Abstract
Background
Cervical cancer is the fourth most common cancer in women worldwide with highest incidence reported in Eastern Africa in 2012. The primary goal of this study was to study the expression of p16INK4a in squamous cell carcinoma (SCC) of the cervix by immunohistochemistry (IHC) and determine relation with clinico-pathological parameters. This study further explored the correlation of p16INK4a immunostaining with another proliferation marker, Ki-67 and to study if human papillomavirus (HPV) IHC can be used as a marker for detection of virus in high-grade dysplasia.
Methods
A total of 90 samples, diagnosed for cervical cancer, were included in the study. Fixed Paraffin Embedded (FFPE) tissue sections were stained with anti-p16INK4a, anti-Ki-67 and anti-HPV antibodies using automated immunohistochemistry platform (ASLink 48-DAKO).
Results
Immunohistochemical protein expression of p16INK4a positivity was found to be highest in SCC (92.2%, n = 71) than other HPV tumors (76.9%, n = 10). The majority of cases (97.4%) were p16INK4a positive in the age group 41–60 years. In addition, a statistically significant difference between p16INK4a and HPV was observed among total cervical tumor cases and SCC cases.
Conclusions
As expected staining of invasive cervical cancer with anti-HPV showed rare positivity because HPV heralds active infection in dysplastic lesions and not of frank cervical carcinoma. In contrast, anti-p16INK4a IHC results showed positive correlation in SCC and other cervical tumors.
Electronic supplementary material
The online version of this article (doi:10.1186/s13027-017-0159-0) contains supplementary material, which is available to authorized users.
Keywords: Cervical cancer, Human Papillomavirus, p16INK4a, Ki-67, Immunohistochemistry, Sudan
Background
Cervical cancer continues to be a major public health problem in less developed regions of the world and ranked at fourth position among various cancers in women with estimated age standardized rates over 30 per 100, 000 women [1]. Global prevalence of cervical cancer is estimated 11.7%, out of which Sub-Saharan Africa was reported most affected region with highest prevalence rates (24%) [2]. It has been well documented that developed nations have significantly reduced the incidence and mortality rates of cervical cancer by successful implementation and sustenance of population wide primary screening programs using the cytological Pap smear and HPV DNA testing [3]. In contrast, data published by the WHO / ICO HPV Information Center reports that frequency of cervical cancer rises steadily in Sudan where 2.7% of women from the total population harbor 82–94% of high-risk HPV subtypes 16, 18, 45, 52 and 58 in the age group ranging between 15 and 44 years of age [4–7]. In Sudan, the current and commonest diagnostic method used to detect high-grade cervical dysplasia is the visual inspection with acetic acid (VIA) test [8, 9]. Though, VIA method showed higher sensitivity to detect high-grade lesions than Pap test but it has a lower specificity and misdiagnosis of cervical cancer ranges ~20–50% of true disease [10, 11]. Currently, molecular assay such as HPV DNA testing has been standardized and being commonly used with cytology.
HPV mediated cervical carcinogenesis is a process divided into two stages: productive and transforming infections. Productive infections are short-term lesions, which regress spontaneously, however transforming infections with certain high risk-HPV (HR-HPV) progress to invasive cancer gradually (20–30 years) if not followed up. Histomorphological types of cervical cancers include squamous cell carcinoma (SCC), adenocarcinomas and neuroendocrine carcinomas. SCC represents the majority of cases diagnosed (80%) while the remainder cases are adenocarcinomas and other types [12, 13].
A previously published study from Sudan has reported that women are diagnosed in late stages with aggressive disease emphasizing lack of efficient screening tests [8]. To facilitate early detection and proper treatment, there is a need to evaluate and introduce robust molecular markers of functional biological relevance, which can track disease progression and help in patient stratification [12, 14].
Among the various biomarkers established to facilitate early screening is the cell cycle regulatory protein known as p16INK4a, which has demonstrated to be highly sensitive and specific marker of high-grade squamous and glandular neoplasia of the cervix due to its overexpression in cancerous and precancerous cervical lesions [3, 15]. Moreover, p16INK4a inhibits the phosphorylation of pRb mediated through cyclin dependent kinases and regulating mitotic transition of cell cycle from G1 to S phase [16, 17]. Alterations in this host cell proliferation pathway is due to interference of viral oncoproteins E6 and E7 causing erratic cell division which leads to enhanced expression and cellular accumulation of p16INK4a in the basal and parabasal epithelial cells of HPV transformed lesions [18]. A recent meta-analysis study has shown that p16INK4a over-expression using immunohistochemistry (IHC) is linked with increased and disease free survival hence proving to be a marker for prognosis [19]. Studies have also shown that HR-HPV DNA presence is elevated in low-grade cervical lesions indicating active viral multiplication in host cell. Immunostaining of anti-HPV protein expression is detectable in initial transient cervical lesions but not in advanced lesions such as invasive SCC where p16INK4a is over-expressed [20].
In addition, cellular proliferation marker that could be used in conjunction with p16INK4a is Ki-67. HPV infected cells/lesions that overexpressed p16INK4a, as a response to viral oncogenes will concomitantly express Ki-67 [18]. Several studies have shown the relation of Ki-67 antibody expression in malignant lesions such as vulva, penis, breast and uterine cervix [21, 22]. Localized in the nucleus, this non-histone cell proliferation antigen is expressed in all cell cycle phases except G0 [23]. Increased Ki-67 expression in the basal layers of the dysplastic epithelium strongly correlates with lesion aggressiveness, and therefore can be used as predicator of proliferation and disease progression [24]. In addition, multiplexing p16INK4a and Ki-67 in both SCC and adenocarcinoma (AC) can be a synergistic approach to quantitate each marker at a cellular level in tumor regions instead of the benign proliferative zones, thereby increasing diagnostic accuracy in both immunohistochemistry studies [25].
The present study aimed to determine the expression of p16INK4a protein in SCC of the cervix by IHC and relate the findings with clinico-pathological characteristics, correlate p16INK4a overexpression with proliferation marker Ki-67 and validate that anti-HPV immunostaining is not suitable for detecting presence of virus in high grade invasive carcinoma.
Methods
Study population and samples
The Joint Institutional Review Board (JIRB) of Weill Cornell Medicine - Qatar (WCM-Q) and Hamad Medical Corporation (HMC) Research Office, Qatar has approved the present study (Protocol no.-10,165/10). This is a retrospective cohort study with a total of 90 formalin-fixed paraffin-embedded (FFPE) cervical carcinoma tumors obtained from patients diagnosed at the pathology laboratory at the National Health Laboratory (NHL), Sudan between years 2004–2008. Clinicopathologic information such as age, tumor differentiation and diagnosis was obtained when available.
Histopathological analysis
All cervical biopsies were fixed in 10% neutral buffered formalin, dehydrated and paraffin embedded. FFPE specimens were sectioned on microtome (4 μm thickness) for both Hematoxylin & Eosin (H&E) staining and immunostaining. H&E stained slides were examined for histopathologic diagnosis by experienced pathologist to record morphological alterations such as presence of koilocytotic cells, extent of keratinization and significant differentiation in the squamous epithelial cells (dysplasia).
Immunohistochemical staining and scoring
Immunostaining of FFPE tissue sections was performed using the Envision Flex™ protocol on ASLink48 (Dako, Glostrup, Denmark), as described previously [26]. The antibody titers and staining parameters were optimized on recommended control tissue according to the manufacturer’s instructions (Dako, Glostrup, Denmark). The antibodies used in this study were: anti-p16INK4a (dilution 1:3, clone E6H4, Roche, Tucson, AZ, USA), anti-HPV (dilution 1:50, clone K1H8, Dako, Glostrup, Denmark) and anti-human Ki-67 (dilution 1:200, clone MIB-1, Dako, Glostrup, Denmark). Briefly, after antigen retrieval the fixed tissue sections were blocked and incubated with primary antibodies for 20 min, washed, and secondary antibody coupled to HRP was added. The reaction was developed using DAB Chromogen (Dako, Glostrup, Denmark) and counterstained with Hematoxylin stain following dehydration in ethanol. Positive and negative tissue controls such as tonsil for p16INK4a, Ki-67 and human condyloma tissue for HPV immunostaining were included in each run to validate the stain localization whereas the negative control was the same tissue section omitting primary antibody.
Scoring of immunostained tumor sections was performed by an experienced pathologist. Strong diffuse nuclear as well as cytoplasmic staining in the basal layers of the squamous epithelium is considered p16INK4a positive. Depending on the distribution of the stain in the epithelium, positivity is scored as strong diffuse or high intensity (3+), strong focal or moderate intensity (2+) and weak sporadic or mild (1+). For anti-HPV, positive staining in the nuclei of infected squamous cells and occasional staining of cytoplasm in koilocytotic & dysplastic epithelial cells was observed. All the cases were scored as positive or negative based if protein expression of HPV capsid protein (VP1) was observed. In case of anti-Ki-67 antigen, depending on the intensity and proportion of mitotic cells in the basal and parabasal cells of the squamous epithelium, scores were assigned as follows: <10- Negative, 10–30 cells- 1+, 40–60 cells- 2+, 70–100- 3+.
Statistical analysis
Immunohistochemical intensity for each of the three markers irrespective of the score was designated as positive for presence and negative if absent. Results were presented using frequencies and percentages. Comparison between different groups was performed using ANOVA test, followed by Student’s t-test. Sensitivity and specificity between p16INK4a and HPV immunostaining were compared using McNemar’s test and positive predictive value (PPV) and negative predictive value (NPV) were recorded. Two tailed test were used for analysis and p-value <0.05 was considered significant. The SPSS statistical package (IBM SPSS version 22.0.) was used for the statistical analysis.
Results
Demographic and clinical characteristics of the study population
This study includes 90 cases of cervical tumor biopsies from Sudan. Based on histology analysis, data in Table 1, 85.6% (n = 77) of women were diagnosed with squamous cell carcinoma (SCC) and 14.4% (n = 13) of other HPV associated tumors. In addition, tumor grade, which is the histopathologic differentiation of cancerous tissue, were classified into well differentiated, moderately and poorly differentiated. The age range among the studied population was 27–80 years with a mean age of 54.2 (±13.5) years. Furthermore, the individuals were categorized into 3 age groups (<40, 41–60, >60 years), in which majority (52.1%) of women were aged 41–60 years. However, age was not reported for seven patients in the study (Table 1). The degree of differentiation, an important classification in SCC cases revealed that the majority of samples were of non-keratinizing 68.8% (n = 53) subtypes, 26% (n = 20) were keratinizing carcinomas. Data were not available for four SCC cases (Table 1 and Fig. 1a, b).
Table 1.
Demographic, clinico-pathological characteristics and frequency of different immunostaining profiles according to diagnosis among women from Sudan
| SCC n = 77a (%) |
Other tumors n = 13 (%) | p value | |
|---|---|---|---|
| Age (years) | |||
| 21–40 | 13 (18.6%) | 3 (23.1%) | 0.134 |
| 41–60 | 36 (51.4%) | 3 (23.1%) | |
| 61–80 | 21 (30.0%) | 7 (53.8%) | |
| Pathological diagnosis | |||
| Keratinizing | 20 (26.0) | NA | |
| Non-keratinizing | 53 (68.8) | ||
| No data | 4 (5.2) | 13 (100) | |
| p16INK4a immunostaining | |||
| Positive | 71 (92.2) | 10 (76.9) | 0.119 |
| Negative | 6 (7.8) | 3 (23.1) | |
| HPV immunostaining | |||
| Positive | 7 (9.1) | 2 (15.4) | 0.384 |
| Negative | 70 (90.9) | 11 (84.6) | |
| Ki-67 immunostaining | |||
| Positive | 47 (61.0) | 8 (61.5) | 0.314 |
| Negative | 5 (6.5) | 2 (15.4) | |
| No data | 25 (32.5) | 3 (23.1) | |
aAge of 7 patients were not available
Fig. 1.
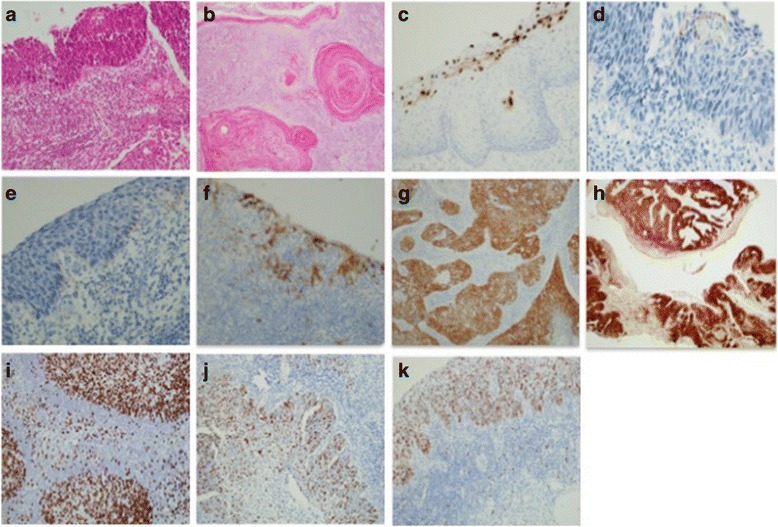
Representative pictures of Hematoxylin & Eosin (H&E) and Immunohistochemical staining for p16INK4a, HPV and Ki-67 in squamous cell carcinoma (SCC). a Non-keratinizing SCC (H&E) (b) Keratinizing SCC (H&E) (c) anti-HPV positive control (human condyloma) (d) anti-HPV positivity in other tumors (e) anti-HPV positivity in SCC (f) p16INK4a positive control (tonsil) (g) p16INK4a moderate intensity (focal) immunostaining in SCC (h) p16INK4a high intensity (diffuse) immunostaining in SCC (i) Ki-67 positive control (tonsil) (j) Ki-67 proliferating epithelial cells in SCC (k) Ki-67 positivity in SCC (a-k: ×20)
Immunostaining
Immunohistochemistry (IHC) of FFPE tissue samples was performed using anti-p16INK4a, Ki-67 and anti-HPV antibodies (Fig. 1c-k). Overexpression of p16INK4a was found to be higher in SCC, 92.2% (n = 71) compared to other HPV tumors, 76.9% (n = 10) (Table 1). Furthermore, based on immunostaining intensity, we extended our analysis to evaluate and compare the test sensitivity between different immunostaining and diagnosis, we found a significant difference in p16INK4a among SCC cases (sensitivity = 79.2%, specificity = 46.1%, PPV = 83.9%, NPV = 27.2%, p = 0.049) (Fig. 2a). However, no significant difference was observed in HPV and Ki-67 immunostaining (p = 0.484 and p = 0.329), respectively (Fig. 2b, c). Additionally, an exact McNemar’s test determined that there was a statistically significant difference between p16INK4a and HPV among cervical tumor cases (p = 0.011) and SCC cases alone (p = 0.049) however; no significant difference was noted in other tumor cases (p = 0.219) (Table 2). Furthermore, no significant correlation was found between tumor grade and p16INK4a immunostaining.
Fig. 2.
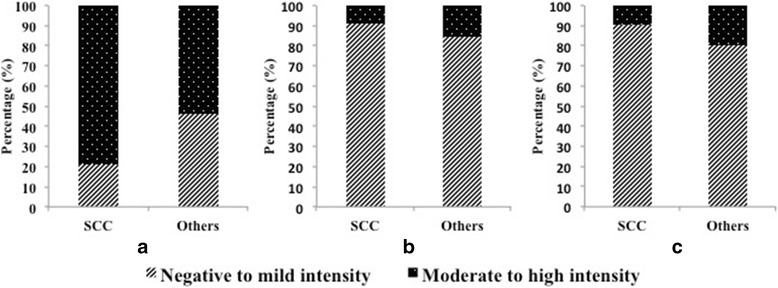
Differential staining of (a) p16INK4a, (b) HPV and (c) Ki-67 between SCC and other tumors
Table 2.
Distribution of p16INK4a versus HPV immunoexpression in 90 cervical tumors cases according to diagnosis in women from Sudan
| Diagnosis | HPV | p16INK4a | p value£ | |
|---|---|---|---|---|
| Negative n (%) |
Positive n (%) |
|||
| SCC | Positive | 3 (18.8) | 4 (6.6) | 0.049£ |
| Negative | 13 (81.2) | 57 (93.4) | ||
| Others | Positive | 1 (16.7) | 1 (14.3) | 0.219 |
| Negative | 5 (83.3) | 6 (85.7) | ||
| Total | Positive | 4 (18.2) | 5 (7.4) | 0.011£ |
| Negative | 18 (81.8) | 63 (92.6) | ||
£significant
Of the total 90 samples collected in this study, Ki-67 immunoreaction was performed only in 68.9% (n = 62) cases (Table 1). Of these, 88.7% (n = 55) were found to be positive for Ki-67 immunostaining (Table 1). Furthermore, to correlate expression of p16INK4a with a proliferation marker, Ki-67 according to the diagnosis, a significant difference was observed in other HPV associated tumors (p = 0.019) however, no significant association was found with SCC cases (Additional file 1: Table S1). According to age groups, the percentage frequency of p16INK4a was significantly higher in the age group 41–60 years (p = 0.001), however, no association with age group was seen in Ki-67 and HPV immunostaining (Fig. 3).
Fig. 3.
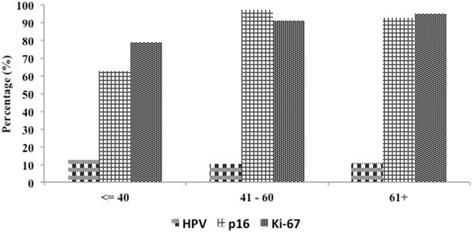
Percentage of HPV, p16 INK4a and Ki-67 immunostaining according to age group. (*p = 0.001)
Discussion
Meta-analysis studies have reported that cervical cancer incidence and mortality rates are rising in sub- Saharan Africa with an estimated prevalence of oncogenic HPV genotypes across all ages at 33.6% specifically in Eastern Africa [2]. The HPV distribution data in Africa reveals two peaks, one at <25 and another ≥45 years of age [2]. In the present study, we observed that 52.1% of women in age group between 41 and 60 years were diagnosed with SCC, a dominant histomorphological form in invasive cervical cancer [12]. According to the WHO guidelines, all women between ages of 35–45 years must be screened annually, which can significantly reduce mortality rates [27]. The degree of differentiation (keratinization) is an important pathological feature in cervical tumors, indicates the site of infection in the cervical mucosal epithelium (squamo-columnar junction) and susceptible to HPV infection. In this study, majority of SCC cases were classified as non-keratinizing SCC, 68.8% and 26% were keratinizing SCC, this corroborates previous findings from Ethiopia [28].
Correlating p16INK4a expression with clinico-pathological parameters, we found that p16INK4a expression was directly proportional to the diagnosis of cervical tumors where 90.1% of SCC cases and 76.9% as Adenocarcinoma (AC) showed positive p16INK4a expression, which is in agreement with previous studies [15, 29, 30]. However, Lin J et al. [19], reports that prognostic significance of p16INK4a overexpression was not significantly associated with tumor grade nor tumor size, however p16INK4a overexpression was highly associated with better prognosis and increased disease-free survival which, corroborates with previous studies [31, 32]. Additionally, in the present study, 9.9% (n = 7) of SCC cases and 23.1% (n = 3) of AC cases were p16INK4a negative which has also been reported in previous studies [33, 34, 35]. Furthermore, absence of p16INK4a expression in the cervical tumors does not indicate lack of HR- HPV or improper IHC technique but rather implicated due to silencing of the gene through mutations in the promoter regions resulting in transcription failure, epigenetic mechanisms and hyper methylation.
Molecular assays are the most sensitive for HPV detection in FFPE samples than IHC [32]. In the present study, the frequency of both p16INK4a and HPV positive expression was seen in age group between 41 and 60 years (n = 3, 3.6%). The positive correlation of HPV expression with p16INK4a intensity and dispersion on cervical carcinoma cases was reported in a study in Saudi Arabia [30]. However, majority (91.5%) of SCC cases were positive for p16INK4a, but negative for HPV-1 derived capsid protein (VP1). The majority of cervical cancers does not have active dysplasia and therefore are limited in protein expression of HR-HPV.
To increase the reproducibility of diagnosis, p16INK4a expression was compared with a cellular proliferation marker, Ki-67. To date, several studies have shown co-expression of p16INK4a and Ki-67 when combined concurrently improve diagnostic accuracy and that Ki-67 expression increases linearly with tumor grade in SCC [25, 34, 36]. In this study, ~61% of SCC and AC cases were positive for Ki-67. Furthermore, the frequency of p16INK4a and Ki-67 positive immunostaining when assessed individually was higher in the age group >40 and 6.6% (n = 4) were both positive for p16INK4a and Ki-67. Several studies have shown that both p16INK4a and Ki-67 proteins are co-expressed in most high-grade squamous lesions, which is also observed in this study. Ki-67 is employed as an objective marker indicating aberrant cellular proliferation that facilitates measurement of advanced disease end point, which is progress of CIN to carcinoma. Hence, direct proportionality in Ki-67 expression in relation to tumor grade along with p16INK4a is involved functionally in the process of HPV induced transformation and overexpressed in the epithelium [29, 36]. Furthermore, Ki-67 expression was found to be significantly different in cervical tumor lesions CIN2 and CIN3 [33] and also expressed in benign proliferative lesions and in basal cells of normal squamous mucosa [25].
In the present study, no correlation was found between p16INK4a and Ki-67 with respect to HPV immunohistochemical protein expression, which is in agreement with previous report [29]. HPV detection rate using in situ hybridization (ISH) varied significantly between cervicitis and low grade SIL; in addition, significant correlation of HR-HPV status with histopathological grade was observed [37]. In contrast, p16INK4a and Ki67 was found useful to detect both LR and HR-HPV in precancerous lesions and distinguish between low grade SIL and high grade SIL [38].
Based on the tumor grade, it has been shown that in low-grade lesions, p16INK4a is diffusely expressed in ~60% of CIN1 and mostly associated with HR-HPV genotypes, but HR-HPV presence was detected in p16INK4a negative tumors [36, 39]. In addition, high incidence of HR-HPV has been observed among young women and most of these infections were transient and regress spontaneously [39]. We therefore concluded that p16INK4a overexpression is already indicative of advanced viral interference progressing towards invasive cancer where aside from active viral replication there is significant morphological change occurring at histology, cellular and molecular level which can be visually scored. However, in invasive cervical cancer, the HPV protein expression was not detectable by IHC and HPV genotyping in FFPE tumor samples.
Currently, in Sudan precancerous cervical lesions are visually detected by VIA [8–10] unlike the Pap test, a primary screening method in industrialized nations, VIA has limitations to distinguish early morphological changes associated with neoplastic transformation. However, Pap test has not been implemented as a screening modality in low resource settings with no national HPV vaccination program [4]. Despite the increasing cancer related incidence and mortality rates, Sudan is burdened with other public health issues such as malaria, leprosy, tuberculosis, HIV/AIDS thereby causing lack of emphasis and knowledge about this disease [40]. Thus, introduction of such molecular biomarkers will prove beneficial in early detection and embarking on screening initiatives and reducing mortality [27]. Recently, WHO has recommended that HPV vaccination should be performed as part of national immunization programs for women between ages 13–26 years to effectively prevent disease [27]. In addition, it was suggested that boys should be included in HPV vaccination programs to overcome cost effectiveness in low resource settings [41]. Current treatment for invasive cervical cancer in sub-Saharan Africa is radiotherapy, which is a major challenge due to lack of both resources and primary screening facilities hence, resulting in progress to advanced disease stage when diagnosed [8, 40]. Thus, there is a need to establish frequent screening intervals of optimal target population as progression from low grade to invasive cervical cancer takes up to 20 years [27] and adopt algorithm models for cervical cancer prevention in developed countries to help in proper management of disease [41].
The strength of the present study lies in the use of IHC technique to study immunochemical staining along with H&E stain of SCC tumors for histopathology assessment. The monoclonal antibody clone used for p16INK4a-E6H4 has been recommended specific antibody for exfoliated dysplastic cells both in histology tissue sections and cytology smears and that normal cervical epithelium, inflammatory or metaplastic lesions were not stained [14].
Limitations of this study are primarily the tissue quality, preservation and fixation of FFPE samples were not suitable to carry out analysis for HPV genotyping study. In addition, cut-off threshold for immune-positivity of p16INK4a varies between pathologists, presenting a challenge as no standard parameters. Limited sample number for Ki-67 hence proper correlation with p16INK4a in SCC cases could not be made. Larger cohort with proper diagnostic, survival data can help to correlate other clinical parameters.
Conclusions
In this preliminary study, we evaluate the clinical utility of p16INK4a as a surrogate marker using IHC technique in invasive cervical carcinoma among women in Sudan. Overall, p16INK4a overexpression in squamous cell carcinoma was significant and differs from expression in other cervical tumors (AC). The immunostaining of p16INK4a and HPV vary in the total sample positive and negative reaction, indicating that p16INK4a is an effective way to detect invasive carcinoma than HPV. Proliferation marker, Ki-67 showed significant correlation with p16INK4a in other HPV associated tumors. Lastly, significant correlation of p16INK4a percentage frequency in age group 41–60 years where active advance transforming CIN occurs concludes that it is an appropriate surrogate marker to help in early screening of cervical neoplasia.
Acknowledgements
We would like to acknowledge the support of the Human Histology Core and its personnel at WCM-Q. We are also grateful to Dr. Imad Bin Mujeeb and his team at the HMC-Anatomic Pathology Department for providing HPV Positive control tissue and facilitating with slides.
Funding
This publication was made possible by NPRP grant [NPRP-5-098-3-021] from the Qatar National Research Fund (a member of Qatar Foundation) and Weill Cornell Medicine-Qatar (WCM-Q) Histology Core grant [BMRP-5726005905]. The findings achieved herein are solely the responsibility of the author [s].
Availability of data and materials
The data will be available on request from the author. Patient’s confidentiality must be respected and adhered to because of the sensitive nature of the information collected from the subjects.
Abbreviations
- AC
Adenocarcinoma
- CIN
Cervical Intraepithelial Neoplasia
- FFPE
Formalin-fixed paraffin embedded
- H&E
Hematoxylin & Eosin
- HMC
Hamad Medical Corporation
- HPV
Human Papillomavirus
- HR
High risk
- IHC
Immunohistochemistry
- LR
Low risk
- NPV
Negative predictive value
- PPV
Positive predictive value
- SCC
Squamous cell carcinoma
- SIL
Squamous Intraepithelial lesion
- SPSS
Statistical package for the Social science
- VIA
Visual inspection with acetic acid
- WCM-Q
Weill Cornell Medicine-Qatar
Additional file
Frequency of p16INK4a/Ki-67 immunostaining according to diagnosis and HPV infection in the 63 cervical tumor cases. (DOCX 51 kb)
Authors’ contributions
SB and AAS contributed to the conceptualization and design of the study. MM, HS, NH and SB assisted in the sample and preliminary clinicopathologic data collection. SB and NH confirmed the pathologic diagnosis. MM, HS and SB generated the data. HS, DB, MM and SB analyzed the data. SB, HS, DB and AAS wrote the manuscript. SB, AAS, MM, DB, NH, HS reviewed the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in the study involving human participants were approved and conducted in accordance with the ethical standards of Joint Institutional Review Board (JIRB) of Weill Cornell Medicine - Qatar (WCM-Q) and Hamad Medical Corporation (HMC) Research Office, Qatar (Protocol no. 10165/10).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13027-017-0159-0) contains supplementary material, which is available to authorized users.
Contributor Information
Hina Sarwath, Email: his2014@qatar-med.cornell.edu.
Devendra Bansal, Email: dbansal2006@gmail.com.
Nazik Elmalaika Husain, Email: nazikhusain@gmail.com.
Mahmoud Mohamed, Email: MMOHD13@hamad.qa.
Ali A. Sultan, Email: als2026@qatar-med.cornell.edu
Shahinaz Bedri, Phone: +974-4492-8387, Email: shahinazbedri@gmail.com, Email: shb2026@qatar-med.cornell.edu.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 3.Sahasrabuddhe VV, Luhn P, Wentzensen N. Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiol. 2011;6:1083–1098. doi: 10.2217/fmb.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ICO Information Centre on HPV and Cancer . Sudan. Human Papillomavirus and related cancers, fact sheet. 2017. [Google Scholar]
- 5.Abate E, Aseffa A, El-Tayeb M, El-Hassan I, Yamuah L, Mihret W, et al. Genotyping of human papillomavirus in paraffin embedded cervical tissue samples from women in Ethiopia and the Sudan. J Med Virol. 2013;85:282–287. doi: 10.1002/jmv.23437. [DOI] [PubMed] [Google Scholar]
- 6.Seoud M. Burden of human papillomavirus-related cervical disease in the extended middle east and north Africa-a comprehensive literature review. J Low Genit Tract Dis. 2012;16:106–120. doi: 10.1097/LGT.0b013e31823a0108. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed EA, Alagib A, Babiker AI. Incidents of cancer in Sudan: past trends and future forecasts. Afr J Math Comput Sci Res. 2013;8:136–142. [Google Scholar]
- 8.Husain N, Helali T, Domi M, Bedri S. Cervical cancer in women diagnosed at the National Health Laboratory, Sudan: a call for screening. Afr J Online (AJOL) 2011;6:183–190. [Google Scholar]
- 9.Ibrahim A, Aro AR, Rasch V, Pukkala E. Cervical cancer screening in primary health care setting in Sudan: a comparative study of visual inspection with acetic acid and pap smear. Int J Womens Health. 2012;4:67–73. doi: 10.2147/IJWH.S28406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan FM, Khirelseed M. Cervical cancer screening among Sudanese women. Gulf J Oncolog. 2009;6:28–34. [PubMed] [Google Scholar]
- 11.Tambouret R. Screening for cervical cancer in low-resource settings in 2011. Arch Pathol Lab Med. 2013;137:782–790. doi: 10.5858/arpa.2011-0695-RA. [DOI] [PubMed] [Google Scholar]
- 12.Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer. 2014;14:395–405. doi: 10.1038/nrc3728. [DOI] [PubMed] [Google Scholar]
- 13.de Freitas AC, Gurgel AP, Chagas BS, Coimbra EC, do Amaral CM. Susceptibility to cervical cancer: an overview. Gynecol Oncol. 2012;126:304–311. doi: 10.1016/j.ygyno.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Burd EM. Human Papillomavirus laboratory testing: the changing paradigm. Clin Microbiol Rev. 2016;29:291–319. doi: 10.1128/CMR.00013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4a as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin-Drubin ME, Crum CP, Munger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci U S A. 2011;108:2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringold F, Serrano M. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35:317–329. doi: 10.1016/S0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 18.von Knebel DM, Reuschenbach M, Schmidt D, Bergeron C. Biomarkers for cervical cancer screening: the role of p16(INK4a) to highlight transforming HPV infections. Expert Rev Proteomics. 2012;9:149–163. doi: 10.1586/epr.12.13. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Albers AE, Qin J, Kaufmann AM. Prognostic significance of overexpressed p16INK4a in patients with cervical cancer: a meta-analysis. PLoS One. 2014;9:e106384. doi: 10.1371/journal.pone.0106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calil LN, Edelweiss MI, Meurer L, Igansi CN, Bozzetti MC. p16INK4a And Ki67 expression in normal, dysplastic and neoplastic uterine cervical epithelium and human papillomavirus (HPV) infection. Pathol Res Pract. 2014;210:482–487. doi: 10.1016/j.prp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Hemalatha A, Suresh TN, Kumar ML. Expression of vimentin inbreast carcinoma, its correlation with Ki67 and other histopathological param-eters. Indian J Cancer. 2013;50:189–194. doi: 10.4103/0019-509X.118724. [DOI] [PubMed] [Google Scholar]
- 22.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste. Costa Rica J Infect Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 23.Ungureanu C, Teleman C, Socolov D, Anton G, Mihailovici MS. Evaluation of p16INK4a and Ki67 proteins expression in cervical intraepithelial neoplasia andtheir correlation with HPV-HR infection. Rev Med Chir Soc Med Nat Iasi. 2014;114:823–828. [PubMed] [Google Scholar]
- 24.Longatto Filho A, Utagawa ML, Shirata NK, Pereira SM, Namiyama GM, Kanamura CT, et al. Immunocytochemical expression of p16INK4A and Ki-67 incytologically negative and equivocal pap smears positive for oncogenic humanpapillomavirus. Int J Gynecol Pathol. 2005;24:118–124. doi: 10.1097/01.RCT.0000157092.44680.25. [DOI] [PubMed] [Google Scholar]
- 25.Samarawardana P, Singh M, Shroyer KR. Dual stain immunohistochemical localization of p16INK4A and ki-67: a synergistic approach to identify clinically significant cervical mucosal lesions. Appl Immunohistochem Mol Morphol. 2011;19:514–518. doi: 10.1097/PAI.0b013e3182167c66. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed M, Sarwath H, Salih N, Bansal D, Chandra P, Husain NE, et al. CD8+ tumor infiltrating lymphocytes strongly correlate with molecular subtype and clinico-pathological characteristics in breast cancer patients from Sudan. Transl Med Commun. 2016;1:4. doi: 10.1186/s41231-016-0005-1. [DOI] [Google Scholar]
- 27.Adewole IF, Abauleth YR, Adoubi I, Amorissani F, Anorlu RI, Awolude OA, et al. Consensus recommendations for the prevention of cervical cancer in sub-Saharan Africa. South Afr J Gynaecol Oncol. 2013;5:47–57. doi: 10.1080/20742835.2013.11441209. [DOI] [Google Scholar]
- 28.Rashed MM, Bekele A. The prevalence and pattern of HPV-16 immunostaining in uterine cervical carcinomas in Ethiopian women: a pilot study. Pan Afr Med J. 2011;8:21. doi: 10.4314/pamj.v8i1.71077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong P, Li J, Gu Y, Liu Y, Wang A, Sun Y, et al. P16 And Ki-67 expression improves the diagnostic accuracy of cervical lesions but not predict persistent high risk human papillomavirus infection with CIN1. Int J Clin Exp Pathol. 2015;8:2979–2986. [PMC free article] [PubMed] [Google Scholar]
- 30.Omran OM, AlSheeha M. Human Papilloma virus early proteins E6 (HPV16/18-E6) and the cell cycle marker P16 (INK4a) are useful PrognosticMarkers in uterine cervical Carcinomasin Qassim region-Saudi Arabia. Pathol Oncol Res. 2015;21:157–166. doi: 10.1007/s12253-014-9801-y. [DOI] [PubMed] [Google Scholar]
- 31.Wang SS, Trunk M, Schiffman M, Herrero R, Sherman ME, Burk RD, et al. Validation of p16INK4a as a marker of Oncogenic HumanPapillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomark Prev. 2004;13:1355–1360. [PubMed] [Google Scholar]
- 32.Kalof AN, Cooper K. p16INK4a Immunoexpression: surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Cheah PL, Looi LM, Teoh KH, Mun KS, Nazarina AR. p16INK4a Is a useful marker of human Papillomavirus integration allowing risk stratification for cervical malignancies. Asian Pacific J Cancer Prev. 2012;13:469–472. doi: 10.7314/APJCP.2012.13.2.469. [DOI] [PubMed] [Google Scholar]
- 34.Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16INK4a Expression correlates with degree of cervical Neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16(7):665–673. doi: 10.1097/01.MP.0000077518.78046.0C. [DOI] [PubMed] [Google Scholar]
- 35.Volgareva G, Zavalishina L, Andreeva Y, Frank G, Krutikova E, Golovina D, et al. Protein p16 as a marker of dysplastic and neoplastic alterations in cervical epithelial cells. BMC Cancer. 2004;4:58. doi: 10.1186/1471-2407-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam EJ, Kim JW, Hong JW, Jang HS, Lee SY, Jang SY, et al. Expression of the p16 and Ki-67 in relation to the grade of cervical intraepithelial neoplasia and high-risk human papillomavirus infection. J Gynecol Oncol. 2008;19:162–168. doi: 10.3802/jgo.2008.19.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zouheir Y, Fechtali T, Elgnaoui N. Human Papillomavirus genotyping and p16INK4a expression in cervical lesions: a combined test to avoid cervical cancer progression. J Cancer Prev. 2016;21:121–125. doi: 10.15430/JCP.2016.21.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim S, Lee MJ, Cho I, Hong R, Lim SC. Efficacy of p16 and Ki-67 immunostaining in the detection of squamous intraepithelial lesions in a high-risk HPV group. Oncol Lett. 2016;11:1447–1452. doi: 10.3892/ol.2015.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negri G, Vittadello F, Romano F, Kasal A, Rivasi F, Girlando S, et al. P16INK4a Expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch. 2004;445:616–620. doi: 10.1007/s00428-004-1127-9. [DOI] [PubMed] [Google Scholar]
- 40.Ntekim A. Cervical Cancer in Sub Sahara Africa, Topics on Cervical Cancer With an Advocacy for Prevention. Dr. R. Rajamanickam (Ed.), ISBN: 978–953–51-0183-3, InTech, 2012. Available at: https://cdn.intechopen.com/pdfs-wm/30747.pdf. Accessed 28 May 2017.
- 41.Kim JJ, Brisson M, Edmunds WJ, Goldie SJ. Modeling cervical cancer prevention in developed countries. Vaccine. 2008;26:76–86. doi: 10.1016/j.vaccine.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on request from the author. Patient’s confidentiality must be respected and adhered to because of the sensitive nature of the information collected from the subjects.


