Abstract
Hepatocellular carcinoma (HCC) is a common malignant tumor of the digestive system, and patients with advanced HCC have a poor outlook, partly due to resistance to chemotherapeutic drugs. Previous studies have implicated microRNAs in the regulation of chemoresistance, and we have previously shown that microRNA (miR)-205-5p is down-regulated in multiple hepatoma cell lines. Here, we investigate whether miR-205-5p is involved in chemotherapeutic resistance in HCC. Expression of miR-205-5p was measured by real-time quantitative reverse transcription PCR and cell viability was determined using a CCK-8 cell viability assay. Expression of proteins in the PTEN/JNK/ANXA3 pathway were assessed via Western blotting. We found that miR-205-5p expression was down-regulated in all HCC cell lines investigated. In addition, miR-205-5p expression was upregulated by 5-fluorouracil (5-Fu) treatment in Bel-7402 (Bel) cells. Interestingly, miR-205-5p expression was increased in multidrug-resistant Bel-7402/5-Fu (Bel/Fu) cells, compared with Bel cells. We next demonstrated that sensitivity to 5-Fu was increased in Bel/Fu cells after treatment with a miR-205-5p inhibitor. Similarly, increased resistance to 5-Fu was observed in Bel cells after transfection with a miR-205-5p mimic. We injected nude mice with Bel/5-Fu cells to promote tumor growth, and found that co-treatment with a miR-205-5p antagomir and 5-Fu slowed tumor growth more than either treatment alone. Finally, we found that these effects were all associated with changes in the PTEN/JNK/ANXA3 pathway. In conclusion, inhibition of miR-205-5p may reverse chemotherapeutic resistance to 5-Fu, and this may occur via the PTEN/JNK/ANXA3 pathway.
Keywords: MicroRNA-205, hepatocellular carcinoma, chemotherapeutic resistance, PTEN
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant tumor of the digestive system and is the second most common cause of cancer-related deaths worldwide [1]. In patients with advanced HCC, traditional therapies have limited efficacy [2], partly due to resistance to the existing anti-cancer agents [3]. This resistance to chemotherapeutic drugs remains a barrier in HCC research, necessitating the identification of novel and feasible treatment strategies.
Small non-coding RNA (microRNAs), are involved in cell growth, differentiation, programmed cell death and immunity [4,5]. In addition, some microRNAs are associated with cancer (oncomiRs) and can elicit tumor-suppressive functions (ts-miRs) by binding to the 3’ untranslated region of target genes [6,7]. Moreover, many studies have reported that microRNAs are related to the modulation of chemosensitivity and chemoresistance in a diverse population of cancer cells [8-12]. We have previously demonstrated that microRNA 205-5p (miR-205-5p) expression is down-regulated in diverse HCC cells. Therefore, the aims of the present study were to determine if miR-205-5p is involved in the chemotherapeutic resistance of HCC and, if so, to investigate the underlying mechanisms.
Materials and methods
Cell culture
The normal liver cell line LO2, the hepatoma cell line Bel-7402 (Bel), the Bel-7402/5-fluorouracil (Bel/Fu), and the SMMC-7721 were purchased from KeyGen Biotech Co., Ltd. (Nanjing, China). HCCLM3, MHCC97-H, MHCC97-L, and Huh-7 cells were obtained from Professor Liming Wang (The Second Affiliated Hospital of Dalian Medical University). All cells were cultured under standard conditions, and Bel/Fu cells were maintained in medium containing 20 µg/mL of the chemotherapeutic drug 5-Fluorouracil (5-Fu).
Reagents
The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo China Co., Ltd. The miRNA Isolation Kit (DP501), miRNA First-Strand cDNA Synthesis Kit (KR201), miRNA qPCR Detection Kit (SYBR Green, FP401), and hsa-miR-205-5p and hsa-U6 primers were purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The 5-Fu (F6627-1G) was obtained from Sigma-Aldrich (St. Louis, MO, USA). The monoclonal rabbit anti-human antibodies for phosphatase and tensin homolog (PTEN; ab32199), c-Jun N-terminal kinase (JNK)1 + JNK2 + JNK3 (ab208035), phospho Thr183 + T183 + T221 (ab124956), JunD (phospho S100) + c-Jun (phospho S73) (ab178858), hypoxia-inducible factor (HIF)-1-alpha (ab51608), P-glycoprotein (P-gp; ab170904), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; ab181602) were all obtained from Abcam Trading Company Ltd (Shanghai, China). The polyclonal rabbit anti-annexin A3 antibody (bs-1631R) was purchased from Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The micrONTM hsa-miR-205-5p mimic (miR-205-5p mimic), micrONTM mimic negative control, micrOFFTM hsa-miR-205-5p inhibitor (miR-205-5p inhibitor), micrOFFTM inhibitor negative control, riboFECTTM CP reagent, micrOFFTM hsa-miR-205-5p antagomir (antogomiR-205-5p) and micrOFFTM antagomir negative control were produced from RiboBio Co., Ltd. (Guangzhou, China).
Cell viability assay
Cell viability was assessed using the CCK-8 assay, per the manufacturer’s instruction. Briefly, cells (5 × 103) were seeded into 96-well plates and cultured in 100 µL of the corresponding culture medium (described above). After 24 h, different concentrations of 5-Fu agent were added to the culture medium. Following incubation at 37°C for 48 h, the medium was exchanged for 100 µL of medium and 10 µL of CCK-8 reagent. Then, cells were again incubated for 2 h at 37°C. Finally, the optical density was measured using an EnSpireTM 2300 Multilabel Reader (PerkinElmer, Inc., Waltham, MA, USA) at 450 nm. Three replicates were prepared for each condition. The IC50 of 5-Fu in HCC cells were counted.
Real-time quantitative reverse transcription PCR (RT-qPCR)
Total miRNA was extracted from cells using the miRNA Isolation Kit, according to the manufacturer’s instructions. To detect mature miR-205-5p, total miRNA was polyadenylated with poly(A) polymerase, then reverse transcription was performed using a poly(A)-tailed total RNA and reverse transcription primer, per the instructions of the manufacturer of the miRNA First-Strand cDNA Synthesis Kit. RT-qPCR was performed using a Bio-Rad Laboratories (Berkeley, CA) sequence detection system and the miRNA qPCR Detection Kit, according to the manufacturer’s instructions. The data were normalized to endogenous U6 small nuclear (sn)RNA. The expression levels of miRNAs were calculated using the equation 2-ΔΔCT in which ΔCT = CT 205-CT U6 [13].
Transfection of miRNA
Cells were cultured in a 6-well plate for 24 hours and then transfected with miRNA. The miRNA transfections were performed using riboFECTTM CP Reagent, according to the manufacturer’s instructions.
Western blotting
Cells were lysed in 1 mL of RIPA buffer (KeyGen Biotech Co., Ltd. Nanjing, China) supplemented with 10 μL phenyl methane sulfonyl fluoride (KeyGen Biotech Co., Ltd. Nanjing, China) and 10 μL of phosphatase inhibitor cocktail (KeyGen Biotech Co., Ltd. Nanjing, China) to obtain all cellular protein. Protein concentrations were determined using a BCA Protein Assay Kit (Beyotime. Shanghai, China) and subsequently boiled for 10 min at 100°C. Samples containing equal protein concentrations were separated by 10% SDS-PAGE gels and then transferred onto polyvinylidene difluoride membranes (Millipore, USA). Finally, protein band intensities were evaluated using a FUSION FX5 Gel Imaging System (VILBER, France) using Western Bright Sirius ECL kit (Advansta, Menlo Park, CA, USA) and normalized to corresponding GAPDH bands. All experiments were performed at least three times.
Animal studies
Only male nude (nu/nu) mice (4-6 weeks old) were used, and all animal experiments were performed in the SPF Laboratory Animal Center at Dalian Medical University. All animal maintenance and procedures were carried out in strict accordance with the guidelines of the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals as well as the recommendations established by the Animal Care and Ethics Committee of Dalian Medical University. The protocol was also approved by the Animal Care and Ethics Committee of Dalian Medical University.
Bel-7402/5-Fu cells (1-5 × 106 in 100 μL of phosphate buffered saline) were injected subcutaneously into the left armpit of the nude mice. After two weeks, when the tumor diameters had reached 4-5 mm, mice were randomly divided into four groups (n = 5 mice/group) which received the following treatments: un-treated (“NS” group), injections of 30 mg/kg 5-Fu in saline buffer (“5-Fu” group), injections of 10 nmol of antagomiR-205-5p (an inhibitor of miR205-5p) in 50 μL of saline buffer (“miR” group), or simultaneous injections of both antagomiR-205-5p and 5-Fu (“5-Fu+miR” group). Tumors were measured with a vernier caliper every four days, and tumor volume was calculated using the formula V = 1/2 a2 × b (where a is the length and b is the width). Five weeks after tumor cell inoculation, all mice were terminated with ether anesthesia and the total weight of the tumors in each mouse was determined.
Statistical analysis
All experiments were repeated three times. The student’s t-test and one-way analysis of variance (ANOVA) were performed, and a p-value of <0.05 was defined as statistical significance. SPSS 20.0 software was used for all statistical analyses.
Results
Expression of miR-205-5p in hepatoma cells
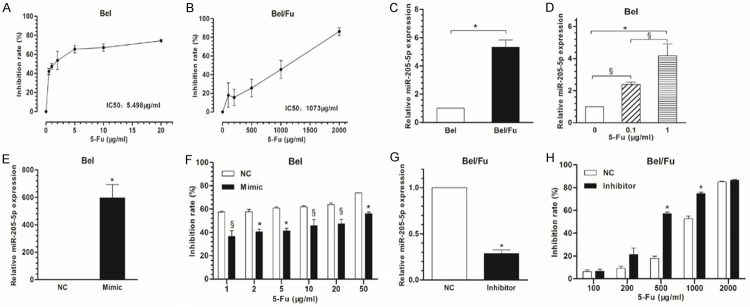
Decreased expression of miR-205-5p was observed in all lines of HCC cells (HCCLM3, MHCC97-H, MHCC97-L, Huh-7, Bel-7402, SMMC-7721 cells) compared with the normal liver cell line, LO2 (Figure 1). The IC50 of 5-Fu was determined in the HCC cell line Bel and the multidrug-resistant cell line Bel/Fu and found to be 5.498 μg/mL and 1073 μg/mL, respectively (Figure 2A, 2B). Interestingly, miR-205-5p expression was increased in Bel/Fu cells compared with normal Bel cells (Figure 2C).
Figure 1.
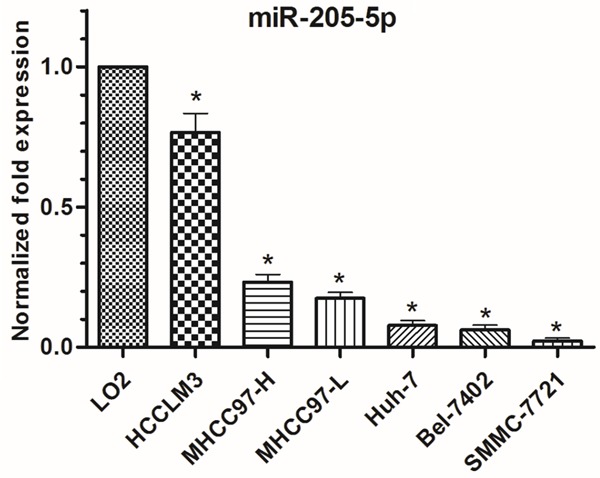
Expression of miR-205-5p was detected in multiple hepatocellular carcinoma cell lines using RT-qPCR and normalized to expression levels in normal liver cells (LO2). The data are presented as the mean ± SD of three independent experiments *p<0.01.
Figure 2.
Expression of miR-205-5p regulates chemotherapeutic resistance. The IC50 of 5-Fu was determined using a CCK-8 cell viability assay in Bel cells and multidrug-resistant Bel/Fu cells (A, B). The expression of miR-205-5p was determined in Bel/Fu cells using RT-qPCR and compared with that of Bel cells (C). Expression of miR-205-5p was reduced in Bel cells by 5-Fu treatment, in a dose-dependent manner (D). Bel cells were transfected with hsa-miR-205-5p, a small double-stranded RNA molecule that mimics mature endogenous miR-205-5p, and the transfection efficiency is shown in (E), compared with the non-transfected control (NC). Transfection with the miR-205-5p mimic increased the resistance of Bel cells to 5-Fu, at all doses tested, as determined by a cell viability assay (F). Multidrug-resistant Bel/Fu cells were transfected with a miR-205-5p inhibitor and the transfection efficiency is shown in (G). Transfection with the miR-205-5p inhibitor decreased the resistance of Bel/Fu cells to 5-Fu (H). The data are presented as the mean ± SD of three independent experiments. *p<0.01, §p<0.05.
Chemotherapeutic resistance is regulated by miR-205-5p expression in vitro
Treatment with 5-Fu increase the expression of miR-205-5p in Bel cells in a dose-dependent manner (Figure 2D). Bel cells were transfected with hsa-miR-205-5p, which mimics mature endogenous miR205-5p. Transfection with the miR-205-5p mimic increased the resistance of Bel cells to 5-Fu, at all doses tested (Figure 2E, 2F). Conversely, transfection of multidrug-resistant Bel/Fu cells with a miR-205-5p inhibitor reversed the chemotherapeutic resistance of these cells to 5-Fu (Figure 2G, 2H).
Inhibition of miR-205-5p reverses chemotherapeutic resistance in vivo
Nude mice were injected with Bel/5-Fu cells to promote the growth of HCC xenografts. Tumor growth was slower in the 5-Fu+miR group (co-treated with a miR-205-5p inhibitor and 5-Fu) than in the 5-Fu or miR groups alone (Figure 3A, 3B). This finding was further corroborated with both tumor volume and weight (Figure 3C, 3D).
Figure 3.
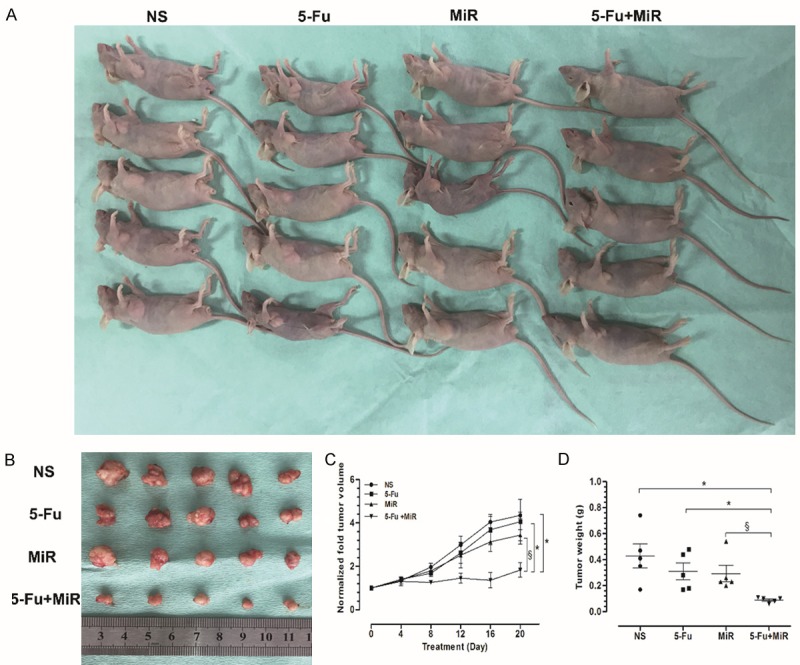
Inhibition of miR-205-5p reverses chemotherapeutic resistance in vivo. Hepatocellular carcinoma xenografts were established by injecting Bel/5-Fu cells into nude mice (A). The growth of tumor xenografts was slower in the combined 5-Fu+miR treatment group than in either the 5-Fu or miR groups alone (B). Tumor volume (C) and weight (D) are shown for each treatment group over time. The data are presented as the mean ± SD of three independent experiments. (NS: control group; 5-Fu: 5-Fu treatment group; miR: antagomiR-205-5p treatment group; 5-Fu+miR: co-treatment with 5-Fu and antagomiR-205-5p). *p<0.01, §p<0.05.
Regulation of chemotherapeutic resistance by miR-205-5p occurs via the PTEN/JNK/ANXA3 pathway
To identify a molecular mechanism by which miR-205-5p elicits its effects, we investigated proteins in the PTEN/JNK/ANXA3 pathway using Western blotting. We found that the expression of PTEN was reduced after 5-Fu treatment in Bel cells. Furthermore, the expression of phosphorylated JNK, along with c-Jun, which is a protein downstream of JNK, was increased. Expression of ANXA3, a member of the annexin family, was also increased by 5-Fu treatment in Bel cells. Finally, the high expression ANXA3 enhanced the expression of HIF-1а and P-gp. These findings were corroborated by our results in Bel cells transfected with a miR-205-5p mimic, and in Bel/Fu cells transfected with a miR-205-5p inhibitor (Figures 4, S1).
Figure 4.
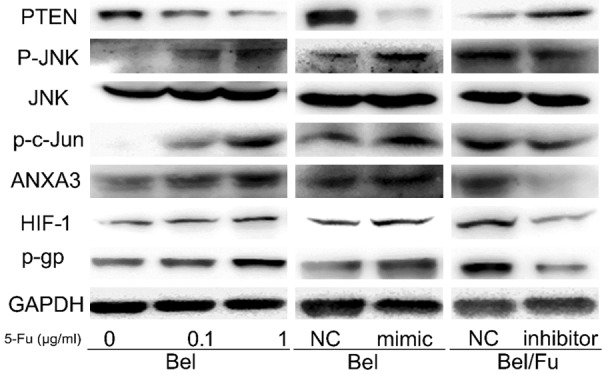
Expression of proteins in the PTEN/JNK/ANXA3 pathway. Protein levels were analyzed by Western botting in Bel cells with the indicated doses of 5-Fu treatment, in Bel cells following transfection of a miR-205-5p mimic, and in Bel/Fu cells following transfection of a miR-205-5p inhibitor. All protein band intensities were normalized to the corresponding GAPDH band. NC: non-transfected control; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; JNK: c-Jun N-terminal kinase; P-JNK: phosphorylated JNK; ANXA3: annexin 3; HIF-1: hypoxia-inducible factor 1; P-gp: P-glycoprotein.
Discussion
Several recent studies have demonstrated that miR-205 functions as an oncogene or tumor suppressor gene in multiple cancer cell lines [14-18]. However, the involvement of miR-205 in HCC remains to be elucidated [19,20]. In our study, we found that expression of miR-205 was suppressed in multiple HCC cells lines; specifically, Bel-7402, MHCCLM3, Huh-7, HCC97-H, HCC97-L and SMMC-7721, compared with a normal liver cell line (LO2).
MicroRNAs have been reported to be involved in diverse biological processes, as well as in carcinogenesis by regulating gene expression. The present study showed that miR-205-5p expression was induced by 5-Fu treatment in Bel cells, and that Bel/Fu cells express miR-205-5p more highly than Bel cells. Therefore, we speculate that miR-205-5p may play an important role in the regulation of the chemotherapeutic resistance of HCC. In addition, we further demonstrated that transfection with a miR-205-5p mimic increased the resistance of Bel cells to 5-Fu. Similarly, transfection of Bel/Fu cells with a miR-205-5p inhibitor increased sensitivity to 5-Fu. Our findings in vitro were in agreement with our in vivo experiments, which showed that tumor growth was slower in mice treated with both antagamiR-205-5p and 5-Fu, compared to either treatment alone. Taken together, our findings show that miR-205-5p is involved in the regulation of chemotherapeutic resistance in HCC.
Previous studies have demonstrated that PTEN is a direct target of miR-205-5p [21-24], which regulates the expression of PTEN by binding to the 3’ untranslated region in multiple cancers. The JNK/c-Jun pathway has also been shown to be involved in the chemoresistance of HCC [25], and phosphorylation of JNK may be associated with PTEN [26]. The protein ANXA3, a member of the annexin family, is downstream of the JNK/c-Jun pathway [27]. A previous study revealed that ANXA3 expression is increased in Bel/Fu cells, compared with Bel cells, by liquid chromatography-mass spectrometry (MS)/MS [28], a high-throughput proteomic technology. Furthermore, ANXA3 promotes resistance to chemotherapy in HCC mainly by enhancing the expression of HIF-1 [29]. In our study, we verified that PTEN expression was reduced, and the expressions of phosphorylated JNK, c-Jun, ANXA3, HIF-1а and p-gp were increased, by 5-Fu treatment in Bel cells. These findings corresponded to our findings in Bel cells following transfection with the miR-205-5p, as well as those in Bel/Fu cells following miR-205-5p inhibitor transfection. Taken together, these results suggest that the PTEN/JNK/ANXA3 pathway may be involved in the regulation of chemotherapeutic resistance in HCC cells.
In conclusion, the present study provides experimental evidence that inhibition of miR-205-5p improves the responsiveness of Bel/Fu cells to 5-Fu treatment. In addition, miR-205-5p may be a novel therapeutic target for HCC.
Acknowledgements
This research was supported by the Liaoning Province Nature Science Foundation of China (No: 201602226).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Zhu AX. Systemic treatment of hepatocellular carcinoma: dawn of a new era? Ann Surg Oncol. 2010;17:1247–1256. doi: 10.1245/s10434-010-0975-6. [DOI] [PubMed] [Google Scholar]
- 3.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z, Liu J, Cui Y, Bian X, Bie P, Qian C. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011;310:160–169. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Giovannetti E, Erozenci A, Smit J, Danesi R, Peters GJ. Molecular mechanisms underlying the role of microRNAs in anticancer drug resistance and implications for clinical practice. Crit Rev Oncol Hematol. 2012;81:103–122. doi: 10.1016/j.critrevonc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Li X, Liao D, Wang X, Wu Z, Nie J, Bai M, Fu X, Mei Q, Han W. Elevated microRNA-23a expression enhances the chemoresistance of colorectal cancer cells with microsatellite instability to 5-fluorouracil by directly targeting ABCF1. Curr Protein Pept Sci. 2015;16:301–309. doi: 10.2174/138920371604150429153309. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Wan G, Spizzo R, Ivan C, Mathur R, Hu X, Ye X, Lu J, Fan F, Xia L, Calin GA, Ellis LM, Lu X. MiR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8:83–92. doi: 10.1016/j.molonc.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmittgen TD, Livak KJ. Analyzing realtime PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 14.Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta P, Valdagni R, Daidone MG, Zaffaroni N. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase cepsilon. Cancer Res. 2009;69:2287–2295. doi: 10.1158/0008-5472.CAN-08-2894. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Zhu S, Mo YY. Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res. 2009;19:439–448. doi: 10.1038/cr.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dar AA, Majid S, de Semir D, Nosrati M, Bezrookove V, Kashani-Sabet M. MiRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. J Biol Chem. 2011;286:16606–16614. doi: 10.1074/jbc.M111.227611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majid S, Saini S, Dar AA, Hirata H, Shahryari V, Tanaka Y, Yamamura S, Ueno K, Zaman MS, Singh K, Chang I, Deng G, Dahiya R. MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal cancer. Cancer Res. 2011;71:2611–2621. doi: 10.1158/0008-5472.CAN-10-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hezova R, Kovarikova A, Srovnal J, Zemanova M, Harustiak T, Ehrmann J, Hajduch M, Sachlova M, Svoboda M, Slaby O. MiR-205 functions as a tumor suppressor in adenocarcinoma and an oncogene in squamous cell carcinoma of esophagus. Tumour Biol. 2016;37:8007–8018. doi: 10.1007/s13277-015-4656-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Zhang J, Cui M, Liu F, You X, Du Y, Gao Y, Zhang S, Lu Z, Ye L, Zhang X. Hepatitis B virus X protein inhibits tumor suppressor miR-205 through inducing hypermethylation of miR-205 promoter to enhance carcinogenesis. Neoplasia. 2013;15:1282–1291. doi: 10.1593/neo.131362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Li J, Guo W, Liu W, Yu J, Song W, Dong L, Wang F, Yu S, Zheng Y, Chen S, Kong Y, Liu C. Targeting the microRNA-21/AP1 axis by 5-fluorouracil and pirarubicin in human hepatocellular carcinoma. Oncotarget. 2015;6:2302–2314. doi: 10.18632/oncotarget.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei L, Huang Y, Gong W. MiR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30:2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 22.Bai J, Zhu X, Ma J, Wang W. MiR-205 regulates A549 cells proliferation by targeting PTEN. Int J Clin Exp Pathol. 2015;8:1175–1183. [PMC free article] [PubMed] [Google Scholar]
- 23.Xin W, Liu X, Ding J, Zhao J, Zhou Y, Wu Q, Hua K. Long non-coding RNA derived miR-205-5p modulates human endometrial cancer by targeting PTEN. Am J Transl Res. 2015;7:2433–2441. [PMC free article] [PubMed] [Google Scholar]
- 24.Jang SJ, Choi IS, Park G, Moon DS, Choi JS, Nam MH, Yoon SY, Choi CH, Kang SH. MicroRNA-205-5p is upregulated in myelodysplastic syndromes and induces cell proliferation via PTEN suppression. Leuk Res. 2016;47:172–177. doi: 10.1016/j.leukres.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Tang PM, Zhang DM, Xuan NH, Tsui SK, Waye MM, Kong SK, Fong WP, Fung KP. Photodynamic therapy inhibits P-glycoprotein mediated multidrug resistance via JNK activation in human hepatocellular carcinoma using the photosensitizer pheophorbide a. Mol Cancer. 2009;8:56. doi: 10.1186/1476-4598-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F, Ciuffreda L. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong M, Fung TM, Luk ST, Ng KY, Lee TK, Lin CH, Yam JW, Chan KW, Ng F, Zheng BJ, Yuan YF, Xie D, Lo CM, Man K, Guan XY, Ma S. ANXA3/JNK signaling promotes self-renewal and tumor growth, and its blockade provides a therapeutic target for hepatocellular carcinoma. Stem Cell Reports. 2015;5:45–59. doi: 10.1016/j.stemcr.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong SW, Yang YX, Hu HD, An X, Ye F, Hu P, Ren H, Li SL, Zhang DZ. Proteomic investigation of 5-fluorouracil resistance in a human hepatocellular carcinoma cell line. J Cell Biochem. 2012;113:1671–1680. doi: 10.1002/jcb.24036. [DOI] [PubMed] [Google Scholar]
- 29.Pan QZ, Pan K, Weng DS, Zhao JJ, Zhang XF, Wang DD, Lv L, Jiang SS, Zheng HX, Xia JC. Annexin A3 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma. Mol Carcinog. 2015;54:598–607. doi: 10.1002/mc.22126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.