deficiency. In this study, we found AA13 improved the spatial memory of scopolamine treated mice in Morris water maze performance. Similar cognitive improving efficacy was also found in the mice model of Aβ-induced memory impairment in our previous study [43]. In vitro study showed increased viability of rat primary astrocytes, which indicated AA13 could increase the proliferation of astrocytes. Besides, Bcl2 up-regulation, Bax down-regulation also support this idea. To figure out if the effect is astrocyte specific, two different cell lines, CHO cell and HeLa were tested. Result shown that the proliferation was not affected in CHO and even slightly suppressed in HeLa cells, which suggests that the effect on proliferation is astrocyte specific (Figure 2).
Then we tested the effects of AA13 on rat primary neurons. Unlike astrocytes, we found that direct-addition of AA13 into the medium of primary cultured neurons could not enhance neuronal survival (Figure 4). Astrocytes were considered as a nutrition of the brain’s nerve cells and neural repair [44]. So, we treated neurons with conditioned medium from the rat primary astrocytes cultures in which AA13 or vehicle was added. Results showed both conditioned medium of AA13 or vehicle treated could significantly increase the survival rate of rat primary neurons. AA13 treated conditioned medium was stronger (Figure 4). These evidences implied some certain component in the culture medium of astrocytes improved neuronal survival, and AA13 could enhance it.
BDNF is prominent in the survival, growth, maintenance of neurons and modulating synaptic-plasticity in brains. BDNF is a ligand for activation of TrkB, which results in PSD-95-TrkB complexes [45], and induces transport of PSD-95 to dendrites and synapses [46]. Our data showed that AA13 markedly enhanced the level of BDNF in cultured rat astrocytes and mice brain tissues (Figure 6). We hypothesize, AA13 exerts neurotrophic role through BDNF which is astrocyte mediated. The effect of AA13-treated conditioned medium from the rat primary astrocytes on rat primary neurons supported this hypothesis to some degree.
In the behavioral experiments, the neuroprotective activity of AA13 on scopolamine-induced memory impairment might also be associated with the increase of BDNF. Scopolamine is a relatively specific antagonist of muscarinic receptors. Hu et al reported that sarsasapogenin increased the muscarinic M1 receptor density during cell aging through CREB [47], which was regulated by BDNF and might contribute to the nootropic effect [47,48]. Researchers also reported that scopolamine-induced memory impairment was improved by the up-regulation of BDNF [49-51].
It’s been reported that synaptic dysfunction takes a part in neurodegenerative disorders. And BDNF is a key neurotrophic factor in synaptic plasticity and synaptogenesis [52,53]. Our data showed that NeuN, a neuronal nuclear antigen used as a biomarker for neurons [54], was similar between groups while the level of PSD95 in mice brains were obviously increased after continuous administration of AA13, which might be related to BDNF enhancement.
In addition, 30 mg/kg of AA13 was a little effective on the scopolamine-induced memory impairment which might indicated an off-target effects. Since there is no significant difference between two dose groups, there can be statistical limitations due to the small sample size. Furthermore, the expression of BDNF and PSD95 in brain tissue of mice was increased after AA13 (30 mg/kg) treatment.
In conclusion, our study suggests that AA13 exerts nootropic and neurotrophic activities through astrocytes mediated upregulation of BDNF secretion. Therefore, AA13 should be further optimized and investigated as a therapeutic possibility of cognitive impairments or even neurodegenerative diseases.
Acknowledgements
This project was supported by grants from the National Natural Science Foundation of China (81072627 and 81230090), the Shanghai Biomedical Technology Support Program (15401901100), the 111 Project of the Chinese Ministry of Education (No B07023), and the Key project from Shanghai Science and Technology Committee (12431900901).
Disclosure of conflict of interest
None.
References
- 1.Couttas TA, Kain N, Suchowerska AK, Quek LE, Turner N, Fath T, Garner B, Don AS. Loss of ceramide synthase 2 activity, necessary for myelin biosynthesis, precedes tau pathology in the cortical pathogenesis of Alzheimer’s disease. Neurobiol Aging. 2016;43:89–100. doi: 10.1016/j.neurobiolaging.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 3.Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- 4.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Jha MK, Lee WH, Suk K. Functional polarization of neuroglia: implications in neuroinflammation and neurological disorders. Biochem Pharmacol. 2016;103:1–16. doi: 10.1016/j.bcp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Parpura V, Verkhratsky A. Homeostatic function of astrocytes: Ca(2+) and Na(+) signalling. Transl Neurosci. 2012;3:334–344. doi: 10.2478/s13380-012-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleiss MM, Sompol P, Kraner SD, Abdul HM, Furman JL, Guttmann RP, Wilcock DM, Nelson PT, Norris CM. Calcineurin proteolysis in astrocytes: implications for impaired synaptic function. Biochim Biophys Acta. 2016;1862:1521–1532. doi: 10.1016/j.bbadis.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matias I, Buosi AS, Gomes FC. Functions of flavonoids in the central nervous system: astrocytes as targets for natural compounds. Neurochem Int. 2016;95:85–91. doi: 10.1016/j.neuint.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martineau M, Parpura V, Mothet JP. Celltype specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci. 2014;6:12. doi: 10.3389/fnsyn.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruol DL. Impact of Increased Astrocyte Expression of IL-6, CCL2 or CXCL10 in Transgenic Mice on Hippocampal Synaptic Function. Brain Sci. 2016:6. doi: 10.3390/brainsci6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A. 2010;107:17005–17010. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu R, Cai WQ, Wu XG, Yang Z. Astrocytederived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience. 2007;144:1229–1240. doi: 10.1016/j.neuroscience.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Mishra M, Ghosh S, Tewari R, Basu A, Seth P, Sen E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Bartus RT, Johnson EM Jr. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: where have we been and what have we learned? Neurobiol Dis. 2017;97:156–168. doi: 10.1016/j.nbd.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Ruozi B, Belletti D, Bondioli L, De Vita A, Forni F, Vandelli MA, Tosi G. Neurotrophic factors and neurodegenerative diseases: a delivery issue. Int Rev Neurobiol. 2012;102:207–247. doi: 10.1016/B978-0-12-386986-9.00009-0. [DOI] [PubMed] [Google Scholar]
- 19.Beach TG, McGeer EG. Lamina-specific arrangement of astrocytic gliosis and senile plaques in Alzheimer’s disease visual cortex. Brain Res. 1988;463:357–361. doi: 10.1016/0006-8993(88)90410-6. [DOI] [PubMed] [Google Scholar]
- 20.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 21.Nagele RG, D’Andrea MR, Lee H, Venkataraman V, Wang HY. Astrocytes accumulate a beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- 22.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 23.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez JJ, Verkhratsky A. Neuroglial roots of neurodegenerative diseases? Mol Neurobiol. 2011;43:87–96. doi: 10.1007/s12035-010-8157-x. [DOI] [PubMed] [Google Scholar]
- 25.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meda L, Baron P, Scarlato G. Glial activation in Alzheimer’s disease: the role of Abeta and its associated proteins. Neurobiol Aging. 2001;22:885–893. doi: 10.1016/s0197-4580(01)00307-4. [DOI] [PubMed] [Google Scholar]
- 27.Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 28.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 29.Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76:347–354. doi: 10.1016/s0169-328x(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 30.Ferrer I, Marin C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Marti E. BDNF and fulllength and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Dong JX, Han GY. A new active steroidal saponin from Anemarrhena asphodeloides. Planta Med. 1991;57:460–462. [PubMed] [Google Scholar]
- 33.Iida Y, Oh KB, Saito M, Matsuoka H, Kurata H, Natsume M, Abe H. Detection of antifungal activity in Anemarrhena asphodeloides by sensitive BCT method and isolation of its active compound. J Agric Food Chem. 1999;47:584–587. doi: 10.1021/jf980707t. [DOI] [PubMed] [Google Scholar]
- 34.Oh JK, Hyun SY, Oh HR, Jung JW, Park C, Lee SY, Park JH, Kim SY, Kim KH, Kim YK, Ryu JH. Effects of anemarrhena asphodeloides on focal ischemic brain injury induced by middle cerebral artery occlusion in rats. Biol Pharm Bull. 2007;30:38–43. doi: 10.1248/bpb.30.38. [DOI] [PubMed] [Google Scholar]
- 35.Liu YW, Zhu X, Lu Q, Wang JY, Li W, Wei YQ, Yin XX. Total saponins from rhizoma anemarrhenae ameliorate diabetes-associated cognitive decline in rats: involvement of amyloid-beta decrease in brain. J Ethnopharmacol. 2012;139:194–200. doi: 10.1016/j.jep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Lee B, Jung K, Kim DH. Timosaponin AIII, a saponin isolated from anemarrhena asphodeloides, ameliorates learning and memory deficits in mice. Pharmacol Biochem Behav. 2009;93:121–127. doi: 10.1016/j.pbb.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Ouyang S, Sun LS, Guo SL, Liu X, Xu JP. Effects of timosaponins on learning and memory abilities of rats with dementia induced by lateral cerebral ventricular injection of amyloid beta- peptide. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:121–126. [PubMed] [Google Scholar]
- 38.Dong D, Zhou NN, Liu RX, Xiong JW, Pan H, Sun SQ, Ma L, Wang R. Sarsasapogenin-AA13 inhibits LPS-induced inflammatory responses in macrophage cells in vitro and relieves dimethylbenzene-induced ear edema in mice. Acta Pharmacol Sin. 2017;38:699–709. doi: 10.1038/aps.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tallant EA, Higson JT. Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia. 1997;19:333–342. [PubMed] [Google Scholar]
- 40.Wang L, Lin F, Wang J, Wu J, Han R, Zhu L, Difiglia M, Qin Z. Expression of mutant N-terminal huntingtin fragment (htt552-100Q) in astrocytes suppresses the secretion of BDNF. Brain Res. 2012;1449:69–82. doi: 10.1016/j.brainres.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 41.He D, Wu H, Wei Y, Liu W, Huang F, Shi H, Zhang B, Wu X, Wang C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur J Pharmacol. 2015;768:96–107. doi: 10.1016/j.ejphar.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C, Dong D, Jiao Q, Pan H, Ma L, Wang R. Sarsasapogenin-AA13 ameliorates Abeta-induced cognitive deficits via improving neuroglial capacity on Abeta clearance and antiinflammation. CNS Neurosci Ther. 2017;23:498–509. doi: 10.1111/cns.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X, Yuan Y, Wang D, Su Z. Heterogeneous astrocytes: active players in CNS. Brain Res Bull. 2016;125:1–18. doi: 10.1016/j.brainresbull.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 46.Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 47.Hu H, Zhang R, Zhang Y, Xia Z, Hu Y. Role of CREB in the regulatory action of sarsasapogenin on muscarinic M1 receptor density during cell aging. FEBS Lett. 2010;584:1549–1552. doi: 10.1016/j.febslet.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Hu Y, Xia Z, Sun Q, Orsi A, Rees D. A new approach to the pharmacological regulation of memory: Sarsasapogenin improves memory by elevating the low muscarinic acetylcholine receptor density in brains of memory-deficit rat models. Brain Res. 2005;1060:26–39. doi: 10.1016/j.brainres.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Min AY, Doo CN, Son EJ, Sung NY, Lee KJ, Sok DE, Kim MR. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem Biol Interact. 2015;242:153–162. doi: 10.1016/j.cbi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Jeon SJ, Lee HJ, Lee HE, Park SJ, Gwon Y, Kim H, Zhang J, Shin CY, Kim DH, Ryu JH. Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology. 2017;113:100–109. doi: 10.1016/j.neuropharm.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Lee B, Sur B, Shim J, Hahm DH, Lee H. Acupuncture stimulation improves scopolamineinduced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2014;14:338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gharami K, Das S. BDNF local translation in viable synaptosomes: implication in spine maturation. Neurochem Int. 2014;69:28–34. doi: 10.1016/j.neuint.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Gusel’nikova VV, Korzhevskiy DE. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae. 2015;7:42–47. [PMC free article] [PubMed] [Google Scholar]



