Abstract
Oral squamous cell carcinoma (OSCC) is a subtype of head and neck cancer with a relatively poor prognosis. The mechanisms underlying the initiation and progression of OSCC are complex and not yet fully understood; however, this information is critical for developing novel therapeutic targets and improving patient outcome. Rab14, a Ras related protein, has been implicated in multiple forms of cancer. In the present study, we confirmed that Rab14 is overexpression in human OSCC tissue, compared with normal oral mucosa samples. In addition, knockdown of Rab14 exerted potent anti-tumor effects by repressing the proliferation and migration of OSCC cell lines. Moreover, knockdown of Rab14 reduced the expression of Cyclin D1 and CXCR4, at the level of protein and mRNA, both in vitro and in vivo. Additionally, abrogation of Rab14 enhanced cisplatin sensitivity in OSCC cells in vitro and in vivo. Taken together, our data provides evidence for Rab14 as a potential therapeutic target in OSCC treatment.
Keywords: Oral squamous cell carcinoma, Rab14, proliferation, migration, chemotherapeutic sensitization
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common malignancies worldwide [1]. Over the last few decades, OSCC patients have benefited from advances in treatment options, such as radiotherapy, chemotherapy, surgery, and targeted therapy; however, overall survival remains limited [2]. In addition, disease recurrence often occurs in single-treatment strategies [3]. Therefore, a better understanding of the mechanisms responsible for this disease is critical to developing more efficacious treatments and increasing the survival rate of patients with OSCC.
A potential mechanism is the Rab family of proteins, which are involved in the regulation of membrane trafficking [4]. In recent years, the Rab family has also been implicated as key proteins in tumor progression, migration, and invasion [5-7]. Moreover, emerging evidence shows that Rab proteins play a pivotal role in the regulation of tumorigenic signaling [8,9]. In addition, aberrant expression of Rab proteins has been shown to occur in multiple forms of cancer, including colon cancer and liver cancer [10,11].
Of the Rab family proteins, only limited number of research studies have focused on the role of Rab14 in human cancers. In recent work, Rab14 was reported to be over-expressed in some human cancers, such as ovarian cancer and non-small cell lung cancer [12,13]. In addition, Rab14 can promote proliferation by regulating the AKT signaling pathway, and inhibition of Rab14 has been shown to induce cell apoptosis and cell cycle arrest in gastric cancer [14]. However, the expression of Rab14 in OSCC, as well as whether it plays a role in the mechanism underlying the disease, remains unclear.
To address this question, in the present study, we performed immunohistochemistry on human OSCC tissue samples to determine Rab14 protein levels. In addition, we investigated the association between Rab14 and clinic-pathological parameters of OSCC, and examined the function of Rab14 in OSCC both in vivo and in vitro.
Material and methods
Ethics statement
All experiments of this study were approved by the Medical Ethics Committee of the People’s Hospital of Zhenhai and performed in the Public Laboratory Platform of Medical School, Ningbo University.
Human OSCC samples
41 cases of human OSCC samples and 13 cases of normal oral mucosa tissues enrolled in this study were collected from the people’s hospital of Zhenhai from 2014 to 2016. Consents were obtained from each patient before surgery. The histological diagnosis, pathological grade and TNM stage were estimated by pathologist according to the WHO classification guidelines.
Immunohistochemistry
Tissue samples were fixed by 4% paraformaldehyde and embedded in paraffin and made into 4 µm thick sections. Antigen retrieval was performed with citrate buffer (pH 6.0) with pressure cooker for 1.5 min. Next, sections were incubated with 0.3% hydrogen peroxide and then incubated with goat serum. SP staining kit (UltrasensitiveTM, Maixin, China) was used to perform immunohistochemical staining. Primary antibodies incubation was performed at 4°C over night. At day 2, sections were incubated with Biotinylated goat anti-rabbit or anti-mouse serum IgG, and followed incubated with HPR conjugated streptavidin-biotin. DAB kit (Maixin, China) was used for section staining. Hematoxylin was used for counterstaining.
RNA extraction and quantitative real-time PCR
Total RNA of cultured cells, nude mice tumor tissues was extracted according to the protocols of the manufacturer (TRIzol, TaKaRa, Japan). The first-strand cDNA was synthesized using 1 μg of total RNA by a Revert Aid First Strand cDNA Synthesis Kit (Fermentas, Lithuania). Reverse transcription-polymerase chain reaction (RT-PCR) was performed by ViiA 7 Real-Time PCR System (Life Technology, USA) with the amplification conditions as follows: 1 cycle of 2 min at 95°C, then 40 cycles of 10 s at 95°C, 30 s at 60°C, and 30 s at 72°C. All experiments were performed in triplicate. The relative quantities of the RNA expression were calculated by 2-ΔΔCt (cycle threshold) values. GAPDH was used as internal control. The primer sequences are listed in Supplementary Table.
Protein extraction and western blotting
Total protein of cultured cells and nude mice tumors was extracted by cell lysis buffer (Pierce, USA). Bradford method was used for protein quantification. 20 μg protein was added into SDS-PAGE and transferred to PVDF membrane (Millipore, USA). Incubation of primary antibodies was performed at 4°C overnight. At day 2, the membrane was incubated with HRP-coupled anti-rabbit IgG antibody (1:3000, CST, USA) at room temperature for 1 h. Protein on the membrane was visualized by ECL kit. β-actin was used as loading control.
Cell culture
Human oral squamous cell carcinoma (OSCC) cell lines SCC4 or Tca8113 were maintained in DMEM/F12 or RPMI-1640 medium (Hyclone) with 10% FBS (fetal bovines serum, Hyclone) at 37°C in a humidified atmosphere with 5% CO2.
Rab14-silenced OSCC cell lines establishment
Two shRNA sequences (shRNA1 and shRNA2) targeting Rab14 and negative shRNA were designed and synthesized (Genepharma, China). To construct human Rab14 shRNA plasmids, the sequences were inserted into the BamHI/EcoRI restriction sites of pGLVU6/Purolentivectors. Next, lentiviral expression vectors and packaging plasmids were co-transduced into 293 T cells. Viral particles were collected, and then infected into SCC4 or Tca8113 cells. Puromycin (2 μg/ml, R&D system, USA) was used to select the infected cells.
CCK-8 proliferation assay
Cell proliferation assay was performed by Cell Counting Kit-8 (CCK8, Dojindo, Japan). 300/well SCC4 and Tca8113 cells transfected with control shRNA or Rab14 shRNA were seeded into 96 well plates for 5 days. Then cells were incubated with CCK-8 solution for 4 h. Plates were read and measured by a reader at the wavelength of 450 nm.
Transwell migration assay
Migration assays were performed with Costar Transwell inserts with 8 μm pore size (Coring, USA). Briefly, SCC4 and Tca8113 cells transfected with control shRNA or Rab14 shRNA were seeded into the upper chamber at a density of 20000 cells per well with 100 µl FBS-free DMEM/F12 or RPMI-1640 medium. Medium with 10% FBS was added in the bottom chamber. After incubation at 37°C for 24 h, the cells in upper chamber were removed. Cells in bottom chamber were fixed and stained with 0.1% crystal violet (Sigma, USA). Migrated cells were photographed and counted.
In vivo studies
All animalsenrolled in this study were purchased from Hunan SJA Laboratory Animal Co. Ltd. (China). Experiments were performed according to institutional guidelines. Briefly, female athymic BALB/c nude mice (18-20 g, 5-6 weeks old) were kept in sterile laminar flow cabinets under appropriate pathogen-free conditions. SCC4 cells transfected with control shRNA or Rab14 shRNA were inoculated into the left flank of the mice. The volumes of tumors were measured by a caliper and calculated with the formula (width2 × length)/2. At the endpoint of the experiment, the mice were euthanized for tumors harvest. For cisplatin treatment, the tumor bearing mice were treated with 10 mg/kg cisplatin by intraperitoneal injection at Day 14. The volume of Tumors from each group (Vector, Vector+Cisplatin and shRab14+cisplatin) were calculated and analyzed.
Reagents and antibodies
Cisplatin was purchased from Selleck (USA). Antibodies used in this study were listed as follows: rabbit Rab14 (Abcam, UK, 1:200 for IHC, 1:1000 for WB), rabbit Ki-67 (Abcam, UK, 1:200 for IHC), rabbit Cyclin D1 (CST, USA, 1:1000 for WB), rabbit CXCR4 (Abcam, UK, 1:1000 for WB), mouse β-actin (Proteintech, USA, 1:2000 for WB).
Statistical analysis
Collected data were analyzed by Graphpad Prism 5.0 software (Graph Pad Software Inc, USA) and expressed as mean ± SEM of repeated experiments in triplicate. The significance of differences was estimated by Student’s t test. P<0.05 were defined as a statistically significant difference. Kaplan-Meier analysis and Log-rank test was performed by SPSS 20.0 (SPSS Inc., USA).
Results
Overexpression of Rab14 in human OSCC tissues
To investigate the expression pattern of Rab14 in tissue samples from patients with OSCC, immunohistochemical staining was performed on human tissue samples representing 41 OSCC cases and 13 cases of normal oral mucosae. Representative images of normal oral mucosa, Rab14-negative OSCC, and Rab14-positive OSCC are displayed in Figure 1A-C, respectively. Rab14 was primarily identified in the cytoplasm of cancerous epithelial cells. We found that 48.8% (20/41) of OSCC samples were positive for Rab14, and that the expression of Rab14 was significantly up-regulated in human OSCC samples compared with normal oral mucosa (p = 0.008; Table 1).
Figure 1.
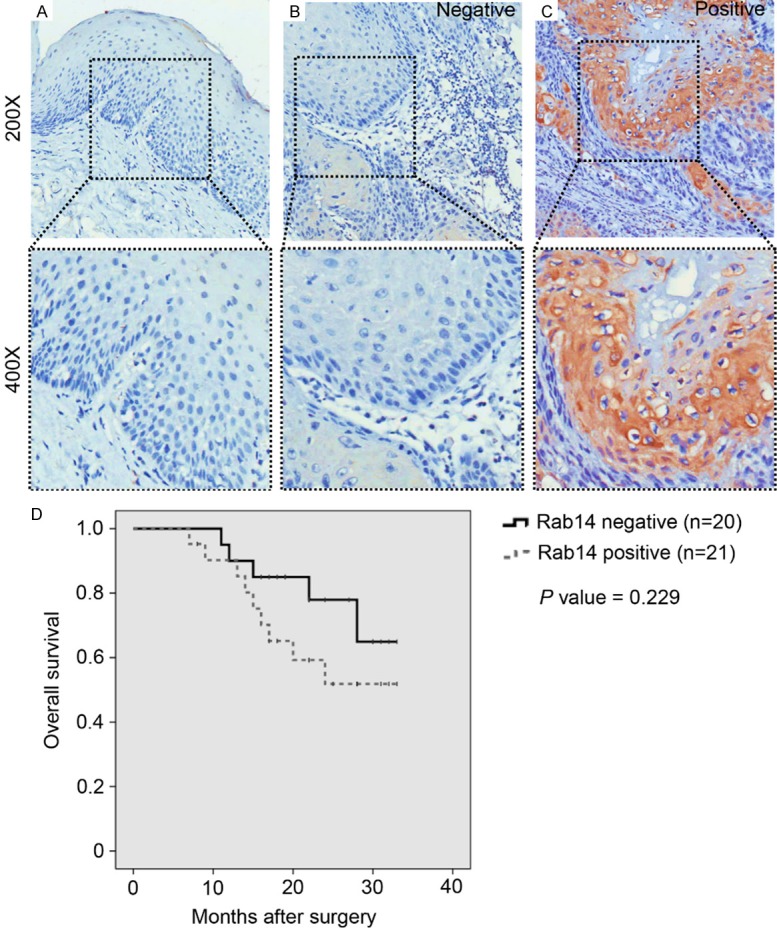
The expression of Rab14 is elevated in human oral squamous cell carcinoma (OSCC) tissues compared with normal oral mucosa. Representative images of Rab14 immunohistochemical staining of (A) normal oral mucosa, (B) Rab14 negative and (C) Rab14 positive human OSCC tissues. Rab14 was mainly located in the cytoplasm of cancerous epithelial tissues (Magnification: upper 200×, lower 400×). (D) No significant overall survival (P = 0.229) was found between Rab14 negative patients (n = 21) and Rab14 positive patients (n = 20) using Kaplan-Meier survival analysis.
Table 1.
The overexpression of Rab14 in human oral squamous cell carcinoma
| Rab14 | P value | |||
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Tissue samples | ||||
| Mucosa | 13 | 12 | 1 | |
| OSCC | 41 | 21 | 20 | 0.008 |
Next, the association between Rab14 expression and clinic-pathological parameters of OSCC was assessed. As shown in Figure 1D, a Kaplan-Meier analysis and Log-rank test were used to investigate the relationship between Rab14 expression and overall survival. We found that overall survival was not significantly different in patients that were positive for Rab14, compared to patients who were negative for Rab14 expression (p = 0.229). In addition, further analysis showed that expression of Rab14 was not significantly correlated with pathological tumor grade (p = 0.294), tumor size (p = 0.948), or lymph node involvement (p = 0.228; Table 2). Taken together, these data suggest that Rab14 is significantly elevated in human OSCC samples, compared with normal oral mucosa, however it is unclear what role Rab14 plays in disease progression.
Table 2.
Association between pathological parameters and Rab14 expression
| Rab14 | P value | |||
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Pathological grade | ||||
| I | 9 | 6 | 3 | |
| II+III | 32 | 15 | 17 | 0.294 |
| Tumor Size | ||||
| T1+T2 | 35 | 18 | 17 | |
| T3 | 6 | 3 | 3 | 0.948 |
| Lymph node involvement | ||||
| Negative | 27 | 12 | 15 | |
| Positive | 14 | 9 | 5 | 0.228 |
Establishment of Rab14-silenced OSCC cell lines
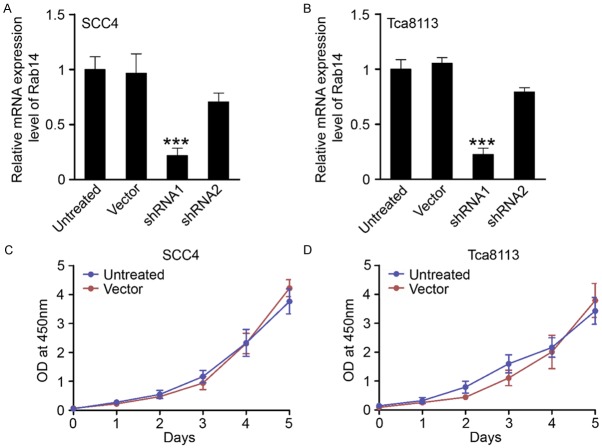
To develop Rab14-silenced OSCC cell lines, we transfected different cell lines with two shRNA sequences (shRNA1 and shRNA2). After the transfection of a negative control, shRNA1 and shRNA2, we found that shRNA1 could significantly inhibit levels of Rab14 mRNA in two oral OSCC cell lines: SCC4 and Tca8113 (Figure 2A and 2B). Importantly, transfection in the negative control did not repress the proliferation of SCC4 and Tca8113 cells, indicating that no cytotoxic effects occurred (Figure 2C and 2D).
Figure 2.
Establishment of Rab14-silenced OSCC cell lines. (A) SCC4 cells and (B) Tca8113 cells were transfected with control shRNA (vector) or Rab14 shRNA (shRNA1 and shRNA2). Real-time PCR indicated that shRNA1 could significantly decrease the mRNA level of Rab14 in both SCC4 and Tca8113 cells (Mean ± SEM, ***, P<0.001, t test). No extra cytotoxic effects were induced by control shRNA (vector) in (C) SCC4 cells and (D) Tca8113 cells using CCK-8 proliferation assays.
Silencing Rab14 suppresses the proliferation and migration of OSCC cell lines
An essential oncogenic role of Rab14 has been reported in multiple studies. Therefore, we investigated the potential function(s) of Rab14 in OSCC cell lines. To this end, we performed CCK-8 cell proliferation assays to assess the role of Rab14 in cell growth. We found that cell proliferation was significantly inhibited in Rab14-silenced SCC4 and Tca8113 cells, compared to control cells (Figure 3A and 3B). Furthermore, we assessed the impact of Rab14 knockdown on cell migration in OSCC cell lines, and found that silencing of Rab14 induced a significant inhibition in migration in SCC4 and Tca8113 cell lines (Figure 3C and 3D). We next analyzed expression of Cyclin D1 and CXCR4 proteins in control cells and Rab14-silenced cells. Our results show that knockdown of Rab14 reduces the expression of Cyclin D1 and CXCR4 in SCC4 and Tca8113 cells (Figure 3E). Finally, real-time PCR assays indicated that knockdown of Rab14 decreases levels of Cyclin D1 and CXCR4 mRNA (Figure 3F). Taken together, these results indicate that Rab14 can regulate the proliferation and migration of OSCC cells.
Figure 3.
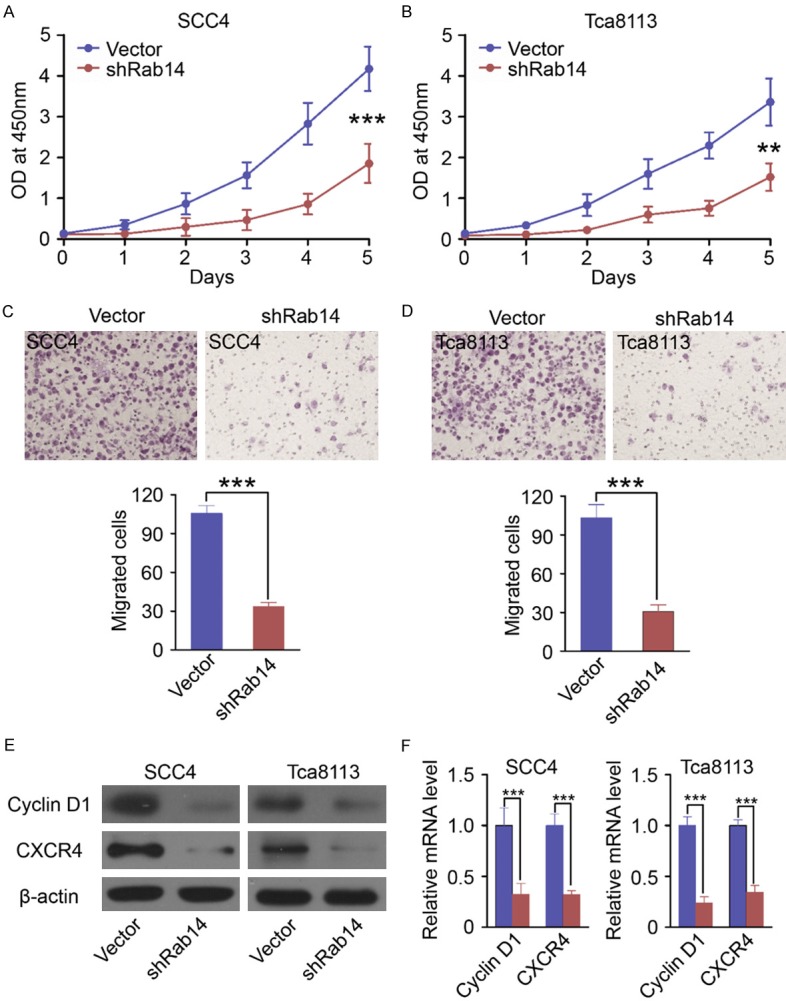
Silencing Rab14 suppresses the proliferation and migration of OSCC cell lines. Silencing Rab14 could induce significant repression of proliferation in (A) SCC4 and (B) Tca8113 cell lines using CCK-8 proliferation assays (Mean ± SEM, **, P<0.01, ***, P<0.001, t test). Transwell migration assays suggested that silencing Rab14 significantly induced inhibition of migration in (C) SCC4 and (D) Tca8113 cell lines (Mean ± SEM, ***, P<0.001, t test). (E) Western Blot showed knockdown of Rab14 downregulated the protein levels of Cyclin D1 and CXCR4 in SCC4 cells and Tca8113 cells. (F) Quantitative real-time PCR revealed that Rab14 silencing could significantly decrease the mRNA level of Cyclin D1 and CXCR4 in SCC4 cells and Tca8113 cells (Mean ± SEM, ***, P<0.001, t test).
Effects of Rab14 silencing on the proliferation of OSCC cells in vivo
To explore whether Rab14 silencing effectstumorigenesis in vivo, SCC4 cells were transfected with negative control shRNA or with Rab14 shRNA. Then, female nude mice (n = 6/group) were inoculated with the transfected cells. We found that tumors derived from Rab14 shRNA-transfected SCC4 cells grew significantly slower (Figure 4A and 4B) than the negative controls. At the end of the experiment, tumor tissue from each group was harvested for further study.
Figure 4.
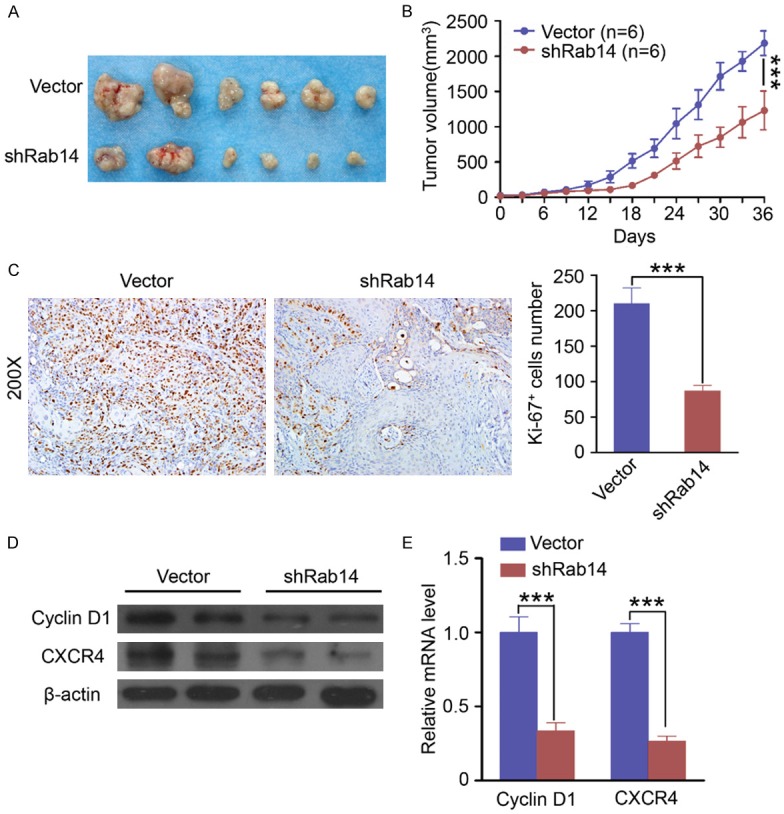
Rab14 silencing inhibits tumor proliferation and blunts tumor growth in vivo. A. The photos of tumors formed from SCC4 cells transfected with control shRNA (Vector, up panel) or Rab14 shRNA (down panel). B. Tumor growth curve of formed from SCC4 cells transfected with control shRNA (Vector, n = 6) or Rab14 shRNA (n = 6). Silencing Rab14 significantly repressed the tumor growth in vivo (Mean ± SEM, ***, P<0.001). C. Knockdown of Rab14 significantly decreased the Ki-67 positive tumor cells in mouse tumor tissue (Mean ± SEM, ***, P<0.001, t test, Magnification: 200×). D. Knockdown of Rab14 in vivo decreased the protein levels of Cyclin D1 and CXCR4 compared with control group (vector). E. Knockdown of Rab14 in vivo decreased the mRNA levels of Cyclin D1 and CXCR4 (Mean ± SEM, ***, P<0.001, t test) compared with control group (vector).
Analysis of Ki-67 expression with immunostaining was performed in resected tumor tissues. The number of Ki-67 positive cells in tumors that developed from Rab14 shRNA-transfected cells was significantly lower than tumors from the negative control group (Figure 4C). Furthermore, we found that Rab14 silencing redu-ced the protein and mRNA levels of both Cyclin D1 and CXCR4 in vivo (Figure 4D and 4E). These results suggest that Rab14 expression is significantly associated with the in vivo proliferation of OSCC cells.
Rab14 silencing enhances cisplatin sensitivity in OSCC cells
To determine if Rab14 silencing influences the sensitivity of OSCC cells to the anti-cancer drug, cisplatin, SCC4 cells were treated with 20 μM cisplatin for 24 h. We found that cisplatin treatment increased the level of Rab14 protein in SCC4 cells, suggesting a potential role of Rab14 in cisplatin resistance (Figure 5A). Additionally, SCC4 cells were transfected with negative control shRNA or Rab14 shRNA and treated with 20 μM cisplatin for 24 h. Then, a CCK-8 proliferation assay was performed to investigate the effect of Rab14 silencing on cisplatin sensitivity in OSCC cells. Our results revealed that cisplatin treatment was more effective on Rab14-silenced cells than on control cells (Figure 5B).
Figure 5.
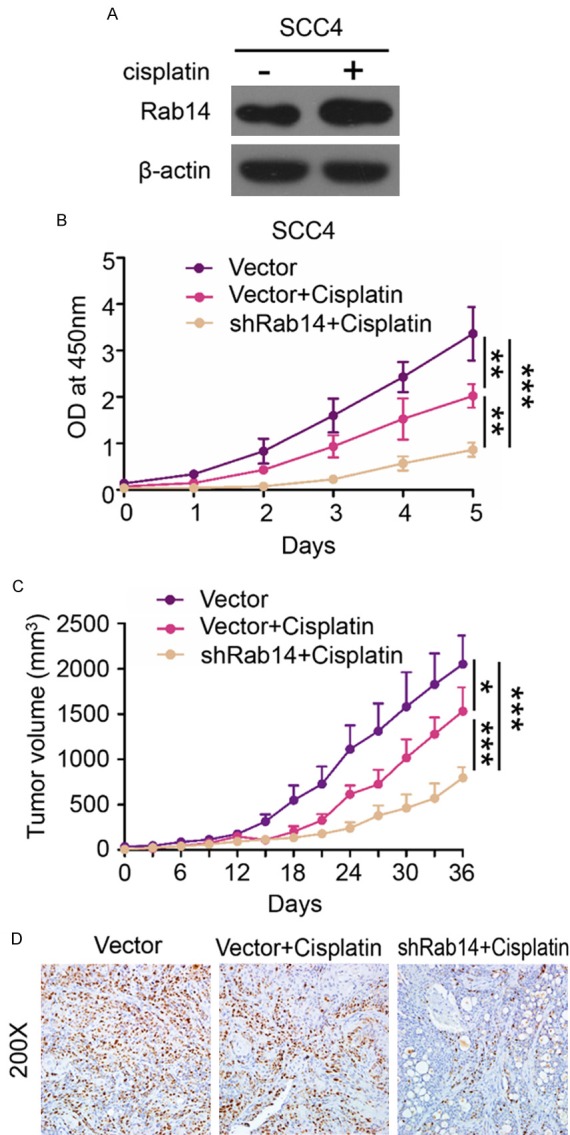
Rab14 silencing could enhance the cisplatin sensitivity in OSCC cells. A. Western Blot suggested ciplatin (20 μM for 24 h) treatment increased the protein level of Rab14 in SCC4 cells. B. CCK-8 proliferation assay indicated that Rab14 silencing significantly enhances the cell growth inhibition effects caused by cisplatin (Mean ± SEM, **, P<0.01, ***, P<0.001). C. The tumor growth curve of control group (Vector), Vector+Cisplatin and shRab14+Cisplatin. Rab14 silencing enhances the sensitivity of cisplatin in vivo (Mean ± SEM, *, P<0.05, ***, P<0.001). D. Representative images of Ki-67 immunohistochemical staining in control group (Vector), Vector+Cisplatin group and shRab14+Cisplatin group. (Magnification: 200×). ShRab14+Cisplatin mouse tumor tissue displayed less Ki-67 positive tumor cells compared with other group.
To investigate cisplatin sensitivity in vivo, female nude mice (n = 6/group) were inoculated with SCC4 cells transfected with either negative control shRNA and Rab14 shRNA. On day 14, mice received 10 mg/kg cisplatin treatment by intraperitoneal injection. As shown in Figure 5C, Rab silencing significantly enhanced the sensitivity of cisplatin in vivo. Furthermore, immunostaining of Ki-67 in the tumors from each group indicated that Rab14 knockdown significantly repressed the proliferation of SCC4 cells in vivo (Figure 5D). Taken together, our results demonstrate that Rab14 silencing enhances cisplatin sensitivity in OSCC cells both in vitro and in vivo.
Discussion
Recent studies have provided evidence for a close relationship between the Rab family of proteins and tumorigenesis in human cancers [15,16]. Importantly, Rab proteins may exert diverse functions in different cancers [17,18], and dysregulated expression of Rab proteins was reported in multiple forms of cancer [19,20]. Therefore, determining the function of Rab proteins and whether they play a role in cancer progression is critical for the development of novel therapeutic strategies.
We chose to focus on Rab14, as a recent study reported high levels of Rab14 mRNA in gastric cancer tissues [14], and elevated levels of Rab14 protein have been observed in human ovarian cancer [12]. In this study, using immunohistochemistry we determined that Rab14 expression is up-regulated in human OSCC samples. In ovarian cancer, overexpression of Rab14 has been associated with the International Federation of Gynecology and Obstetrics (FIGO) stage, indicating a potential function of Rab14 in tumor progression [12]. In addition, Rab14 overexpression has been correlated with poor outcome in breast cancer [21]. However, in our study, we found that expression of Rab14 was not significantly related with prognosis, pathological grade, tumor size, or involvement of the lymph nodes. This discrepancy may be due to a small sample size and short follow-up period. Therefore, a future study should be conducted with a larger number of samples and a longer follow-up, to resolve this question.
Next, we carried out functional assays to evaluate the function of Rab14 in OSCC cell lines. Our in vitro study revealed that knockdown of Rab14 inhibits the proliferation and migration of OSCC and downregulates Cyclin D1 and CXCR4 protein. This may have important implications in disease progression, as Cyclin D1 plays a vital role in uncontrolled proliferation [22], and high expression of CXCR4 promotes tumor cell metastasis [23,24]. The results of our in vivo study confirmed a function of Rab14 in proliferation, as we found that knockdown of Rab14 suppressed tumor growth and decreased Ki-67 expression. These results are consistent with previous studies, which have shown that Rab14 can regulate proliferation and migration in multiple forms of cancer. For example, in ovarian cancer, depletion of Rab14 inhibits cell proliferation by decreasing Cyclin E and upregulating p21, through a Wnt signaling-dependent mechanism [12]. Moreover, in gastric cancer, Rab14 promotes cell proliferation by activating the Ser473 site of Akt [14]. In non-small cell lung carcinoma, ectopic miR-338-3p expression has been shown to regulate proliferation partially through downregulation of Rab14 [13]. Finally, miR-320a has been suggested to modulate cell proliferation, migration, and invasion by targeting Rab14 [21].
Resistance to chemotherapeutic drugs reduces the efficacy of chemotherapy, resulting in unsuccessful treatment [25,26]. In recent years, several of the mechanisms underlying drug resistance have been identified [27-29]. Here, we investigated a role of Rab14 in the resistance of OSCC to the chemotherapeutic drug, cisplatin. We found that levels of Rab14 protein were increased after cisplatin treatment. Moreover, the results of our CCK-8 assay and in vivo study showed that Rab14 knockdown enhances the cytotoxic effects of cisplatin. Interestingly, in renal cancer, miR-148a has been shown to enhance cisplatin sensitivity by negatively regulating Rab14 [30] and, in nasopharyngeal carcinoma, miR-451 inhibits Rab14 expression and increases sensitivity to radiotherapy [31]. Taken together with the literature, our results indicate that Rab14 may play a crucial role during chemotherapy or radiotherapy.
In summary, our data showed overexpression of Rab14 in human OSCC tissue, compared with normal oral mucosa samples. The association between Rab14 and clinic-pathological parameters was also analyzed. Both our in vitro and in vivo studies showed that knockdown of Rab14 can suppress the proliferation and migration of OSCC cell lines. However, interestingly, silencing Rab14 enhances sensitivity to cisplatin. Together, our data provides evidence for a role of Rab14 in OSCC, and highlights Rab14 as a potential target for OSCC treatment.
Acknowledgements
This work was supported by General Medical Research Program of Department of Health of Zhejiang Province (2013KYB247) to X.J. Li.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 3.Huang SH, O’Sullivan B. Oral cancer: Current role of radiotherapy and chemotherapy. Med Oral Patol Oral Cir Bucal. 2013;18:e233–240. doi: 10.4317/medoral.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 5.Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX, Xia SL, Yang SX, Zheng B. Rab11-FIP2 promotes colorectal cancer migration and invasion by regulating PI3K/AKT/MMP7 signaling pathway. Biochem Biophys Res Commun. 2016;470:397–404. doi: 10.1016/j.bbrc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Hou Q, Wu YH, Grabsch H, Zhu Y, Leong SH, Ganesan K, Cross D, Tan LK, Tao J, Gopalakrishnan V, Tang BL, Kon OL, Tan P. Integrative genomics identifies RAB23 as an invasion mediator gene in diffuse-type gastric cancer. Cancer Res. 2008;68:4623–4630. doi: 10.1158/0008-5472.CAN-07-5870. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Liu W, Lu X, Fu Y, Li L, Luo Y. High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget. 2015;6:11125–11138. doi: 10.18632/oncotarget.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzeng HT, Wang YC. Rab-mediated vesicle trafficking in cancer. J Biomed Sci. 2016;23:70. doi: 10.1186/s12929-016-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Ren J, Wu B, Feng S, Cai G, Tuluc F, Peranen J, Guo W. Activation of Rab8 guanine nucleotide exchange factor Rabin8 by ERK1/2 in response to EGF signaling. Proc Natl Acad Sci U S A. 2015;112:148–153. doi: 10.1073/pnas.1412089112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas JD, Zhang YJ, Wei YH, Cho JH, Morris LE, Wang HY, Zheng XF. Rab1A is an mTORC1 activator and a colorectal oncogene. Cancer Cell. 2014;26:754–769. doi: 10.1016/j.ccell.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu BH, Li XX, Yang Y, Zhang MY, Rao HL, Wang HY, Zheng XF. Aberrant amino acid signaling promotes growth and metastasis of hepatocellular carcinomas through Rab1A-dependent activation of mTORC1 by Rab1A. Oncotarget. 2015;6:20813–20828. doi: 10.18632/oncotarget.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou R, Jiang L, Yang Z, Wang S, Liu Q. Rab14 is overexpressed in ovarian cancers and promotes ovarian cancer proliferation through Wnt pathway. Tumour Biol. 2016 doi: 10.1007/s13277-016-5420-4. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Feng X, Gao S, Xiao Z. microRNA-338-3p functions as a tumor suppressor in human nonsmallcell lung carcinoma and targets Ras-related protein 14. Mol Med Rep. 2015;11:1400–1406. doi: 10.3892/mmr.2014.2880. [DOI] [PubMed] [Google Scholar]
- 14.Guo B, Wang W, Zhao Z, Li Q, Zhou K, Zhao L, Wang L, Yang J, Huang C. Rab14 Act as Oncogene and induce proliferation of gastric cancer cells via AKT signaling pathway. PLoS One. 2017;12:e0170620. doi: 10.1371/journal.pone.0170620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler DB, Zoncu R, Root DE, Sabatini DM, Sawyers CL. Identification of an oncogenic RAB protein. Science. 2015;350:211–217. doi: 10.1126/science.aaa4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang BL, Ng EL. Rabs and cancer cell motility. Cell Motil Cytoskeleton. 2009;66:365–370. doi: 10.1002/cm.20376. [DOI] [PubMed] [Google Scholar]
- 17.Tong M, Chan KW, Bao JY, Wong KY, Chen JN, Kwan PS, Tang KH, Fu L, Qin YR, Lok S, Guan XY, Ma S. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 2012;72:6024–6035. doi: 10.1158/0008-5472.CAN-12-1269. [DOI] [PubMed] [Google Scholar]
- 18.Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, Aronow BJ, Yeatman TJ, Bhartur SG, Calhoun BC, Condie B, Manley NR, Beauchamp RD, Coffey RJ, Goldenring JR. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. 2010;120:840–849. doi: 10.1172/JCI40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo ML, Gong C, Chen CH, Hu H, Huang P, Zheng M, Yao Y, Wei S, Wulf G, Lieberman J, Zhou XZ, Song E, Lu KP. The Rab2A GTPase promotes breast cancer stem cells and tumorigenesis via Erk signaling activation. Cell Rep. 2015;11:111–124. doi: 10.1016/j.celrep.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva P, Mendoza P, Rivas S, Diaz J, Moraga C, Quest AF, Torres VA. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget. 2016;7:29548–29562. doi: 10.18632/oncotarget.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Wang L, Yang H, Ding D, Zhang L, Wang J, Chen Q, Zou Q, Jin Y, Liu X. Rab14 suppression mediated by MiR-320a inhibits cell proliferation, migration and invasion in breast cancer. J Cancer. 2016;7:2317–2326. doi: 10.7150/jca.15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Cui J, Yu Q, Wu X, Pan A, Li L. Evaluation of CCND1 amplification and CyclinD1 expression: diffuse and strong staining of CyclinD1 could have same predictive roles as CCND1 amplification in ER positive breast cancers. Am J Transl Res. 2016;8:142–153. [PMC free article] [PubMed] [Google Scholar]
- 23.Yu T, Wu Y, Huang Y, Yan C, Liu Y, Wang Z, Wang X, Wen Y, Wang C, Li L. RNAi targeting CXCR4 inhibits tumor growth through inducing cell cycle arrest and apoptosis. Mol Ther. 2012;20:398–407. doi: 10.1038/mt.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur J Cancer. 2011;47:452–459. doi: 10.1016/j.ejca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, Ramena G, Elble RC. The role of cancer stem cells in relapse of solid tumors. Front Biosci (Elite Ed) 2012;4:1528–1541. doi: 10.2741/e478. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 27.Broxterman HJ, Lankelma J, Hoekman K. Resistance to cytotoxic and anti-angiogenic anticancer agents: similarities and differences. Drug Resist Updat. 2003;6:111–127. doi: 10.1016/s1368-7646(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 28.Swick AD, Prabakaran PJ, Miller MC, Javaid AM, Fisher MM, Sampene E, Ong IM, Hu R, Iida M, Nickel KP, Bruce JY, Wheeler DL, Kimple RJ. Co-targeting mTORC and EGFR signaling as a therapeutic strategy in HNSCC. Mol Cancer Ther. 2017;16:1257–1268. doi: 10.1158/1535-7163.MCT-17-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousquet G, El Bouchtaoui M, Sophie T, Leboeuf C, de Bazelaire C, Ratajczak P, Giacchetti S, de Roquancourt A, Bertheau P, Verneuil L, Feugeas JP, Espie M, Janin A. Targeting autophagic cancer stem-cells to reverse chemoresistance in human triple negative breast cancer. Oncotarget. 2017;8:35205–35221. doi: 10.18632/oncotarget.16925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EA, Kim TG, Sung EG, Song IH, Kim JY, Doh KO, Lee TJ. miR-148a increases the sensitivity to cisplatin by targeting Rab14 in renal cancer cells. Int J Oncol. 2017;50:984–992. doi: 10.3892/ijo.2017.3851. [DOI] [PubMed] [Google Scholar]
- 31.Zhang T, Sun Q, Liu T, Chen J, Du S, Ren C, Liao G, Yuan Y. MiR-451 increases radiosensitivity of nasopharyngeal carcinoma cells by targeting ras-related protein 14 (RAB14) Tumour Biol. 2014;35:12593–12599. doi: 10.1007/s13277-014-2581-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.