Abstract
Background: Roasted seeds of Amazonian guarana (Paullinia cupana var. sorbilis; Sapindaceae) are popular in South America due to their stimulant activity on the central nervous system (CNS). Rich in purine alkaloids, markedly caffeine, the seeds are extensively used in the Brazilian beverage industry for the preparation of soft drinks and as additives in energy drinks. Methods: To investigate the putative anti-aging and antioxidant activity of guarana, we used the model organism Caenorhabditis elegans. Chemical analyses were performed using high-performance liquid chromatography (HPLC) and electrospray ionization-mass spectrometry (ESI-MS/MS). Results: When tested in the model system Caenorhabditis elegans, the water extract from roasted guarana seeds enhanced resistance against oxidative stress, extended lifespan and attenuated aging markers such as muscle function decline and polyQ40 aggregation. Conclusions: In the current study, we demonstrate that guarana extracts can work as a powerful antioxidant in vivo; moreover, guarana extracts exhibit anti-aging properties. Our results suggest that the biological activities of guarana go beyond the extensively reported CNS stimulation.
Keywords: C. elegans, lifespan, oxidative stress, anti-aging, Paullinia cupana, guarana
1. Introduction
Plants that produce purine alkaloids like caffeine, theophylline and theobromine are widely used due to their stimulant activity on the central nervous system (CNS). Among the most popular plants we can list coffee (Coffea arabica), tea (Camellia sinensis), mate (Ilex paraguariensis) and cocoa (Theobroma cacao) which are mainly consumed as beverages and teas. They are also found included in sweets, bakery and desserts all over the world. The stimulant effects of purine alkaloids are related to their ability to inhibit cAMP phosphodiesterases and adenosine receptors promoting, among others, an increase in mental alertness, physical performance and reduction of fatigue [1,2]. Due to their pharmacological properties, isolated purine alkaloids are active ingredients of several medical preparations, as well as nutraceuticals and energy drinks of high popularity among those who search for natural products to achieve health and wellness goals. However, indiscriminate consumption is not free of adverse effects [3].
Guarana [Paullinia cupana var. sorbilis (Mart.) Kunth; Sapindaceae], widely used in the Brazilian beverage industry, is reported to have the highest concentration of caffeine (more than 6% in the seeds) observed in nature so far. The seeds are famous in Brazil for their stimulant effects. Popularly, roasted seed powder is used in preparations to enhance memory and to attenuate mental and physical stress [4].
Guarana is native to the Amazonia forest and was domesticated by indigenous people of the ethnic group Sateré Mawé about 500 years ago. In the wild, it grows as a woody vine that can reach 10 m in length, but when domesticated it grows as a bush reaching approximately two to three meters in height. It bears a characteristic red colored fruit that when ripe partially opens, exhibiting up to three black seeds covered at their lower half with a thick white aril, resembling an eye [5].
Espinola et al. [6] assessed the effects of guarana on the physical and cognitive performance of rats. The anti-fatigue effect was demonstrated in a forced swimming test; surprisingly, the group treated with pure caffeine or ginseng did not show a significant difference from the untreated control. Additionally, the authors demonstrated that guarana is readily able to reverse the amnesic effect of scopolamine. Otobone et al. [7] tested the effects of guarana seed extract on rats submitted to a Morris water maze test and reported significantly lower latency to escape among treated animals in comparison with untreated ones. Otobone et al. [8] demonstrated the antidepressant effect of guarana in rats and Rangel et al. [9] provided evidence for anxiolytic effects. Besides the effects on the CNS, guarana can attenuate markers of metabolic syndrome, like waist circumference and cholesterol levels; this effect was demonstrated among seniors self-referred as guarana drinkers. The authors additionally reported a significant association between lower levels of advanced oxidative protein products and guarana consumption [10]. Ruchel et al. [11] reported the ability of guarana to reduce total cholesterol and LDL-cholesterol to basal levels in hypercholesterolemic rats and highlighted the association between high guarana intake and reduction of the inflammatory process in those hypercholesterolemic rats.
Other activities frequently credited to guarana include analgesic, aphrodisiac, astringent, anorectic, anti-inflammatory, anti-platelet, cardiotonic, bronchorelaxant, gastrostimulant, immunostimulant, thermogenic and diuretic properties [12]. Carvalho et al. [13] reported antiproliferative activity of guaraná crude extract in HL-60 cells.
Considering the high amount of caffeine in guarana, it sounds reasonable to credit its biological effects to the purine alkaloids. However, guarana also contains significant amounts of phenolics, especially tannins like catechin, epicatechin and their polymers and proanthocyanidins, which might also contribute to its properties that go beyond the CNS stimulant effect [14,15,16].
In the current study, we have investigated a water extract and an alkaloid extract from roasted seeds of guarana (P. cupana var. sorbilis) with regard to its putative antioxidant and anti-aging properties. To determine antioxidant activity, the guarana extract was analyzed in vitro by DPPH method, a standard antioxidant assay. The in vivo antioxidant activity was investigated using the nematode C. elegans that is a powerful system in this context [17,18,19]. Furthermore, we evaluated a potential protective effect of the guarana extract against induced oxidative stress, its influence on the cellular accumulation of reactive oxygen species (ROS) and in the expression of important stress response genes like hsp-16.2 and sod-3, which is regulated by DAF-16/FOXO transcription factor. To explore a potential anti-aging activity, we investigated the influence of guarana extract on the lifespan of C. elegans, age-related muscle function decline and formation of polyQ40 plaques, which are considered as sensitive and relevant aging markers.
2. Material and Methods
2.1. Plant Material and Extraction
Guarana extract (GE) was prepared from the powder of roasted seeds purchased from a local trader in Manaus-AM (Brazil). The powder was weighted and exhaustively extracted with distilled water (5 × 1 L) at room temperature during an overall extraction period of 5 d. Using a rotary evaporator, the extract was reduced to approximately ¼ of the initial volume at low pressure, 40 °C, and subsequently frozen at −80 °C. The frozen extract was lyophilized yielding a fine dried powder. The alkaloid extract (AlkE) was obtained through standard alkaline dichloromethane extraction.
2.2. Chemical Characterization of the Extract
2.2.1. Characterization of Alkaloids
The alkaloid content (caffeine, theobromine and theophylline) of GE was determined by high-performance liquid chromatography-ultraviolet/visible spectroscopy (HPLC-UV/VIS) using an Agilent 1200 series HPLC system (Agilent, Santa Clara, CA, USA), provided with a column Synergi 4 μm Polar-RP 80 Å, 250 × 4.6 mm (Phenomenex, Torrance, CA, USA). Solvent system A—0.1% formic acid, solvent B—acetonitrile. The gradient profile was: 2–12% B over 5 min, 12–30% B over 15 min, 30–90% B over 3 min, isocratic 90% B for 8 min, 90–2% over 1 min, isocratic 2% B for 3 min. The flow rate was 0.8 mL/min and the column temperature was kept at 40 °C, the injection volume was 20 μL. The sample concentration was 1 mg/mL diluted in water. The UV-detection wavelength was 270 nm. The measurements were carried out in duplicate. The standard addition calibration method was used to determine the concentration of the analyzed compounds. Pure reference alkaloids were available and used for peak matching.
2.2.2. Characterization of Catechins (ESI-MS/MS)
Catechins and procyanidins in the guarana extract were identified with an Agilent 1100 HPLC system (Santa Clara, CA, USA) equipped with a degasser, quaternary pump, autosampler, column compartment, and VW-detector coupled to a PE Sciex API 2000 triple quad mass spectrometer (Sciex, Framingham, MA, USA). HPLC conditions were partly adapted from (Bonoli, et al., 2003). HPLC conditions: column Luna 5 μm C18 100 Å, 250 × 4.6 mm (Phenomenex, Torrance, CA, USA); column temperature: 20 °C; solvent system: A-H2O/methanol/formic acid: 74.7/25/0.3 (v/v/v), B—acetonitrile/formic acid: 99.7/0.3 (v/v); gradient profile: isocratic 0% B for 8 min, 100% B over 24 min, isocratic 100% B for 6 min, 0% B over 4 min, isocratic 0% B for 6 min; flow rate: 0.5 mL/min; injection volume: 20 μL; sample concentration: 1 mg/mL in H2O. ESI-MS/MS conditions: positive ion mode; ion spray voltage: 5500 V; temperature 450 °C; declustering potential: 61 V; focusing potential: 370 V; entrance potential: 12 V; collision cell entrance potential: 14 V [20].
2.2.3. Total Phenol Content
The Folin-Ciocalteu method was adapted to 96-well microplate format. Briefly, 100 μL of Folin-Ciocalteu reagent (Merck, Darmstadt, Germany) was added to 20 μL of sample; 5 min later, 80 μL of sodium carbonate (7.5% solution) were added. The reaction ran in the dark at room temperature for 2 h. The absorbance was measured at 750 nm using a microplate reader (Tecan Group Ltd., Männedorf, Switzerland). All measurements were carried out in triplicate and at least three times. The total phenol content is expressed as gallic acid equivalents (GAE/mg of sample).
2.3. Antioxidant Activity in Vitro (DPPH Assay)
A method described by Blois [21] was adapted to a 96-well microplate format. Briefly, 100 μL of 200 μM DPPH (2,2-diphenyl-1-picrylhydrazyl; Sigma-Aldrich GmbH, Steinheim, Germany) was added to 100 μL of sample. The reaction ran in the dark at room temperature (25 °C) for 30 min and the absorbance was measured at 517 nm using a microplate reader. The scavenging activity was calculated according the following equation:
| DPPH scavenging effect (%) = [(A0 – A1)/A0] × 100 |
where A0 means the absorbance of the control reaction and A1 is the absorbance in the presence of AE. All measurements were performed in triplicate. The EC50 value was estimated by sigmoid non-linear regression and is presented in μg/mL.
2.4. Caenorhabditis Elegans: Strains and Culture
The strains N2 (wt), CF1038 [daf-16(mu86)I], GR1307 [daf-16(mgDf50)-I], CF1553 {muls84 [pAD76(sod-3::GFP)]}, TJ375 (gpIs1[hsp-16-2::GFP]), BA17 [(fem-1(hc17)IV] and AM141 [rmIs133 (unc-54p::Q40::YFP)] were purchased from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN, USA). Worms were cultured on solid nematode growth media (NGM) plates, fed on living Escherichia coli OP50 and maintained at 20 °C in a temperature-controlled incubator [22]. Prior to the assays, the eggs were isolated to obtain age synchronized cultures and kept in M9 buffer for hatching as described by Strange et al. [23]. For all assays, after 24 h at 20 °C the larvae (L1 stage) were transferred to liquid S-medium inoculated with living E. coli OP50 (O.D.600 = 1.0) and treated according to the correspondent group.
2.5. Antioxidant Activity in Vivo
2.5.1. Survival Assay under Oxidative Stress
Age synchronized worms (strains N2, CF1038 and GR1307), grown in S-medium at L1 larval stage, were sorted into groups of 75 worms and treated with GE or AlkE for 48 h, except the control. Then, the groups were exposed to 80 μM of the pro-oxidant juglone (5-hydroxy-1,4-naphthoquinone; Sigma-Aldrich GmbH, Steinheim, Germany) and the number of dead and live worms was counted for 24 h after juglone treatment. The worms were considered dead when they did not respond to a gentle touch with a platinum wire. The assay was carried out in triplicate and results are presented as mean ± SEM, compared by one-way analysis of variance (ANOVA) followed by Bonferroni (post-hoc).
2.5.2. Intracellular ROS Accumulation
Age synchronized N2 worms (L1 stage, grown in S-medium) were sorted into groups and treated with GE or AlkE for 48 h, except the control group. Afterwards, the worms were washed with M9 buffer and incubated with 1 mL 50 μM CM-H2DCFDA solution (1 h at 20 °C) (6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; Fluka Chemie GmbH, Buchs, Switzerland), washed again with M9 and mounted on a glass slide, and a drop of 10 mM sodium azide (AppliChem GmbH, Darmstadt, Germany) was added to paralyze the nematodes. Live images were captured from at least 30 worms per group, using a fluorescence microscope (Keyence Deutschland GmbH, Neu-Isenburg, Germany; λEx 480/20 nm; λEm 510/38 nm). The relative fluorescence of the whole body was determined densitometrically using ImageJ software version 1.48 (National Institutes of Health, Bethesda, MD, USA). The results are presented as mean fluorescence intensity (mean ± SEM) and were compared by one-way ANOVA followed by Bonferroni (post-hoc). The assay was repeated three times.
2.6. Expression of Stress Response Genes
2.6.1. Quantification of hsp-16.2::GFP Expression
Age synchronized worms (TJ375, L4 stage, grown in S-medium) which carry a GFP reporter fused with hsp-16.2 were sorted into groups and treated with GE or AlkE for 48 h, except the control group. Subsequently, 20 μM juglone was added to the medium and 24 h later the worms were submitted to fluorescence microcopy as described before.
2.6.2. Quantification of Sod-3::GFP Expression
Age synchronized worms (CF1553, L1 stage, grown in S media) which carry a GFP reporter fused with sod-3, were treated with GE or AlkE for 48 h, except the control group, and submitted to fluorescence microscopy as described above.
2.7. Aging Markers
2.7.1. Longevity Assay
Age synchronized worms (BA17, grown in S-medium, at day 1 of adulthood) were sorted and treated with GE, except the control group. Live worms were transferred every second day to fresh medium and counted during the transfer. The worms exhibiting internally hatched progeny or extruded gonads were scored as censored worms and excluded from the assay. Dead worms, those that did not respond to a gentle touch with the platinum wire, were scored and removed from the assay. The results are presented as percentage of survival and the statistical significance was determined by Log-rank (Mantel-Cox) tests followed by Gehan-Breslow-Wilcoxon Test.
2.7.2. Pharyngeal Pumping Rate
Age synchronized worms (N2, day 1 of adulthood, grown in S-medium) were sorted and placed on NGM agar plates seeded with E. coli OP50. The bacterial lawn was supplemented with GE in the treated groups. The adults were transferred daily to separate them from their progeny; after day 5 of adulthood, the transfer was carried out one day before the analyses of the pumping activity, which were taken on day 5, 8 and 10 of adulthood. To score the pumping rate, each worm was observed for 1 min when crawling on the bacterial lawn using a stereoscope. Each group had a minimum of 10 worms. The results are presented as pumps/min (mean ± SEM) and were compared by two-way ANOVA followed by Bonferroni (post-hoc).
2.7.3. Quantification of PolyQ40::YFP Aggregates
Age synchronized worms (transgenic strain AM141), cultured at 20 °C in S-medium inoculated with living E. coli OP50, at L1 stage, were separated in groups and treated for 48 h with GE, except the control group. Then, the worms were mounted onto a glass slide with a drop of 10 mM sodium azide for paralysis and submitted to fluorescence microscopy (λEx 480/20 nm; λEm 510/38 nm). The results are presented as number of PolyQ40::YFP aggregates (mean ± SEM) and were compared by one-way ANOVA followed by Bonferroni (post-hoc).
2.8. Toxicity Assay: C. Elegans Brood Size
Age synchronized worms (N2, L4 stage, grown in S-medium) were sorted and placed individually on NGM agar plates seeded with E. coli OP50. The bacterial lawn was supplemented with GE or AlkE in the treatment groups. From day 1 of adulthood, the worms were transferred to fresh NGM plates supplemented according to their groups and the eggs at the former NGM plates were scored. The procedure was repeated for 5 days. The mean brood size was compared by two-way ANOVA followed by Bonferroni (post-hoc).
3. Results and Discussion
3.1. Chemical Characterization of Guarana Extract
Using HPLC-UV/Vis, three compounds were identified in guarana alkaloid extracts, namely caffeine, theobromine and theophylline. The quantification showed a high concentration of caffeine (102.8 mg/g), followed by theophylline (2.3 mg/g) and theobromine (1.0 mg/g). ESI-MS/MS analysis detected the presence of catechin and epicatechin (Table 1). Our results thus agree with the published chemical composition of guarana [14,15,24] and highlight the high concentration of caffeine as a hallmark of the species.
Table 1.
Determination of guarana extract (GE) chemical content. The high concentration of caffeine is a hallmark of this species.
| Compound | Concentration (mg/g) |
|---|---|
| Caffeine | 102.8 |
| Theophyline | 2.3 |
| Thebromine | 1.0 |
| Catechin | + |
| Epicatechin | + |
+ Detected by ESI-MS/MS analysis but not quantified.
The Folin-Ciocalteu method demonstrates a high phenolic content (1250 GAE/g of sample) for the roasted seed extract. Total phenolic content correlates with the antioxidant activity observed in vitro and in vivo.
3.2. In Vitro Antioxidant Activity of Guarana
Guarana extract was evaluated regarding its antioxidant activity in vitro through DPPH assay (Table 2) and exhibited moderate activity when compared with standard dietary antioxidants such as vitamin C and epigallocatechin gallate (EGCG). The alkaloid extract (AlkE) however, exhibited no antioxidant activity (Table 2).
Table 2.
In vitro antioxidant activity of GE as compared to the alkaloid extract from guarana (ALK) and standard natural antioxidants.
| Sample | DPPH * |
|---|---|
| GE | 40 |
| EGCG | 1.03 |
| Vitamin C | 2.12 |
| ALK | - |
* EC50 values expressed in μg/mL sample; - no antioxidant activity detected.
3.3. In Vivo Antioxidant Activity of Guarana
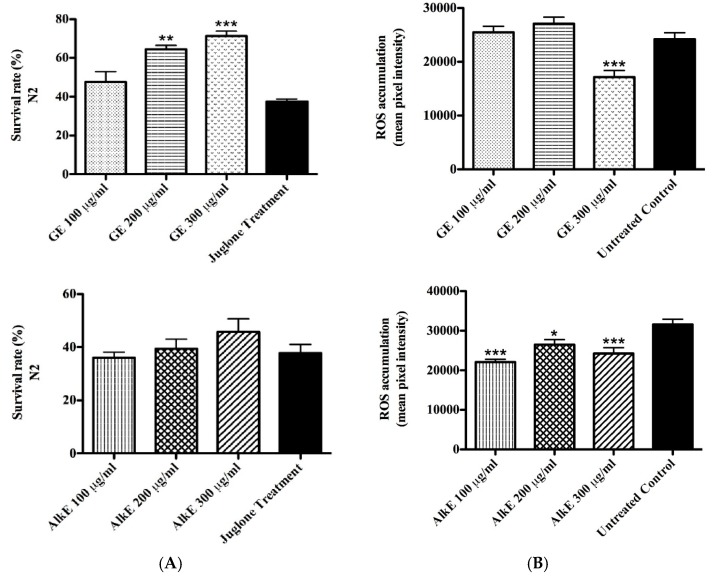
The antioxidant effects of GE were investigated in vivo in C. elegans. Worms were treated with a lethal dose of the toxic pro-oxidant juglone and analyzed 24 h later regarding the score of dead and live individuals. Worms pre-treated with GE exhibited a significantly higher survival rate as compared to the untreated control. At the highest tested concentration of 300 μg/mL GE, the survival rate was up to 71%, while in untreated control worms it was about 37% (adjusted p-value < 0.0001) (Figure 1A). The alkaloid extract was tested as well, but no significant difference was observed between AlkE treated and untreated worms (Figure 1B).
Figure 1.
Effect of GE and AlkE on stress resistance of wild-type worms. (A) Survival rate of N2 (wt) worms under juglone-induced oxidative stress. Survival rate of N2 worms was significantly enhanced in the groups treated with 200 and 300 μg/mL of GE; no significant difference was observed among worms treated with AlkE and untreated worms. (B) Intracellular reactive oxygen species (ROS) accumulation in N2 (wt) worms after treatment with GE. ROS accumulation was significantly decreased by GE and AlkE treatment compared to the untreated control. The results are presented as mean ± SEM from three independent experiments. * p < 0.05, ** p < 0.01 *** p < 0.0001 compared to the untreated control by one-way analysis of variance (ANOVA) followed by Bonferroni’s method (post-hoc).
To evaluate the influence of GE on the basal level of ROS accumulation in vivo, N2 (wild type) worms were exposed to the molecular probe H2DCFDA; the probe is sensitive to oxidation by H2O2 produced during the cellular aerobic metabolism. Once oxidized, it converts into DCF, which is a highly fluorescent compound. Analyses of the emitted florescence indicated a significant difference between GE treated and untreated worms; a decrease of up to 30% was observed in worms treated with 300 μg/mL GE compared to the untreated control group (adjusted p-value < 0.0001) (Figure 1C). The result adds up to the demonstrated capacity of GE to protect worms against juglone induced oxidative stress, supporting the role of GE as antioxidant in vivo. Interestingly, the AlkE extract that was inactive in the survival assay was able to decrease ROS accumulation by about 30% in worms treated with 300 μg/mL compared to the untreated control group (adjusted p-value < 0.0001) (Figure 1D).
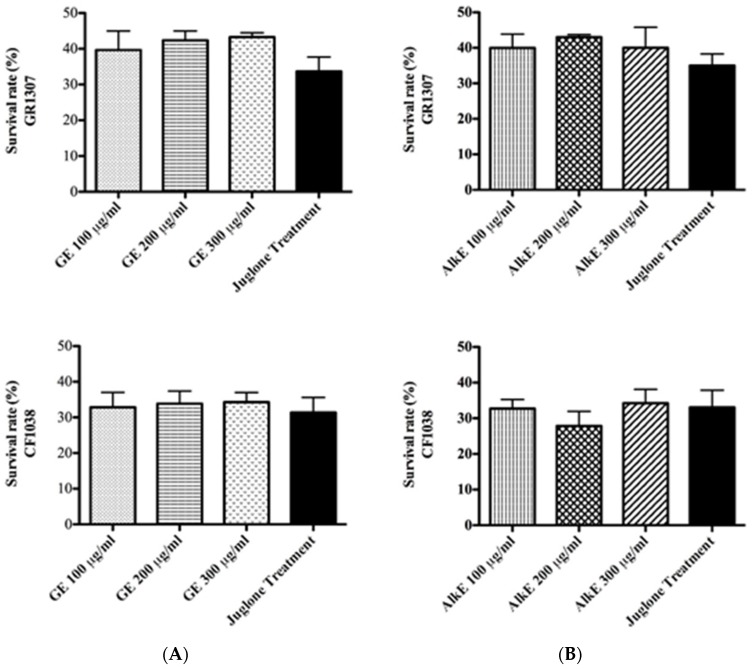
To assess whether the antioxidant effect of GE is based on its capacity to scavenge radicals or if it involves a molecular mechanism of stress resistance, we carried out the survival assay using mutant worms which lack the daf-16 gene (CF1038 ([daf-16(mu86)I]), GR1307 ([daf-16(mgDf50)])). By this approach, the inability of the extract to counteract the toxic effect of juglone could be demonstrated (Figure 2A), in contrast to the result with N2 (wt) worms. The alkaloid extract was tested as well; but, no significant difference was observed between AlkE treated and untreated worms (Figure 2B). Therefore, we assume that the antioxidant activity of GE is an outcome of its capacity to modulate molecular pathways of stress resistance, particularly involving the DAF-16 transcription factor.
Figure 2.
Effect of GE (A) or AlkE (B) treatment on the survival rate of the mutant strains CF1038 and GR1307 under juglone-induced oxidative stress. The DAF-16 mutant worms (GR1307 [daf-16(mgDf50) I] and CF1038 [daf-16(mu86) I]) did not respond to GE or AlkE treatment. Each bar represents the mean ± SEM from three independent experiments. The treatment groups are compared with the untreated control by one-way ANOVA followed by Bonferroni’s method (post-hoc).
DAF-16 is the C. elegans homologue of the mammalian FOXO transcription factor, which regulates the expression of several genes involved in stress resistance and longevity. Furthermore, mammalian FOXO proteins have been associated with aging phenotypes and aging related diseases, such as Alzheimer’s disease, cancer and diabetes type II [25].
3.4. Quantification of the Expression of Stress Response Genes after Guarana Treatment
A suitable approach to evaluate the efficacy of antioxidants in vivo is to assess the expression pattern of stress response genes like hsp-16.2 and sod-3; in this context, C. elegans is an appropriate model organism as several relevant mutant strains with a GFP reporter gene fusion are available.
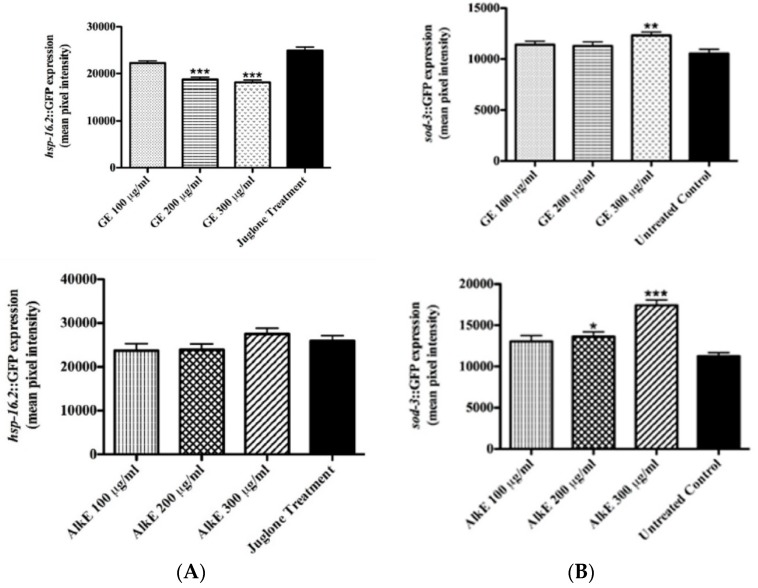
To assess the influence of GE on hsp-16.2 expression, TJ375 worms expressing the target gene fused with a GFP reporter, pre-treated with GE or AlkE, were submitted to mild oxidative stress induced by 20 μM juglone. Analyses of the emitted fluorescence revealed a significant difference among the groups: GE treated worms exhibited up to 23% lower fluorescence intensity compared to the untreated control worms (adjusted p-value < 0.0001) (Figure 3A). No significant effect was observed among worms treated with AlkE extract (Figure 3B). hsp-16.2 expression is frequently referred as a stress sensor due to the fact that it takes place only when the organism is facing stress conditions, such as heating or oxidants [26].
Figure 3.
Effect of GE or AlkE treatment on the expression of stress resistance related genes. (A) Heat shock protein 16.2 (HSP-16.2) expression in the mutant strain TJ375 [hsp-16.2::GFP(gplsI)] under juglone-induced oxidative stress. The hsp-16.2 expression levels were significantly decreased by GE treatment compared to the untreated control. No significant effect was observed among worms treated with the alkaloid extract, AlkE. (B) Superoxide dismutase 3 (SOD-3) expression in the mutant strain CF1553 after treatment with GE and AlkE. Worms [(pAD76)sod-3p::GFP + rol-6] treated with 300 μg/mL of GE showed higher levels of SOD-3 expression compared to the control. Similar results were observed among worms treated with the alkaloid extract, AlkE. The results are presented as mean ± SEM from three independent experiments. * p <0.05, ** p <0.01, *** p < 0.0001 compared to the untreated control by one-way ANOVA followed by Bonferroni’s method (post-hoc).
The expression of sod-3, a gene that codes for the mitochondrial antioxidant enzyme superoxide dismutase 3, was investigated using the mutant strain CF1553 (muIs84 ([(pAD76)sod-3p::GFP+rol-6]). The results showed significantly higher fluorescence intensity among mutants treated with 300 μg/mL GE as compared to the untreated control group (adjusted p-value = 0.0033) (Figure 3C). Similar results were obtained from worms treated with the alkaloid extract (Figure 3D). At the highest tested concentration of 300 μg/mL AlkE, the GFP expression was up 40% higher in comparison with the untreated control (adjusted p-value < 0.0001).
The demonstrated ability of GE to counteract juglone toxicity (down-regulate hsp-16.2) and to up-regulate sod-3 reinforces the assumption that the extract effectively promotes antioxidant activity in vivo. AlkE treatment also promoted an increase in the expression of sod-3::GFP, but did not influence the expression pattern of hsp-16.2::GFP. Although similar, the effect promoted by GE seems more interesting with regard of the antioxidant activity than the alkaloid extract.
3.5. Anti-Aging effects
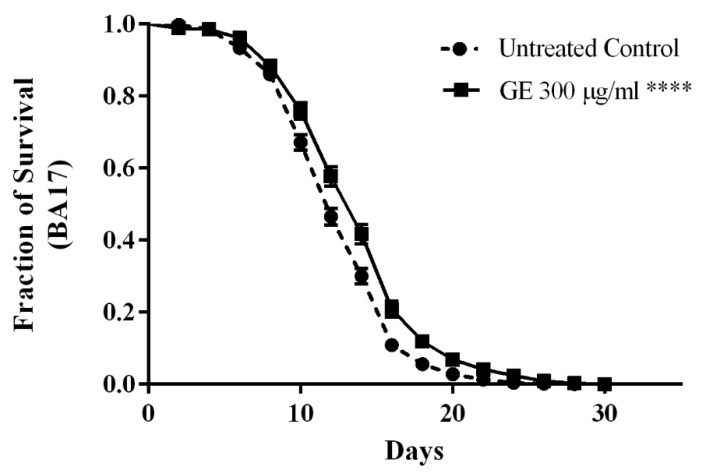
The putative ability of guarana to extend the lifespan was explored in C. elegans similarly to other polyphenol rich extracts like green tea and rooibos tea [18,27]. For the assay, feminized mutants BA17 (fem-1(hc17), which are unfertile when cultured at 25 °C, were selected. A significant extension of the lifespan by 14% was scored in worms treated with 300 μg/mL GE as compared to untreated control worms (p-value p < 0.0001) (Figure 4).
Figure 4.
Influence of GE treatment on longevity of the mutant worms BA17. GE 300 μg/mL increased the lifespan significantly as compared to the control group. The results are presented as percentage of survival and the statistical significance was determined by Log-rank (Mantel-Cox) tests followed by Gehan-Breslow-Wilcoxon Test. **** p < 0.0001.
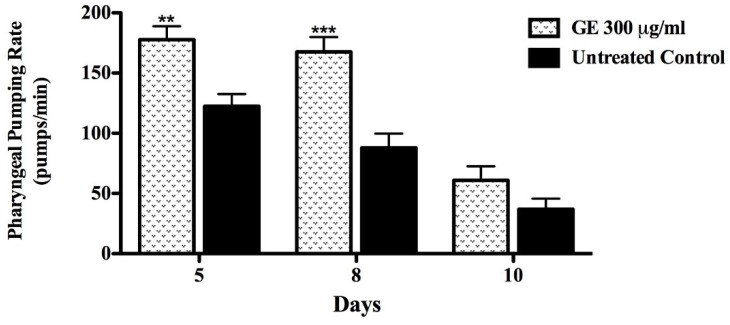
Caloric restriction (CR) is known as a potent lifespan enhancer [28]. In order to investigate whether worms treated with GE underwent caloric restriction during the course of the experiment, the pharyngeal pump rate was measured (which would decrease in case of CR). The pumping activity was scored in GE treated and untreated N2 (wt) worms at day 5, 8 and 10 of adulthood. The results obtained indicate a positive effect of GE throughout all periods of observation. The pumping activity of the GE treated group was 60% higher at day 10 compared to the untreated control group (p-value < p < 0.0001) (Figure 5).
Figure 5.
Pharyngeal pumping rate in N2 wt worms after GE treatment. The treatment with GE 300 μg/mL significantly attenuated the age-associated decline in the muscle function of pharynx. Data are presented as mean ± SEM. ** p <0.01,*** p < 0.0001 related to the control by a two-way ANOVA.
No impairment of the pumping activity of the pharynx was observed among GE treated worms; instead, the pumping rate was significantly higher compared to that scored among the untreated control. We assume that GE lifespan extension effect is not due to CR influence. Moreover, the attenuation of the age-related muscle function decline, assessed in C. elegans by the activity of the pharynx, is indicative for the anti-aging property of guarana extracts [29,30]. C. elegans pharynx muscle structure undergoes progressive deterioration and function decline with age, similarly to the sarcopenia which accompanies human aging; the damage seems to be at least partially the result of a lifetime stress promoted by increased ROS generation due to the high ATP demand of those muscles [31].
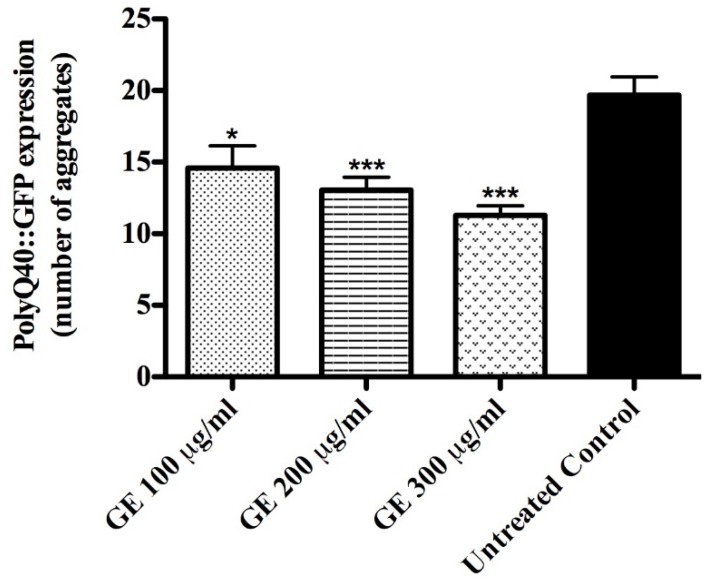
Genetic studies with C. elegans showed a positive association between aging delay and neuroprotection[32]; correspondingly, the number of polyQ40 aggregates, scored using the mutant strain AM141 ([rmIs133 (unc-54p::Q40::YFP)]), was significantly lower among GE treated worms compared to the untreated control group. When tested at a concentration of 300 μg/mL GE, the number of aggregates was 30% lower than in the corresponding control group (adjusted p-value < 0.0001) (Figure 6); the data supports the anti-aging property of guarana.
Figure 6.
PolyQ40::YFP aggregate accumulation in mutant worms AM141 after GE treatment. Worms treated with GE exhibited significantly lower numbers of polyQ40::GFP aggregates compared to the control group. Data are presented as mean ± SEM. * p <0.05, ** p < 0.01 and *** p < 0.0001 related to the control by a one-way ANOVA followed by Bonferroni (post-hoc).
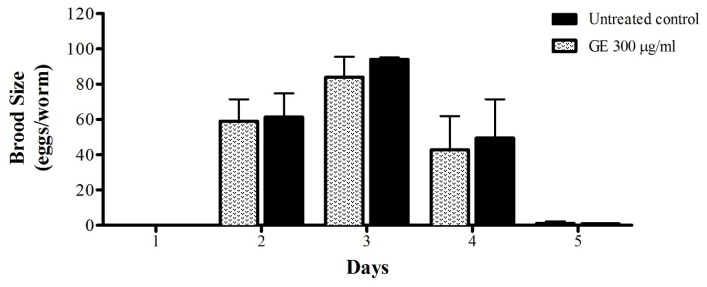
Bridi et al. [33] demonstrated the lifespan-prolonging effect of caffeine in C. elegans. However, the mentioned effect was accompanied by delayed larval development and impaired fertility rate. Sutphin et al. [34] reported the ability of caffeine to attenuate age-associated markers, including formation of polyQ40 plaques and highlighted that the ability of caffeine to extend the lifespan is temperature-dependent; the substance significantly shortened the lifespan of wild-type worms cultured at 25 °C. Guarana extract has a high caffeine content and also exhibited a lifespan prolonging effect and attenuation of aging markers in our study; however, no significant impairment in the fertility rate during the entire fertile period was observed (Figure 7).
Figure 7.
Brood size of N2 (wt) worms after GE treatment. Treatment with GE 300 μg/mL had no effect on egg laying activity. The results are expressed as mean ± SEM from three independent experiments. Treatment groups are compared to the untreated control by two-way ANOVA.
Caffeine might contribute to the antioxidant and longevity-promoting properties of guarana but it is not expected to be the sole active compound in that extract. The purine alkaloids may play a synergistic role in the antioxidant activity of guarana together with the known antioxidant phenolic components, including tannins (catechin, epicatechin and their polymers) and proanthocyanidins.
4. Conclusions
In summary, the current work demonstrated substantial antioxidant and anti-aging activity of guarana in C. elegans and indicates a new utilization for the fruit that is already well known due to its stimulant effects. The water extract from guarana can apparently enhance stress resistance though a pathway, which is dependent on the activity of the transcription factor DAF-16/FOXO. In addition, the extract can extend the lifespan and attenuate markers of aging, such as the age-related muscle function decline, assessed through the pharynx activity, and the formation of polyQ40 aggregates. However, further studies are needed to understand the underlying mechanisms and to identify the possible health benefits of guarana consumption throughout the human aging process.
Acknowledgments
H.P. thanks the National Counsel of Technological and Scientific Development (CNPq) for the PhD scholarship.
Author Contributions
H.P. and M.R. wrote the paper, designed and performed the experiments, analyzed the data; T.R. and E.R. performed the chemical characterization of the extract; X.W. wrote the paper and analyzed data; M.W. conceived and designed the project.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Satoskar R., Rege N., Bhandarkar S. Pharmacology and Pharmacotherapeutics. Elsevier Health Sciences APAC; Berlin, Germany: 2015. [Google Scholar]
- 2.Wink M., Van Wyk B.-E. Mind-Altering and Poisonous Plants of the World. Timber Press; Portland, OR, USA: 2008. [Google Scholar]
- 3.Lüde S., Vecchio S., Sinno-Tellier S., Dopter A., Mustonen H., Vucinic S., Jonsson B., Müller D., Veras Gimenez Fruchtengarten L., Hruby K. Adverse effects of plant food supplements and plants consumed as food: Results from the poisons centres-based plantlibra study. Phytother. Res. 2016;30:988–996. doi: 10.1002/ptr.5604. [DOI] [PubMed] [Google Scholar]
- 4.Smith N., Atroch A.L. Guarana’s journey from regional tonic to aphrodisiac and global energy drink. Evid. Based Complement. Altern. Med. 2010;7:279–282. doi: 10.1093/ecam/nem162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henman A.R. Guarana (Paullinia cupana var. sorbilis): Ecological and social perspectives on an economic plant of the central Amazon basin. J. Ethnopharmacol. 1982;6:311–338. doi: 10.1016/0378-8741(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 6.Espinola E., Dias R., Mattei R., Carlini E. Pharmacological activity of guarana (Paullinia cupana Mart.) in laboratory animals. J. Ethnopharmacol. 1997;55:223–229. doi: 10.1016/S0378-8741(96)01506-1. [DOI] [PubMed] [Google Scholar]
- 7.Otobone F.J., Sanches A.C., Nagae R.L., Martins J.V.C., Obici S., Mello J.C.P.D., Audi E.A. Effect of crude extract and its semi purified constituents from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) lucke] on cognitive performance in Morris water maze in rats. Braz. Arch. Biol. Technol. 2005;48:723–728. [Google Scholar]
- 8.Otobone F., Sanches A., Nagae R., Martins J., Sela V., De Mello J., Audi E. Effect of lyophilized extracts from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) ducke] on behavioral profiles in rats. Phytother. Res. 2007;21:531–535. doi: 10.1002/ptr.2089. [DOI] [PubMed] [Google Scholar]
- 9.Rangel M.P., de Mello J.C.P., Audi E.A. Evaluation of neurotransmitters involved in the anxiolytic and panicolytic effect of the aqueous fraction of Paullinia cupana (guaraná) in elevated t maze. Rev. Bras. Farmacogn. 2013;23:358–365. doi: 10.1590/S0102-695X2013005000024. [DOI] [Google Scholar]
- 10.Da Costa Krewer C., Ribeiro E.E., Ribeiro E.A.M., Moresco R.N., da Rocha M.I.U.M., Montagner G.F.F.S., Machado M.M., Viegas K., Brito E., Da cruz I. Habitual intake of guaraná and metabolic morbidities: An epidemiological study of an elderly amazonian population. Phytother. Res. 2011;25:1367–1374. doi: 10.1002/ptr.3437. [DOI] [PubMed] [Google Scholar]
- 11.Ruchel J., Rezer J., Thorstenberg M., Santos C., Cabral F., Lopes S., Silva C., Machado A., Cruz I., Schetinger M. Hypercholesterolemia and ecto-enzymes of purinergic system: Effects of Paullinia cupana. Phytother. Res. 2016;30:49–57. doi: 10.1002/ptr.5499. [DOI] [PubMed] [Google Scholar]
- 12.Hamerski L., Somner G.V., Tamaio N. Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plants Res. 2013;7:2221–2229. [Google Scholar]
- 13.Do Nascimento Carvalho L.V., Cordeiro M.F., e Lins T.U.L., Sampaio M.C.P.D., de Mello G.S.V., da Costa V.D.C.M., Marques L.L.M., Klein T., de Mello J.C.P., Cavalcanti I.M.F., et al. Evaluation of antibacterial, antineoplastic, and immunomodulatory activity of Paullinia cupana seeds crude extract and ethyl-acetate fraction. Evid. Based Complement. Altern. Med. 2016;2016 doi: 10.1155/2016/1203274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx F. Analysis of guarana seeds ii.Studies on the composition of the tannin fraction. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1990;190:429–431. doi: 10.1007/BF01202562. [DOI] [Google Scholar]
- 15.Meurer-Grimes B., Berkov A., Beck H. Theobromine, theophylline, and caffeine in 42 samples and products of guarana (Paullinia cupana, Sapindaceae) Econ. Bot. 1998;52:293–301. doi: 10.1007/BF02862148. [DOI] [Google Scholar]
- 16.Van Wyk B.-E., Wink M. Phytomedicines, Herbal Drugs, and Poisons. University of Chicago Press; Chicago, IL, USA: 2015. [Google Scholar]
- 17.Peixoto H., Roxo M., Krstin S., Röhrig T., Richling E., Wink M. An anthocyanin-rich extract of acai (Euterpe precatoria Mart.) increases stress resistance and retards aging-related markers in Caenorhabditis elegans. J. Agric. Food Chem. 2016;64:1283–1290. doi: 10.1021/acs.jafc.5b05812. [DOI] [PubMed] [Google Scholar]
- 18.Abbas S., Wink M. Green tea extract induces the resistance of Caenorhabditis elegans against oxidative stress. Antioxidants. 2014;3:129–143. doi: 10.3390/antiox3010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W., Müller D., Richling E., Wink M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the daf-16/foxo transcription factor. J. Agric. Food Chem. 2013;61:3047–3053. doi: 10.1021/jf3054643. [DOI] [PubMed] [Google Scholar]
- 20.Bonoli M., Pelillo M., Toschi T.G., Lercker G. Analysis of green tea catechins: Comparative study between HPLC and HPCE. Food Chem. 2003;81:631–638. doi: 10.1016/S0308-8146(02)00565-4. [DOI] [Google Scholar]
- 21.Blois M. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 22.Stiernagle T. In: Maintenance of C. elegans. Wormbook, editor. The C. elegans Research Community, Wormbook; Pasadena, CA, USA: 2006. [Google Scholar]
- 23.Strange K., Christensen M., Morrison R. Primary culture of Caenorhabditis elegans developing embryo cells for electrophysiological, cell biological and molecular studies. Nat. Protoc. 2007;2:1003–1012. doi: 10.1038/nprot.2007.143. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguti-Sasaki E., Ito L.A., Canteli V.C.D., Ushirobira T.M.A., Ueda-Nakamura T., Nakamura C.V., Palazzo de Mello J.C. Antioxidant capacity and in vitro prevention of dental plaque formation by extracts and condensed tannins of Paullinia cupana. Molecules. 2007;12:1950–1963. doi: 10.3390/12081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesp K., Smant G., Kammenga J.E. Caenorhabditis elegans daf-16/foxo transcription factor and its mammalian homologs associate with age-related disease. Exp. Gerontol. 2015;72:1–7. doi: 10.1016/j.exger.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Strayer A., Wu Z., Christen Y., Link C.D., Luo Y. Expression of the small heat-shock protein hsp 16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB J. 2003;17:2305–2307. doi: 10.1096/fj.03-0376fje. [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Sudji I.R., Wang E., Joubert E., van Wyk B.-E., Wink M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine. 2013;20:380–386. doi: 10.1016/j.phymed.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Fontana L., Partridge L., Longo V.D. Extending healthy life span—From yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman J.H., Singson A. Caenorhabditis elegans: Cell Biology and Physiology. Volume 107 Academic Press; Cambridge, MA, USA: 2012. [Google Scholar]
- 30.Herndon L.A., Schmeissner P.J., Dudaronek J.M., Brown P.A., Listner K.M., Sakano Y., Paupard M.C., Hall D.H., Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing. C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 31.Chow D.K., Glenn C.F., Johnston J.L., Goldberg I.G., Wolkow C.A. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp. Gerontol. 2006;41:252–260. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper J.F., Dues D.J., Spielbauer K.K., Machiela E., Senchuk M.M., Van Raamsdonk J.M. Delaying aging is neuroprotective in Parkinson’s disease: A genetic analysis in C. elegans models. NPJ Parkinson Dis. 2015;1 doi: 10.1038/npjparkd.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridi J.C., de Almeida Barros A.G., Sampaio L.R., Ferreira J.C.D., Soares F.A.A., Romano-Silva M.A. Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front. Aging Neurosci. 2015;7 doi: 10.3389/fnagi.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutphin G.L., Bishop E., Yanos M.E., Moller R.M., Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev. Healthspan. 2012;1 doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]