Abstract
Background: Cisplatin is a common used anti-tumor drug in ovarian cancer therapy with potent effect. Studies have reported that autophagy works as a cell-survival process in cancer, chloroquine has been added to various chemotherapeutic drugs. In the current study, we aim to evaluate whether chloroquine can enhance the effects of cisplatin in treating ovarian cancer. Methods: CCK-8 assay was used to detect cell viability. Transwell assay was used to examine cell migration and invasion. Flow cytometry assay was applied to evaluate cell apoptosis. Western-blot assay was used to detect proteins related to apoptosis, autophagy and the AKT/mTOR pathway. Results: In the current study, we showed that low concentration of chloroquine alone did not affect cell viability, migration or invasion, but it could enhance the efficacy of cisplatin in inhibiting cell viability, migration and invasion in both SKOV3 and hey cells. Afterwards, we observed that cisplatin triggered apoptosis and autophagy in both SKOV3 and hey cells in a dose-dependent manner. After treatment of cisplatin, SKOV3 and hey cells showed increased apoptotic rate in flow cytometry assay, increased protein levels of cleaved caspase 3, cleaved PARP and Bax, and decreased protein levels of Bcl-2 and Bcl-XL. Cisplatin also induced the formation of autophagosomes and increased autophagy-related proteins ATG 5, ATG 7, Beclin 1 and LC3B II/LC3B I. Meanwhile, cisplatin activated the AKT-mTOR pathway in both SKOV3 and hey cells. Next, chloroquine was added to ovarian cancer cells, flow cytometry assay revealed that chloroquine alone did not affect cell apoptosis and expressions of apoptosis-related proteins, while chloroquine plus cisplatin induced more apoptotic rate than cisplatin alone (p < 0.05). Meanwhile, apoptosis-related proteins had the same change trend. In vivo experiment demonstrated that chloroquine plus cisplatin was more effective than cisplatin alone in suppressing the growth of xenograft tumors, with lower ki-67 expression and higher cleaved caspase 3 expression. Conclusion: Based on our study, we propose that cisplatin activates the AKT/mTOR signaling pathway, which subsequently induces cytoprotective autophagy in ovarian cancer cells. Meanwhile, inhibition of autophagy via chloroquine enhances the anti-tumor effect of cisplatin.
Keywords: Chloroquine, cisplatin, ovarian cancer, autophagy, AKT/mTOR signaling pathway
Introduction
Ovarian cancer is one of the most common malignant gynecologic tumors. As most patients are in advanced stages when they are firstly diagnosed, patients with ovarian cancer have low survival rates and high mortality rates [1-3]. In the present, main treatments for ovarian cancer is complete surgical staging and maximal resection with additional chemotherapy. Chemotherapy is effective in cancer therapy, as it can not only inhibit tumor cell growth, but also induce cell apoptosis. For ovarian cancer, platinum-based combination therapy is the first-line chemotherapy. However, chemoresistance of platinum-based therapy can reduce chemotherapeutic efficacy and lead to tumor recurrence. In spite of progresses have been made in surgery and chemotherapy agents in the past years, 5-year survival rates of ovarian cancer is around 40% [4,5].
At present, recognized mechanisms related to the resistance of ovarian cancer include pharmacological resistance, biochemical resistance, apoptosis resistance and microenvironment resistance [6]. As a common agent in ovarian cancer therapy, cisplatin has a potent effect, and it can have synergistic effects with other anti-tumor agents. As reported, after entrance into cells, cisplatin can form complexes with DNA, thus inhibiting DNA replication, RNA transcription and cell cycle arrest [7,8]. Due to its cytotoxicity, cisplatin has severe toxic and side effects on patients, so it is refined in some clinical conditions. Therefore, increasing the susceptibility of tumor cells to cisplatin is of great significance for drug resistance inversion.
Chloroquine (CQ) has been used for the treatment of malaria and rheumatism for many years, as it could inhibit lysosomal enzymes and regulate immunity [9,10]. Moreover, it has been thought to have antiviral effect, as it could suppressing protein glycosylation which was needed for viral function [11]. Via increasing PH of lysosome, chloroquine can interfering with the fusion of autophagosome and lysosome, thus inhibiting autophagy. More recently, due to its ability to block autophagy, further interest has been generated into other fields, such as cancer treatment [9,12-14].
Autophagy is a kind of protein degradation process in eukaryotic cells, dependent on lysosome. Autophagy has paradoxical effects in cancer development and progression. As reported by some experts, inhibiting autophagy could promote tumorigenesis [15]. However, according to other reports, autophagycould provide a protective effect for cancer cells when they were exposed to pressure, thus even increasing tumor metastasis [16]. Since various studies have reported that autophagy can work as a cell-survival process in cancer, chloroquine has been added to various chemotherapeutic drugs. In some kinds of cancer such as pancreatic cancer and prostate cancer, chloroquine could augment the anti-tumor efficacy via inhibiting autophagy [17,18]. Oppositely, in other cancers such as small-cell lung cancers, chloroquine could not enhance the efficacy of chemotherapy orra dioth erapy [19,20]. Therefore, we propose that whether chloroquine could enhance the efficacy of chemotherapy and radiotherapy is depend on the context and neoplasms type.
Till now, whether chloroquine can boost the effects of cisplatin in treating ovarian cancer is unclear. In the current study, we presented experimental evidence showing that cisplatin could trigger protective autophagy in ovarian cancer and adding chloroquine to cisplatin could significantly increase its cytotoxicity via promoting apoptosis.
Materials and methods
Cell lines and reagents
We obtained the epithelial ovarian cancer cell lines SKOV3 and hey cells from ATCC. These cells were grown in RPMI 1640 (Hyclone, USA) with 10% fetal bovine serum (Hyclone, USA) at 37°C and 5% CO2. Cisplatin and chloroquine were obtained from Sigma Chemical Co. (USA) and dissolved in saline. 5 weeks old male Nude BALB/c mice were purchased from SLRC Laboratory Animal Company (China).
Cell viability assay by the CCK-8 method
Cell viability as previously described was measured by the CCK-8 assay kit (Dojindo Japan) [1]. Briefly, approximately 5 × 103 cells were seeded in 96-well plates. After adherence overnight, cells were starved in FBS-free medium for 12 hours before cisplatin or chloroquine treatment for indicated time. At the time of harvest, after adding 10 μl CCK-8 reagent to 100 ul medium, cells were cultured at 37°C for 1 hour. Afterwards, a spectrophotometer was used to detect the absorbance of each well at 450 nm.
Migration assay
Ovarian cancer cell lines were starved in FBS-free medium for 12 hours, then a total of 1 × 105 cells/well in 200 ul of serum-free medium were seeded into the upper Transwell chamber (Corning, USA) with indicated drugs. Meantime, 600 ul medium with 20% FBS was added to the lower chamber of a 24-well plate. 24 hours later, cells on the upper surface of the chamber were wiped with a cotton swab. Afterwards, cells on the lower surface were fixed with 4% paraformaldehyde for 15 minutes, and then stained with 0.1% crystal violet for 15 minutes. Finally, an inverted microscope was used to photograph five random areas of the lower membrane, and cells were counted for measurement.
Invasion assay
Similarly to the migration assay, cells were starved in FBS-free medium for 12 hours. The transwell chamber was pre-applied with 50 ul Matrigel (BD, USA), then a total of 1 × 105 cells/well in 200 ul of serum-free medium were seeded into the upper Transwell chamber (Corning, USA) with indicated drugs. Meantime, 600 ul medium with 20% FBS was added to the lower chamber of 24-well plate. 48 hours later, cells on the upper surface of the chamber were wiped with a cotton swab. Afterwards, cells on the lower surface were fixed with 4% paraformaldehyde for 15 minutes, and then stained with 0.1% crystal violet for 15 minutes. Finally, an inverted microscope was used to photograph five random areas of the lower membrane, and cells were counted for measurement.
Apoptosis assay
PE Annexin V Apoptosis Detection Kit I (BD, USA) was used to detect apoptotic cells. After cells were treated with indicated drugs for 24 hours, they were washed twice with ice-cold PBS and resuspended in 1X Binding Buffer at a concentration of 1 × 106 cells/ml. Afterwards, 5 μl PE Annexin V and 5 μl 7-AAD were added to the Binding Buffer and cells were incubated at RT for 15 mins in the dark. Afterwards, apoptotic cells were detected by flow cytometry. The PE Annexin V positive, 7-AAD negative or PE Annexin V positive, 7-AAD positive cell populations were considered as the apoptotic cells.
Western-blot analysis
After cells were treated with indicated drugs for 24 hours, total protein was extracted and quantified using BCA method (Beyotime, China). Then, 30 ug proteins were separated by 10% SDS-PAGE gels and transferred to PVDF membrane. Afterwards, the PVDF membrane was blocked for 2 h using 5% none-fat milk at room temperature, and primary antibodies were added to incubate the PVDF membrane overnight at 4°C, and secondary antibodies were added to incubate for 1 h at 37°C, followed by band development via the ECL detection system. First antibodies anti-Bcl-2, anti-Bcl-XL, anti-Bax, anti-cleaved PARP, anti-cleaved caspase 3, anti-p-AKT, anti-t-AKT, anti-p-mTOR, anti-t-mTOR, anti-ATG 5, anti-ATG 7, anti-LC3B, anti-Beclin 1 and anti-GAPDH were purchased from CST technology (USA). Secondary antibodies goat anti-mouse HRP antibody and goat anti-rabbit HRP antibody were obtained from Beyotime (China). The experiment was repeated three times.
Tandem mCherry-Wassabi confocal microscopy
Lentivirus with mCherry-Wassabi-LC3B were obtained from Gene-Chem Co. Ltd (Shanghai, China). SKOV3 and hey cells stably transfected with mCherry-Wassabi-LC3B were treated with indicated drugs for 24 hours. At the time of harvest, cells were fixed with 4% paraformaldehyde and then washed with PBS three times. Afterwards, cells were examined using a confocal laser scanning microscope (Leica TCS SP5 Confocal Microscope, Germany).
Mouse xenograft assay and immunohistochemistry staining
5 weeks old female Nude BALB/c mice were fed in standard environment and the protocol was approved by the Animal Care and Research Committee of Fudan University. 1 × 107 SKOV3 cells suspended in 0.2 ml of PMI 1640 were inoculated subcutaneously in the flank of each mouse. At the time that tumors reached 50-100 mm3, mice were divided into 4 groups at random (n = 6 per group). Mice were continuously injected intraperitoneally with PBS, cisplatin (5 mg/kg/6 days), chloroquine (60 mg/kg/day), and combination of cisplatin and chloroquine for 21 days. We measured tumor sizes using calipers every three days. Tumor volumes were calculated with the following formula: volume = 0.5 × width2 × length. After treatment for 21 days, the mice were sacrificed and tumors were removed carefully, photographed, and fixed with 4% paraformaldehyde for the following immunochemistry assay. Antibodies against ki-67 were purchased from Abcam (USA), and antibodies against cleaved caspase 3 were purchased from CST Technology (USA). Images were obtained using the microscope (Olympus BX53; Olympus, Japan). Five images at 400X magnification were selected at random to evaluate the average number of Ki-67 and cleaved caspase 3 positive cells.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. The experimental data were presented as mean ± standard deviation. T test was used for the comparison of means between two groups, and one-way ANOVA test was used for the comparison of more than two groups. *P < 0.05 and **P < 0.01 suggested that the difference was statistically significant.
Results
Chloroquine promotes the effect of cisplatin in inhibiting ovarian cancer cell growth, migration and invasion
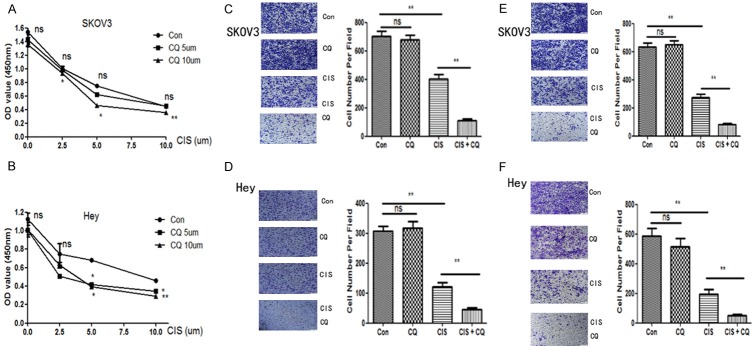
The effect of chloroquine and/or cisplatinon cell viability was examined using the CCK-8 assay (Figure 1A and 1B). 48 hours after drug treatment, cells in the control andchloroquine groups still maintained healthy, and nosignificant difference in the cell viability between the control and chloroquine groups were found, whereas cells in the cisplatin and cisplatin + chloroquine groups had decreased viability (p < 0.05). Furthermore, as shown in Figure 1, 10 uM chloroquine could promote the inhibiting effect of cisplatin (2.5 uM, 5 uM or 10 uM) on SKOV3 cells (p < 0.05). While in hey cells, both 5 uM or 10 uM chloroquine could make cells more sensitive to cisplatin (5 uM or 10 uM) treatment (p < 0.05). Therefore, we assume that low concentration of chloroquine had no effect on the viability of ovarian cancer cells, but the concomitant treatment (cisplatin + chloroquine) was more efficient at suppressing both SKOV3 and hey cellviability thancisplatin alone. Moreover, transwell assay was used to test cell migration and invasion in SKOV3 and hey cells. As shown in Figure 1C and 1D, chloroquine alone had no effect on cell migration, while cisplatin alone could inhibit cell migration (p < 0.05). Moreover, the combination of cisplatin and chloroquinesignificantly enhanced the inhibiting effect as compared to cisplatinalone (p < 0.01). Meanwhile, the same phenomenon was seen in the invasion assay. As shown in Figure 1E and 1F, the combination of cisplatin and chloroquine significantly enhanced the inhibiting effect on cell invasion as compared to cisplatin alone (p < 0.01) in both SKOV3 and hey cells.
Figure 1.
Effects of cisplatin and/or chloroquine on cell viability, migration and invasion ability in SKOV3 and hey cell lines. A and B. SKOV3 and hey cells were cultured with cisplatin, and/or chloroquine for 48 hours. Cell viability was assessed by CCK-8 assay. Results are mean of three independent experiments. *, P < 0.05 and **, P < 0.01 for comparisons between cisplatin + chloroquine group and cisplatin alone group. C and D. Migration was evaluated with transwell assay in SKOV3 and hey cells after treatment with cisplatin (5 uM for both SKOV3 and hey cells) and/or chloroquine (10 uM for SKOV3 cells and 5 uM for hey cells) for 24 hours. **, P < 0.01 for comparisons between groups. E and F. Invasion was evaluated with transwell assay in SKOV3 and hey cells after treatment with cisplatin (5 uM for both SKOV3 and hey cells) and/or chloroquine (10 uM for SKOV3 cells and 5 uM for hey cells) for 48 hours. **, P < 0.01 for comparisons between groups.
Cisplatin induces ovarian cancer cell apoptosis in concentration-dependent manner
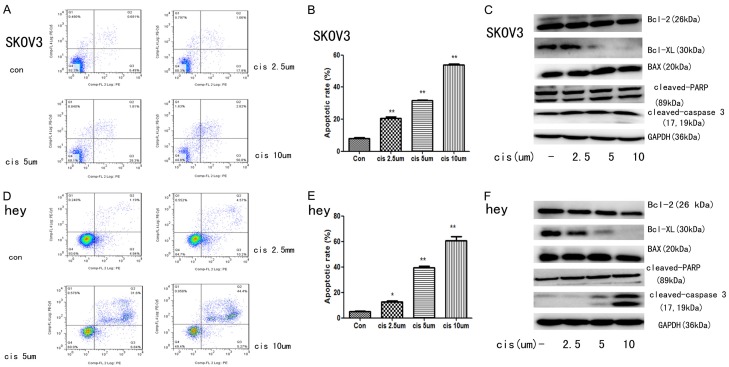
To determine the apoptosis rate after the treatment of cisplatin, flow cytometry was used. As it was shown in Figure 2A, 2B, 2D and 2E, after 24 hours treatment of cisplatin (2.5 um, 5 um and 10 um), both SKOV3 and hey cells had increased apoptotic rate in concentration-dependent manner as compared to the control group (p < 0.05). Moreover, western blot analysis was used to detect apoptosis-related proteins. As shown in Figure 2C and 2F, cisplatin treatment could increase protein expression of cleaved-PARP, cleaved-caspase 3 and BAX. Meanwhile, the expressions of anti-apoptosis protein Bcl-2 and Bcl-XL were both decreased after treatment of cisplatin in both SKOV3 and hey cells. Taken together, there data suggested that cisplatin could induce ovarian cancer cell apoptosis in concentration-dependent manner.
Figure 2.
Effects of cisplatin on apoptosis in SKOV3 and hey cell lines. Apoptosis was evaluated with PE Annexin V and 7-ADD staining in SKOV3 and hey cells after treatment with cisplatin (2.5 uM, 5 uM and 10 uM) for 24 hours. (A and D) Representative dot plots illustrating the data near the mean of the groups in (B and E) *, P < 0.05 and **, P < 0.01 for comparisons between cells treated with cisplatin and untreated cells. (C and F) Western blotting analysis of cleaved-caspase 3, cleaved PARP, Bax, Bcl-XL and Bcl-2. Western blotting of GAPDH was included as aloading control.
Cisplatin induces autophagy in ovarian cancer cells via the AKT/mTOR pathway
SKOV3 and hey cells were used to determine whether cisplatin could induce autophagy. Firstly, we examined LC3B-II formation and the level of ATG-5, ATG-7 and Beclin 1 in ovarian cancer cells treated with cisplatin. After treatment with cisplatin at the indicated concentration for 24 h (Figure 3A and 3B), LC3B II/ LC3B I, ATG-5, ATG-7 and Beclin 1 levels were increased in SKOV3 and hey cells in dose-dependent manner. These results indicated that cisplatin may induce autophagy in ovarian cancer cells. Afterwards, we also monitored the formation of autophagosomes in these ovarian cancer cell lines stably transfected with tandem mCherry-Wassabi-LC3B. In these cells, early autophagosomes displayed both green signal and red signal. Autophagolysosome displayed only red fluorescence since the Wassabi signal is sensitive to the acidic conditions in the lysosome lumen while the mCherry signal is more stable. After cells treatment with cisplatin, autophagosomes were detected by confocal microscopy. The number of yellow puncta (both green and red positive, G+R+) and red only puncta (G-R+) increased significantly in cells treated with 5 um cisplatin compared with untreated control cells (Figure 3A and 3C). These results proved that cisplatin could induce autophagy in ovarian cancer cells.
Figure 3.

Effects of cisplatin on autophagy and the AKT/mTOR pathway in SKOV3 and hey cell lines. A and C. SKOV3 and hey cells stably expressing mCherry-Wassabi-LC3B were treated without or with cisplatin (5 uM) for 24 hours, then subjected to confocal microscope analysis. Green-positive, red-positive puncta (G+R+) are autophagosomes; green-negative, red positive puncta (G-R+) are autophagolysosomes. B and D. SKOV3 and hey cells were treated without or with cisplatin (2.5 uM, 5 uM and 10 uM) for 24 hours. Western blotting analysis of p-AKT, t-AKT, p-mTOR, t-mTOR, ATG-5, ATG-7, Beclin 1 and LC3B. Western blotting of GAPDH was included as a loading control.
To investigate the regulatory mechanism by which cisplatin induces autophagy, we focused onthe AKT/mTOR signaling, which is a well-documented upstream regulator of autophagy [21]. As shown in Figure 3B and 3D, we detected the status of AKT and mTOR activation after cisplatintreatment at 24 h via western blot analysis.Results showed that cisplatin induced significant AKT phosphorylationat 24 h, whereas the phosphorylation of mTOR, was significantly decreased. Overall, these data demonstrated thatcisplatin could induce autophagy in ovarian cancer cells via the AKT/mTOR pathway.
Inhibition of autophagy by chloroquine attenuates the cytoprotective effect of cisplatin in ovarian cancer cells
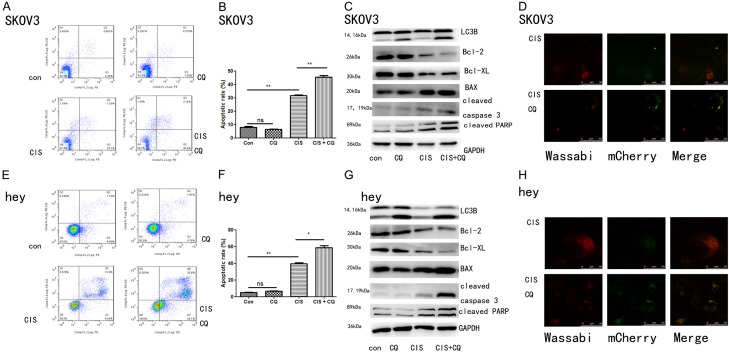
Chloroquine is a lysosomotropic agent which prevents endosomal acidification, thus inhibiting lysosomal enzymes and causing accumulation of LC3B-II [22]. Western blot showed that cisplatin combined with chloroquine induced more LC3B II/LC3B I expression than cisplatin alone, proving that chloroquine could inhibit lysosomal enzymes and cause accumulation of LC3B-II (Figure 4C and 4G). Afterwards, we also examined the effect of cisplatin combined with chloroquine on LC3B puncta formation in SKOV3 and hey cells using confocal microscopy. As shown in Figure 4D and 4H, chloroquine increased the number of yellow spots and reduced the number of red spots, indicating that chloroquine induced accumulation of autophagosomes while reducing the formation of autophagolysosomes. To further investigate whether autophagy was involved in the protective effect of cisplatin against apoptosis and whether targeting autophagy by chloroquine was the reason it could make ovarian cancer cells more sensitive to cisplatin, chloroquine was added for treatment and apoptotic rate was monitored. As shown in Figure 4A, 4B, 4E and 4F, flow cytometry analysis suggested that cisplatin could induced cell apoptosis (p < 0.01), and this effect was significantly promoted by chloroquine (p < 0.05) in both SKOV3 and hey cells. However, chloroquine alone had no significant effect in apoptosis of ovarian cancer cells. In addition, Western blotting further suggested that autophagy plays a role in cisplatin-mediated protection against apoptosis (Figure 4C and 4G). Moreover, western blot demonstrated that cisplatin combined with chloroquine had a more significant effect in increasing protein expression of cleaved-PARP, cleaved-caspase 3 and BAX than cisplatin alone. Meanwhile, the expressions of anti-apoptosis protein Bcl-2 and Bcl-XL was lower in the cisplatin combined with chloroquine group than the cisplatin alone group. Taken together, these results suggested that chloroquine could inhibited the protective autophagy effect of cisplatin and promoting apoptosis, thus increasing the cytotoxicity of cisplatin on ovarian cancer cells.
Figure 4.
The combination of chloroquine and cisplatin on cell apoptosis and autophagy in SKOV3 and hey cells. Apoptosis was evaluated with PE Annexin V and 7-ADD staining in SKOV3 and hey cells after treatment with cisplatin (5 uM for both SKOV3 and hey cells) and/or chloroquine (10 uM for SKOV3 cells and 5 uM for hey cells) for 24 hours. (A and E) Representative dot plots illustrating the data near the mean of the groups in (B and F). *, P < 0.05 and **, P < 0.01 for comparisons between groups. (C and G) SKOV3 and hey cells were treated with cisplatin (5 uM for both SKOV3 and hey cells) and/or chloroquine (10 uM for SKOV3 cells and 5 uM for hey cells) for 24 hours. Western blotting analysis of LC3B, Bcl-2, Bcl-XL, Bax, cleaved caspase 3 and cleaved PARP. Western blotting of GAPDH was included as a loading control. (D and H) SKOV3 and hey cells stably expressing mCherry-Wassabi-LC3B were treated with cisplatin (5 uM for both SKOV3 and hey cells) and/or chloroquine (10 uM for SKOV3 cells and 5 uM for hey cells) for 24 hours, then subjected to confocal microscope analysis. Green-positive, red-positive puncta (G+R+) are autophagosomes; green-negative, red positive puncta (G-R+) are autophagolysosomes.
Chloroquine enhances the therapeutic efficacy of cisplatin in a mouse xenograft model in vivo
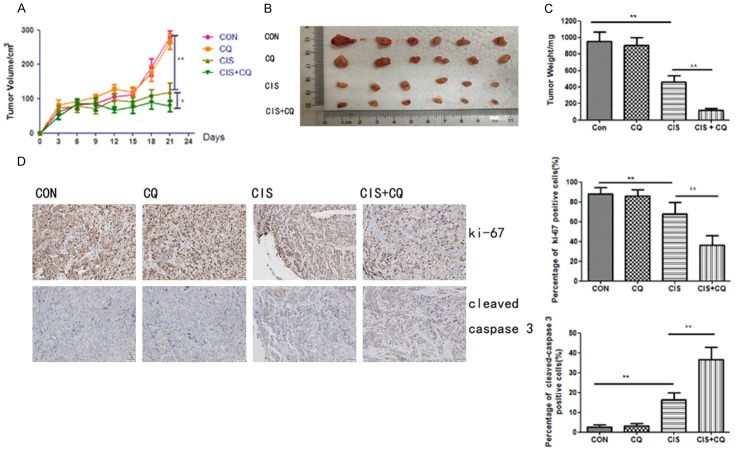
Nude mice with SKOV3 cells tumor xenografts were continuously injected intraperitoneally with PBS, cisplatin (5 mg/kg/6 days), chloroquine (60 mg/kg/day), and combination of cisplatin with chloroquine until study termination. After the administration of the treatment for 21 days, combined treatment with cisplatin and chloroquine significantly reduced the tumor volume and tumorweight, compared to vehicle treated group and cisplatin treated group (Figure 5A and 5C). Meanwhile, the results of immunochemistry assay suggested that the combined treatment increased the level of cleaved caspase 3 while reduced Ki-67 expression (Figure 5D). These data suggested that autophagy inhibition by chloroquine alsoenhanced the cytotoxicity of cisplatin in SKOV3 cells in vivo. Collectively, these results indicated that autophagy protected ovarian cancer cells from cisplatin-induced cell death.
Figure 5.
The combination of chloroquine and cisplatinon tumor growth in a SKOV3 mouse xenograft model. A. Average tumor volume in vehicle control mice, mice treated with chloroquineor cisplatin or a combination of chloroquine and cisplatin. **, P < 0.01 for comparisons between control group and cisplatin treatment, *, P < 0.05 for comparisons between cisplatin alone group and cisplatin + chloroquine group. B. The tumor pictures of xenografts for each group after mice sacrifice. C. The tumor weight of xenografts for each group. **, P < 0.01 for comparisons between groups. D. Tumor tissues were sectioned and subjected to immunohistochemistry for evaluating ki-67 and cleaved caspase 3. **, P < 0.01 for comparisons of ki-67 or cleaved caspase 3 positive cells between groups.
Discussion
Autophagy was firstly proposed by Christian de Duve and he was rewarded the Nobel Prize because of this achievement in 1963 [23]. Autophagy has been classified as type II programmed cell death as it is a self-digestion procedure in cells, and it is in parallel with apoptosis, the type I programmed cell death [24]. In the recent years, autophagy has drawn a lot of attention in cancer therapy, but its role in cancer therapy is always controversial. Various reports have proved that many anticancer drugs can induce autophagy, it remains unclear if this autophagy is a protective effect for cancer cells against stress caused by anticancer agents, if it is another way of cell death aroused when apoptosis does not work, if it cannot affect cell viability at all, or these three effects happen in different conditions.
Cisplatin is one of the most common used chemotherapeutic drugs for the treatment of ovarian cancer, but chemotherapy resistance remains a major problem that restrains its efficacy [25]. Till now, various studies have reported that chloroquine, an autophagy inhibitor, has the ability to reverse drug resistance and enhance the sensitivity of chemotherapy and radiotherapy in some kinds of human tumors [26-28]. In addition, chloroquine has been used in some clinical trials and shows its therapeutic effect. Based on the report of Sotelo et al, at the same time of regular chemotherapy and radiotherapy, oral administration of 150 mg chloroquine per day for 12 months can prolong the median survival of patients with glioblastoma compared to placebo group in a Phase II clinical trial [29]. Boone et al reported that in a phase 1/2 trial, hydroxychloroquine (HCQ) was given combined with gemcitabine for patients with pancreatic adenocarcinoma, results showed that preoperative treatment of HCQ plus gemcitabine is safe and can increase CA 199 response and surgical oncologic outcomes [30]. Till now, little has been reported about whether chloroquine can increase the sensitivity of ovarian cancer cells to cisplatin treatment and its related mechanisms, and this is the key points of our study.
In our study, we first examined the effect of chloroquine and/or cisplatinon cell viability, results showed that low concentration of chloroquine did not affect cell viability, but the concomitant treatment (chloroquine + cisplatin) was more efficient at suppressing both SKOV3 and hey cell viability than cisplatin alone. Moreover, transwell assay showed that the combination of chloroquine and cisplatin significantly enhanced the inhibiting effects of migration and invasion as compared to cisplatin alone. Afterwards, to explore the related mechanisms of this phenomena, two popular ways of programmed cell death, apoptosis and autophagy, were both detected after treatment of cisplatin on ovarian cancer cells. As shown in Figures 2, 3, cisplatin could induce both cell autophagy and apoptosis in SKOV3 and hey cells in concentration-dependent manner, suggesting that cisplatin-induced apoptosis would cause ovarian cancer cell autophagy. mTOR, a kind of serine/threonine protein kinase, has been reported to play an important part in a lot of cell activities, including cell survival, proliferation, metabolism and so on [31,32]. The PI3K/AKT/mTOR pathway has been widely recognized as a classical pathway of autophagy. In this current study, western blot showed that AKT phosphorylation at Ser473 was increased, while mTOR phosphorylation at Ser2448 was decreased after cisplatin treatment in SKOV3 and hey cells. These data suggested that cisplatin could induce autophagyin ovarian cancer cells via the AKT/mTOR pathway.
Although it is still unclear what is the precise mechanism how chloroquine has anticancer effect, one possible mechanism is that it can inhibit autophagy [33]. In the chloroquine alone group, increased number of yellow spots and reduced number of red spots were seen, indicating that chloroquine induced accumulation of autophagosomes while reducing the formation of autophagolysosomes. Flow cytometryrevealed that chloroquine alone did not affect cell apoptosis and expressions of apoptosis related proteins, suggesting that chloroquine could inhibit autophagy of ova + rian cancer cells, but had no effect in inhibiting cell viability by itself. Nevertheless, chloroquine plus cisplatin induced more apoptotic rate than cisplatin alone (p < 0.05). Meanwhile, apoptosis related proteins had the same change trend.
According to the aforementioned results, cisplatin could induce protective autophagy to antagonize cisplatin-induced apoptosis in ovarian cancer cells, which meant protective autophagy led to declined sensitivity to chemotherapy. In consequence, autophagy inhibition by chloroquine could enhance the efficacy of cisplatin via promoting apoptosis. To further verify our conclusion, we also used a xenograft tumor nude mice model to evaluate the efficacy of cisplatin and/or chloroquine. As shown in Figure 5, though chloroquine alone had no influence on the growth of xenograft tumor, cisplatin plus chloroquine was more potent in inhibiting the growth of xenograft tumor than cisplatin alone. In addition, the combined treatment increased the level of cleaved caspase 3 while reduced Ki-67 expression than cisplatin alone. The results demonstrated that chloroquine could increase the sensitivity to chemotherapy in vivo.
Chloroquine has been used in the treatment for malaria for many years. Till now, the precise mechanisms of its antimalarial effect is unclear. Some reports have suggested that it may due to its weak-base lysosome-tropic feature [34]. In recent years, accumulating evidences have suggested that chloroquine increases sensitivity to radiotherapy and chemotherapy. In this current study, we provided more evidence to the hypothesis that inhibiting autophagy by chloroquine can sensitize cancer cells to anticancer drugs in ovarian cancer. Other than inhibiting autophagy, chloroquine has been reported to reinforce the efficacy of chemotherapy by other mechanisms [35]. According to the report of Maes et al, via normalizing tumor vessels, chloroquine could suppress cancer invasion and metastasis, as well as enhance sensitivity to chemotherapy. Moreover, Lee et al reported that chloroquine could increase the endosome PH and drug penetration, thus enhancing the efficacy of chemotherapeutic drugs [36]. Therefore, more studies should be made to explore detailed mechanisms of chloroquine in enhancing the efficacy of cisplatin for the treatment of ovarian cancer.
In conclusion, based on our study, we propose that cisplatin activates the AKT/mTOR signaling pathway, which subsequently induces cytoprotective autophagy in ovarian cancer cells (Figure 6). Meanwhile, inhibition of autophagy by chloroquine enhances the anti-tumor effect of cisplatin. Thus, we predict that combination treatment with cisplatin and pharmacological autophagy inhibitors will be an effective therapeutic strategy for ovarian cancer.
Figure 6.

Schematic diagram of hypothetical mechanisms underlying chloroquine enhances sensitivity to cisplatin in ovarian cancer cells. Cisplatin activated the AKT/mTOR pathway, thus leading to the induction of autophagosome formation. Inhibition of autophagy with autophagy inhibitors chloroquine enhances cisplatin-induced cell death in ovarian cancer cells.
Acknowledgements
This work was supported by the Shanghai Science and Technology Department Funds (No. 14ZR1404100) to Hong Sun and Shanghai Science and Technology Department Funds (No. 16411963600) to Hong Sun. Moreover, this work was funded by the Shanghai (China) science committee (No. 15ZR1404800) to Jing Zhu.
Disclosure of conflict of interest
None.
References
- 1.Zhu J, Zheng Y, Zhang H, Sun H. Targeting cancer cell metabolism: the combination of metformin and 2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am J Transl Res. 2016;8:4812–4821. [PMC free article] [PubMed] [Google Scholar]
- 2.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Jeon SY, Hwang KA, Choi KC. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol. 2016;158:1–8. doi: 10.1016/j.jsbmb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Eckel R, Schubert-Fritschle G, Kerr J, Kuhn W, Diebold J, Kimmig R, Rehbock J, Holzel D. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002;38:2435–2445. doi: 10.1016/s0959-8049(02)00495-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Zhang P, Shi B, Zhou M, Jiang H, Zhang H, Pan X, Gao H, Sun H, Li Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014;5:e991. doi: 10.1038/cddis.2013.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren F, Shen J, Shi H, Hornicek FJ, Kan Q, Duan Z. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta. 2016;1866:266–275. doi: 10.1016/j.bbcan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Fokkema E, Groen HJ, Helder MN, de Vries EG, Meijer C. JM216-, JM118-, and cisplatininduced cytotoxicity in relation to platinum-DNA adduct formation, glutathione levels and p53 status in human tumour cell lines with different sensitivities to cisplatin. Biochem Pharmacol. 2002;63:1989–1996. doi: 10.1016/s0006-2952(02)00983-8. [DOI] [PubMed] [Google Scholar]
- 8.Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol. 2016;77:1103–1124. doi: 10.1007/s00280-016-2976-z. [DOI] [PubMed] [Google Scholar]
- 9.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Lesiak A, Narbutt J, Sysa-Jedrzejowska A, Lukamowicz J, McCauliffe DP, Wozniacka A. Effect of chloroquine phosphate treatment on serum MMP-9 and TIMP-1 levels in patients with systemic lupus erythematosus. Lupus. 2010;19:683–688. doi: 10.1177/0961203309356455. [DOI] [PubMed] [Google Scholar]
- 11.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, Lam KS, Kung HJ. Autophagy blockade sensitizes prostate cancer cells towards Src Family kinase inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zinn RL, Gardner EE, Dobromilskaya I, Murphy S, Marchionni L, Hann CL, Rudin CM. Combination treatment with ABT-737 and chloroquine in preclinical models of small cell lung cancer. Mol Cancer. 2013;12:16. doi: 10.1186/1476-4598-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristol ML, Emery SM, Maycotte P, Thorburn A, Chakradeo S, Gewirtz DA. Autophagy inhibition for chemosensitization and radiosensitization in cancer: do the preclinical data support this therapeutic strategy? J Pharmacol Exp Ther. 2013;344:544–552. doi: 10.1124/jpet.112.199802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang WR, Li TT, Jing T, Li YX, Yang XF, He YH, Zhang W, Lin R, Zhang JY. SIRT1 regulates the inflammatory response of vascular adventitial fibroblasts through autophagy and related signaling pathway. Cell Physiol Biochem. 2017;41:569–582. doi: 10.1159/000457878. [DOI] [PubMed] [Google Scholar]
- 22.Zhao F, Huang W, Zhang Z, Mao L, Han Y, Yan J, Lei M. Triptolide induces protective autophagy through activation of the CaMKKbeta-AMPK signaling pathway in prostate cancer cells. Oncotarget. 2016;7:5366–5382. doi: 10.18632/oncotarget.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740–743. doi: 10.4161/auto.6398. [DOI] [PubMed] [Google Scholar]
- 24.Levine B, Klionsky DJ. Development by selfdigestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F, Yang X, Zhang C. PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int J Oncol. 2017;50:835–846. doi: 10.3892/ijo.2017.3873. [DOI] [PubMed] [Google Scholar]
- 26.Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, Qiu SJ, Fan J. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 27.Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Corominas-Faja B, Cuyas E, Lopez-Bonet E, Martin-Castillo B, Joven J, Menendez JA. The anti-malarial chloroquine overcomes primary resistance and restores sensitivity to trastuzumab in HER2-positive breast cancer. Sci Rep. 2013;3:2469. doi: 10.1038/srep02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao XG, Sun RJ, Yang XY, Liu DY, Lei DP, Jin T, Pan XL. Chloroquine-enhanced efficacy of cisplatin in the treatment of hypopharyngeal carcinoma in xenograft mice. PLoS One. 2015;10:e0126147. doi: 10.1371/journal.pone.0126147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotelo J, Briceno E, Lopez-Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 30.Boone BA, Bahary N, Zureikat AH, Moser AJ, Normolle DP, Wu WC, Singhi AD, Bao P, Bartlett DL, Liotta LA, Espina V, Loughran P, Lotze MT, Zeh HJ 3rd. Safety and biologic response of Pre-operative autophagy inhibition in combination with gemcitabine in patients with pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22:4402–4410. doi: 10.1245/s10434-015-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, Westerterp M, Wang C, Zhu Y, Ai D. Macrophage mTORC1 disruption reduces inflammation and insulin resistance in obese mice. Diabetologia. 2014;57:2393–2404. doi: 10.1007/s00125-014-3350-5. [DOI] [PubMed] [Google Scholar]
- 33.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahinas D, Folefoc A, Taldone T, Chiosis G, Crandall I, Pillai DR. A purine analog synergizes with chloroquine (CQ) by targeting Plasmodium falciparum Hsp90 (PfHsp90) PLoS One. 2013;8:e75446. doi: 10.1371/journal.pone.0075446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8:200–212. doi: 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CM, Tannock IF. Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration. Br J Cancer. 2006;94:863–869. doi: 10.1038/sj.bjc.6603010. [DOI] [PMC free article] [PubMed] [Google Scholar]