Abstract
Oxidative stress and cell apoptosis play important roles in the pathogenesis of asthma. Specific immunotherapy (SIT) is the only curative approach for asthma and is effective at decreasing asthmatic oxidation and cell apoptosis, but the mechanisms remain unclear. In this study, by using in vivo and in vitro models, we indirectly demonstrated that SIT alleviated the apoptosis and oxidative stress of bronchial epithelial cells in an asthma model through regulating interleukin (IL)-25. Female BALB/c mice were used for an asthma model induced by exposure to house dust mite (HDM) extracts as allergens. Prior to the challenge, the mice were either given the SIT vaccine or N-Acetyl-L-cysteine (NAC). Results: Compared with that in asthma models, SIT administration decreased (1) airway hyper-responsiveness; (2) the production of cytokines, including IL-4, IL-5, IL-13, and IL-25, as well as serum HDM-specific IgE and IgG1, as shown by ELISA; and (3) lipid oxidative species, such as reactive oxidative species (ROS) and malondialdehyde (MDA), in the lung tissue. Moreover, TUNEL staining showed that SIT alleviated pulmonary cell apoptosis. In vitro, flow cytometry showed that human recombinant IL-25 (rIL-25) led to increased cell apoptosis and ROS in the human epithelial cell line 16HBE in a dose and time-dependent fashion. In conclusion, in vivo, SIT reduced asthmatic Th2 cytokine levels and the production of IL-25 and alleviated oxidative stress and cell apoptosis in the lung tissue. In vitro, IL-25 increased the number of apoptotic cells and the production of ROS in16HBE cells.
Keywords: Asthma, specific immunotherapy (SIT), IL-25, reactive oxygen species (ROS), epithelial cells
Introduction
Allergic asthma is a chronic airway disease characterized by airway hyper-responsiveness (AHR), high mucus secretion, airway remodeling, and airway narrowness, which is mediated by dysfunction of immune system regulation [1]. Currently, the control of asthma symptoms commonly relies on the administration of hormones and β2 receptor agonists.
Specific immunotherapy (SIT), first developed at St Mary’s Hospital London in the 19th century, was thought to be the only method to cure asthma [2]. For SIT, specific allergen extracts are given to patients with different modified treatment plans to revise the immunity imbalance and, in the end, abolish the symptoms.
In the clinic, asthma is commonly believed to be associated with T cell differentiation from Th0 to Th2 [3,4] and is characterized by the predominate presence of CD4+Th2 cells [5]. The pathogenesis of asthma is normally believed to be mediated by the secretion of Th2 cytokines such as interleukin (IL)-4, IL-5, and IL-13, which can be induced by cytokines, such as granulocyte/macrophage colony-stimulating factor (GM-CSF), thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 [6,7], secreted by airway epithelial cells. Airway epithelial cells, as the outside barrier of the lung, are commonly where immune responses are initiated [8]. Epithelial cells are critical in the process of allergen contact and sensitization and asthma stimulation [9]. However, whether there are differences in the expression of these epithelial cell-secreted cytokines in response to SIT treatment in asthma is not yet known.
IL-25 is a member of the IL-17 family, termed IL-17E. High expression of IL-25 promotes a Th2-type immune response, an increase in IgE and the number of eosinophils in the serum, and the pathogenesis of lung tissue damage [10,11]. However, whether SIT regulates IL-25 secretion in an asthmatic mouse model in unclear.
The generation of reactive oxygen species (ROS) has been shown to be critical in asthma [12]. Oxidative stress may be associated with allergic reactions and inflammatory cascade amplification. Indicators of oxidative stress, potentially in response to allergen stimulation or the release of ROS and reactive nitrogen species (RNS) upon pollutant stimulation, have been found in asthma patients [13]. N-acetyl-L-cysteine (NAC) is a thiol compound that has the potential to interact either directly as a free radical scavenger or indirectly as a precursor of reduced glutathione [14]. The anti-oxidant protective effect of NAC can attenuate inflammation in experimental asthma [15] and in various inflammatory pulmonary diseases [16-18].
Airway epithelial cells are the target of many pulmonary diseases. The apoptosis of these cells associated with the activation of ROS is an initial step in the pathogenesis of multiple pulmonary diseases and is always accompanied by pathogenic processes such as airway remodeling and pro-inflammatory cytokine secretion. Thus, a deeper understanding of airway epithelial apoptosis may provide a new therapeutic asthma treatment.
In this study, we report that SIT alleviates the asthmatic oxidative stress reaction and down-regulates the secretion of IL-25 in lung tissue. We then indirectly prove that this down-regulation of IL-25 affects oxidative stress and apoptosis of the airway epithelial cells.
Materials and methods
Animals, cells, and reagents
Six to eight-week-old female BALB/c mice were purchased from the Animal Center of Guangdong Province. All experimental protocols and procedures were approved by the Animal Care and Use Committee of Shenzhen University. The human bronchial epithelial cell line was purchased from the Cell Culture Institute of Xiangya Medical College, Zhongnan University. Cell culture medium was supplemented with RPMI 1640, 10% fetal calf serum, 200 mg/mL streptomycin and 200 U/mL penicillin, and these reagents were purchased from Invitrogen (Carlsbad, CA, USA). NAC was purchased from Sigma-Aldrich Inc (Saint Louis, MO, USA). The NAC solution for intragastric administration was prepared with sterile distill water with an adjusted PH value of 7.4.
HDM extract collection
Four grams of Dermatophagoidesfarinae (Der. f) was ground in a mortar with a pestle for 30 minutes in the presence of liquid nitrogen. Then, 10 mL PBS was added prior to ultrasonic disruption for 1 h on ice. After the solution was re-suspended by centrifugation at 10000 rpm at 4°C, the supernatant was collected and stored at -80°C.
Study design
The mouse model was designed based on previous studies [19,20], and the scheme of the study is shown in Figure 1A. For the asthma group, BALB/c mice were sensitized by intraperitoneal injection with a thorough mixture of Der. f extracts (50 μg per mouse) and the same amount of aluminum hydroxide adjuvant (Thermo, USA) on day 0, 7 and 14. From day 32 to 38, the mice were intranasally challenged with 50 μg Der. f extracts daily for a week. The PBS control group was treated with PBS in all procedures. The SIT group consisted of another asthma group that received SIT vaccine treatment by subcutaneous injection of 50 μg Der. f extracts per mouse on day 21 and 25. The NAC group consisted of another asthma group that received intragastric administration of NAC solution (3 mmol/kg) everyday from day 25 to day 31.
Figure 1.
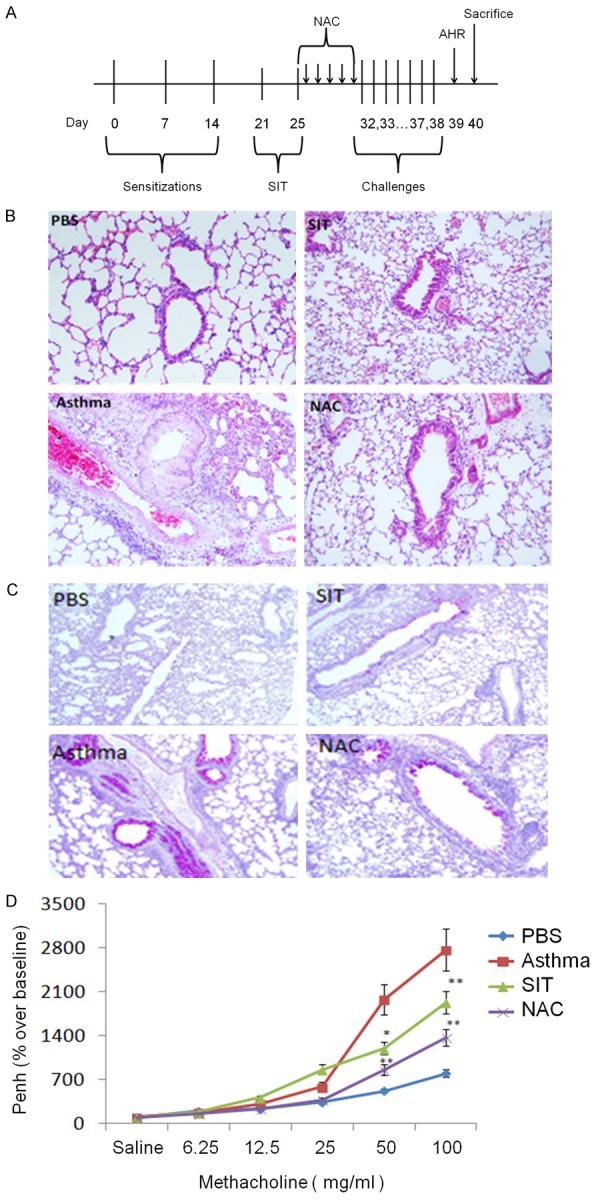
SIT and NAC alleviate asthmatic symptoms in a mouse model. The design of the model is shown in (A). Histology of a lung stained with H&E (B) and PAS (C) at 10× magnification. The AHR of the four groups is shown in (D). The results are representative of 3 independent experiments. The statistics of the results were performed by student t-test. *means P<0.05, **means P<0.01.
AHR and airway inflammation
Twenty-four hours after the final challenge, Penh values were evaluated by whole-body plethysmography (Buxco Europe Ltd, Winchester, UK). The baseline response was recorded for 5 minutes in the event of stabilized respiration after 10 minutes of adaptation. Mice were exposed to increasing doses of methacholine (Mch) (i.e., 6.25, 12.5, 25, 50, 100 mg/mL, Sigma, St Louis, USA) or PBS. Tests at two different concentrations were temporally separated to allow the respiratory intensity to drop back to baseline. The percentage curves for Penh values at different Mchdoses were plotted, starting with PBS stimulation. Mice were sacrificed on day 40, and the lungs were removed and processed for hematoxylin-eosin (H&E) staining. The levels of IgE and IgG1 in the serum and the levels of IL-4, IL-5, IL13 and IL-25 in the bronchoalveolar lavage fluid (BALF) of each group were measured by ELISA. BAL fluid was centrifuged at 1500 rpm for 5 min at 4°C, and the supernatants were stored at -80°C for cytokine analyses. Lung tissues were fixed in 10% formalin for 24 h and then embedded in paraffin wax after dehydration in alcohol. Tissue slides were prepared for H&E, periodic acid-Schiff (PAS), and TUNEL staining.
TUNEL staining
Lung tissue sections were dewaxed, hydrated, incubated with 0.1% Triton X-100 for 5 min, and washed twice with PBS. After incubation with proteinase K (BiolegendInc.) at 37°C for 10 min, the sections were washed with PBS, incubated with the TUNEL reaction mixture at 37°C in darkness for 1 h, washed with PBS, incubated with converter-peroxidase (POD) at 37°C for 30 min, and incubated with diaminobenzidine (DAB) was for 10 min. The sections were then washed with PBS 3 times and stained with hematoxylin before observation under a microscope.
Determination of cytokines and allergen-specific IgE and IgG1
The levels of IgE and IgG1 in the serum were measured by ELISA. In brief, a 96-well plate was coated with 100 ng house dust mite (HDM) extract in a total volume of 100 μl carbonate-bicarbonate buffer overnight at 4°C. Then, the plate was blocked with 5% BSA for 2 h at 37°C, followed by 10% (v/v) serum solution diluted with blocking buffer. BiotinylatedIgE was then added to the plate for 2 h. After the plate was washed 5 times and incubated at 37°C for 1 h, 100 μL of streptavidin-labeled horseradish peroxidase (HRP; 1:5000 dilution) was added. The plate was then developed for 10 minutes, and the reactions were stopped by adding 2 mM H2SO4. The plate was then read with a microplate reader at an absorbance of 450 nm.
ROS of BALF and lung tissue
BALF was centrifuged at 1200 rpm for 5 min and resuspended with 100 μL PBS. The fluorescentprobe, 2’-7’-dichlorofluorescein (DCF-DA), was then added to a final concentration of 25 μM. After incubation at 37°C for 15 min, the sample was observed under a microscope. The lung tissue was also collected and stored at -80°C. Frozen lung sections were sliced 4 μm thick, and DCF-DA was added to a final concentration of 25 μM per slice. After incubation at 37°C for 15 min and a 3-min wash, the sample was observed under a microscope.
Immunofluorescent staining
The frozen lung tissue sections and cells in culture dishes were fixed with 4% para-formaldehyde for 30 min, blocked with 0.1% BSA for 30 min, and incubated with FITC-conjugated goat anti-rabbit or TRITC-conjugated goat anti-rabbit antibodies to bind the primary antibodies. Confocal microscopy was used for observation.
Flow cytometry of Tregs
Mouse spleen tissues were homogenized and filtered through a 200-mesh screen. The splenocytes were suspended in 1640 culture medium and erythrocyte lysate and then centrifuged at 1500 rpm at room temperature for 5 minutes. Mononuclear cells (MCs) were isolated with lymphocyte separation medium. We then measured the frequency of CD4+CD25+Foxp3+Treg cells as in previous studies [21,22]. Here, 106 cells of every sample were stained with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD4, allophycocyanin (APC)-conjugated anti-CD25, and phycoerythrin (PE)-conjugated anti-Foxp3 (all from eBioscience).
Cell culture
16HBE cells were cultured in minimum essential medium (Gibco, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 100 μunits/mL penicillin, and 100 μg/mL streptomycin and incubated at 37°C in a humidified chamber with 5% CO2. Cells were digested and passaged two to three times per week with 0.02% (w/v) ethylenediaminetetraacetic acid (EDTA) and 0.25% (w/v) trypsin.
Cell apoptosis experiment (Annexin V-FITC/PI apoptosis detection)
Here, 106 16HBE cells were seeded in a sterile 24-well plate and co-cultured with human recombinant IL-25 (rIL-25; SizhengboInc, China) in a final concentration of 0.1 mg/mL or 1 mg/mL for 12 h or 24 h. An Annexin V-FITC/PI Apoptosis Detection kit was purchased from BD Inc. After the cells were digested with 0.2% trypsin without EDTA for 1 min, they were washed in cold PBS, and 5×105 cells were re-suspended in 100 μL binding buffer. Afterwards, 5 μL AnnexinV-FITC and 5 μL PI staining solution were added, followed by incubation in darkness at room temperature for 10 min. Then, the cells were processed for flow cytometry analysis.
Statistical analysis
All data are expressed as the mean ± SD. All of the experiments were repeated 3 times. Statistical significance between different groups was determined using the SPSS 13.0 software with student t-test. A P<0.05 was statistically significant.
Results
To compare the effect of SIT and NAC treatment on a murine asthma model, a lung histological study was performed by H&E and PAS staining. In Figure 1B, the H&E staining shows that there was an obvious infiltration of inflammatory cells around the airway and hyperplasia of the airway smooth muscle in the asthma group. In contrast, in the SIT and NAC groups, the infiltration of the inflammatory cells was significantly reduced, and no obvious hyperplasia of the airway smooth muscle was seen. The PAS-stained lung tissue of the asthma group (Figure 1C) exhibited a large amount of light purple staining within the airway, indicating that the airway secreted a large amount of mucus. However, dust mite SIT and anti-oxidative NAC significantly reduced airway mucosal secretion.
To investigate AHR, the Penh value of the mouse model was tested after stimulation with a serial concentration of methacholine. Figure 1D shows that the Penh value of the asthma group was significantly higher than control (P>0.01). The Penh values of the SIT group and NAC group were significantly lower than that of the asthma group (P>0.01), indicating that dust mite SIT and anti-oxidative therapy alleviated the asthmatic AHR.
To test if SIT or NAC treatment reduced cytokine secretion in the murine asthma model, serum was collected from mice from each group, and the cytokine concentration was tested by ELISA. As shown in Figure 2A, SIT significantly reduced the levels of IL-4, IL-5, and IL-13 compared with those in the asthma model. Interestingly, the level of IL-25, which is generally considered to be secreted by lung tissue, was also reduced after SIT treatment.
Figure 2.
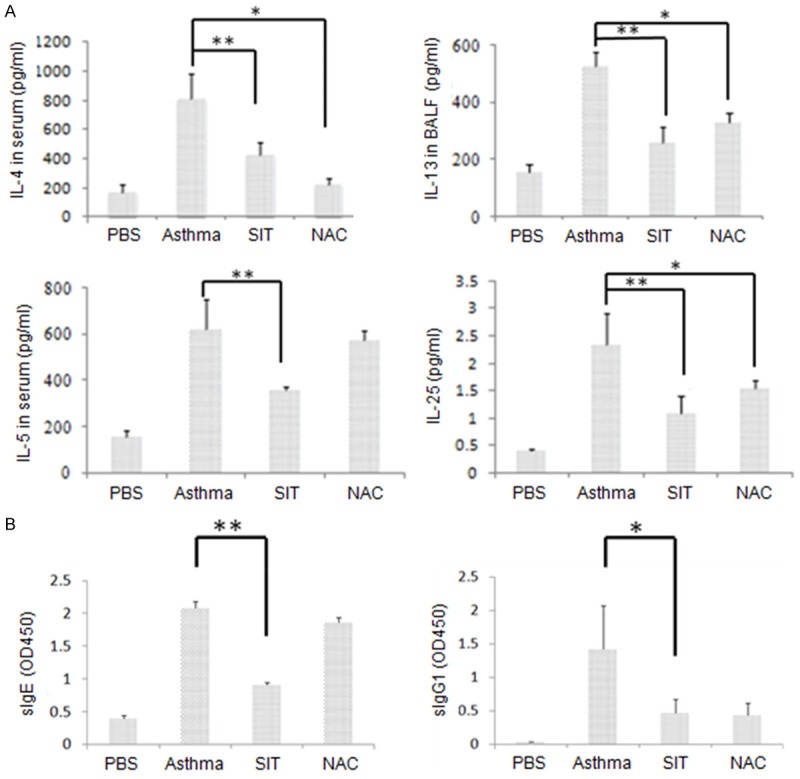
In the mouse model, compared with those in the asthma group, SIT reduces the levels of Th2 cytokines and IL-25 as well as sIgE and sIgG1 in the serum. A: The levels of IL-4 and IL-5 in serum, IL-13 in the BALF, and IL-25 in the homogenized lung tissue were measured by ELISA. B: The levels of HDM-specific IgE and IgG1 were measured by ELISA, and the value is shown as the optical density read at 450 nm (OD450). The results are representative of 3 independent experiments. The statistics of the results were performed by student t-test. *means P<0.05, **means P<0.01.
Murine serum was tested for the level of dust mite-specific IgE and IgG1. Figure 2B shows significantly higher levels of both antibodies in the asthmatic model group than in the PBS-treated group. Furthermore, significantly lower levels of serum IgE (sIgE) and sIgG1 were seen in the SIT group than in the asthma group (*P<0.05, **P<0.01).
To test if SIT can reduce the oxidative stress of lung tissue, the level of lipid oxidation in the murine lung tissue homogenate was tested by examining malondialdehyde (MDA). As shown in Figure 3A, the level of MDA from asthmatic lung tissue was significantly higher than in control tissue. Additionally, the SIT and NAC group had significantly lower levels of MDA than the asthma group (P<0.05).
Figure 3.

In the mouse model, dust mite SIT alleviates the oxidative stress of lung tissue. (A) The level of MDA in the homogenized murine lung tissue was measured. (B and C) The ROS level in the BALF was measured by flow cytometry. One of the 3 results are shown in the histogram (B) and the ratio of cells with ROS in the BALF was analyzed (C). (D) Frozen lung section stained by the fluorescent ROS antibody observed at 10× magnification. The results are representative of 3 independent experiments. The statistics of the results were performed by student t-test. *means P<0.05, **means P<0.01.
The ROS level in the BALF was tested by flow cytometry after staining cells for ROS. As shown in Figure 3B and 3C, the number of ROS-stained cells was significantly higher in the asthma group than in the PBS-treated group, whereas the generation of ROS in the SIT group and anti-oxidative NAC group was significantly reduced (P<0.05).
Then, frozen sections of lung tissue from the asthmatic mouse model were prepared and fluorescently stained with ROS-sensitive dye. Figure 3D shows that the ROS signal in the asthmatic mice was greater than the signal in the PBS group. However, the ROS signals in the SIT group and NAC group were significantly reduced, indicating that both SIT and anti-oxidative therapy reduced the generation of ROS in the lung.
To determine if SIT can alleviate apoptosis in lung tissue, murine lung tissue was collected and processed with TUNEL staining. Figure 4A shows that there were significantly more apoptotic cells in asthmatic mice than in PBS-treated mice, while the number of apoptotic cells in the SIT and NAC groups was smaller than in the asthma group.
Figure 4.
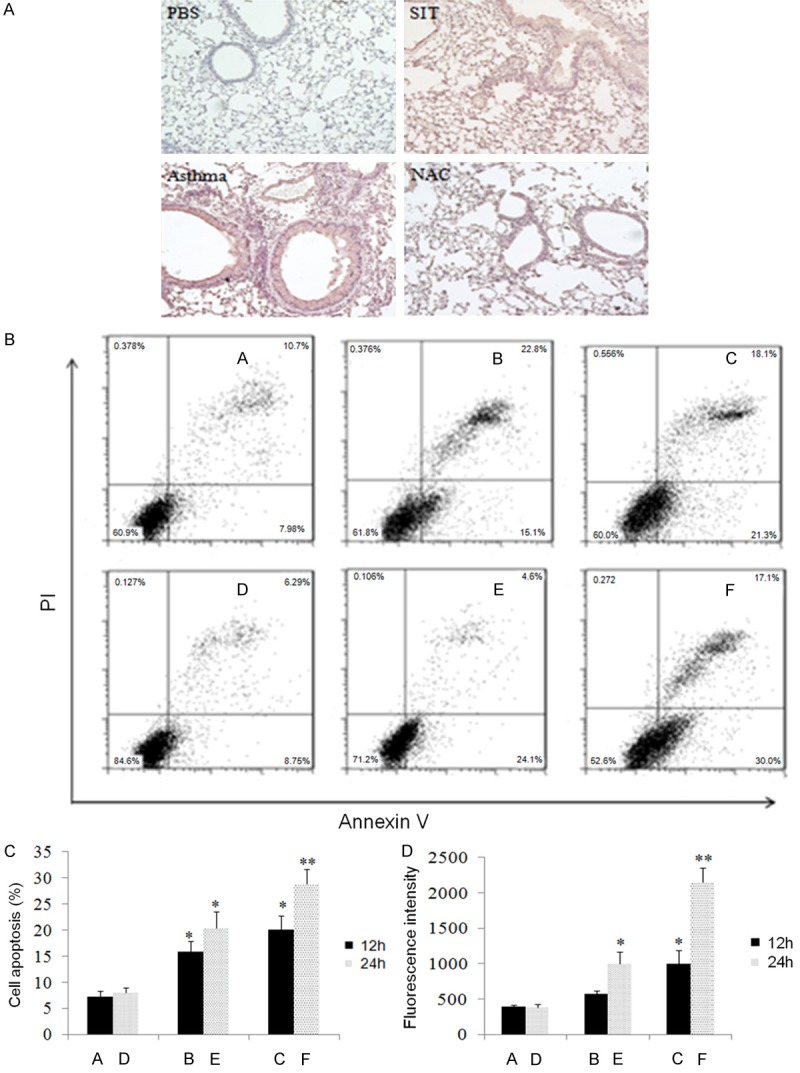
Dust mite SIT reduces apoptosis in pulmonary tissue. In vivo, SIT reduced lung apoptosis, and in vitro, IL-25 increased the apoptosis and ROS of 16HBE cells in a time and dose-dependent fashion. A: In the mouse model, the apoptosis of lung tissue was shown with TUNEL staining observed at 10× magnification. B and C: In vitro, apoptosis of 16HBE cells was shown with Annexin V-FITC/PI detection after exposure to various doses of human rIL-25 for different durations (A: control for 12 h; B: 0.1 mg/ml for 12 h; C: 0.1 mg/ml for 24 h; D: control for 24 h; E: 1 mg/ml for 12 h; F: 1 mg/ml for 24 h). D: The levels of ROS in 16HBE cells of the above groups were also tested using flow cytometry. The results are shown as the fluorescence intensity and are representative of 3 independent experiments. The statistics of the results were performed by student t-test. *means P<0.05, **means P<0.01.
To further test if IL-25 may upregulate apoptosis of human airway epithelial cells, the human bronchial epithelial cell line, 16HBE, was exposed to various concentrations of human rIL-25. As shown in Figure 4B and 4C, the percentage of apoptotic cells was significantly higher with an increase in the dose of rIL-25 and culture time. At the high concentration of rIL-25 (1 μg/mL), the percentage of apoptotic cells was significantly higher after culture for 24 hours than 12 hours (P<0.05). Using a similar co-culture system, we also studied the production of ROS in the 16HBE cells. As shown in Figure 4D, in parallel with the previous apoptosis results, the production of ROS of 16HBE increased following the increase in the rIL-25 dose and/or culture time. The greatest ROS fluorescence intensity was shown in the cells cultured with the highest concentration of rIL-25 (1 μg/ml) for 24 hours (P<0.01).
Treg polarization and anti-inflammatory suppression is generally accepted to be an important mechanism of the curative effect of SIT. Therefore, we measured the proportion of Tregs in the splenocytes of the four groups of mice using flow cytometry. Figure 5A and 5B show that the SIT group contained the most significant proportion of Tregs (8.98%) compared with that in the PBS control group (4.34%), asthma group (3.43%), and NAC group (5.22%) (P<0.01).
Figure 5.
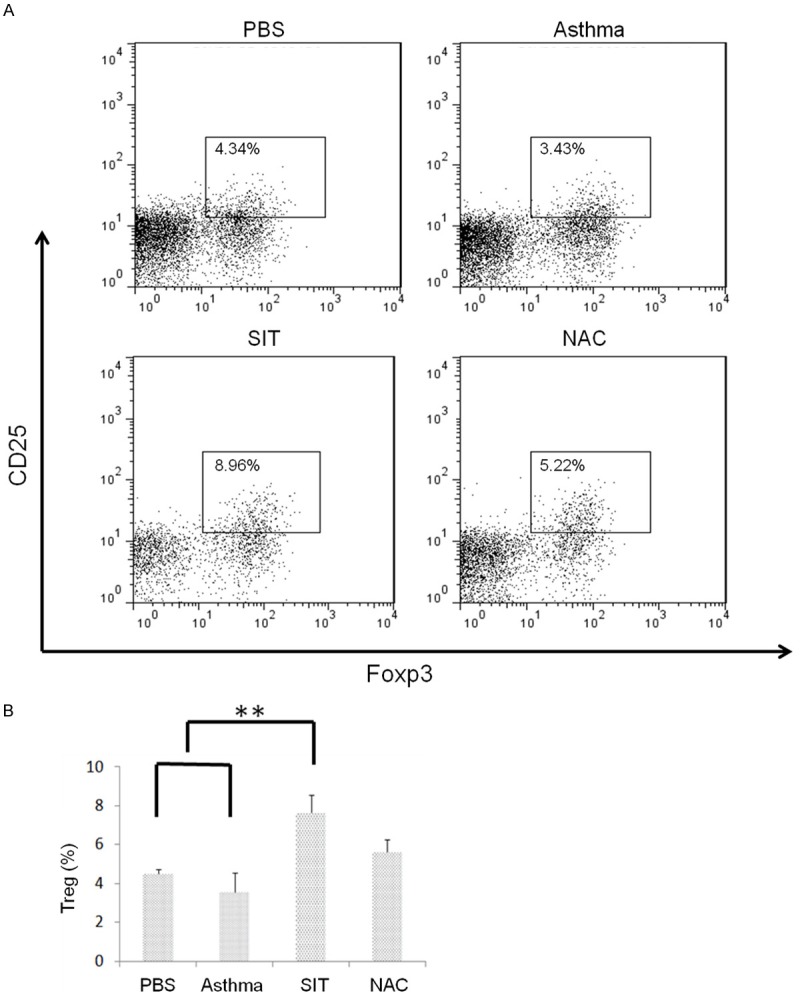
Dust mite SIT upregulates the number of Tregs in the spleen. The spleen from mouse models were homogenized, and the percentage of Tregs (CD4+CD25+Foxp3+) in CD4+T cells was tested by flow cytometry; the results showed (A) the representative and (B) the analysis of 3 independent experiments. The statistics of the results were performed by student t-test. **means P<0.01.
Discussion
Bronchial asthma is a chronic disease that features eosinophilic infiltration and high levels of Th2 cytokine secretion. In recent years, massive evidence has proven that oxidative stress plays an important role in the development and progression of the pathogenesis of asthma, especially in AHR and mucus secretion. SIT is the only curative approach to allergic asthma. This investigation aimed to investigate whether SIT could relieve the oxidative stress of asthma and to examine the potential mechanism.
Oxidative stress is a known risk factor for the morbidity of asthma and its pathogenic exacerbation. Many aerial hazardous substances, such as cigarettes, pollutant particles, or respiratory viruses, stimulate asthma by initiating oxidative stress. Some patients are highly sensitive to oxidative stress due to a deficiency in anti-oxidative mechanisms [23-25]. Oxidative stress may alter the accessibility of chromatin through its actions on transcriptional factor NF-E2-related factor 2 (NRF2) or acetylated albumin and accordingly activate an internal anti-oxidative mechanism [26-28].
This study used HDM extracts to establish an allergic mouse model and prepare the SIT vaccine. In this experiment, SIT significantly alleviated AHR, decreased the infiltration of inflammatory cells in the lungs, and decreased the level of Th2-type cytokines, IL-4, IL-5, and IL-13 in the BALF. SIT also significantly increased the number of Tregs, which is a known mechanism of SIT [29,30]. These results demonstrated the effectiveness of SIT in an animal model.
The AHR, pulmonary infiltration of inflammatory cells, Th2 cytokines, IL-4, IL-5, and IL-13, and airway mucus secretion were also significantly lower in the NAC group than in the asthma group, indicating that the oxidative stress response affects asthmatic pathogenesis and anti-oxidative therapy relieves the asthmatic symptoms.
To investigate whether SIT affects the oxidative stress of asthma, we showed that SIT reduced 1) the generation of ROS in BALF, 2) the generation of ROS in lung tissue, and 3) the level of MDA.
The anti-oxidative therapy, NAC, had a more pronounced anti-oxidative effect than the SIT vaccine, whereas SIT therapy had a more significant therapeutic effect than NAC on AHR symptoms and inflammatory cell infiltration. Additionally, in the TUNEL apoptosis experiment, SIT treatment decreased apoptosis in the lung tissue compared with that observed in mice with asthma. In summary, SIT not only alleviated the oxidative stress response but also inhibited the apoptosis of pulmonary epithelial cells.
Next, we investigated the mechanisms by which SIT down-regulated the oxidative reaction in asthma. When exposed to an allergen, murine pulmonary airway epithelial cells can secrete IL-25, TSLP, and IL-33. IL-25 and TSLP may promote the pathogenesis of asthma by promoting Th2 cytokine secretion; therefore, we hypothesized that SIT might regulate pulmonary ROS through IL-25. In the experiment, the amount of IL-25 in the BALF was significantly lower in the SIT group than the asthma group [31]. Therefore, we suggest that SIT treatment alleviates oxidative stress and the apoptosis of airway epithelial cells via down-regulation of IL-25.
Studies have reported a massive death of airway columnar epithelial cells in patients with asthma, as well as a high expression of epithelial cell growth factors such as epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 1 (HER1) [32,33]. Airway epithelial cells are the targets of oxidative agents such as O3, N2, and SO2; meanwhile, inflammatory cells, i.e., eosinophils and neutrophils, are producers of internal oxidation and tumor necrosis factor alpha (TNF-α), which is known to induce cell apoptosis. Fabio and colleagues have shown that the oxidative stress of airway epithelial cell leads to the apoptosis of airway epithelial cells [34]. Although there is a massive death of airway epithelial cells in patients with severe asthma, the mechanism of the apoptosis is unclear [35].
In an in vitro assay, human airway epithelial cells were exposed to various concentrations of IL-25, and IL-25 significantly increased the generation of ROS by airway epithelial cells. When applying the Annexin V-FITC/PI dual-staining apoptosis assay, we found that IL-25 increased the apoptosis of human airway epithelial cells.
In conclusion, in the pathogenesis of asthma, IL-25 exacerbates pulmonary oxidative stress, and increases the generation of oxidative ROS. Furthermore, IL-25 exacerbates the apoptosis of airway epithelial cells and asthma. Our data suggest that SIT down-regulates the secretion of IL-25, a promoter of the oxidative stress response and the apoptosis of airway epithelial cells.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 91442118, 91542104, 31400786, 2016YFC0905802), The Medical Scientific Research Foundation of Guangdong Province, China (No. 2014B090901041, 2014A020212466, 2013B031800023, 2016A020216029, 2014A020212422), The State Key Laboratory of Respiratory Disease Foundation (No. SKLRD2016ZJ001), Shenzhen Scientific Technology Basic Research Projects (No. JCYJ20160328144536436, JCYJ20150525092941036, JCYJ20160429171931438), and the Shenzhen Health and family planning system research project (No. 201604130418).
Disclosure of conflict of interest
None.
References
- 1.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Frew AJ. Allergen immunotherapy. J Allergy ClinImmunol. 2010;125:S306–S313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 3.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Gregory LG, Causton B, Murdoch JR, Mathie SA, O’Donnel lV, Thomas CP, Priest FM, Quint DJ, Lloyd CM. Inhaled house dust mite induces pulmonary T helper 2 cytokine production. Clin Exp Allergy. 2009;39:1597–1610. doi: 10.1111/j.1365-2222.2009.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulek K, Swaidani S, Aronica M, Li X. Epithelium: the interplay between innate and Th2 immunity. Immunol Cell Biol. 2010;88:257–268. doi: 10.1038/icb.2009.113. [DOI] [PubMed] [Google Scholar]
- 9.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Intratracheal instillation of lipopolysaccharide in mice induces apoptosis in bronchial epithelial cells: no role for tumor necrosis factor-alpha and infiltrating neutrophils. Am J Respir Cell Mol Biol. 2001;24:569–576. doi: 10.1165/ajrcmb.24.5.4156. [DOI] [PubMed] [Google Scholar]
- 10.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 11.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 12.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 13.Chung KF, Marwick JA. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann N Y Acad Sci. 2010;1203:85–91. doi: 10.1111/j.1749-6632.2010.05600.x. [DOI] [PubMed] [Google Scholar]
- 14.CotgreaveI A. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 15.Blesa S, Cortijo J, Mata M, Serrano A, Closa D, Santangelo F, Estrela JM, Suchankova J, Morcillo EJ. Oral n-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur Respir J. 2003;21:394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- 16.Millman M, Millman FM, Goldstein IM, Mercandetti AJ. Use of acetylcysteine in bronchial asthma--another look. Ann Allergy. 1985;54:294–296. [PubMed] [Google Scholar]
- 17.Bylin G, Hedenstierna G, Lagerstrand L, Wagner PD. No influence of acetyl cysteine on gas exchange and spirometry in chronic asthma. Eur J Respir Dis. 1987;71:102–107. [PubMed] [Google Scholar]
- 18.Davreux CJ, Soric I, Nathens AB, Watson RW, Mcgilvray ID, Suntres ZE, Shek PN, Rotstein OD. N-acetyl cysteine attenuates acute lung injury in the rat. Shock. 1997;8:432–438. [PubMed] [Google Scholar]
- 19.Shirinbak S, Taher YA, Maazi H, Gras R, van Esch BC, Henricks PA, Samsom JN, Verbeek JS, Lambrecht BN, van Oosterhout AJ, Nawijn MC. Suppression of Th2-driven airway inflammation by allergen immunotherapy is independent of B cell and Ig responses in mice. J Immunol. 2010;185:3857–3865. doi: 10.4049/jimmunol.0903909. [DOI] [PubMed] [Google Scholar]
- 20.Maazi H, Shirinbak S, Willart M, Hammad HM, Cabanski M, Boon L, Ganesh V, Baru AM, Hansen G, Lambrecht BN, Sparwasser T, Nawijn MC, van Oosterhout AJ. Contribution of regulatory T cells to alleviation of experimental allergic asthma after specific immunotherapy. Clin Exp Allergy. 2012;42:1519–1528. doi: 10.1111/j.1365-2222.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya P, Fan J, Haddad C, Essani A, Gopisetty A, Elshabrawy HA, Vasu C, Prabhakar BS. A novel pancreatic β-cell targeting bispecific-antibody (BsAb) can prevent the development of Type 1 diabetes in NOD mice. Clin Immunol. 2014;153:187–198. doi: 10.1016/j.clim.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Kim SJ, Chamberlain ND, Pickens SR, Volin MV, Volkov S, Arami S, Christman JW, Prabhakar BS, Swedler W, Mehta A, Sweiss N, Shahrara S. The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis. J Immunol. 2013;190:5256–5266. doi: 10.4049/jimmunol.1201675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14:90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama Wong LS, Aung HH, Lamé MW, Wegesser TC, Wilson DW. Fine particulate matter from urban ambient and wildfire sources from California’s San Joaquin Valley initiate differential inflammatory, oxidative stress, and xenobiotic responses in human bronchial epithelial cells. Toxicol In Vitro. 2011;25:1895–1905. doi: 10.1016/j.tiv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Nel AE. Role of the NRF2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 27.Mercado N, Thimmulappa R, Thomas CM, Fenwick PS, Chana KK, Donnelly LE, Biswal S, Ito K, Barnes PJ. Decreased histone deacetylase 2 impairs NRF2 activation by oxidative stress. Biochem Biophys Res Commun. 2011;406:292–298. doi: 10.1016/j.bbrc.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Cavkaytar O, Akdis CA, Akdis M. Modulation of immune responses by immunotherapy in allergic diseases. Curr Opin Pharmacol. 2014;17:30–37. doi: 10.1016/j.coph.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Akdis M, Akdis CA. Mechanisms of allergenspecific immunotherapy. J Allergy ClinImmunol. 2007;119:780–789. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- 33.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 34.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanović R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 35.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FASL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]


