Abstract
Background
Urinary lipoarabinomannan (LAM) has limited sensitivity for diagnosing active human immunodeficiency virus (HIV)-associated tuberculosis (TB) disease, but LAM screening at HIV diagnosis might identify adults with more severe clinical disease or greater risk of mortality.
Methods
We enrolled antiretroviral therapy-naive HIV-infected adults from 4 clinics in Durban. Nurses performed urine LAM testing using a rapid assay (Determine TB LAM) graded from low (1+) to high (≥3+) intensity. Urine LAM results were not used to guide anti-TB therapy. We assessed TB-related symptoms and obtained sputum for mycobacterial smear and culture. Participants were observed for 12 months, and we used multivariable Cox proportional hazard models to determine hazard ratios for all-cause mortality.
Results
Among 726 HIV-infected adults with median CD4 of 205 cells/mm3 (interquartile range, 79–350 cells/mm3), 93 (13%) were LAM positive and 89 (12%) participants died during the follow-up period. In multivariable analyses, urine LAM-positive participants had a mortality hazard ratio (MHR) of 3.58 (95% confidence interval [CI], 2.20–5.81) for all-cause mortality. Among participants with mycobacterial-confirmed TB, urine LAM-positivity had a 2.91 (95% CI, 1.26–6.73) MHR for all participants and a 4.55 (95% CI, 1.71–12.1) MHR for participants with CD4 ≤100 cell/mm3. Participants with LAM-positive TB had significantly more clinical signs and symptoms of disease, compared with participants with LAM-negative TB disease.
Conclusions
Among HIV-infected adults, urinary LAM-positive patients had more clinical disease severity and a 3-fold increase in 12-month mortality compared with those who were LAM negative.
Keywords: HIV/AIDS, lipoarabinomannan (LAM), South Africa, tuberculosis, urine
Tuberculosis (TB) is the leading infectious cause of mortality worldwide and a leading cause of human immunodeficiency virus (HIV)-related mortality [1, 2]. The World Health Organization (WHO)’s “End TB Strategy” seeks to end the TB epidemic (incidence <10 cases/100000 people) by the year 2035 [3]. The strategy relies on screening all HIV-infected persons for 4 TB-related symptoms: current cough, fever, night sweats, and weight loss [4, 5]. The WHO recommends that people living with HIV be systematically screened for active TB at each visit to a health facility [5]. Those who have any TB-related symptom should undergo appropriate diagnostic testing with a test that has high diagnostic specificity, such as the Xpert MTB/RIF assay [5].
Some HIV-infected people with active TB may not experience typical TB symptoms, which leads to poor accuracy of TB screening algorithms. A meta-analysis of 12 observational studies reported that the subjective symptom-based screening algorithm had a diagnostic sensitivity of 79% and specificity of 50% among HIV-infected adults worldwide [6]. Furthermore, although symptom-based screening can improve early detection of active TB, symptom-based screening has not been demonstrated to directly impact health outcomes, compared with passive case finding [4]. Better screening “triage” tests are being evaluated [7, 8], and an alternative clinic-based approach could use a rapid diagnostic test that has better prognostic capacity.
Lipoarabinomannan (LAM), a glycolipid within the cell wall of TB, may be a useful pathogen biomarker to identify patients with active HIV-associated TB infections who have a greater risk of death [9]. In a recent meta-analysis, rapid LAM testing was associated with greater mortality risk, but these studies were predominantly among sick, hospitalized patients [10]. In several clinic-based studies, a rapid LAM assay has demonstrated poor diagnostic sensitivity for use as a stand-alone TB screening test at HIV diagnosis [11–15], and the WHO recommended against using urine LAM as an ambulatory TB screening test among HIV-infected persons [16]. However, because we have demonstrated that a rapid LAM assay can be easily performed by nurses in an ambulatory clinical setting [11], we sought to determine whether urine LAM positivity might identify adults with more severe clinical disease or be a viable prognostic marker of mortality among newly diagnosed HIV-infected adults in a TB-endemic region. If urinary LAM positivity reflects greater disease severity or mortality risk, then LAM screening might be beneficial at HIV diagnosis to identify people who need accelerated anti-TB therapy initiation and/or more intensive clinical monitoring.
METHODS
Study Design and Participants
We conducted a prospective, clinic-based, cohort study by enrolling consecutive, newly diagnosed, antiretroviral therapy (ART)-naive HIV-infected adults in the ambulatory clinical areas of 2 hospitals and 2 municipal health centers in KwaZulu-Natal, South Africa from October 2011 to January 2014 [17, 18]. The study was designed to assess the diagnostic accuracy and prognostic value of point-of-care urinary LAM screening, regardless of TB-related symptoms, at the time of HIV diagnosis in an outpatient setting, and we have previously reported the diagnostic accuracy results [11, 12]. Eligible participants were adults (≥18 years) who presented to the clinic for voluntary HIV testing and counseling, not known to be pregnant, and not having received anti-TB therapy within the prior 3 months. Ethics committees of the 2 local hospitals (McCord Hospital and St. Mary’s Hospital) and Partners HealthCare in Boston (no. 2006-P-001379/40) approved the study, and all study participants provided written informed consent. Participants were offered ART and TB treatment in accordance with local and South African Department of Health guidelines [19], and urinary LAM results were not used to guide therapeutic decisions.
Procedures
Study nurses, who had advanced training in TB diagnosis, care, and treatment, collected demographic, socioeconomic, and clinical information, including TB-related symptoms at enrollment. Participants were asked to provide a sputum sample, and those unable to provide an expectorated sample underwent induction with 3% nebulized hypertonic saline. Participants were then asked to provide a urine sample in a sterile container. Nurses tested urine samples using a rapid LAM assay and interpreted the result within 25–35 minutes in the ambulatory clinical setting. Participants were offered baseline CD4 count testing and received chest radiography as clinically indicated. Urine LAM test results were blinded from clinicians and not used to guide therapeutic decisions. All participants were followed for 12 months from study enrollment.
Sputum for Tuberculosis Testing
A certified technologist, who was blinded to clinical information and urinary LAM results, performed sputum smear microscopy on decontaminated samples using both Ziehl-Neelson and Auramine stains. Before staining for acid-fast bacilli (AFB), sputum samples were decontaminated with N-acetyl-l-cysteine and NaOH to a final concentration of 1.25% before being centrifuged at 3000 revolutions for 20 minutes and resuspended in 1 mL 7H9 broth. Decontaminated sputum samples were inoculated into Bactec 960 mycobacterial growth indicator tubes (MGIT; BD, Franklin Lakes, NJ) and solid culture Middlebrook 7H11 agar medium, and TB was confirmed using niacin and nitrate testing.
Participants were considered smear acid-fast positive if either Ziehl-Neelson or Auramine stains were positive, and they were considered culture positive if TB was identified from either liquid or solid culture media. Microbiologically confirmed TB was defined as having either positive sputum smear AFB microscopy or mycobacterial culture. Participants were considered to have evidence of active TB disease if they had either microbiologically confirmed TB or were started on anti-TB therapy for presumptive TB based on clinical judgment.
Urinary Lipoarabinomannan Testing
Urine specimens were considered LAM positive if colorimetric bands were visible at both the control and test lines. Halfway through the study, based on operational feedback, nurses began graded positive urine LAM results from low-band intensity (1+) to high-band intensity (5+), according to the manufacturer’s original reference card with 5 positive categories. Rapid LAM tests that did not have a positive control line were repeated using the same urine sample.
Before study commencement, a representative from Alere Inc. conducted a training session for study nurses to review procedures and interpretation of the Determine TB LAM assay (Alere Inc., Waltham, MA), and study nurses practiced until comfortable. During the study, regular oversight and observation of the nurses’ LAM testing procedures by the principal investigator and study coordinator ensured continued testing competence and proficiency. All urine LAM tests (manufacturer lot numbers 110512, 120215, and 120222) and reagents were maintained in a sealed pouch and stored out of direct sunlight at room temperature (15–26°C), as recommended.
The nurse performed 2 rapid urine LAM tests on each fresh urine specimen. However, our previous analyses found no significant benefit with the second urine LAM test [12]. Therefore, we restricted these analyses to only the first urine LAM test result. Among the subgroup with graded LAM results, nurses were allowed to grade a test as a “faint” positive if a line was visible but not dark enough to be considered a 1+ grade. Our previous analyses found this interpretation to be a false-positive result when compared with sputum culture for pulmonary TB [12], so “faint” positive results were reclassified as a negative LAM test result for these analyses.
Outcome
The primary outcome was all-cause mortality within 12 months after the baseline assessment. We used the South African National Death Registry to determine survival status and the date of death during the 12-month follow-up period, and all participants were linked to the Registry. Participants who survived the follow-up period were censored at 12 months and contributed to the final outcome ascertainment.
Statistical Analysis
We categorized patients into one of the following 3 groups: (1) no evidence of TB and urine LAM-negative; (2) evidence of TB by either sputum culture or presumptive TB by clinical judgment, but urine LAM-negative; or (3) urine LAM-positive. We used these discrete categories, because our objective was to determine whether LAM positivity identifies a subset of TB-infected patients who have a higher clinical severity of disease and a greater risk of mortality, which may represent an important clinical distinction. We used Cox proportional hazard models to determine the association between baseline LAM result and the time to death within the 12-month, follow-up period. We compared Kaplan-Meier survival curves within the 12-month, follow-up period using a log-rank test. We stratified analyses by participants with CD4 >200 cells/mm3, CD4 101–200 cells/mm [3], and ≤100 cells/mm3, because these categories have been previously used and form the basis of WHO recommendations [12, 16]. We performed separate Cox proportional hazard models to compare urine LAM-positive participants against urine LAM-negative participants among those with evidence of clinically active TB disease, among participants with microbiologically confirmed TB, and among participants who were started on anti-TB therapy. We evaluated the mortality hazard ratio (MHR) by urine LAM grade, which included a test for trend. We evaluated hazard mortality ratios by other screening modalities—presence of a cough, any TB-related symptom, and sputum AFB smear-positive. All multivariable survival analyses were adjusted by age, sex, education, and smoking status.
To evaluate LAM as a marker of clinical disease severity, we used Fisher’s exact test and t tests to compare demographic, clinical, laboratory, and treatment outcomes between LAM-negative and LAM-positive adults, where appropriate. We conducted univariate and multivariable logistic regression analyses to compare participants with LAM-positive versus LAM-negative TB disease. We first conducted unadjusted linear regression models for each independent variable. Factors with P < .2 were then included together in a single multivariable model. Factors that were no longer significant at the 0.2 level in the full multivariable model were removed one at a time. Finally, factors not selected based on the unadjusted analyses were included one at a time in the current multivariable model to assess their importance in adjusted analyses. We calculated 95% confidence intervals (CIs), reported 2-tailed P values (α = 0.05), and used SAS 9.4 (Cary, NC).
RESULTS
Cohort Characteristics and Outcomes
We enrolled 796 newly diagnosed, ART-naive HIV-infected adults. After excluding 31 (4%) participants unable to provide a urine specimen and 39 (5%) participants who were receiving anti-TB therapy, our analyses included 726 HIV-infected adults (Table 1). Mean age was 34 years (standard deviation 10 years), 59 (8%) participants reported a prior TB infection, and 169 (23%) participants were current smokers. Overall, each TB-related symptom was reported by 31%–38% of participants. Among the 627 (86%) participants who completed CD4 testing, the median CD4 count was 205 cells/mm3 (interquartile range, 79–350 cells/mm3).
Table 1.
Cohort Characteristics of ART-Naive HIV-Infected Adults
| Characteristics | Mean ± SD or N (%) |
|---|---|
| Demographic and Education | |
| Age (years) | 34 ± 10 |
| Male sex | 385 (53) |
| Completed high school | 274 (38) |
| Clinical | |
| Prior TB infection | 59 (8) |
| Currently smoke tobacco | 169 (23) |
| Taking a diuretic medication | 3 (<1) |
| TB-Related Symptoms | |
| Current cough | 255 (35) |
| Fever | 227 (31) |
| Night sweats | 227 (31) |
| Weight loss | 277 (38) |
| Having any of the above TB-related symptoms | 449 (62) |
| CD4 Cell Count [median/IQR]/mm3 (N = 627) | 205 [79–350] |
| >200 cells/mm3 | 319 (51) |
| 101–200 cells/mm3 | 118 (19) |
| ≤100 cells/mm3 | 190 (30) |
| Rapid Urine LAM Testing | |
| Positive by first rapid urine LAM test | 93 (13) |
| Positive by second rapid urine LAM test | 103 (14) |
| Positive by either rapid urine LAM test | 106 (15) |
| Tuberculosis Test Results | |
| Sputum smear acid-fast bacilli positive | 37 (5) |
| Culture-confirmed pulmonary TB positive | 123 (18) |
| Microbiologically confirmed TB | 139 (19) |
| TB Diagnosis by Category | |
| No evidence of TB and LAM-negative | 504 (69) |
| Evidence of TBa and LAM-negative | 129 (18) |
| LAM-positive | 93 (13) |
| TB Therapy and Mortality | |
| Started anti-TB therapy | 119 (16) |
| Deaths during 12-month follow-up | 89 (12) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; LAM, lipoarabinomannan; SD, standard deviation; TB, tuberculosis.
aEvidence of TB was defined as either microbiologically TB+ or being empirically stated on anti-TB therapy.
The observed prevalence of culture-confirmed pulmonary TB was 18% (95% CI, 15–21%). Ninety-three (13%) participants were urinary LAM positive and 37 (5%) participants were sputum smear positive for AFB. Among those who were LAM-positive, 38 (46%) participants had culture-confirmed pulmonary TB, and 34 of those 38 people initiated anti-TB therapy. Among those LAM-negative, 129 (18%) participants either had evidence of active TB or were initiated on anti-TB therapy. Eighty-nine (12%) participants died during the 12-month follow-up period.
Urinary Lipoarabinomannan and Mortality Risk Among Entire Cohort
Urine LAM-positive participants had a greater mortality hazard compared with LAM-negative participants without evidence of diagnosed or presumptive active TB disease (Table 2). The overall mortality rate during the 12-month, follow-up period was 31.2% among those urine LAM positive, 14.0% among those with urine LAM-negative TB disease, and 8.3% among those without evidence of TB disease and LAM negative. In univariate analyses, participants who screened urine LAM positive had an MHR of 4.26 (95% CI, 2.65–6.84), compared with participants who were LAM negative and had no evidence of active TB disease. Urine LAM-positive participants with CD4 ≤100 cells/mm3 also had a significantly greater mortality hazard, compared with participants with CD4 ≤100 cells/mm3 who were LAM negative and had no evidence of active TB disease.
Table 2.
Hazard Ratio of Mortality by TB and Urinary LAM Status Among ART-Naive HIV-Infected South African Adults
| Screening Test | Number of Deaths/ Number at Risk (%) | Unadjusted Models | Adjusted Modelsa | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| All Participants | |||||
| No evidence of TB and LAM-negative | 42/504 (8.3) | Ref. | — | Ref. | — |
| Evidence of TBb and LAM-negative | 18/129 (14.0) | 1.73 (0.99–3.00) | .05 | 1.54 (0.89–2.69) | .13 |
| Urine LAM-positive | 29/93 (31.2) | 4.26 (2.65–6.84) | <.0001 | 3.58 (2.20–5.81) | <.0001 |
| CD4 >200 cells/mm3 | |||||
| No evidence of TB and LAM-negative | 9/271 (3.3) | Ref. | — | Ref. | — |
| Evidence of TBb and LAM-negative | 3/30 (10.0) | 3.17 (0.86–11.7) | .08 | 3.46 (0.85–14.1) | .08 |
| Urine LAM-positive | 2/18 (11.1) | 3.41 (0.74–15.8) | .12 | 4.99 (0.98–25.4) | .05 |
| CD4 101–200 cells/mm3 | |||||
| No evidence of TB and LAM-negative | 6/69 (8.7) | Ref. | — | Ref. | — |
| Evidence of TBb and LAM-negative | 7/31 (22.6) | 2.74 (0.92–8.16) | .07 | 2.80 (0.91–8.57) | .07 |
| Urine LAM-positive | 4/18 (22.2) | 2.80 (0.79–9.92) | .11 | 2.72 (0.76–9.76) | .13 |
| CD4 ≤100 cells/mm3 | |||||
| No evidence of TB and LAM-negative | 27/164 (9.3) | Ref. | — | Ref. | — |
| Evidence of TBb and LAM-negative | 8/68 (11.8) | 0.70 (0.32–1.53) | .37 | 0.65 (0.29–1.43) | .28 |
| Urine LAM-positive | 23/57 (40.4) | 2.80 (1.61–4.89) | .0003 | 2.38 (1.34–4.21) | .003 |
| Participants With Microbiologically Confirmed TB | |||||
| Urine LAM-negative | 14/99 (14.1) | Ref. | — | Ref. | — |
| Urine LAM-positive | 15/40 (37.5) | 3.01 (1.45–6.23) | .003 | 2.91 (1.26–6.73) | .01 |
| CD4 ≤100 cells/mm3 | |||||
| Urine LAM-negative | 10/71 (14.1) | Ref. | — | Ref. | — |
| Urine LAM-positive | 14/34 (41.2) | 3.48 (1.54–7.84) | .003 | 4.55 (1.71–12.1) | .003 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; Ref., ; TB, tuberculosis.
aAdjusted by age, sex, education, and smoking status.
bEvidence of TB was defined as either microbiologically postive for TB or being started empirically on anti-TB therapy.
In multivariable hazard models, participants who had either culture-confirmed or presumptive TB disease, but were urine LAM-negative, had a 1.54 (95% CI, 0.89–2.69) greater mortality hazard, compared with participants who were LAM negative and had no evidence of active TB disease (Table 2). Participants who had a positive LAM screening test had a 3.58 (95% CI, 2.20–5.81) greater hazard of mortality, compared with LAM-negative participants who had no evidence of TB. Among those with CD4 ≤100 cells/mm3, participants with a LAM-positive screening test had a 2.38 (95% CI, 1.34–4.21) greater mortality hazard, compared with those who were LAM negative and without evidence of TB.
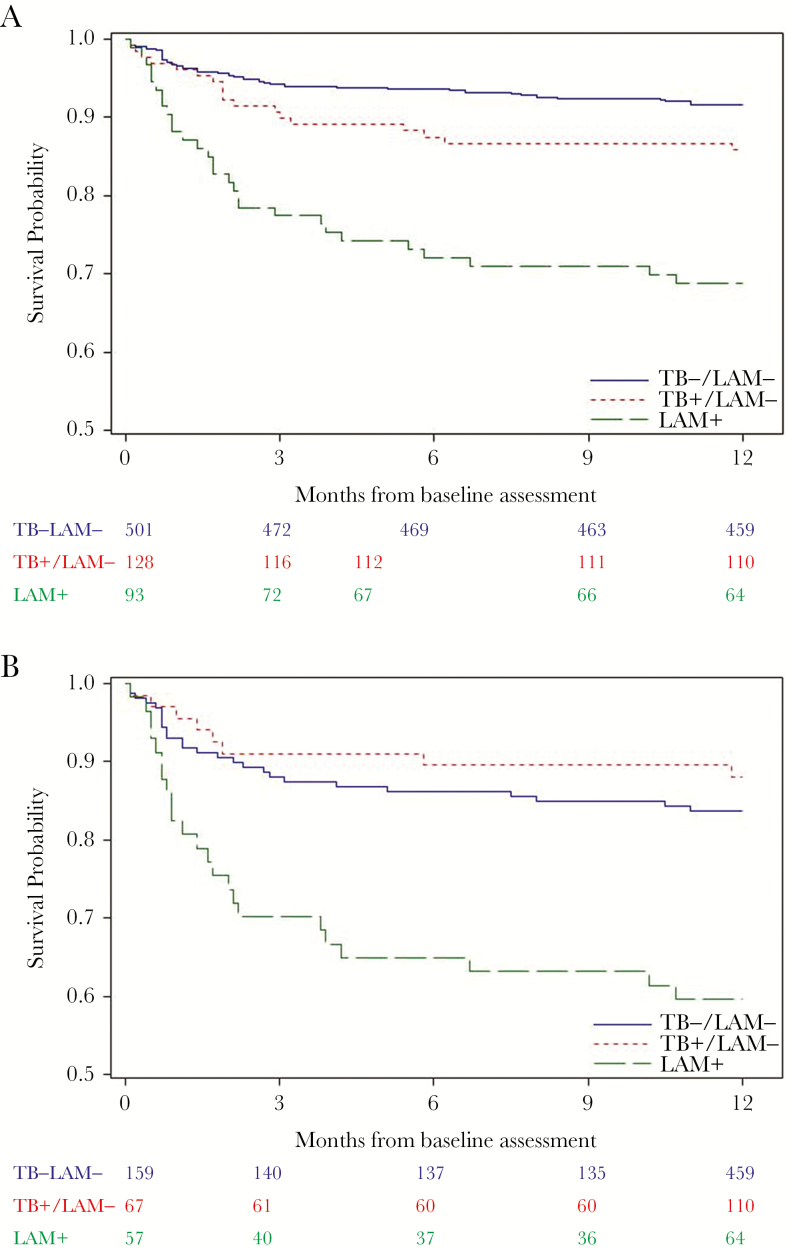
The multivariable survival curve by TB category is shown for all participants (Figure 1A) and among immunosuppressed participants with CD4 ≤100 cells/mm3 (Figure 1B). Among all participants with censorship at 12 months, the median survival time was 8.2 (standard error [SE] = 0.4) months for LAM-positive participants, 10.5 (SE = 0.3) months for LAM-negative participants with evidence of TB, and 10.4 (SE = 0.1) months for LAM-negative participants with no evidence of active TB disease. Among participants with CD4 ≤100 cells/mm3, the median survival time was 7.4 (SE = 0.6) months for LAM-positive participants, 10.8 (SE = 0.4) months for LAM-negative participants with evidence of TB, and 9.6 (SE = 0.3) months for LAM-negative participants with no evidence of active TB disease. The differences between the 3 strata for all-cause mortality was highly significant for all participants (P < .0001) and when restricted to participants with CD4 ≤100 cells/mm3 (P < .0001).
Figure 1.
(A) Survival by tuberculosis (TB) category at baseline assessment for antiretroviral therapy (ART)-naive human immunodeficiency virus (HIV)-infected participants. (B) Survival by TB category at baseline assessment for ART-naive HIV-infected participants with CD4 ≤100 cells/mm3. LAM, lipoarabinomannan.
Urinary Lipoarabinomannan and Mortality Risk Among All Tuberculosis (TB)-Positive and Microbiologically Confirmed TB
We then restricted our survival analyses to those who had either microbiological or clinical evidence of TB and to those with only microbiologically confirmed TB. Among participants with clinical or microbiological TB, the overall mortality rate was 14.0% and 31.2% among those who screened urine LAM negative and positive, respectively. In a multivariable model, participants with urine LAM-positive TB disease had a 2.33 (95% CI, 1.28–4.24) greater hazard of mortality, compared with urine LAM-negative TB disease. Among participants with CD4 ≤100 cells/mm3, LAM-positive TB-infected adults had a 3.73 (95% CI, 1.65–8.42) greater risk of death in an adjusted model, compared with LAM-negative TB-infected adults.
Urine LAM-positivity remained a significant predictor of mortality when analyses were further restricted to the participants with microbiologically confirmed TB (Table 2). Overall, urine LAM-positive TB disease had a 3-fold greater hazard of death compared with urine LAM-negative TB disease. In a multivariable analysis, urine LAM-positive participants with confirmed TB had an MHR of 2.91 (95% CI, 1.26–6.73), regardless of CD4 count, and a 4.55 (95% CI, 1.71–12.1) greater mortality hazard when restricting analyses to those with CD4 ≤100 cells/mm3, compared with LAM-negative participants with confirmed TB.
Urine Lipoarabinomannan (LAM) and Mortality Risk by Grade of LAM Test
In separate subanalyses, participants with a higher LAM test grade had a greater hazard of mortality (Table 3). Overall, in a multivariable model, participants with a weak positive LAM result (1+) had a 2.57 (95% CI, 0.79–8.38) greater hazard of mortality, compared with LAM-negative participants who had no evidence of TB. Participants with a 2+ LAM grade and highly positive LAM result (≥3+) had a 3.41 (95% CI, 1.22–9.57) and 5.02 (95% CI, 2.48–10.17) greater hazard of mortality, respectively. In separate multivariable models restricted to participants with CD4 ≤200 cells/mm3 and CD4 ≤100 cells/mm3, a 2+ LAM grade and highly positive LAM result (≥3+) remained significantly associated with a greater hazard of mortality. In all scenarios, a test for trend indicated that a higher grade of LAM positivity carried a significantly greater MHR.
Table 3.
Hazard Ratio of Mortality by Urinary LAM Grade Among ART-Naive HIV-Infected South African Adults
| Screening Test | Number of Deaths/ Number at Risk (%) | Unadjusted Models | Adjusted Modelsa | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | P Value for Trend | Hazard Ratio (95% CI) | P Value | P Value for Trend | ||
| All Participants | <.0001 | <.0001 | |||||
| No evidence of TB and LAM-negative | 42/504 (8.3) | Ref. | — | Ref. | — | ||
| Evidence of TB and LAM-negative | 18/129 (14.0) | 1.71 (0.99–2.98) | .06 | 1.54 (0.88–2.68) | .13 | ||
| Urine LAM 1+ grade | 3/14 (21.4) | 2.74 (0.85–8.83) | .09 | 2.57 (0.79–8.38) | .12 | ||
| Urine LAM 2+ grade | 4/13 (30.8) | 4.12 (1.48–11.5) | .007 | 3.41 (1.22–9.57) | .02 | ||
| Urine LAM ≥3+ grade | 10/23 (43.5) | 6.88 (3.45–13.7) | <.0001 | 5.02 (2.48–10.2) | <.0001 | ||
| Participants with CD4 ≤200 cells/mm3 | <.0001 | .0005 | |||||
| No evidence of TB and LAM-negative | 33/233 (14.2) | Ref. | — | Ref. | — | ||
| Evidence of TB and LAM-negative | 15/100 (15.0) | 1.06 (0.57–1.94) | .86 | 0.99 (0.53–1.83) | .96 | ||
| Urine LAM 1+ grade | 3/12 (25.0) | 1.83 (0.56–5.95) | .32 | 1.74 (0.53–5.72) | .36 | ||
| Urine LAM 2+ grade | 4/10 (40.0) | 3.17 (1.12–8.95) | .03 | 2.71 (0.95–7.71) | .06 | ||
| Urine LAM ≥3+ grade | 9/19 (47.4) | 4.51 (2.15–9.43) | <.0001 | 3.61 (1.69–7.71) | .0009 | ||
| Participants With CD4 ≤100 cells/mm3 | .0003 | .003 | |||||
| No evidence of TB and LAM-negative | 27/164 (16.5) | Ref. | — | Ref. | — | ||
| Evidence of TB and LAM-negative | 8/69 (11.6) | 0.69 (0.31–1.51) | .35 | 0.65 (0.29–1.43) | .31 | ||
| Urine LAM 1+ grade | 3/8 (37.5) | 2.45 (0.74–8.09) | .13 | 2.10 (0.63–7.05) | .21 | ||
| Urine LAM 2+ grade | 4/8 (50.0) | 3.51 (1.23–10.0) | .02 | 2.96 (1.01–8.70) | .05 | ||
| Urine LAM ≥3+ grade | 8/17 (47.1) | 3.70 (1.68–8.14) | .001 | 3.04 (1.34–6.91) | .008 | ||
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; Ref., ; TB, tuberculosis.
aAdjusted by age, sex, education, and smoking status.
Urinary Lipoarabinomannan in Comparison to Other Tuberculosis Screening Modalities
Among single screening assessments, participants with a current cough, any TB-related symptom, and urine LAM positivity had a higher rate of mortality (Table 4). In separate multivariable models adjusted for age, sex, education, and smoking status, presence of any TB-related symptom and urine LAM positivity had a significant hazard ratio for mortality. Urine LAM positivity (≥1+) and urine LAM ≥2+ grade had hazard ratios of 3.18 (95% CI, 2.02–5.00) and 3.34 (95% CI, 1.73–6.46) for mortality, and they were the highest hazard ratios among single screening assessments.
Table 4.
Hazard Ratio of Mortality by Tuberculosis Screening Method Among Newly Diagnosed HIV-Infected South African Adults
| Screening Test | Number of Deaths/ Number at Risk (%) | Unadjusted Models | Adjusted Modelsa | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| All Participants | |||||
| Absence of Cough | 46/471 (9.8) | Ref. | — | Ref. | — |
| Presence of Cough | 43/255 (16.9) | 1.80 (1.18–2.72) | .006 | 1.68 (1.10–2.55) | .02 |
| No TB-related symptoms | 17/277 (6.1) | Ref. | — | Ref. | — |
| Any TB-related symptom | 72/449 (16.0) | 2.76 (1.63–4.68) | .0002 | 2.41 (1.41–4.12) | .001 |
| Sputum AFB smear-negative | 75/650 (11.5) | Ref. | — | Ref. | — |
| Sputum AFB smear-positive | 6/37 (16.2) | 1.43 (062-3.29) | .40 | 1.22 (0.53–2.82) | .64 |
| Urine LAM-negative | 60/632 (9.5) | Ref. | — | Ref. | — |
| Urine LAM-positive | 29/93 (31.2) | 3.72 (2.39–5.79) | <.0001 | 3.18 (2.02–5.00) | <.0001 |
| Urine LAM <2+ grade | 30/313 (9.6) | Ref. | — | Ref. | — |
| Urine LAM ≥2+ grade | 14/36 (38.9) | 4.74 (2.51–8.95) | <.0001 | 3.34 (1.73–6.46) | .0003 |
| Participants With Evidence of TBb | |||||
| Absence of Cough | 31/90 (34.4) | Ref. | — | Ref. | — |
| Presence of Cough | 44/85 (51.8) | 1.82 (1.14–2.89) | .01 | 1.67 (1.04–2.69) | .03 |
| No TB-related symptoms | 16/39 (41.0) | Ref. | — | Ref. | — |
| Any TB-related symptom | 59/136 (43.4) | 1.22 (0.70–2.13) | .47 | 1.07 (0.61–1.88) | .82 |
| Urine LAM-negative | 42/124 (33.9) | Ref. | — | Ref. | — |
| Urine LAM-positive | 33/51 (64.7) | 2.51 (1.58–4.00) | <.0001 | 2.48 (1.55–3.98) | .0002 |
| Participants With Evidence of TBb and CD4 <200/mm3 | |||||
| Absence of Cough | 25/64 (39.1) | Ref. | — | Ref. | — |
| Presence of Cough | 36/66 (54.6) | 1.63 (0.97–2.73) | .06 | 1.42 (0.83–2.41) | .20 |
| No TB-related symptoms | 11/25 (44.0) | Ref. | — | Ref. | — |
| Any TB-related symptom | 50/105 (47.6) | 1.22 (0.64–2.35) | .55 | 1.09 (0.56–2.12) | .81 |
| Urine LAM-negative | 33/87 (37.9) | Ref. | — | Ref. | — |
| Urine LAM-positive | 28/43 (65.1) | 2.45 (1.47–4.10) | .0006 | 2.29 (1.35–3.88) | .002 |
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; Ref., ; TB, tuberculosis.
aAdjusted by age, sex, education, and smoking status.
bEvidence of TB was defined as either microbiologically TB+ being empirically stated on anti-TB therapy.
In subanalyses among TB-infected participants, presence of a cough and urine LAM positivity were each associated with a greater risk of mortality. In multivariable models, participants with presence of a cough had a 1.42 (95% CI, 0.83–2.41) greater hazard of mortality, whereas urinary LAM-positive participants had a 2.48 (95% CI, 1.55–3.98) greater hazard of mortality. When restricted to TB-infected participants with CD4 <200 cells/mm3, only urine LAM screening was associated with a higher mortality risk (MHR = 2.29; 95% CI, 1.35–3.88). When including symptom screening and urinary LAM in the same multivariate model, urinary LAM remained a significant predictor of mortality.
Urinary Lipoarabinomannan by Anti-Tuberculosis Therapy Initiation
Among the 119 participants who initiated anti-TB therapy, LAM-positive participants had a 2.37 (95% CI, 0.91–6.14) greater hazard of mortality compared with LAM-negative participants. In a multivariable model, LAM-positive participants had a 2.44 (95% CI, 0.92–6.49) greater hazard of mortality. When restricting the analyses to the 95 participants with CD4 ≤200 cells/mm3 who started anti-TB therapy, LAM-positive participants had a 2.29 (95% CI, 0.83–6.34) greater mortality hazard in a univariate model and 2.48 (95% CI, 0.85–7.20) greater mortality hazard in a multivariable model.
Urinary Lipoarabinomannan as a Biomarker of Severity of Clinical Disease
To examine urinary LAM as a biomarker of clinical disease severity, we compared clinical measures, laboratory results, and outcomes between those with LAM-positive versus LAM-negative TB disease (Table 5). Participants with LAM-positive TB had significantly more tachycardia (>100 heart beats/minute), lower systolic and diastolic blood pressures, more liquid, or solid positive TB culture result. The rate of TB treatment outcomes (failed, defaulted, or not completed treatment) was similar between the 2 groups.
Table 5.
Characteristics Between LAM-Negative and LAM-Positive HIV-Associated Tuberculosis (N = 194)
| Characteristics | LAM-Negative TB (N = 138) |
LAM-Positive TB (N = 56) |
P Value |
|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | ||
| Demographic and Education | |||
| Age (years) | 35.3 ± 10.4 | 34.0 ± 7.5 | .30 |
| Male sex | 83 (60.1) | 34 (60.7) | 1.0 |
| Completed high school | 50 (36.5) | 15 (26.8) | .24 |
| Clinical | |||
| Prior TB infection | 9 (6.5) | 5 (8.9) | .55 |
| Currently smoke tobacco | 32 (23.2) | 8 (14.3) | .24 |
| Body temperature | 36.5 ± 1.2 | 36.5 ± 1.5 | .77 |
| Fever (temperature >38.3°C) | 8 (9.4) | 5 (10.2) | 1.0 |
| Heart rate | 97.7 ± 22.9 | 115.2 ± 26.0 | .0002 |
| Tachycardia (>100 heart beats/minute) | 39 (44.8) | 32 (65.3) | .03 |
| Systolic blood pressure | 113.5 ± 16.0 | 104.7 ± 21.0 | .01 |
| Diastolic blood pressure | 73.2 ± 16.3 | 68.3 ± 12.3 | .06 |
| TB-Related Symptoms | |||
| Current cough | 60 (43.5) | 32 (57.1) | .11 |
| Fever | 58 (42.0) | 23 (41.1) | 1.0 |
| Night sweats | 59 (42.8) | 28 (50.0) | .43 |
| Weight loss | 75 (54.4) | 30 (53.6) | 1.0 |
| Having any of 4 TB-related symptoms | 104 (75.4) | 46 (82.1) | .35 |
| Having ≥2 TB-related symptoms | 72 (52.2) | 36 (64.3) | .15 |
| CD4 Cell Count | |||
| Median [IQR] CD4 cells/mm3 | 115 [48–227] | 61 [20–146] | .002 |
| Low CD4 (<100 cells/mm3) | 58 (45.3) | 36 (66.7) | .01 |
| Sputum AFB Microscopy Results | |||
| AFB negative | 106 (80.9) | 41 (77.4) | .37 |
| AFB positive with “scant” or 1+ | 20 (15.3) | 6 (11.3) | |
| AFB positive with ≥2+ | 5 (3.8) | 6 (11.3) | |
| Sputum TB Culture Results | |||
| Liquid culture (MGIT) positive | 78 (60.9) | 38 (74.5) | .09 |
| Solid culture positive | 42 (32.1) | 33 (64.7) | .0002 |
| Either culture positive | 82 (62.6) | 41 (80.4) | .02 |
| Treatment Outcomes | |||
| Failed, defaulted, or not completed treatment | 39 (47.6) | 21 (56.8) | .35 |
| Death during 12-month follow-up period | 19 (13.8) | 19 (33.9) | .001 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; LAM, lipoarabinomannan; SD, standard deviation; TB, tuberculosis.
In univariate logistic regression models, LAM-positive TB disease was associated with having more tachycardia, lower blood pressures, CD4 <100 cells/mm3, and having TB-culture positive disease, compared with LAM-negative TB disease (Table 6). In multivariable analyses, LAM-positive TB disease was most strongly associated with lower systolic blood pressure, having TB-culture positive disease, and having a current cough.
Table 6.
Univariate and Multivariable Logistic Regression of Risk Factors for LAM-Positive HIV-Associated TB, Compared With LAM-Negative HIV-Associated TB (N = 194)
| Characteristics | Univariate Odds Ratio (95% CI) | P Value | Multivariate Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Demographic and Education | ||||
| Age (years) | 0.98 (0.95–1.02) | .37 | — | — |
| Male sex | 1.02 (0.54–1.93) | .94 | — | — |
| Completed high school | 0.64 (0.32–1.26) | .20 | — | — |
| Clinical | ||||
| Currently smoke tobacco | 0.55 (0.24–1.29) | .17 | — | — |
| Tachycardia (>100 beats/minute) | 2.32 (1.12–4.78) | .02 | 1.85 (0.79–4.33) | .16 |
| Systolic blood pressure (mmHg) | 0.97 (0.95–0.99) | .01 | 0.97 (0.95–0.99) | .006 |
| Diastolic blood pressure (mmHg) | 0.98 (0.95–1.00) | .08 | — | — |
| TB-Related Symptoms | ||||
| Current cough | 1.73 (0.93–3.25) | .09 | 2.33 (1.01–5.39) | .05 |
| Having any of 4 TB-related symptoms | 1.50 (0.69–3.30) | .31 | — | — |
| Having ≥2 TB-related symptoms | 1.65 (0.87–3.13) | .13 | — | — |
| CD4 Cell Count | ||||
| Low CD4 (<100 cells/mm3) | 2.41 (1.24–4.69) | .009 | — | — |
| TB Testing Results | ||||
| Sputum smear acid-fast bacilli positive | 1.24 (0.57–2.70) | .59 | — | — |
| TB culture positive | 2.45 (1.13–5.33) | .02 | 2.71 (1.05–7.00) | .04 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; LAM, lipoarabinomannan; TB, tuberculosis.
DISCUSSION
In this high TB-burden setting, ART-naive adults with a positive clinic-based urine LAM screening test had significantly greater risk of death within the 12 months after HIV diagnosis. The risk of death was greatest for adults with a CD4 ≤100 cells/mm3 and among participants with highly positive (≥2+) urine LAM test result. In addition, urinary LAM-positive participants had more severe clinical signs and symptoms of disease. Urinary LAM testing was strongly associated with clinical disease severity and all-cause mortality, whereas symptom-based screening was not associated with mortality among adults with evidence of TB. These results suggest that LAM screening among ART-naive adults at HIV diagnosis might identify immunocompromised adults who have a greater risk of death and who might benefit from accelerated initiation of anti-TB therapy and/or more intensive clinical monitoring.
Current WHO guidelines recommend diagnostic urine LAM testing in sick, HIV-infected, hospitalized patients, and do not recommend urinary LAM screening in ambulatory clinics due to limited supportive evidence [16]. In a recent hospital-based, randomized, controlled trial, urine LAM testing performed by trained research nurses and used to expedite initiation of anti-TB therapy led to a relative risk reduction of 17% for 8-week all-cause mortality among HIV-infected adults with at least 1 TB-related symptom [20]. Several other hospital-based studies have demonstrated that urinary LAM testing can be predictive of mortality [21–24]. One combined hospital and outpatient participant study used fresh urine samples to predict mortality in Ghana [14]. Thus far, 2 smaller outpatient studies have used frozen urine samples tested in a laboratory setting to assess mortality risk, and both studies reported higher mortality rates with 3–6 months follow-up among LAM-positive participants [15, 25]. To our knowledge, our study represents the largest longitudinal study of nurses performing real-time urinary LAM testing on fresh urine samples in outpatient ambulatory clinics with a direct association to clinical disease severity and having more than 6 months of participant follow-up.
In clinical settings, the accuracy of symptom-based screening to identify HIV-associated active TB disease has been poor. A meta-analysis of 12 studies, only 3 of which were conducted in a high TB-burden setting (>10% prevalence), found symptom-based TB screening missed nearly one quarter of active TB cases [6]. Inaccurate symptom-based screening among HIV-infected adults can lead to treatment delays, inadequate provision of isoniazid preventive therapy and overprescribing of empiric TB therapy, and high loss to follow-up rates [1, 5]. Consequently, a WHO TB diagnostic group identified a screening test and algorithm as one of its highest priorities [7].
In our study, the current urine LAM assay predicted all-cause mortality at initial HIV diagnosis, which may be useful as a prognostic biomarker. The majority of the deaths in our cohort occurred within the first 3 months, which reflects a period of high risk for a vulnerable group. We have previously shown the urine LAM assay to be associated with more severe disease states and predict longer-term outcomes among patients who have completed the intensive 2-month period of initial anti-TB therapy [26, 27]. Together, these findings suggest that urine LAM-positive patients with a greater risk of mortality may have had a higher bacillary burden of TB. When compared with existing screening modalities for HIV-associated TB disease, urinary LAM screening outperformed symptom-based screening and AFB smear microscopy.
One prospective clinic-based study, the TB Fast Track study, evaluated urinary LAM testing as a triage tool in combination with TB-symptom screening, body mass index (BMI), and hemoglobin measurement [28, 29]. The study found no overall difference in mortality, which may have been due to the low sensitivity of urine LAM testing, and poor specificity of BMI and hemoglobin testing, to identify active TB disease. Because the LAM assay has inadequate sensitivity as a stand-alone screening test [11–13], several groups are working to develop a more sensitive LAM assay. One promising approach using high-avidity monoclonal antibodies reported a 50-fold increase in LAM detection and 93% diagnostic sensitivity of urine LAM testing in an Ethiopian cohort [30].
Our study had several strengths and limitations. Primary strengths were evaluating the urine LAM assay when used by trained nurses in ambulatory settings, which is similar to a real-world application, assessing both liquid and solid mycobacterial culture, enrolling participants on the day of HIV diagnosis and before ART initiation, assessing urine LAM among adults with a range of CD4 counts, and adjusting multivariable models for tobacco smoking and age, both of which influence TB outcomes. Primary limitations included using an observational study design, only grading half of the LAM test strips, as well as not evaluating participants for diabetes, low BMI, or alcohol dependence, which can also influence TB-related mortality. We also excluded participants who were unable to provide an expectorated or induced sputum sample, which could have biased the results. Although we were not able to ascertain the cause of death for participants, most deaths occurred within the first 3 months of LAM testing, suggesting that mortality was related to active HIV-associated TB disease.
CONCLUSIONS
In conclusion, clinic-based urine LAM screening predicted mortality among ART-naive HIV-infected adults, which may have been due to detecting greater severity of clinical disease. A prospective randomized clinical trial is required to determine whether clinic-based urine LAM screening with accelerated anti-TB therapy can reduce mortality rates among HIV-infected adults in TB-endemic regions, particularly if a highly sensitive, second-generation, point-of-care urinary LAM assay becomes available.
Acknowledgments
We thank the women and men who participated in this study, the clinical sites for sharing their space, and our research staff and nurses who conducted the study.
Disclaimer. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies.
Financial support. This work was funded by the US National Institutes of Health; the Harvard Global Health Institute (to P. K. D.); the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988; to P. K. D.); the Infectious Disease Society of America Education and Research Foundation and National Foundation for Infectious Diseases (to P. K. D.); Massachusetts General Hospital Executive Committee on Research (to P. K. D.); the Program in AIDS Clinical Research Training Grant (T32 AI007433; to P. K. D.); the Harvard University Center for AIDS Research (P30 AI060354; to P. K. D.); the National Institute of Allergy and Infectious Diseases (K23 AI108293 [to P. K. D.] and R01AI058736 [to K. A. F.]); and the National Institute of Mental Health (R01 MH090326; to I. V. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2. World Health Organization. WHO Policy on Collaborative TB/HIV Activities. Geneva: World Health Organization; 2012. [Google Scholar]
- 3. World Health Organization, Stop TB. “End TB Strategy.” Geneva: World Health Organization; 2014. [Google Scholar]
- 4. World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 5. World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living With HIV in Resource-Constrained Settings. Geneva: World Health Organization; 2011. [Google Scholar]
- 6. Getahun H, Kittikraisak W, Heilig CM et al. . Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med 2011; 8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denkinger CM. High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Geneva: World Health Organization; 2014. [Google Scholar]
- 8. Denkinger CM, Kik SV, Cirillo DM et al. . Defining the needs for next generation assays for tuberculosis. J Infect Dis 2015; 211(Suppl 2):S29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis 2012; 12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta-Wright A, Peters JA, Flach C, Lawn SD. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med 2016; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drain PK, Losina E, Coleman SM et al. . Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed HIV-infected adults: a prospective, clinic-based study. BMC Infect Dis 2014; 14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drain PK, Losina E, Coleman SM et al. . Value of urine lipoarabinomannan grade and second test for optimizing clinic-based screening for HIV-associated pulmonary tuberculosis. J Acquir Immune Defic Syndr 2015; 68:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 2012; 12:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bjerrum S, Kenu E, Lartey M et al. . Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 2015; 15:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balcha TT, Winqvist N, Sturegård E et al. . Detection of lipoarabinomannan in urine for identification of active tuberculosis among HIV-positive adults in Ethiopian health centres. Trop Med Int Health 2014; 19:734–42. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV; Policy Guidance. Geneva: World Health Organization; 2015. [Google Scholar]
- 17. Bassett IV, Giddy J, Chaisson CE et al. . A randomized trial to optimize HIV/TB care in South Africa: design of the Sizanani trial. BMC Infect Dis 2013; 13:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bassett IV, Coleman SM, Giddy J et al. . Sizanani: a randomized trial of health system navigators to improve linkage to HIV and TB care in South Africa. J Acquir Immune Defic Syndr 2016; 73:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Department of Health, Republic of South Africa. The South African Antiretroviral Treatment Guidelines 2013. Pretoria: Department of Health; 2013. [Google Scholar]
- 20. Peter JG, Zijenah LS, Chanda D et al. . Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 21. Lawn SD, Kerkhoff AD, Burton R et al. . Systematic investigation for tuberculosis in HIV-infected patients on the first day of admission to a South African hospital: incremental diagnostic yield, accuracy and prognostic value of a urine LAM lateral-flow assay. In: 45th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (The Union) Barcelona, Spain, 2014. (HIV Late Breaker Oral Presentation [HIV_LB04]). [Google Scholar]
- 22. Peter J, Theron G, Chanda D et al. . Test characteristics and potential impact of the urine LAM lateral flow assay in HIV-infected outpatients under investigation for TB and able to self-expectorate sputum for diagnostic testing. BMC Infect Dis 2015; 15:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manabe YC, Nonyane BA, Nakiyingi L et al. . Point-of-care lateral flow assays for tuberculosis and cryptococcal antigenuria predict death in HIV infected adults in Uganda. PLoS One 2014; 9:e101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peter JG, Theron G, Dheda K. Can point-of-care urine LAM strip testing for tuberculosis add value to clinical decision making in hospitalised HIV-infected persons? PLoS One 2013; 8:e54875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Clinical significance of lipoarabinomannan detection in urine using a low-cost point-of-care diagnostic assay for HIV-associated tuberculosis. AIDS 2012; 26:1635–43. [DOI] [PubMed] [Google Scholar]
- 26. Drain PK, Gounder L, Grobler A et al. . Urine lipoarabinomannan to monitor antituberculosis therapy response and predict mortality in an HIV-endemic region: a prospective cohort study. BMJ Open 2015; 5:e006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drain PK, Gounder L, Sahid F, Moosa MY. Rapid urine LAM testing improves diagnosis of expectorated smear-negative pulmonary tuberculosis in an HIV-endemic region. Sci Rep 2016; 6:19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fielding KL, Charalambous S, Hoffmann CJ et al. . Evaluation of a point-of-care tuberculosis test-and-treat algorithm on early mortality in people with HIV accessing antiretroviral therapy (TB Fast Track study): study protocol for a cluster randomised controlled trial. Trials 2015; 16:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tlali M, Charalambous S, Fielding K et al. . TB Fast Track: a study to evaluate the effect of a point-of-care TB test-and-treat algorithm on early mortality in people with HIV accessing ART, a trial with randomisation at clinic level. In: South African HIV Clinicians Society Meeting, May 2016. [Google Scholar]
- 30. Hamasur B, Bruchfeld J, van Helden P et al. . A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS One 2015; 10:e0123457. [DOI] [PMC free article] [PubMed] [Google Scholar]