Abstract
We investigated the protective effects exerted by oridonin, the main active constituent of the Chinese medicinal herb Rabdosiarubescens, against lipopolysaccharide (LPS)/D-galactosamine (D-Gal)-induced acute liver injury (ALI). An ALI model was induced in mice using LPS (40 μg/0.5 ml) and D-Gal (5 mg/0.5 ml). The mice were randomly divided into the following five groups of six mice each: one control group (a), one ALI group (b), two oridonin treatment groups (c and d), and one oridonin control group (e). Oridonin (0.2 mg/0.5 ml) was administered once 1 h prior to the LPS/D-Gal challenge in group c and a total of three times over a period of four days, with the last dose given at 1 h before the LPS/D-Gal challenge, in group d. Pretreatment with oridonin improved the survival rate, alleviated histopathological abnormalities, and suppressed plasma aminotransferases in the LPS/D-Gal-challenged mice. Importantly, oridonin attenuated LPS/D-Gal-induced apoptosis in hepatocytes by reducing pro-apoptotic signals (P<0.05), such as tumor necrosis factor-α (TNF-α) and c-Jun N-terminal kinases (JNK). Furthermore, JNK-associated mitochondrial pro-apoptotic proteins were also suppressed by pretreatment with oridonin. Taken together, these data show that oridonin exerts protective effects against LPS/D-Gal-induced ALI in mice via a mechanism that may involve the suppression of the pro-apoptotic cytokine TNF-α and JNK-associated pro-apoptotic signaling.
Keywords: Lipopolysaccharide (LPS), D-galactosamine (D-Gal), drug-induced liver injury, oridonin, apoptosis
Introduction
The liver plays an important, pivotal role in the regulation of body metabolism and detoxification, so injuries to this organ must be rapidly and efficiently remedied [1,2]. Liver injury is induced by hepatotoxins, such as alcohol, acetaminophen, lipopolysaccharide (LPS), and D-galactosamine (D-Gal), and manifests as hepatocyte apoptosis, necrosis, and dysfunction of the liver [2,3]. An animal model of acute liver injury (ALI), established using a combination of LPS and D-Gal, has been used as a mature platform for investigating the mechanisms underlying clinical liver disease and evaluating the effects of hepato-protective agents [4]. Hepatocyte apoptosis, a crucial manifestation of the early stages of ALI [5] and a potential target for drug therapies [6], can be examined with this animal model of ALI.
Oridonin is a well-known tetracyclic diterpenoid (Figure 1A) that isisolated from the Chinese medicinal herb Rabdosiarubescens (dong ling cao in Chinese) [7]. Oridonin has been used for years to treat gastric cancer in traditional Chinese medicine [8] and has attracted considerable attention in recent decades because of its effects against cancer and inflammation-related diseases [9,10]. However, no previous studies have explored the hepato-protective capacity of oridonin. We therefore evaluated the protective effects and mechanisms of action of oridonin in an LPS/D-Gal-induced ALI mouse model. In particular, we focused on the ability of oridonin to inhibit apoptosis.
Figure 1.
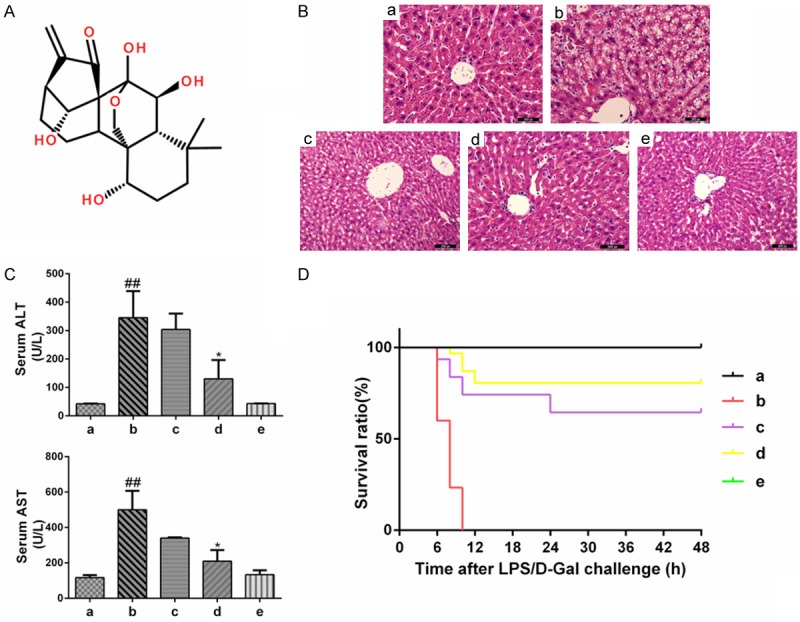
Protective effects of oridonin on LPS/D-Gal-induced ALI. A. The chemical structure of oridonin. B. The levels of ALT and AST in plasma were determined 6 h after LPS/D-Gal exposure using an automated multi-parametric analyzer. The serum ALT and AST levels, which were significantly increased by the LPS/D-Gal challenge, were reduced upon oridonin treatment (P<0.05). C. Representative liver histology of mice in each group (n=6/per group). Liver sections were stained with H&E (original magnification ×200). The severe histological abnormalities were clearly ameliorated in the oridonin treatment groups. D. Survival curve of mice (n=30 per group) following administration of LPS/D-Gal with or without oridonin treatment. Survival of the mice was assessed every 6 h for 2 days, and the cumulative survival curve was constructed using the Kaplan-Meier method. Oridonin pretreatment significantly improved the survival rate of mice with ALI induced by LPS/D-Gal. The survival rate of the model group was 0, and the survival rate in oridonin treatment groups c and d were increased to 64.52% and 80.65%, respectively (P<0.01). The data are expressed as the mean ± SD. Legend: (##) P<0.01 compared with the control group a; (*) P<0.05 compared with the model group b; (a) control; (b) LPS/D-Gal; (c) oridonin/1+LPS/D-Gal; (d) oridonin/3+LPS/D-Gal; (e) oridonin.
Materials and methods
Reagents
Oridonin (empirical formula, C20H28O6; molecular weight, 360.42) was purchased from Selleck (Houston, TX, USA). The purity of the oridonin (isolated from Rabdosiarubescens) was confirmed by HPLC to be >99.81%. The oridonin was initially dissolved in dimethyl sulfoxide (DMSO), stored at -20°C, and thawed before use. LPS (Escherichiacoli, 0111:B4) and D-Gal were purchased from Sigma (Ronkonkoma, NY, USA). A ReverTra Ace qPCR RT Kit was purchased from Toyobo (Osaka, Japan). A SYBR@ Green Real-time PCR Master Mix was obtained from ExCell Bio (Shanghai, China). An In Situ Cell Death Detection Kit was purchased from Roche (Branchburg, NJ, USA). RIPA lysis buffer was obtained from KeyGen Biotech (KGP702-100, Nanjing, Jiangsu, China). Antibodies against c-Jun N-terminal kinases (JNK), phospho-JNK, cytochrome c, caspase-8, caspase-9, caspase-3, bax, bcl-xl, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies were obtained from Beyotime (Shanghai, China).
Animals
The animals were cared for and treated according to the institutional animal care policies of the Shanghai Institute of Planned Parenthood Research. Female C57BL/6 mice (specific pathogen-free), with body weights ranging from 20 to 22 g, were purchased from SIPPR-BK Animal Co., Ltd. (Shanghai, China). All mice were fed a standard laboratory diet and provided with free access to water. The animals were housed under normal laboratory conditions (21±2°C, 12-h light-dark cycle) and acclimated for at least 2 weeks before use.
Experimental protocols
ALI was induced in C57BL/6 mice by an intraperitoneal (i.p.) injection of LPS (40 μg/0.5 ml) plus D-Gal (5 mg/0.5 ml). The mice were randomly divided into the following five groups (n=6 per group): one control group (a), one ALI group (b), two oridonin treatment groups (c and d), and one oridonin control group (e). Oridonin (0.2 mg/0.5 ml) was administered 1 h prior to LPS/D-Gal challenge in group c. In group d, oridonin (0.2 mg/0.5 ml) was administered every 4 days for a total of three times, with the last dose given at 1 h before the LPS/D-Gal challenge. In the ALI and control groups, the mice were injected with the same volume of sterile 0.9% sodium chloride instead of oridonin using the same treatment protocol. All mice were sacrificed by decapitation 6 h after the LPS/D-Gal challenge. Blood samples and livers were then harvested for further analysis. The right lobes of the livers were fixed in 4% paraformaldehyde for morphological analysis, and the remaining liver tissues were stored at -80°C until needed.
To evaluate the potential effects of oridonin on the survival rate in LPS/D-Gal-challenged mice, another set of mice were allocated to the same five groups (n=30 per group) described previously. Survival was assessed in the mice every 6 h for 2 days, and a cumulative survival curve was constructed using the Kaplan-Meier method.
Histological analysis and determination of serum enzymes
Four percent paraformaldehyde-fixed specimens were embedded in paraffin and stained with hematoxylin and eosin using a standard protocol for conventional morphological analysis under a light microscope (Olympus, Tokyo, Japan). The levels of serum enzymatic activity of alanine aminotransferase(ALT) and aspartate aminotransferase (AST) were assessed using a Hitachi 7180 automated multi-parametric analyzer (Hitachi, Tokyo, Japan).
Quantitative real-time polymerase chain reaction (PCR)
The mRNA level of tumor necrosis factor-α (TNF-α) in the livers was determined using quantitative real-time PCR. Briefly, total RNA was isolated from liver samples using TRIzol reagent. Complementary DNA (cDNA) was synthesized according to the manufacturer’s instructions. Real-time PCR was performed using SYBR green PCR Master Mix. The following primers were used for amplification: TNF-α sense 5’-CTCCCAGGTATATGGGCTCA-3’, TNF-α anti-sense 5’-CCAGGTTCTCTTCAAGGGAC-3’, GAPDH sense 5’-TCC AAG GAG TAA GAA ACC CTG GAC-3’, and GAPDH anti-sense 5’-GTTATTATGGGGGTCTGGGATGG-3’. The mRNA level of TNF-α was normalized to that of GAPDH.
Terminal deoxynucleotidyl transferase-mediated nucleotide nick-end labeling (TUNEL) assay
An In Situ Cell Death Detection Kit was used to assess the liver apoptosis according to the manufacturer’s instructions (Branchburg, NJ, USA). Under light microscopy, the number of TUNEL-positive cells (indicated by dark brown precipitate) was counted in 10 randomly selected high-power fields (400× magnification).
Protein isolation and western blot
Total intracellular protein was isolated using RIPA lysis buffer supplemented with a protease inhibitor cocktail. Protein concentrations were determined using the bicinchoninic acid (BCA) method. Equal amounts of protein were loaded into gels to analyze the levels of JNK, phospho-JNK, cytochrome c, caspase-8, caspase-3, caspase-9, bax, and bcl-xl. GAPDH was used as a control in the western blots. The relative density of protein expressions was quantitated using Image J software.
Statistical analysis
All numerical results are expressed as the mean ± S.D. and represent data from a minimum of three independent experiments. The student’s t-test was used to analyze differences between two groups. A Chi-square test was used to compare sample rates among multiple samples. All analyses were performed using GraphPad Prism 6.0 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). The level of statistical significance was set at P<0.05.
Results
Oridonin exerted protective effects on an LPS/D-Gal-induced ALI model in mice
The severe histological abnormalities induced by LPS/D-Gal included the destruction of liver structures, cytoplasmic vacuolization, hemorrhage, and inflammatory cell infiltration. These effects were clearly ameliorated in the oridonin-treated groups (Figure 1B). The levels of serum ALT and AST activities, two important indicators of liver function, were markedly higher at 6 h after LPS/D-Gal exposure than the levels observed in the control group, indicating that severe liver injury was induced in the LPS/D-Gal-challenged mice. Pretreatment with oridonin reduced the extent of ALT and ASL activity increase in a dose-dependent manner. In the oridonin-treated group d, serum ALT and AST activities were significantly lower than in the ALI group (P<0.05, Figure 1C).
Because reduced liver damage is correlated with enhanced survival, we also sought to determine whether pretreatment with oridonin increased the survival rate in mice. After the mice were injected with LPS/D-Gal, they were observed every 6 h to determine the survival rate. The results show that mice pretreated with oridonin had a significantly higher survival rate (Figure 1D). These results indicate that oridonin was effective in protecting mice from LPS/D-Gal-induced ALI.
Oridonin suppressed LPS/D-Gal-induced pro-apoptotic signals
LPS/D-Gal-induced liver injury has been associated with higher levels of hepatic TNF-α expression in mice [5]. Consistent with previous studies, our results show that higher levels of hepatic TNF-α were induced in the mice treated with LPS/D-Gal than in the control group. However, pretreatment with oridonin significantly reduced the level of TNF-α (Figure 2A). Another crucial pro-apoptotic signal, JNK (Figure 2B and 2C), was also expressed at markedly lower levels in LPS/D-Gal model animals pretreated with oridonin [11]. Treatment with oridonin alone did not induce the expression of any pro-apoptotic signals (Figure 2B).
Figure 2.
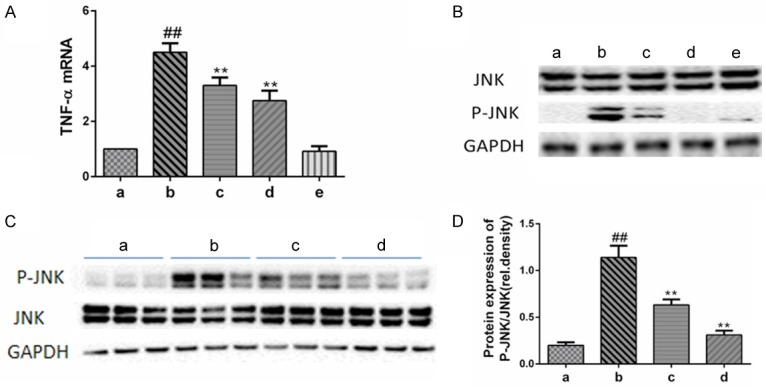
Oridonin suppressed the LPS/D-Gal-induced pro-apoptotic signals. A. The expression of hepatic TNF-α mRNA was determined by real-time PCR. Pretreatment with oridonin significantly inhibited the release of TNF-α induced by LPS/D-Gal (P<0.01). B. The levels of phosphorylated JNK (p-JNK) and total JNK (JNK) in the liver were determined by immunoblot analysis. Pretreatment with oridonin significantly inhibited the phosphorylation of JNK induced by LPS/D-Gal (P<0.01). C. Representative data of JNK/p-JNK from three independent experiments. D. The band intensity in western blots among the four groups was quantified by Image J software. Legend: (##) P<0.01 compared with the control group (a); (**) P<0.01 compared with the model group (b); (a) control; (b) LPS/D-Gal; (c) oridonin/1+LPS/D-Gal; (d) oridonin/3+LPS/D-Gal; (e) oridonin.
Oridonin inhibited LPS/D-Gal-induced hepatocyte apoptosis
Hepatocyte apoptosis may be an early and important event in acute liver injury [6], so we examined the degree of hepatocyte apoptosis in ALI mice in this study. We used TUNEL staining to characterize the apoptotic response and to locate and quantify the number of apoptotic cells in liver sections obtained from ALI mice. There were few apoptotic cells (i.e., TUNEL-positive cells) in the oridonin control group. Furthermore, the number of apoptotic cells in the livers of the LPS/D-Gal-induced ALI mice was significantly higher than the number in the control group. Pretreatment with oridonin effectively reduced the number of apoptotic cells, indicating that cell apoptosis was markedly inhibited (Figure 3A and 3B).
Figure 3.

Oridonin inhibited LPS/D-Gal-induced hepatocyte apoptosis. A. Apoptotic cells were determined by TUNEL assay. TUNEL-positive cells exhibited a dark brown nucleus. Representative liver sections of each group are shown (original magnification ×200). B. Statistical analysis of TUNEL-positive cells. Random counting of 10 fields of view at 400× magnification was performed to calculate the apoptosis rate. Compared with the control group a, the hepatocyte apoptosis rate of mice in the model group b was significantly increased (36.40±1.84 vs. 1.40±0.46, respectively; P<0.01). Pretreatment with oridonin effectively reduced the hepatocyte apoptosis rate to 19.41±3.34% and 11.39±0.29% in groups c and d, respectively (P<0.01). The hepatocyte apoptosis rate of mice in the oridonin control group was only 1.17±0.46%. Legend: (##) P<0.01, compared with the control group a; (**) P<0.01, compared with the model group b; (a) control; (b) LPS/D-Gal; (c) oridonin/1+LPS/D-Gal; (d) oridonin/3+LPS/D-Gal; (e) oridonin.
Oridonin inhibited the expression of apoptosis pathway-related proteins
The most important process during the induction of apoptosis is the activation of a caspase cascade [12]. Our results show that oridonin significantly suppressed the LPS/D-Gal-induced up-regulation of cleaved caspase-9 and cleaved caspase-3. However, the level of cleaved caspase-8 was not affected by oridonin (Figure 4A and 4B). Furthermore, immunoblot analyses showed that bax and cytochrome c expression were increased, indicating the involvement of the mitochondrial apoptotic pathway in LPS/D-Gal-treated mice. Hence, oridonin exerted its beneficial effect by inhibiting bax and the release of cytochrome c, which subsequently inhibited the activation of caspase-9 and caspase-3 and increased the expression of the anti-apoptotic protein bcl-xl (Figure 4A and 4B).
Figure 4.
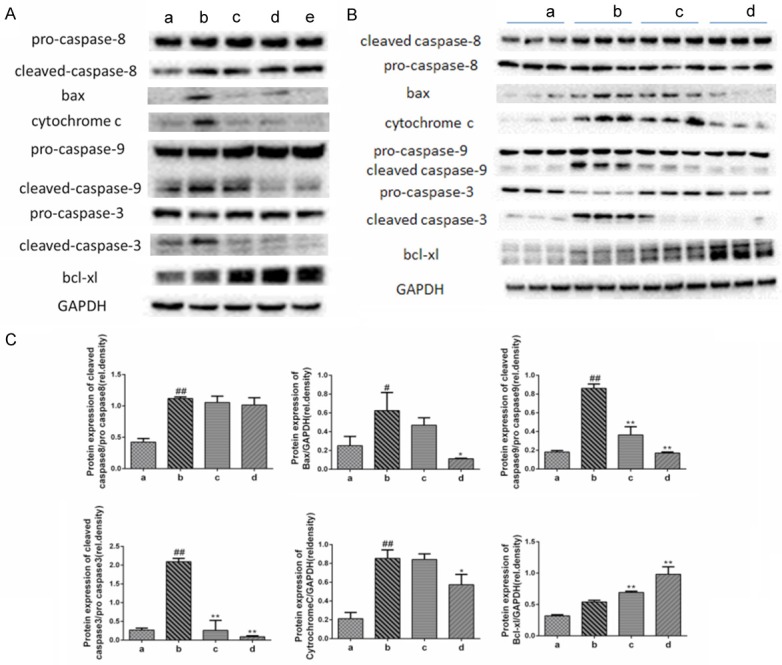
Oridonin inhibited the expression of mitochondrial-related apoptosis proteins. A. Compared with the normal control group a, LPS/D-Gal-induced the activation of caspase-8, caspase-9, bax, cytochrome c, and caspase-3, whereas oridonin intervention significantly inhibited the levels of mitochondrial-related pro-apoptotic proteins and up-regulated the expression of the anti-apoptotic protein bcl-xl (P<0.05). Levels of caspase-8 were not affected by oridonin. B. Representative data of apoptosis-related proteins from three independent experiments. C. The band intensity in western blot among the four groups was quantified by Image J software. Legend (#) P<0.01 and (##) P<0.01 compared with the control group a; (*) P<0.05 and (**) P<0.01 compared with the model group b; (a) control; (b) LPS/D-Gal; (c) oridonin/1+LPS/D-Gal; (d) oridonin/3+LPS/D-Gal; (e) oridonin.
Discussion
Co-administration of D-Gal and the bacterial endotoxin LPS is an established method for inducing ALI in mice that results in a pathological process similar to that of clinical ALI. This model is also an important platform for screening new drugs to treat ALI [13]. Our results show that oridonin improved the survival rate in mice with induced ALI, indicating that oridonin exerts a significant protective effect against ALI. We infer from this result that prompt treatment with oridonin may be beneficial for ALI patients.
Hepatocyte apoptosis, a short-term pathological process, is not only a pivotal pathological manifestation of ALI but also an important target for drug therapies [6,14]. Taking into account the rapid progression and variable courses of ALI, a therapeutic window must be established to improve the prognosis of ALI patients. Therefore, prior to LPS/D-Gal challenge, we pretreated the mice with oridonin. One concern that should be noted is that early endotoxin tolerance resulting from LPS contamination during pretreatment can mitigate the response to direct stimulation with LPS/D-Gal [4]. Hence, we ensured that all of the LPS-related reagents, including oridonin, that were used in these experiments were free of endotoxins. Our results confirm that exposure to LPS/D-Gal induced a wide variety of hepatocyte apoptosis. Oridonin intervention significantly reduced apoptosis in liver cells, and this effect was accompanied by improvements in liver histopathology and serum amino transferases activity. In addition, treatment with oridonin alone induced few apoptotic cells. These results collectively indicate that the protective effects of oridonin may involve the inhibition of hepatocyte apoptosis.
Many types of stimulation induce apoptosis in hepatic cells, including inflammatory factors (such as TNF-α), mitochondrial damage, and hypoxia. These factors often overlap during the course of ALI, resulting in the promotion of the progress of the disease. Death receptor pathways (i.e., those initiated by TNF-α and FasL) and mitochondrial apoptotic pathways (those regulated by the bcl-2 family proteins) are the two classic apoptosis pathways [15]. TNF-α is a macrophage-derived major deleterious pro-apoptotic factor that plays an important role in the pathophysiology of LPS/D-Gal-induced liver damage. However, a number of lines of evidence indicate that natural killer (NK) and natural killer T (NKT) cells generate the TNF-α that is responsible for D-Gal-sensitized LPS lethality [16]. NKT cells must be considered with care because humans and mice may not have comparable resident NKT populations in the liver. However, regardless of its source, TNF-α is absolutely required for D-Gal-sensitized lethality [4]. Numerous studies have demonstrated that the hepatic injury response induced by LPS/D-Gal is essentially eliminated in both TNF-α-deficient and in TNF-α receptor 1 (TNFR1)-deficient mice [14]. Binding to TNFR1 may induce caspase-8-initiated apoptotic cascades [17]. The results presented here show that the induction of TNF-α was suppressed by pretreatment with oridonin in LPS/D-Gal-challenged mice. Consistent with this result, we found that caspase-3 was activated at lower levels after oridonin treatment, whereas caspase-8 expression was not influenced by oridonin. We infer from these results that the protective effects of oridonin may involve decreasing the production of the pro-apoptotic factor TNF-α but may not involve caspase-8.
The sustained activation of JNK is one mechanism involved in LPS/D-Gal-induced liver cell apoptosis [18]. In addition, JNK is over activated before the onset of hepatic apoptosis [11]. JNK is a mitogen-activated protein kinase (MAPK) family member that is thought to be activated by a variety of cell stressors and inflammatory cytokines [19]. These stimulating factors typically activate JNK by inducing TNF-α to bind to TNFR1 [20]. Based on JNK1 and JNK2 null mice or a specific JNK inhibitor, several studies show that JNK exerts its pro-apoptotic effects by inducing bid cleavage, changing the permeability of the mitochondria membrane, and releasing cytochrome c [11,21]. Therefore, TNF-α not only can directly induce apoptosis but can also activate the JNK-mediated mitochondrial apoptotic pathway, which significantly amplifies the apoptosis-promoting effect of TNF-α.
To shed additional light on the protective mechanisms of oridonin, we examined the expression of JNK and mitochondrial apoptotic pathways related to pro-apoptotic and anti-apoptotic proteins. Our results indicate that the increase of TNF-α in LPS/D-Gal-induced ALI mice may be the promoter of hepatocyte apoptosis, and the mitochondrial apoptotic pathway mediated by JNK activation is the key to a large amount of apoptosis. Oridonin intervention can significantly reverse the activation of JNK, down-regulate the levels of mitochondrial-related pro-apoptotic proteins including bax, cytochrome c, and caspase-9, and up-regulate the expression of the anti-apoptotic protein bcl-xl in LPS/D-Gal-induced ALI mice. Furthermore, the whole liver lysate apoptotic profile was unchanged by oridonin alone. Thus, the protective effects of oridonin may be closely associated with inhibition of JNK-related pro-apoptotic signals.
In the past few decades, there has been a growing interest in oridonin due to its anti-tumor and anti-fibrosis activity. Regardless of its anti-tumor or anti-fibrosis activity, both activities are related to the effect of oridonin on inducing cancer cell or hepatic stellate cell apoptosis [10,22,23]. The benefits of oridonin in our study are also related to the ability of oridonin to inhibit hepatocyte apoptosis in the ALI mouse model, supporting the concept that the modulatory effect of oridonin on apoptosis is important and depends on the circumstances.
Our results showed that oridonin has protective effects against ALI induced in mice, which may be related to a reduced production of the pro-apoptotic cytokine TNF-α and suppressed activation of JNK-mediated mitochondrial pro-apoptotic signals. Although the protective mechanisms of oridonin in LPS/D-Gal-induced ALI in mice require more intensive study, the present results portend that oridonin may have wide prospects for application in clinical treatment of ALI.
Acknowledgements
We are indebted to all individuals who participated in or helped with this research project. This research was supported by grants from the National Natural Science Foundation of China (NO: 81570549, NO: 31300959, NO: 81400631); Shanghai Municipal Health Bureau Key Disciplines Grant (ZK2015A24); Natural Science Foundation of the Science and Technology Commission of Shanghai Municipality (14411973700); and Shanghai Municipal Health Bureau, (20134100).
Disclosure of conflict of interest
None.
References
- 1.Adams DH, Ju C, Ramaiah SK. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaeschke H, Gores GJ, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H. Acetaminophen: dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig Dis. 2015;33:464–471. doi: 10.1159/000374090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res. 2004;10:147–162. doi: 10.1179/096805104225004879. [DOI] [PubMed] [Google Scholar]
- 5.Josephs MD, Bahjat FR, Fukuzuka K, Ksontini R, Solorzano CC, Edwards CK 3rd, Tannahill CL, MacKay SL, Copeland EM 3rd, Moldawer LL. Lipopolysaccharide and D-galactosamineinduced hepatic injury is mediated by TNF-α and not by Fas ligand. Am J Physiol Regul Integr Comp Physiol. 2000;278:1196–1201. doi: 10.1152/ajpregu.2000.278.5.R1196. [DOI] [PubMed] [Google Scholar]
- 6.Luo M, Zhao A, Li J. Acute liver injury attenuation of a novel recombinant sTNFR through blocking hepatic apoptosis. Immunopharmacol Immunotoxicol. 2015;37:295–300. doi: 10.3109/08923973.2015.1035390. [DOI] [PubMed] [Google Scholar]
- 7.Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Robert W, Chan L, Richard K. Is there a role for second-line chemotherapy in advancedgastric cancer? Lancet Oncol. 2009;10:903–912. doi: 10.1016/S1470-2045(09)70136-6. [DOI] [PubMed] [Google Scholar]
- 9.Guo W, Zheng P, Zhang J. Oridonin suppresses transplant rejection by depleting T cells from the periphery. Int Immunopharmacol. 2013;17:1148–1154. doi: 10.1016/j.intimp.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Zhen T, Wu CF, Liu P, Wu HY, Zhou GB, Lu Y, Liu JX, Liang Y, Li KK, Wang YY, Xie YY, He MM, Cao HM, Zhang WN, Chen LM, Petrie K, Chen SJ, Chen Z. Targeting of AML1-ETO in t(8;21) leukemia by oridonin generates a tumor suppressor-like protein. Cancer Biol Ther. 2012;4:127–38. doi: 10.1126/scitranslmed.3003562. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Singh R, Lefkowitch JH. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Med. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuñón MJ. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086. doi: 10.3748/wjg.15.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK 3rd, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-α and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 2000;278:1202–1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- 15.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 16.Vinay DS, Choi BK, Bae JS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharideinduced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–4229. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 17.Schwabe RF. Mechanisms of liver injury. I. TNF--induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G9. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 18.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-α-induced apoptosis in liver injury. J Cell Mol Med. 2004;8:445–454. doi: 10.1111/j.1582-4934.2004.tb00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwabe RF, Uchinami H, Brenner DA. Differential requirement for c-Jun NH2-terminal kinase in TNF-α- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004;18:720–743. doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 22.Bohanon FJ, Wang X, Ding C. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res. 2014;190:55–63. doi: 10.1016/j.jss.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DL, Bu HQ, Jin HM. Enhancement of the effects of gemcitabine against pancreatic cancer by oridonin via the mitochondrial caspase-dependent signaling pathway. Mol Med Rep. 2014;10:3027–3034. doi: 10.3892/mmr.2014.2584. [DOI] [PubMed] [Google Scholar]


