Abstract
Revision anterior cruciate ligament (ACL) reconstruction is substantially more challenging than primary reconstruction. Management of previously malpositioned or widened tunnels often requires innovative approaches for managing bony defects. Massive osteolysis with poor bone stock and convergence or overlapping of revision tunnels into the previously placed tunnels may necessitate a staged revision procedure. In this surgical technique description, we describe a method for the management of bony deficiencies using allograft bone dowels in staged revision ACL reconstruction.
Anterior cruciate ligament (ACL) injuries have become increasingly common in the United States, numbering 200,000 cases per year,1, 2 and approximately 120,000 to 150,000 of these undergo reconstruction.3, 4 Although long-term functional stability and symptom relief can be achieved in the majority of patients following ACL reconstruction, approximately 2% to 10% of patients will eventually require revision ACL reconstruction.5 The number of patients undergoing ACL reconstruction appears to be increasing annually; therefore the need for revision surgery is also likely to increase. It is estimated that between 2,900 and 13,000 patients will require a revision ACL reconstruction each year.6
There is no single standard revision procedure, but it is well-known that revision ACL reconstruction is substantially more challenging than primary surgery. Management of previously malpositioned or widened tunnels often requires innovative approaches for managing bony defects.7, 8, 9 Revision surgery can be performed in a one-stage or a 2-stage fashion. Achieving stable graft fixation is extremely difficult in the setting of tunnel convergence with the expansion or overlapping of revision tunnels into previous tunnels and with massive osteolysis with poor bone stock.10 To address these problems, staged ACL revision surgery with an initial procedure for tunnel grafting to ensure adequate bone stock for proper tunnel placement at a later revision surgery should be considered.11, 12 Several techniques for bone grafting the tunnels in a staged ACL revision procedure have been described, including allograft chips, struts, and autografts from the iliac crest.11, 13, 14, 15 Bone dowels are structural allografts commercially available in different lengths and diameters to fill the bone defect with varying sizes. They are easy to use and avoid donor site morbidity. Furthermore, they afford sufficient stability for the graft fixation at the second-stage revision. In this surgical technique description, we describe a method for the management of the bone deficiencies using allograft bone dowels in staged revision ACL reconstruction.
Surgical Technique
Preoperative Evaluation
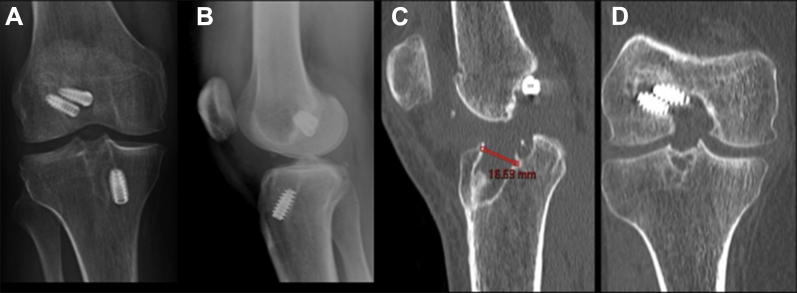
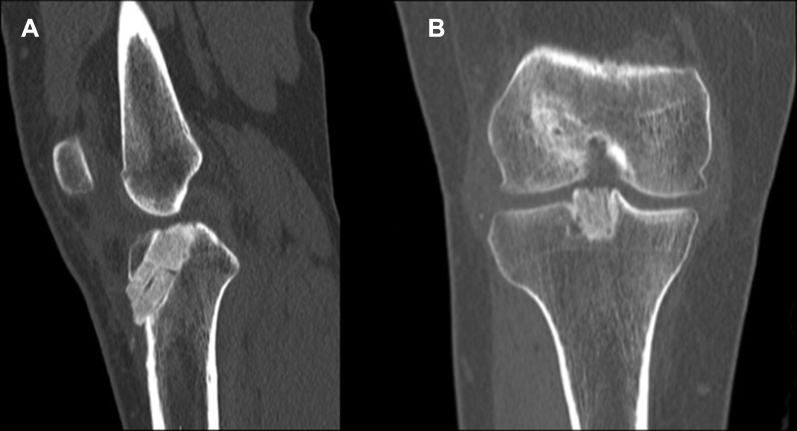
When a revision is planned for a failed ACL reconstruction, reviewing the previous operative notes, imaging studies, and arthroscopic images can provide important information about the previously used technique, fixation methods, and implant location. Previous fixation hardware and location of tunnels should be assessed on anteroposterior and lateral radiographs in full extension. If there is any concern about tunnel osteolysis, it is helpful to obtain a noncontrast computed tomography (CT) scan preoperatively. CT scans with/without three-dimensional reconstructions may provide further information regarding the amount of osteolysis, as plain films may underestimate the defect's size (Fig 1). Recent studies demonstrate CT scans are the most reliable imaging modality for evaluation of ACL bone tunnels when compared with magnetic resonance imaging and radiographs, with superior intra- and interobserver reliability.16, 17 Furthermore, the degree of osteolysis is crucial to determine whether a 2-stage reconstruction with bone grafting is required. In the setting of prior anatomic or nonanatomic tunnel placement, greater than 14 mm of tunnel osteolysis is a general guideline for staged reconstruction.18
Fig 1.
(A and B) Anteroposteral/lateral radiographs, right knee. Note the nonuniform defect size and shape within the tibial tunnel. (C and D) Sagittal/coronal computed tomography images, right knee. Note the nonuniform defect size and shape within the tibial tunnel, which measured 16.59 mm at its widest point orthogonal to the axis of the tunnel.
Surgical Technique
The patient is placed in the supine position on the operating table with a thigh tourniquet and standard arthroscopic set-up. An Alvarado leg holder (Zimmer, Warsaw, IN) is applied to hold the patient's knee in the desired position during the surgery. After performing a routine diagnostic arthroscopy to address concomitant pathology as indicated (i.e. loose body removal, addressing any chondral lesions, partial meniscectomy, or meniscal repair), the femoral tunnel osteolysis can be addressed first if a previous independent tunnel technique has been used. Otherwise, previous transtibial surgical techniques can allow the surgeon to address any femoral lesion through the prior tibial tunnel, which can not only be technically easier from an instrumentation perspective, but also can allow passage of the allograft dowel through the tibial tunnel, once prepared, into the femoral defect. More recently, prior independent femoral tunnel drilling techniques have been used, which increases tunnel obliquity given a lower placement on the wall. In this setting, hyperflexion of the knee to obtain colinear instrumentation placement is paramount. The prior femoral tunnel/ACL graft origin is identified. The prior hardware is removed as needed. If the hardware can be used as a void filler or is a biocomposite and can be drilled through, then removal is not entertained. After debriding the remaining soft-tissue graft present by arthroscopic shaver and biter (Smith and Nephew Endoscopy, Andover, MA), an appropriately sized tunnel dilator (Depuy, Raynham, MA) is centered into the cavitary defect present. This dilator is used to center the placement of a standard Beath pin into the prior femoral tunnel and pass it retrograde, exiting on the superolateral aspect of the thigh. The femoral defect is then sequentially reamed to debride all prior soft tissue. Great care is taken to directly view the posterior wall and the new tunnel through the anteromedial portal after each successive reaming. Sometimes fluoroscopy maybe helpful to review the new tunnel as well. This is to ensure no cortical wall blowout, which could potentially jeopardize the bone dowel graft passage as line to line. The key to this line-to-line fit technique is to obtain a perfectly cylindrical wall in the reamed tunnels. Next a prefashioned allograft cylindrical Cloward dowel by LifeNet (MatriGRAFT, Virginia Beach, VA), which comes prepackaged as different lengths, 15 to 30 mm, and diameters, ranging from 10 to 20 mm, is selected as a line-to-line fit for impaction. The dowel's edge is slightly bulleted for easier insertion into the tunnel. If an independent femoral drilling tunnel was used then the anteromedial portal or possible accessory anteromedial portal, if used, will need to be widened to pass the graft with ease. Otherwise, as previously mentioned, the tibial tunnel after being prepared in the below listed fashion can be used to pass the femoral graft safely as well if a prior transtibial technique was used during the index procedure. The dowel comes partially cannulated, and so it is completed with a 3/32″ pin so that it can easily slide over the Beath pin. Using an in-house machined cannulated bone tamp that can accommodate a Beath pin, the dowel is impacted into the freshly reamed femoral tunnel as a line-to-line fit (Fig 2). The Beath pin is removed, and final adjustments are made with a bone tamp so that it is flush to the wall's edge (Fig 3). Great care is taken when making final adjustments not to crack the dowel or fragment its edge, which can affect the bone tunnel-graft occupation ratio and destabilize the graft. If a second dowel is needed for length, which usually is not found to be the case as it is on the femoral side, then it is stacked, and both can be inserted at the same time over the Beath pin during impaction. If any significant protuberance of bone is present and if any further impaction would result in graft fragmentation, then the arthroscopic burr is used to resect this prominence so the graft is flush to the wall's edge.
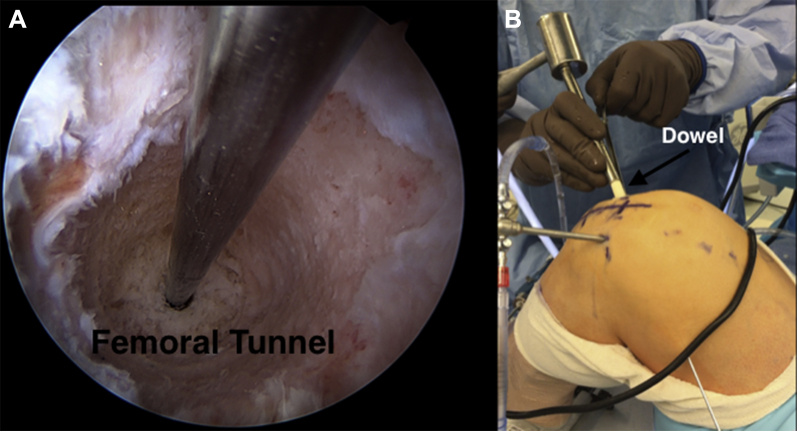
Fig 2.
(A) Arthroscopic image, anteromedial viewing portal, left knee. The Beath pin is placed along the long axis of the femoral defect, and the defect is sequentially reamed to obtain a perfectly cylindrical, contained wall. (B) Intraoperative image, left knee. A cannulated dowel is slid over the Beath pin and tamped into the freshly reamed femoral tunnel through the far medial portal.
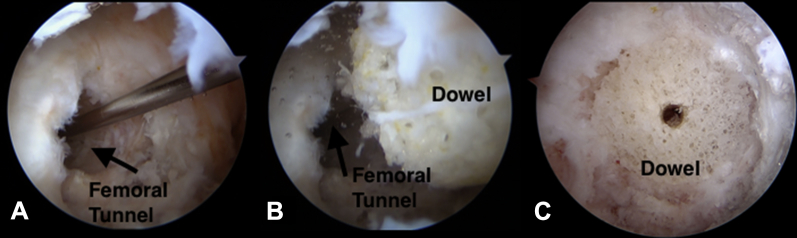
Fig 3.
(A-C) Arthroscopic images, right knee, anterolateral viewing portal. The dowel is placed over the Beath pin and is gently impacted into the defect. The Beath pin is removed, and final adjustments are made with the bone tamp so that it is flush to the wall's edge.
Prior surgical skin incisions are routinely used for the tibial exposure. If previous hardware is encountered, then it is removed. The prior tibial tunnel is identified, and a similar technique of placing an appropriately sized tunnel dilator (Depuy) into the aperture is used. Next a Beath pin is advanced through the dilator and viewed arthroscopically to be exiting in what would be the previous tibial tunnel/ACL insertion point. If there is a bony block that limited dilator insertion, then the standard tibial guide is used to place a 3/32″ guide pin into the center of the prior tunnel as viewed arthroscopically. Preoperative imaging can be used to calculate the previous tibial tunnel angle measurement on sagittal CT scan so the same angle measurement can be used through the tibial aiming guide (Smith and Nephew Endoscopy) to place the 3/32″ guide pin into the center of the footprint/insertion. Intraoperative fluoroscopy is then used to confirm adequate placement. Once confirmed, sequential reaming is performed to the desired diameter to adequately debride the remnant soft-tissue graft from the tunnel. Again the arthroscope is used to view inside the tunnel to review the process of tunnel preparation, namely, the production of a cylindrical tunnel without evidence of eccentric reaming or prior graft presence. The guide pin is adjusted intra-articularly with a Kocher clamp to direct the reamer as needed for debridement of previous soft-tissue graft and to create the cylindrical bone tunnel. Using the same technique, a line-to-line allograft bone dowel is selected, 2 if needed for length, and impacted into place, taking care to not overinsert the graft while viewing arthroscopically (Fig 4). Any excess graft intra-articularly is addressed once again by arthroscopic burr, and an oscillating saw is used to resect any graft extending beyond the tibial cortex. Intraoperative fluoroscopy is used to assess the overall grafting of the prior osseous defects in the femur and tibia to ensure adequate fit and fill. The wounds are thoroughly irrigated with copious normal saline, and layers are closed in successive fashion (Video 1). The pearls and pitfalls of this surgical technique are summarized in Table 1.
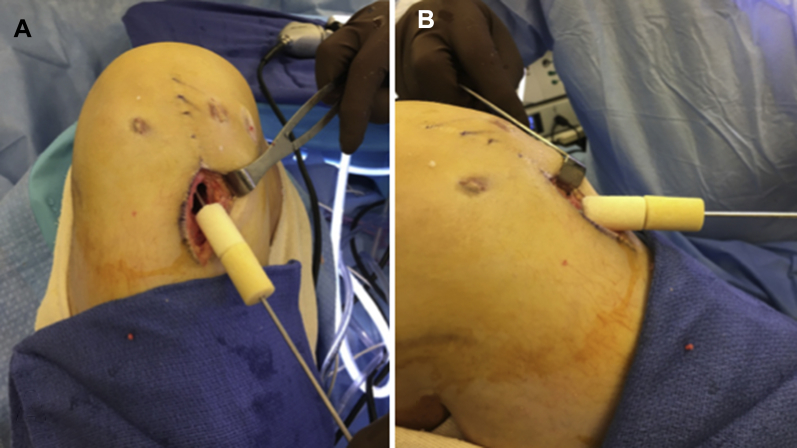
Fig 4.
(A and B) Intraoperative images, right knee. For long tibial bone deficiencies, dowels can be stacked on top of each other to fill the defect along its axis.
Table 1.
Pearls and Pitfalls
| Pearls |
| Preoperatively assess and measure the extent of osteolysis on computed tomography scans. |
| Sequential reaming to remove fibrous and sclerotic tissue. |
| Place the scope inside the tunnel to check tunnel preparation after finished reaming. |
| Retain previous hardware as a void filler if it does not interfere with the new tunnel. |
| Measure the tunnel length to select adequate allograft dowel length. |
| If necessary, use multiple dowels to ensure completely filling the defect. |
| Impact dowels in a line-to-line fashion. |
| Resect prominence of dowels with an arthroscopic burr. |
| Pitfalls |
| Secure Beath pin position during sequential reaming. |
| Avoid excessive force during impaction tamping the dowels. |
| In postoperative period, avoid high-impact activities. |
Postoperative Regimen
Patient age, athletic demands, and size of tunnel grafting required should be considered when planning a rehabilitation program. After surgery, a supervised rehabilitation protocol is instituted immediately to control pain and swelling. Patients are instructed to continue with early range of motion and quadriceps reconditioning exercises. Weight bearing is not limited and allowed as tolerated. Treadmill walking, stationary bicycle, and aquatic therapy are stressed gradually to increase knee motion, strengthen the extremity, and begin gait training as part of an initial regimen. We do not recommend the use of functional braces given no existing braces have been successfully validated in the literature to restore normal anterior stability to the ACL-deficient knee.19 At 5 to 6 weeks after surgery, patients are started on an elliptical trainer and conventional weight machines with closed-chain exercises. Recommendations are made against any high-impact and pivoting activities given the ACL-deficient knee between first and second stage of revision ACL reconstruction.
Routine postoperative radiographs are obtained at 2 weeks and 3 months to assess the overall total graft incorporation. For a more detailed assessment and to ascertain the amount of graft incorporation, which aids in the determination of the timing of revision ACL reconstruction, a CT scan is obtained at 4 to 6 months postoperatively (Fig 5).
Fig 5.
(A and B) Sagittal/coronal postoperative computed tomography (CT) images, right knee. Note the excellent integration and incorporation of allograft dowels to host bone with excellent fit and fill on 4-month postoperative CT scan.
Discussion
It is clear that femoral or tibial tunnel malposition generates excessive stress in the ACL graft as the knee moves through its arc of motion, resulting in graft failure.20, 21 Furthermore, locations and angles of tunnels are thought to correlate with tunnel enlargement because of windshield-wiper or bungee-cord motion of the graft, which may be enhanced by changing tension in the graft due to tunnel malposition.22 These wholly expansive, nonuniform lesions can make graft hardware fixation and bone tendon healing difficult to optimize. In the setting of these bony defects, a 2-stage ACL reconstruction should be entertained to first adequately replenish the bone stock, and once incorporated, then proceed with the revision ACL reconstruction.
Several investigators have described different kinds of grafting material and techniques for grafting previous tunnels in the setting of revision ACL surgery. Thomas et al. reported on their results using autograft iliac grafts in the form of dowels for grafting the tibial tunnels. Laxity measurements achieved with revision ACL reconstruction using a 2-stage technique with bone grafting of the tibial tunnels were similar to those achieved after primary ACL reconstructions.11 Said et al. described a technique using the OATS grafting instrumentation (Osteochondral Autologous Transfer System; Arthrex, Naples, FL) for femoral and tibial tunnel impaction grafting in 2-stage ACL revisions. The appropriately sized OATS harvester is chosen to be 1 mm larger than the tunnel size and is used to harvest bone graft from the iliac crest through a percutaneous approach.13 To overcome iliac crest donor site morbidity, Franceschi et al. proposed a technique to fill femoral tunnels using grafts from the tibial metaphysis using an OATS harvester.23 Although Lysholm and International Knee Documentation Committee scores were significantly improved compared with preoperative levels, these 2 techniques have a limitation in the size of grafts obtained secondary to the OATS harvester size restrictions.
The technique presented here is a simple method and may have some advantages compared with previous techniques (Table 2). Using a cannulated allograft dowel eliminates the need for concurrent graft harvesting. Sequential reaming of the tunnel to the widest diameter of the irregular cavity will allow the surgeon to achieve a newly prepared, cylindrical tunnel that can be filled with off-the-shelf commercially available dowels with different widths and lengths. When properly placed, an allograft bone dowel affords sufficient stability to allow use of the surgeons' choice of graft fixation, including interference screws.9 Furthermore, particularly for long tibial bone deficiencies, dowels can be placed in a stacked fashion to fill the defect along its longitudinal axis. A potential limitation of this technique is that the maximum diameter of dowels is 20 mm, which may limit the use of these allografts in larger defects. In these circumstances, a fashioned femoral head allograft is preferred for filling the defects. Another limitation of this technique, especially in femoral tunnels, is that the dowel may crack if an excessive stress is imparted while tamping the graft. To avoid this complication, the surgeon must be wary of removing any fibrous tissue remnants or sclerotic tissues along the tunnels before grafting with the allograft dowels and bulleting the ends of the dowel to aid in insertion. We believe that impacting a dense, structural allograft dowel into freshly reamed femoral and tibial tunnels is a useful technique to adequately replenish bone stock for the anticipated revision surgery. Further studies are needed to describe the long-term outcomes of this technique, to include the histology and radiographic incorporation rates of the dowels. This will provide further guidance in order to optimize the local host bone stock and environment for revision ACL reconstruction.
Table 2.
Advantages and Limitations
| Advantages |
| No donor site morbidity |
| Reconstitution of bone stock |
| Unimpeded placement of new tunnels |
| Management of massive osteolysis |
| Relatively simple and time-sparing technique |
| Limitations |
| Cost of allograft |
| Two-stage surgery |
| Additional cost of second surgery |
| Activity limitation between 2 surgeries |
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.D.M. receives support from Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This video demonstrates the described methods for a 2-stage revision ACL reconstruction with large tunnel osteolysis on both the femur and tibia in a left knee. By centralizing a guide pin in each defect with sequential reaming, a contained cylindrical tunnel is created that allows for the placement of a cannulated structural allograft bone dowel into the defect in a line-to-line manner.
References
- 1.Brophy R.H., Wright R.W., Matava M.J. Cost analysis of converting from single-bundle to double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37:683–687. doi: 10.1177/0363546508328121. [DOI] [PubMed] [Google Scholar]
- 2.Spindler K.P., Wright R.W. Clinical practice. Anterior cruciate ligament tear. N Engl J Med. 2008;359:2135–2142. doi: 10.1056/NEJMcp0804745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mall N.A., Chalmers P.N., Moric M. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42:2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 4.Kim S., Bosque J., Meehan J.P., Jamali A., Marder R. Increase in outpatient knee arthroscopy in the United States: A comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 5.Wright R.W., Magnussen R.A., Dunn W.R., Spindler K.P. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: A systematic review. J Bone Joint Surg Am. 2011;93:1159–1165. doi: 10.2106/JBJS.J.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leroux T., Wasserstein D., Dwyer T. The epidemiology of revision anterior cruciate ligament reconstruction in Ontario, Canada. Am J Sports Med. 2014;42:2666–2672. doi: 10.1177/0363546514548165. [DOI] [PubMed] [Google Scholar]
- 7.Noyes F.R., Barber-Westin S.D. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. J Bone Joint Surg Am. 2001;83:1131–1143. doi: 10.2106/00004623-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi F., Papalia R., Di Martino A., Rizzello G., Allaire R., Denaro V. A new harvest site for bone graft in anterior cruciate ligament revision surgery. Arthroscopy. 2007;23:558.e1–558.e4. doi: 10.1016/j.arthro.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Battaglia T.C., Miller M.D. Management of bony deficiency in revision anterior cruciate ligament reconstruction using allograft bone dowels: Surgical technique. Arthroscopy. 2005;21:767. doi: 10.1016/j.arthro.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Kamath G.V., Redfern J.C., Greis P.E., Burks R.T. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:199–217. doi: 10.1177/0363546510370929. [DOI] [PubMed] [Google Scholar]
- 11.Thomas N.P., Kankate R., Wandless F., Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33:1701–1709. doi: 10.1177/0363546505276759. [DOI] [PubMed] [Google Scholar]
- 12.Oetgen M.E., Smart L.R., Medvecky M.J. A novel technique for arthroscopically assisted femoral bone tunnel grafting in two-stage ACL revision. Orthopedics. 2008;31:16–18. doi: 10.3928/01477447-20080101-31. [DOI] [PubMed] [Google Scholar]
- 13.Said H.G., Baloch K., Green M. A new technique for femoral and tibial tunnel bone grafting using the OATS harvesters in revision anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:796.e1–796.e3. doi: 10.1016/j.arthro.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Chahla J., Dean C.S., Cram T.R. Two-stage revision anterior cruciate ligament reconstruction: Bone grafting technique using an allograft bone matrix. Arthrosc Tech. 2016;5:e189–e195. doi: 10.1016/j.eats.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilde J., Bedi A., Altchek D.W. Revision anterior cruciate ligament reconstruction. Sports Health. 2014;6:504–518. doi: 10.1177/1941738113500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchant M.H., Jr., Willimon S.C., Vinson E., Pietrobon R., Garrett W.E., Higgins L.D. Comparison of plain radiography, computed tomography, and magnetic resonance imaging in the evaluation of bone tunnel widening after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1059–1064. doi: 10.1007/s00167-009-0952-4. [DOI] [PubMed] [Google Scholar]
- 17.Meuffels D.E., Potters J.W., Koning A.H.J., Brown C.H., Jr., Verhaar J.A.N., Reijman M. Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: Reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta Orthop. 2011;82:699–703. doi: 10.3109/17453674.2011.623566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayr R., Rosenberger R., Agraharam D., Smekal V., El Attal R. Revision anterior cruciate ligament reconstruction: An update. Arch Orthop Trauma Surg. 2012;132:1299–1313. doi: 10.1007/s00402-012-1552-1. [DOI] [PubMed] [Google Scholar]
- 19.Smith S.D., Laprade R.F., Jansson K.S., Aroen A., Wijdicks C.A. Functional bracing of ACL injuries: Current state and future directions. Knee Surg Sports Traumatol Arthrosc. 2014;22:1131–1141. doi: 10.1007/s00167-013-2514-z. [DOI] [PubMed] [Google Scholar]
- 20.Chen J.L., Allen C.R., Stephens T.E. Differences in mechanisms of failure, intraoperative findings, and surgical characteristics between single- and multiple-revision ACL reconstructions: a MARS cohort study. Am J Sports Med. 2013;41:1571–1578. doi: 10.1177/0363546513487980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dargel J., Gotter M., Mader K., Pennig D., Koebke J., Schmidt-Wiethoff R. Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies Trauma Limb Reconstr. 2007;2:1–12. doi: 10.1007/s11751-007-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segawa H., Omori G., Tomita S., Koga Y. Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2001;9:206–210. doi: 10.1007/s001670100201. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi F., Papalia R., Del Buono A. Two-stage procedure in anterior cruciate ligament revision surgery: A five-year follow-up prospective study. Int Orthop. 2013;37:1369–1374. doi: 10.1007/s00264-013-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates the described methods for a 2-stage revision ACL reconstruction with large tunnel osteolysis on both the femur and tibia in a left knee. By centralizing a guide pin in each defect with sequential reaming, a contained cylindrical tunnel is created that allows for the placement of a cannulated structural allograft bone dowel into the defect in a line-to-line manner.