Abstract
Metformin is commonly used for treating type II diabetes and has recently been reported to possess anti-proliferative properties that can be exploited for the prevention and treatment of a variety of cancers. Ginsenosides are the main effective biological components of ginseng. It has been reported that ginsenoside-Rb2 inhibit the invasiveness of endometrial cancer cells (ECC). The aim of this study was to investigate whether protopanaxadiol (PPD, a metabolite of ginsenosides) and metformin could synergistically regulate the biological behavior of ECC and analyze its possible mechanism. We here found that either metformin or PPD treatment led to a decreased viability and increased apoptosis and autophagy levels in ECC lines (Ishikawa and RL95-2 cells), and combination of PPD and metformin could enhance these effects induced by metformin or PPD in vitro. PPD and metformin significantly decreased the expression of estrogen receptor alpha (ERα) in Ishikawa and RL95-2 cells. Estrogen promoted the viability and restricted the apoptosis and autophagy of Ishikawa and RL95-2 cells, and PPD and metformin reversed these effects. In vivo trials showed that combination of PPD and metformin had the strongest activity of anti-tumor growth compared with PPD alone and metformin alone. These data suggest that PPD and metformin can be used together to play a more powerful anti-EC effect. Our study provides a scientific basis for the clinical application of PPD and metformin in the treatment of EC, especially in estrogen-dependent patients.
Keywords: Metformin, protopanaxadiol, estrogen, endometrial cancer cells, growth, autophagy
Introduction
As one of one of the most common gynecological malignancies, the incidence of endometrial cancer (EC) is increasing worldwide. This increase is attributed to the rising prevalence of nulliparity and obesity [1]. Although the prognosis of low-risk EC is generally favorable, chemotherapeutic options for high-risk EC patients are limited. Therefore, exploring novel therapeutic strategies is necessary to improve EC prognosis.
EC can be divided into two types: type I, estrogen-dependent EC; and type II, estrogen-independent EC [2,3]. The type I EC usually has favorable prognosis while type II EC is more aggressive and presented poor prognosis [4]. The type I EC is hormone-sensitive and approximately occupied 80% cases, and those patients almost have risk of obesity, which is usually well-differentiated [5]. As the primary risk factor for development of EC, Estrogen can contribute to the growth of EC [6,7]. In addition, obesity and diabetes are related-risk factors for type I EC [8]. With the increase of obese population, the levels of endogenous hormones are changed, the occurrence of EC are gradually increased [3,9].
As one of the most commonly used hypoglycemic agents in the management of type II diabetes, metformin, has anti-tumor effects on breast, prostate, ovarian, and EC cells by alterations of glucose metabolism and inhibition of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) and insulin/insulin-like growth factor (IGF)-I signaling pathways [10-12]. Metformin can prevent and inhibit ECs, especially patients with diabetes. It can also enhance the anti-tumors effects of chemotherapy drugs, such as carboplatin [13,14]. A phase II/III study is currently underway to evaluate metformin vs. placebo in advanced or recurrent EC patients receiving paclitaxel and carboplatin (NCT02065687).
As a medicinal herb, ginseng is widely used in Asian countries and the North American. Ginsenosides are main components extracted from ginseng. Ginsenosides have been reported to exert anti-tumor activities for various types of cancer, such as lung cancer and prostate cancer [15-17]. It has reported that ginsenoside-Rb2 inhibits invasiveness to the basement membrane possibly via down-regulation of MMP-2 in EC [18]. Protopanaxadiol (PPD) is the metabolite of ginsenoside, also exhibit activity against a variety of cancer cells [17,19,20]. Ginsenosides structurally contain a steroidal backbone. They can mediate their cellular activities by binding to the active sites of steroid receptors [21], for example, PPD suppresses estrogen-stimulated gene expression and cell proliferation in ER-positive breast cancer cells [22]. Besides that, PPD synergistically enhances cytotoxicity of tamoxifen in an ER-independent manner, probably by down-regulating Akt activity [22]. However, it’s still unclear whether PPD has anti-EC effects in a synergistic fashion with metformin.
Therefore, this study was performed to investigate the effects of PPD and metformin on the viability, apoptosis and autophagy levels, and estrogen receptor α (ERα) expression in ECC in vitro and in vivo.
Materials and methods
Reagents
The anti-human Beclin-1, P62, LC3B and Actin antibodies (Abs) were purchased from R&D Systems (USA); the Annexin V/7-AAD Apoptosis Detection Kit was purchased from BD Biosciences (San Jose, CA, USA); the anti-human ERα Abs were purchased from Abcam (USA); the Cell Counting Kit-8 (CCK-8) were purchased from Dojindo (Japan), the peridininchlorophylla protein cyanine 5.5 (PerCP-Cy™5.5)-conjugated anti-human Ki-67 and allophycocyanin (APC)-conjugated anti-human Bcl-2 Abs were purchased from BD Biosciences; PE-conjugated Bcl-xL Abs were purchased from Cell Signaling Technology; APC-conjugated Fas, PE-conjugated Fas Ligand (FasL) and fluorescein isothiocyanate (FITC)-conjugated anti-human Cytokeratin (CK7) Abs were purchased from Biolegend (San Diego, CA, USA); and 17β-estrogen (E2), PPD and metformin were purchased from Sigma-Aldrich Co. LLC., (USA).
Cell culture
The human endometrial carcinoma cell lines Ishikawa and RL95-2 were obtained from the cell bank of Chinese Academy of Science (Shanghai, China) and grown in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/mL penicillin and 100 mg/mL streptomycin. The cells were incubated under normoxic conditions (21% O2, 5% CO2, 74% N2) at 37°C in a humidified incubator (Heal Force, HF 100, Shanghai, China).
CCK-8 assay
The Ishikawa cells and RL95-2 cells were seeded in 96-well plates (4×103 cells/well), and then treated with different concentration of PPD (0, 10, 20, 40, 80 or 160 uM), Metformin (0, 1, 5, 10, 20, 40 or 80 mM) or metformin (20 mM) plus PPD (0, 2.5, 5, 10, 20 or 40 uM) for 24 h. Subsequently, these cells were collected and detected the viability by the cell-counting Kit-8. According to the manufacturer’s protocol, the CCK-8 regent was added to each well 10 ul and 90 ul culture solution then total cells was incubated at 37°C for 1 h. The absorbance (optical density) at 450 nm was measured and used to represent the viability of cells. Each experiment was performed in eight parallel wells, and all experiment repeated three times.
Annexin V/7-AAD apoptosis assay
The Ishikawa and RL95-2 cells were seeded in 24-well flat-bottom microplates at the density of 1×105 cells/well, and then treated with PPD (40 uM), metformin (20 mM), or PPD plus metformin for 24 h, then these cells were digested by 0.25% trypsin without EDTA, then centrifuged at 1200 rpm for 5 min, re-suspended with 200 ul binding buffer, and labeled by Annexin V and 7-AAD according to the protocol. Flow cytometry assay was performed to detect the percentage of early apoptotic cells and total apoptotic cells. The experiment were carried out triplicate, and repeated three times.
Protein extraction and western blotting
Cell were washed in phosphate buffered saline (PBS), detached with the cell scraper and centrifuged for 20 min at 12000 rpm at 4°C. The pellet was re-suspended in high efficiency cell tissue rapid lysis buffer (RIPA; Beyotime, Shanghai, China) containing 1% phenylmethanesulfonyl fluoride (PMSF; Beyotime) proteinase and 1% phosphatase inhibitors (Roche, USA). Cell lysates were boiled for 10 min at 95°C and then stored at -80°C. Protein concentrations were quantified using the BCA protein assay kit (Beyotime). Total proteins (20 ug) were electrophoresed in SDS-PAGE gels (EpiZyme scientific) on a Miniprotein III system (Bio-Rad, USA), and transferred into PVDF membrane (Millipore) at 2 h, overnight incubated with primary antibody against Beclin-1, p62, LC3b, Actin and ERα at 4°C, then the PVDF membrane washed three times by TBST solution, and incubated at room temperature for 1 h in peroxidase-conjugated goat anti-rabbit IgG secondary antibodies (1:5000; Bioworld Technology, co. Ltd. USA). After then the membrane were washed three times and processed for chemiluminescence with Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore).
Exposure with E2
Ishikawa cells and RL95-2 cells were treated with E2 (10-7 M), E2 plus PPD (40 uM), E2 plus metformin (20 mM), E2 plus PPD and metformin for 24 h, we collected these cells, and analyzed the viability, apoptosis and autophagy levels by CCK8, apoptosis and western blotting assays, respectively.
In vivo experiments
Nude mice of 4-5 weeks age were inoculated subcutaneously under the scruff on day 0 with 200 ul of 1×107 Ishikawa cells. PPD and or metformin were injected 100 mg/kg by intraperitoneal once a day after xentransplantation. Tumor growth was monitored by measuring the tumor volume every three day. Tumor volume was determined using the formula: volume (mm3) =1/2(L×W×W). After 19 days, mice were euthanized, and the tumor tissues were collected.
Flow cytometry
The tumor tissues of nude mice were perfused thoroughly with cold PBS before cell collection, then tissues were minced on ice and digested with an enzyme mix of Liberase and Dispase (Invitrogen). Then we collected these cells and evaluated the expression of Ki-67, Fas, FasL, Bcl-xL and Bcl-2 by flow cytometry. The samples were analyzed using a FACS Calibur flow cytometer (Becton Dickinson, USA) and Cellquest software (Becton Dickinson).
Statistics
All values were shown as the means ± SEM. The data were analyzed with GraphPad Prism version 5 by t-test or one-way ANOVA. Differences were considered statistically significant at P<0.05.
Results
PPD enhances inhibitory effect of metformin on the viability of ECC
To detect whether PPD and metformin regulate the viability of ECC, the CCK8 assay was performed to analyze the effect of PPD and metformin on the viability of ECC lines Ishikawa and RL95-2 cells. As shown in Figure 1, PPD significantly decreased the viability of Ishikawa and RL95-2 cells in a dose-dependent manner, especially at concentration higher than 40 uM (P<0.001) (Figure 1A). Treatment with metformin also led to an obviously decreaseof the viability of Ishikawa and RL95-2 cells (P<0.01 or P<0.001) (Figure 1B). When the concentration was higher than 10 mM, the inhibition efficiency of metformin was the most significant (P<0.0001) (Figure 1B). In addition, PPD further amplified the inhibitory effect of metformin on the viability of Ishikawa and RL95-2 cells (P<0.05, P<0.01 or P<0.001) (Figure 1C).
Figure 1.
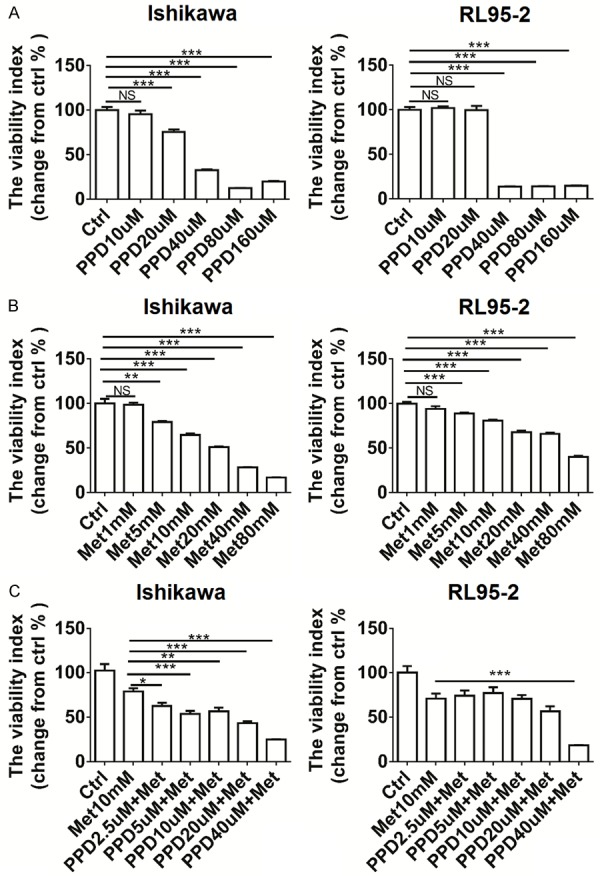
PPD enhances inhibitory effect of metformin on the viability of ECC. (A) Ishikawa and RL95-2 cells were treated with PPD at different concentration of 0, 10, 20, 40, 80 or 160 uM for 24 h, (B) Ishikawa and RL95-2 cells were treated with metformin at different concentration of 0, 1, 5, 10, 20, 40 or 80 mM for 24 h, (C) Ishikawa and RL95-2 cells were treated with metformin (20 mM) plus PPD (0, 2.5, 5, 10, 20 or 40 uM) for 24 h, then a CCK-8 assay was performed to detect the viability of Ishikawa and RL95-2 cells. Met: metformin. The data were expressed as means ± SEM. *P<0.05, **P<0.01 or ***P<0.001 (one-way ANOVA). NS: no significant difference.
PPD and metformin cooperate in induction of ECC’s apoptosis and autophagy
Next, the results of apoptosis assay showed that both PPD and metformin promoted the early apoptosis (Annexin V+7-ADD-), late apoptosis or necrosis (Annexin V+7-ADD+) of Ishikawa and RL95-2 cells (P<0.01 or P<0.001) (Figure 2A-C). Compared with PPD alone and metformin alone, treatment with PPD plus metformin resulted in higher levels of early apoptotic, late apoptotic or necrotic Ishikawa and RL95-2 cells (P<0.01 or P<0.001) (Figure 2A-C).
Figure 2.
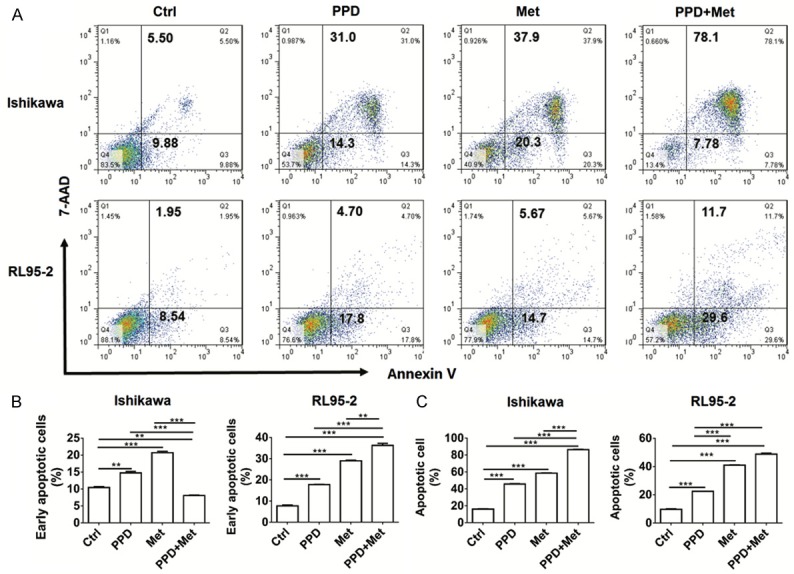
PPD and metformin cooperate in induction of ECC’s apoptosis. A-C: Ishikawa and RL95-2 cells were treated with PPD (40 uM) and or metformin (20 mM) for 24 h, and then the Annexin-V/7-AAD assay was used to analyze the apoptosis of Ishikawa and RL95-2 cells. Early apoptotic cells: Annexin V+/7-AAD- cells; late apoptotic or necrotic cells: Annexin V+/7-AAD+ cells. The data were expressed as means ± SEM. **P<0.01 or ***P<0.001 (one-way ANOVA).
Beclin-1 is essential in the induction process of autophagy [23], and considers as a “platform protein” that provides a framework for other autophagy-related (Atg) proteins and class III phosphoinositide 3-kinase (PI3K, Vps34), which initiate macroautophagic activity together. The p62, also called sequestosome 1 (SQSTM1), is an autophagy adaptor protein [24]. The p62 protein is itself degraded by autophagy and may serve to link ubiquitinated proteins to the autophagic machinery to enable their degradation in the lysosome. Therefore, p62 accumulates when autophagy is inhibited. The half-life of LCB3-II is very short and Atg4 may cleave LC3-II at the phosphatidylethanolamine (PE) and release back the LC3-I form. The enhanced LC3B-II/LC3B-I ratio indicates more intense formation of autophagy [25]. To explore the possible role of PPD and metformin in the autophagy of EEC, we analyzed the expression of autophagy-associated proteins (Beclin-1, p62 and LC3B) [26] in Ishikawa and RL95-2 cells after treatment with PPD and or metformin. As shown, either PPD or metformin led to higher levels of Beclin-1 and LC3B II/LC3B I, and low level of p62 expression in Ishikawa and RL95-2 cells compared with control group (Figure 3A, 3B). Inaddition, PPD and metformin had synergistic effects on the expression of these autophagy-associated proteins (Figure 3A, 3B). These data above indicate that there is a synergistic effect between PPD and metformin in promoting apoptosis and autophagy in ECC.
Figure 3.
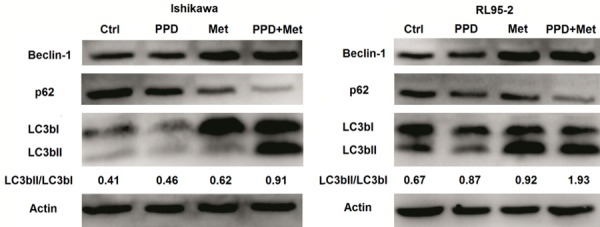
PPD and metformin corporately trigger ECC’s autophagy. A, B: Ishikawa and RL95-2 cells were treated with PPD (40 uM) and or metformin (20 mM) for 24 h, and then the expression of autophagy-associated proteins Beclin-1, p62 and LC3b was analyzed by western blotting. In addition, the ratio of LC3b II to LC3B was counted by Quantity One analysis soft. Data were representative immune blots of assays.
PPD and metformin down-regulates the expression of ERα in ECC
To investigate whether PPD and metformin regulate ERα expression in ECC, we detected the expression of ERα in Ishikawa and RL95-2 cells after treatment with or without PPD and metformin. The results of western blotting showed that exposure with PPD or metformin down-regulated the expression levels of ERα in Ishikawa and RL95-2 cells, especially exposure with PPD plus metformin (Figure 4A, 4B). These results suggest that PPD and metformin may synergistically regulate the effect of estrogen on EEC by down-regulating the expression of ERα.
Figure 4.
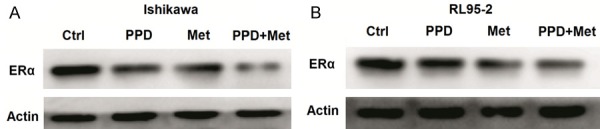
PPD and metformin down-regulates the expression of ERα in ECC. A, B: Ishikawa and RL95-2 cells were treated with PPD (40 uM) and or metformin (20 mM) for 24 h, and then the expression of ERα was analyzed by western blotting. Data were representative immune blots of assays.
PPD and metformin restrict the effects of estrogen on ECC’s growth and autophagy
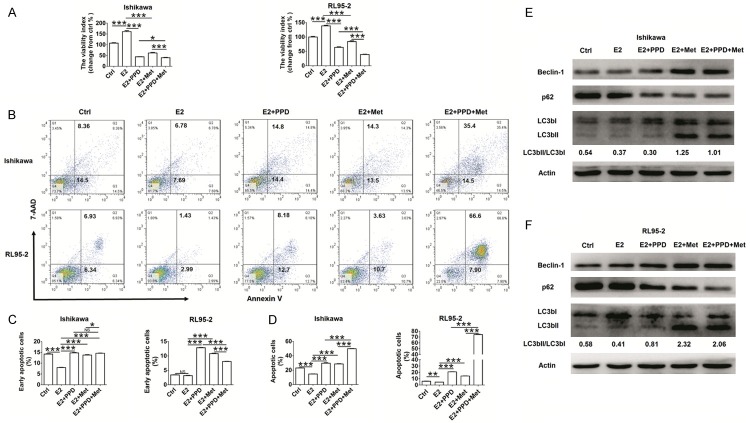
Subsequently, we found that estrogen significantly promoted the viability of Ishikawa and RL95-2 cells (P<0.001) (Figure 5A), and these effects could be markedly reversed by PPD and or metformin (P<0.001) (Figure 5A). In contrast, exposure with estrogen obviously decreased the early apoptotic, late apoptotic or necrotic levels of Ishikawa and RL95-2 cells (P<0.01 or P<0.001) (Figure 5B-D), PPD and or metformin also suppressed these effects (P<0.001) (Figure 5B-D). Further analysis showed that PPD and or metformin could inhibit the role of estrogen on the expression of Beclin-1 and p62 and the ratio of LC3B II to LC3B I in Ishikawa and RL95-2 cells (Figure 5E, 5F), especially combination of PPD and metformin. These data reveal that PPD and metformin remarkably antagonize the regulatory effect of estrogen on ECC’s growth and autophagy, and this effect may be dependent on inhibition of ERα.
Figure 5.
PPD and metformin restrict the effects of estrogen on ECC’s growth and autophagy. Ishikawa cells and RL95-2 cells were treated with E2 (10-7 M), E2 plus PPD (40 uM), E2 plus metformin (20 mM), E2 plus PPD and metformin for 24 h, then we collected these cells, and analyzed the viability, apoptosis and autophagy levels by CCK8 (A), apoptosis (B-D) and western blotting (E, F) assays, respectively. E2: 17β-estrogen. The data were expressed as means ± SEM. *P<0.05, **P<0.01 or ***P<0.001 (one-way ANOVA).
PPD and metformin synergistically inhibit EC growth in vivo
To probe into whether PPD and metformin play the anti-EC activity in vivo, Ishikawa cells were inoculated subcutaneously under the scruff of nude mice, and treated with PPD and or metformin. As shown, the tumor volumes of PPD and metformin groups were smaller than that of control group since 13d later (P<0.001) (Figure 6A). In addition, the tumor volume in combination group of PPD and metformin was minimal compared with PPD alone or metformin alone (P<0.05) (Figure 6A).
Figure 6.
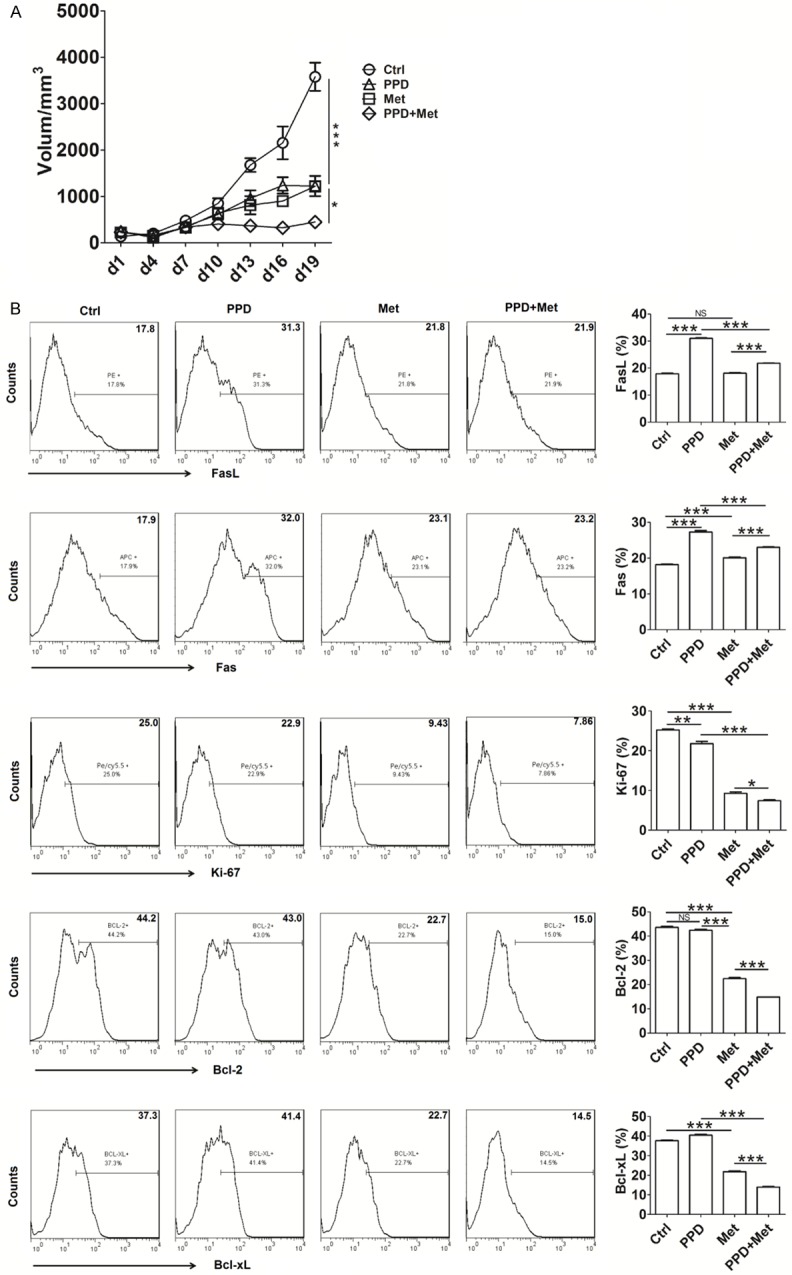
PPD and metformin synergistically inhibit EC growth in vivo. Ishikawa cells were inoculated subcutaneously under the scruff of nude mice (8 mice/group), and then PPD and or metformin were injected 100 mg/kg by intraperitoneal once a day after xentransplantation. Tumor growth was monitored by measuring the tumor volume every three day (A). After 19 days, mice were euthanized, and the tumor tissues were collected, and digested for analyzing the expression of Fas, FasL, Ki-67, Bcl-2 and Bcl-xL by flow cytometry (B). The data were expressed as means ± SEM. *P<0.05, **P<0.01 or ***P<0.001 (one-way ANOVA).
Both PPD and metformin promoted the expression of pro-apoptotic molecules Fas and FasL, and inhibited the expression of pro-proliferation molecule Ki-67, and anti-apoptotic molecules Bcl-2 and Bcl-xL in CK7+ ECC from tumor tissues (P<0.01 or P<0.001) (Figure 6B). Moreover, the effects of combined application of PPD and metformin on these proliferation and apoptosis-related molecules were most significant (P<0.05 or P<0.001) (Figure 6B).
Discussion
Studies of EC have shown that it’s the fourth common gynecological tumors in the developed countries [27]. In this study, we found that both PPD and metformin inhibited viability, and promoted apoptosis and autophagy of Ishikawa and RL95-2 cells. Moreover, PPD and metformin synergistically inhibited the stimulatory effect of estrogen on viability and the inhibitory effect of estrogen on apoptosis and autophagy in Ishikawa and RL95-2 cells, and this process may be associated with the down-regulation of ERα.
As a hormone-dependent disease, type I EC is sensitive to endogenous and exogenous estrogens. Estrogen binding to their receptors may stimulate MAPK/ERK1/2 pathways, which leads to ER phosphorylation and consecutive nuclear translocation to regulate gene transcription (e.g. cell proliferation, migration, differentiation) [28].
The potential role of metformin in treating EC has been uncovered in many studies [29,30]. Metformin exerts its anti-tumorigenic effects possibly through indirect mechanisms by increasing insulin sensitivity, inhibiting liver gluconeogenesis, and reducing hyperglycemia and insulin levels [31], and direct mechanisms by activating AMP-activated protein kinase (AMPK), and further inhibiting the mammalian target of rapamycin (mTOR) pathway [32] and extracellular signal-regulated kinase (ERK) signaling [33]. In addition, metformin inhibits estrogen-dependent type I EC proliferation by activate AMPK-FOXO1 signal pathway [34]. Here, we found that metformin directly down-regulated the expression of ERα in Ishikawa and RL95-2 cells, and promoted the expression pro-apoptosis molecules Fas and FasL and decreased the expression of pro-proliferation molecule Ki-67 and anti-apoptosis molecules Bcl-2 and Bcl-xL. These data suggest the regulatory effects of metformin on these proliferation and apoptosis molecules may be associated with the inhibition of ERα and its downstream singling. The possible mechanism for this process needs to further research.
A lot of researches have reported that ginsenosides and its metabolites have a wide variety of antitumor activities [21]. PPD has anti-oxidative stress (e.g. up-regulation of ROS reproduction) [35,36] anti-fatigue [37] properties. It also mediates mitochondrial apoptosis of tumor cell by cytochrome c/caspase-9 [38] or PI3K/AKT singling pathway [39,40], stimulates acute lymphoblastic leukemia cells differentiate [41], and inhibits matrix remodeling and cell metastasis (e.g. down-regulation of MMP9 and MMP2 expression) [42,43]. Furthermore, PPD are reported to change Ca2+ and NO levels in endothelia cells through glucocorticoid and ER for prevention and treatment of vascular diseases associated with endothelial cell dysfunction [44]. Here, we found PPD significantly inhibited the viability, promoted the apoptosis and autophagy of ECC. Moreover, it can cooperate with metformin to limit activities of estrogen on ECC’s viability, apoptosis and autophagy. However, the exact mechanism for these processes remains to be further studied.
Some studies have revealed that metformin triggers autophagy of several tumor cells through CEBPD [45] or AMPKα [46]. However, little is reported about the regulation of PPD in cell autophagy. A recent study has showed that PPD induces autophagy and apoptosis in human melanoma via AMPK/JNK phosphorylation [47]. Therefore, in combination with our findings, we hypothesize that the synergistic inhibition of PPD and metformin on autophagy of EEC may be associated with regulation of AMPK signaling [48].
Autophagy takes place at the basal level but is also regulated developmentally and/or by environment stimuli, such as nutrient/energy availability, hypoxia and reactive oxygen species [48]. Our previous work also has confirmed that estrogen suppresses the autophagy of endometrial stromal cells from endometriosis by up-regulating CXCL12/CXCR4 signaling pathway [49]. In current study, both PPD and metformin could markedly down-regulated the expression of ERα in Ishikawa and RL95-2 cells, and restricted the anti-autophagy activation of estrogen, suggesting the stimulation activation of PPD and metformin may result from the inhibition of ERα and its downstream singling. However, we still cannot rule out the potential effects of PPD and metformin on autophagy by anti-oxidative stress. Further studies are needed to confirm these possible hypotheses.
Here, in vivo experiment further proved an evidence for the synergistic anti-EC activity of PPD and metformin. Therefore, it can be concluded that PPD and metformin have a synergistic potential of anti-EC, especially for the estrogen-sensitive and diabetes patients. In addition, it has been reported that metformin overcomes progesterone resistance in EC by targeting autophagy [50]. Thus, PPD and metformin may also have the potential to assist progesterone in the treatment of EC by creating an endocrine environment with antagonist of estrogen. However, the clinical value for the combination of PPD, metformin and progesterone in anti-EC still needs further study and evaluation.
Acknowledgements
This study was supported by the Major Research Program of National Natural Science Foundation of China (NSFC) 91542108, the NSFC 81471513, the NSFC 31671200, the Shanghai Rising-Star Program (16QA1400800), the Development Fund of Shanghai Talents (201557), the Oriented Project of Science and Technology Innovation from Key Lab. of Reproduction Regulation of NPFPC (CX2017-2), and the Program for Zhuoxue of Fudan University (all to M-Q Li), the Shanghai Natural Science Foundation 17ZR1403200 to F Xie and the Research Program for Maternal and Child of Jiangsu Province (No. F201660) to Jin-Jin Yu.
Disclosure of conflict of interest
None.
References
- 1.Anderson AS, Key TJ, Norat T, Scoccianti C, Cecchini M, Berrino F, Boutron-Ruault MC, Espina C, Leitzmann M, Powers H. European Code against Cancer 4th Edition: Obesity, body fatness and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S34–S45. doi: 10.1016/j.canep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 3.Mcalpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother’s cancer. Cancer. 2016;122:2787–2798. doi: 10.1002/cncr.30094. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA. Uterine papillary serous carcinoma: what have we learned over the past quarter century? Gynecol Oncol. 2005;98:341–343. doi: 10.1016/j.ygyno.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 6.He YY, Du GQ, Cai B, Yan Q, Zhou L, Chen XY, Lu W, Yang YX, Wan XP. Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2. J Cancer Res Clin Oncol. 2012;138:775–783. doi: 10.1007/s00432-011-1133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike MC, Peters RK, Cozen W, Probst-Hensch NM, Felix JC, Wan PC, Mack TM. Estrogenprogestin replacement therapy and endometrial cancer. J Natl Cancer Inst. 1997;89:1110–1116. doi: 10.1093/jnci/89.15.1110. [DOI] [PubMed] [Google Scholar]
- 8.Chia VM, Newcomb PA, Trenthamdietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17:441–446. doi: 10.1111/j.1525-1438.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 9.Arora V, Quinn MA. Endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2012;26:311–324. doi: 10.1016/j.bpobgyn.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 11.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinasedependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 12.Sarfstein R, Friedman Y, Attias-Geva Z, Fishman A, Bruchim I, Werner H. Metformin downregulates the insulin/IGF-I signaling pathway and inhibits different uterine serous carcinoma (USC) cells proliferation and migration in p53-dependent or -independent manners. PLoS One. 2013;8:e61537. doi: 10.1371/journal.pone.0061537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erices R, Bravo ML, Gonzalez P, Oliva B, Racordon D, Garrido M, Ibañez C, Kato S, Brañes J, Pizarro J, Barriga MI, Barra A, Bravo E, Alonso C, Bustamente E, Cuello MA, Owen GI. Metformin, at concentrations corresponding to the treatment of diabetes, potentiates the cytotoxic effects of carboplatin in cultures of ovarian cancer cells. Reprod Sci. 2013;20:1433–46. doi: 10.1177/1933719113488441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu P, Su W, Miao ZH, Niu HR, Liu J, Hua QL. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with nonsmall cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–35. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- 17.Xu FY, Shang WQ, Yu JJ, Sun Q, Li MQ, Sun JS. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016;8:1708–18. [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto J, Sakaguchi H, Aoki I, Toyoki H, Khatun S, Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. Eur J Gynaecol Oncol. 2001;22:339–41. [PubMed] [Google Scholar]
- 19.Zhu GY, Li YW, Tse AK, Hau DK, Leung CH, Yu ZL, Fong WF. 20(S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur J Pharmacol. 2011;668:88–98. doi: 10.1016/j.ejphar.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Rayburn ER, Hao M, Zhao Y, Hill DL, Zhang R, Wang H. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008;68:809–19. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- 21.Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–72. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y, Zhou Q, Hang Y, Bu X, Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007;109:2374–2382. doi: 10.1002/cncr.22659. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YC, Athar M, Chaudry IH. When apoptosis meets autophagy: deciding cell fate after trauma and sepsis. Trends Mol Med. 2009;15:129–138. doi: 10.1016/j.molmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 25.Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 26.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talhouk A, Mcalpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract. 2016;3:14. doi: 10.1186/s40661-016-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuffa LG, Lupi-Junior LA, Costa AB, Amorim JP, Seiva FR. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids. 2017;118:93–108. doi: 10.1016/j.steroids.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, Yang Y, Liu N, Zhao X, Santin AD, Taylor H, Huang Y. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34:3076–84. doi: 10.1038/onc.2014.236. [DOI] [PubMed] [Google Scholar]
- 30.Tan BK, Adya R, Chen J, Lehnert H, Sant Cassia LJ, Randeva HS. Metformin treatment exerts antiinvasive and antimetastatic effects in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2011;96:808–16. doi: 10.1210/jc.2010-1803. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viollet B, Guigas B, Sanz GN, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci. 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queiroz EA, Puukila S, Eichler R, Sampaio SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB, Khaper N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One. 2014;9:e98207. doi: 10.1371/journal.pone.0098207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou J, Hong L, Luo C, Li Z, Zhu Y, Huang T, Zhang Y, Yuan H, Hu Y, Wen T, Zhuang W, Cai B, Zhang X, Huang J, Cheng J. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK-FOXO1 signal pathway. Cancer Sci. 2016;107:1806–1817. doi: 10.1111/cas.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CZ, Li B, Wen XD, Zhang Z, Yu C, Calway TD, He TC, Wei D, Yuan CS. Paraptosis and NF-κB activation are associated with protopanaxadiol-induced cancer chemoprevention. BMC Complement Altern Med. 2013;13:2. doi: 10.1186/1472-6882-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh HA, Kim DE, Choi HJ, Kim NJ, Kim DH. Anti-fatigue effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in mice. Biol Pharm Bull. 2015;38:1415–1419. doi: 10.1248/bpb.b15-00230. [DOI] [PubMed] [Google Scholar]
- 37.Oh SJ, Kim K, Lim CJ. Photoprotective properties of 20(S)-protopanaxatriol, an aglycone of ginseng saponins: protection from ultraviolet-B radiation-induced oxidative stress in human epidermal keratinocytes. Mol Med Rep. 2016;14:2839–45. doi: 10.3892/mmr.2016.5581. [DOI] [PubMed] [Google Scholar]
- 38.Jin YH, Yim H, Park JH, Lee SK. Cdk2 activity is associated with depolarization of mitochondrial membrane potential during apoptosis. Biochem Biophys Res Commun. 2003;305:974–80. doi: 10.1016/s0006-291x(03)00870-2. [DOI] [PubMed] [Google Scholar]
- 39.Gao JL, Lv GY, He BC, Zhang BQ, Zhang H, Wang N, Wang CZ, Du W, Yuan CS, He TC. Ginseng saponin metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting multiple cancer signaling pathways. Oncol Rep. 2013;30:292–298. doi: 10.3892/or.2013.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YL, Zhang R, Xu HL, Yu XF, Qu SC, Sui DY. 20(S)-protopanaxadiol triggers mitochondrial-mediated apoptosis in human lung adenocarcinoma A549 cells via inhibiting the PI3K/Akt signaling pathway. Am J Chin Med. 2013;41:1137–52. doi: 10.1142/S0192415X13500778. [DOI] [PubMed] [Google Scholar]
- 41.Sun L, Wang Q, Liu X, Brons NHC, Wang N, Steinmetz A, Lv Y, Liao Y, Zheng H. Anticancer effects of 20(S)-protopanoxadiol on human acute lymphoblastic leukemia cell lines Reh and RS4;11. Med Oncol. 2011;28:813–21. doi: 10.1007/s12032-010-9508-1. [DOI] [PubMed] [Google Scholar]
- 42.Park MT, Cha HJ, Jeong JW, Kim SI, Chung HY, Kim ND, Kim OH, Kim KW. Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT1080 human fibrosarcoma cells. Mol Cells. 1999;9:476–483. [PubMed] [Google Scholar]
- 43.Li G, Wang Z, Sun Y, Liu K, Wang Z. Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 cells. Basic Clin Pharmacol Toxicol. 2006;98:588–92. doi: 10.1111/j.1742-7843.2006.pto_415.x. [DOI] [PubMed] [Google Scholar]
- 44.Leung KW, Leung FP, Mak NK, Tombran-Tink J, Huang Y, Wong RN. Protopanaxadiol and protopanaxatriol bind to glucocorticoid and oestrogen receptors in endothelial cells. Br J Pharmacol. 2009;156:626–637. doi: 10.1111/j.1476-5381.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai HH, Lai HY, Chen YC, Li CF, Huang HS, Liu HS, Tsai YS, Wang JM. Metformin promotes apoptosis in hepatocellular carcinoma through the CEBPD-induced autophagy pathway. Oncotarget. 2017;8:13832–13845. doi: 10.18632/oncotarget.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z, He W, Yang J, Dai C. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci Rep. 2016;6:23975. doi: 10.1038/srep23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang S, Kim JE, Song NR, Jung SK, Lee MH, Park JS, Yeom MH, Bode AM, Dong Z, Lee KW. The ginsenoside 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol induces autophagy and apoptosis in human melanoma via AMPK/JNK phosphorylation. PLoS One. 2014;9:e104305. doi: 10.1371/journal.pone.0104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng J, Baba M, Nair U, Klionsky DJ. Quantitative analysis of autophagy-related protein stoichiometry by fluorescence microscopy. J Cell Biol. 2008;182:129–140. doi: 10.1083/jcb.200711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30:1677–1689. doi: 10.1093/humrep/dev100. [DOI] [PubMed] [Google Scholar]
- 50.Zhuo Z, Wang A, Yu H. Metformin targeting autophagy overcomes progesterone resistance in endometrial carcinoma. Arch Gynecol Obstet. 2016;294:1055–1061. doi: 10.1007/s00404-016-4148-0. [DOI] [PubMed] [Google Scholar]