Abstract
Background
Myelofibrosis (MF) is associated with a variety of burdensome symptoms and reduced survival compared with age-/sex-matched controls. This analysis evaluated the long-term survival benefit with ruxolitinib, a Janus kinase (JAK)1/JAK2 inhibitor, in patients with intermediate-2 (int-2) or high-risk MF.
Methods
This was an exploratory analysis of 5-year data pooled from the phase 3 COMFORT-I and -II trials. In both trials, patients could cross over to ruxolitinib from the control group (COMFORT-I, placebo; COMFORT-II, best available therapy). All continuing patients in the control groups crossed over to ruxolitinib by the 3-year follow-up. Overall survival (OS; a secondary endpoint in both trials) was evaluated using pooled intent-to-treat data from patients randomized to ruxolitinib or the control groups. OS was also evaluated in subgroups stratified by baseline anemia and transfusion status at week 24.
Results
A total of 528 patients were included in this analysis; 301 were originally randomized to ruxolitinib (COMFORT-I, n = 155; COMFORT-II, n = 146) and 227 to control (n = 154 and n = 73, respectively). The risk of death was reduced by 30% among patients randomized to ruxolitinib compared with patients in the control group (median OS, 5.3 vs 3.8 years, respectively; hazard ratio [HR], 0.70 [95% CI, 0.54–0.91]; P = 0.0065). After correcting for crossover using a rank-preserving structural failure time (RPSFT) method, the OS advantage was more pronounced for patients who were originally randomized to ruxolitinib compared with patients who crossed over from control to ruxolitinib (median OS, 5.3 vs 2.3 years; HR [ruxolitinib vs RPSFT], 0.35 [95% CI, 0.23–0.59]). An analysis of OS censoring patients at the time of crossover also demonstrated that ruxolitinib prolonged OS compared with control (median OS, 5.3 vs 2.4 years; HR [ruxolitinib vs censored at crossover], 0.53 [95% CI, 0.36–0.78]; P = 0.0013). The survival benefit with ruxolitinib was observed irrespective of baseline anemia status or transfusion requirements at week 24.
Conclusions
These findings support ruxolitinib treatment for patients with int-2 or high-risk MF, regardless of anemia or transfusion status. Further analyses will be important for exploring ruxolitinib earlier in the disease course to assess the effect on the natural history of MF.
Trial registration
ClinicalTrials.gov identifiers, NCT00952289 and NCT00934544.
Keywords: Ruxolitinib, Myelofibrosis, Overall survival, Anemia, Transfusion
Background
Myelofibrosis (MF) is associated with progressive bone marrow fibrosis, splenomegaly [1], burdensome symptoms [2], and reduced survival compared with age- and sex-matched controls [3]. Anemia [4] and transfusion dependence [5] are associated with shortened overall survival (OS) in patients with MF. However, the effects of transfusion status on OS have not been evaluated in patients receiving ruxolitinib.
Ruxolitinib is a Janus kinase (JAK)1/JAK2 inhibitor approved by the European Medicines Agency for the treatment of disease-related splenomegaly or symptoms in adult patients with primary MF (PMF), post–polycythemia vera MF (PPV-MF), and post–essential thrombocythemia MF (PET-MF) [6], and by the US Food and Drug Administration for intermediate or high-risk MF, including PMF, PPV-MF, and PET-MF [7]. Primary results from two long-term, pivotal phase 3 clinical trials (COMFORT-I and COMFORT-II) demonstrated that ruxolitinib reduced spleen volume, improved MF-related symptoms and quality-of-life measures, and was associated with prolonged OS in patients with intermediate-2 (int-2) or high-risk MF compared with controls [8–10]. Exploratory analyses of pooled 3-year data from the COMFORT trials showed that OS favored ruxolitinib regardless of baseline anemia status or development of new or worsening anemia post baseline [11].
Here, we report the findings from exploratory analyses of long-term OS benefit with ruxolitinib using pooled 5-year data from the COMFORT trials. In addition, OS was also evaluated in subgroups stratified by baseline anemia and week 24 transfusion status.
Methods
The double-blind COMFORT-I and open-label COMFORT-II trials (ClinicalTrials.gov identifiers, NCT00952289, NCT00934544) were randomized phase 3 studies described previously [8, 10]. Briefly, all patients were ≥ 18 years of age with int-2 or high-risk PMF, PPV-MF, or PET-MF. The ruxolitinib starting dose was 15 or 20 mg twice daily based on baseline platelet counts (100–200 or > 200 × 109/L, respectively). Dose modifications were permitted for safety and efficacy. Patients could cross over to ruxolitinib from the control group for progressive splenomegaly (COMFORT-I, ≥ 25% increase in spleen volume from baseline; COMFORT-II, study nadir) or for select protocol-defined progression events; crossover was mandatory following treatment unblinding in COMFORT-I. The control group in COMFORT-I received placebo. The control group in COMFORT-II received best available therapy; the three most common were hydroxyurea (47%), no medication (33%), and prednisone/prednisolone (12%). All continuing patients in the control groups crossed over to ruxolitinib by the 3-year follow-up [9, 12].
This report includes exploratory analyses of OS (a secondary endpoint in both studies) using pooled intent-to-treat (ITT) data from patients randomized to ruxolitinib and the control groups. OS was also evaluated in subgroups stratified by baseline anemia and transfusion status at week 24, defined as follows:
Baseline anemia: receiving any units of red blood cells (RBCs) within 12 weeks before baseline measurement or having baseline hemoglobin < 10 g/dL.
Baseline nonanemic: not meeting criteria for anemia.
Transfusion independence at week 24: absence of RBC transfusions and hemoglobin levels ≥ 8 g/dL during weeks 13 to 24.
Not transfusion independent at week 24: requiring RBC transfusions or hemoglobin levels < 8 g/dL during weeks 13 to 24.
Transfusion dependence at week 24: requiring ≥ 4 units of RBCs or hemoglobin levels < 8 g/dL during weeks 17 to 24.
Not transfusion dependent at week 24: requiring < 4 units of RBCs and hemoglobin levels ≥ 8 g/dL during weeks 17 to 24.
Transfusion independence/dependence subgroup status was defined separately from baseline transfusion status (e.g., patients who were transfusion independent at week 24 did not necessarily require RBC transfusions before baseline). Two analyses were performed based on the definitions of independence and dependence, comparing patients who were (1) transfusion independent at week 24 versus not independent and (2) transfusion dependent at week 24 versus not dependent.
Overall survival was evaluated using a stratified log-rank test and Cox proportional hazards model that estimated the treatment effect stratified by clinical trial and International Prognostic Scoring System (IPSS) risk [4]. The crossover-corrected treatment effect was estimated using a rank-preserving structural failure time (RPSFT) method and through censorship of survival time at the time of crossover. The effect of transfusion status on OS was evaluated using the Landmark approach, which included patients completing ≥ 24 weeks of study treatment, and the stratified log-rank test, which included study, IPSS risk, and baseline anemia status as stratification variables.
Results
Disposition and baseline anemia
This pooled analysis included 528 patients; 301 were originally randomized to ruxolitinib (COMFORT-I, n = 155; COMFORT-II, n = 146) and 227 were randomized to control (n = 154 and n = 73, respectively). Most patients in the control group crossed over to ruxolitinib during the study (69.6%). At data cutoff, 27.2% of patients in the ruxolitinib group versus 0 in the control group remained on treatment. Similar proportions of patients in each pooled treatment group had anemia at baseline (ruxolitinib, 45.8%; control, 49.8%); 39.3 and 67.5% of patients in the ruxolitinib and control groups, respectively, had ≥ 1 transfusion in the 28 days before baseline.
Overall survival: ITT analysis and subgroups by IPSS risk status
At the 5-year ITT analysis, 128 patients (42.5%) in the ruxolitinib group had died compared with 117 (51.5%) in the control group. The risk of death was reduced by 30% among patients randomized to ruxolitinib compared with patients in the control group (median OS, 5.3 vs 3.8 years, respectively; hazard ratio [HR; ruxolitinib vs control], 0.70 [95% CI, 0.54–0.91]; P = 0.0065; Fig. 1a [13]). After correcting for crossover using RPSFT, the OS advantage was more pronounced in patients who were originally randomized to ruxolitinib compared with patients who crossed over from control to ruxolitinib (median OS, 5.3 vs 2.3 years; HR [ruxolitinib vs RPSFT], 0.35 [95% CI, 0.23–0.59]; Fig. 1b). An analysis of OS censoring patients at the time of crossover also demonstrated that ruxolitinib prolonged survival compared with control (median OS, 5.3 vs 2.4 years; HR [ruxolitinib vs censored at crossover], 0.53 [95% CI, 0.36–0.78]; P = 0.0013; Fig. 1c).
Fig. 1.
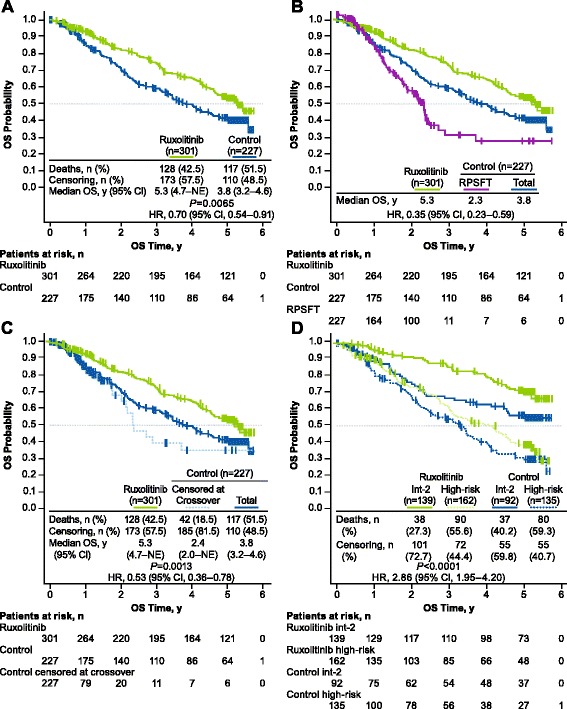
Overall survival: 5-year pooled data. OS analysis of 5-year pooled data from the COMFORT-I and -II trials. Data are presented a for the ITT population, b corrected for crossover with the RPSFT model, c censored at crossover, and d stratified by IPSS risk status. Originally presented at the American Society of Hematology 58th Annual Meeting [13]. HR, hazard ratio; int-2, intermediate-2; IPSS, International Prognostic Scoring System; ITT, intent-to-treat; NE, not evaluable; OS, overall survival; RPSFT, rank-preserving structural failure time
Among patients randomized to ruxolitinib, those with int-2 MF had longer median OS than those with high-risk MF (median OS, int-2 not reached, estimated, 8.5 years; high-risk, 4.2 years; HR [high vs int-2], 2.86 [95% CI, 1.95–4.20]; P < 0.0001; Fig. 1d).
Overall survival: subgroups by baseline anemia status and week 24 transfusion status
Overall survival was not significantly different between ruxolitinib-treated patients who were transfusion independent and not independent at week 24 (P = 0.1322; Fig. 2a, e [13]), whereas there was a statistically significant difference in the control-treated subgroups (P = 0.0004; Fig. 2b, f). Among patients who were not transfusion independent at week 24, median OS favored ruxolitinib versus control in those with baseline anemia (200 vs 137 weeks) and those without baseline anemia (271 vs 166 weeks; overall P = 0.002).
Fig. 2.
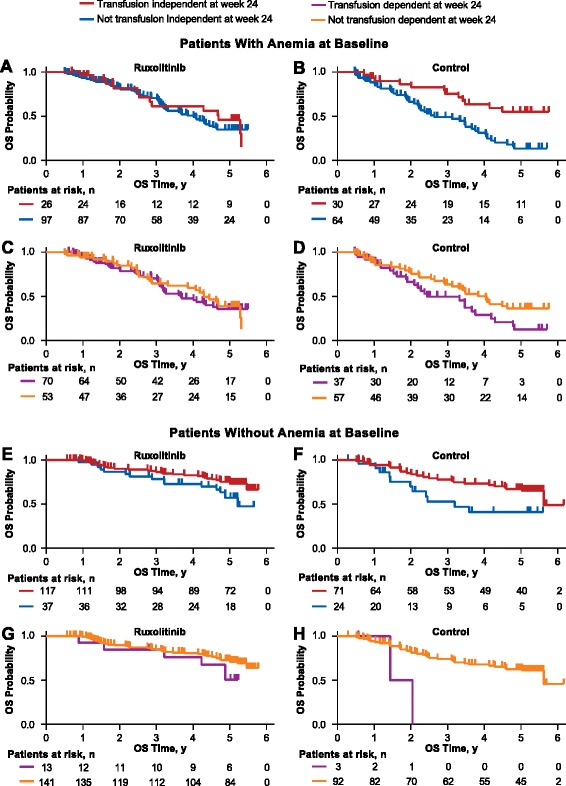
Overall survival: 5-year pooled data stratified by baseline anemia status and week 24 transfusion status. OS analysis of 5-year pooled data from the COMFORT-I and -II trials stratified by baseline anemia status and week 24 transfusion status. Patients in the ruxolitinib and control groups were stratified by anemia status at baseline and (a, b, e, f) transfusion independence status at week 24 or (c, d, g, h) transfusion dependence status at week 24. OS probability in the ruxolitinib group was not significantly affected by transfusion status at week 24 (transfusion independent vs not independent, P = 0.1322*; transfusion dependent vs not dependent, P = 0.4547*), but was significantly affected in the control group (transfusion independent vs not independent, P = 0.0004*; transfusion dependent vs not dependent, P = 0.0323*). Baseline anemia was defined as receiving any units of RBCs within 12 weeks before baseline measurement or having baseline hemoglobin < 10 g/dL; nonanemic was defined as not meeting criteria for anemia. Transfusion independence at week 24 was defined as the absence of RBC transfusions and hemoglobin levels ≥ 8 g/dL during weeks 13 to 24; not transfusion independent at week 24 was defined as requiring RBC transfusions or hemoglobin levels < 8 g/dL during weeks 13 to 24. Transfusion dependence at week 24 was defined as requiring ≥ 4 units of RBCs or hemoglobin levels < 8 g/dL during weeks 17 to 24; not transfusion dependent at week 24 was defined as requiring < 4 units of RBCs and hemoglobin levels ≥ 8 g/dL during weeks 17 to 24. Originally presented at the American Society of Hematology 58th Annual Meeting [13]. IPSS, International Prognostic Scoring System; OS, overall survival; RBC, red blood cell. *Stratified by study, IPSS risk, and baseline anemia status
Overall survival in the ruxolitinib group was similar between patients who were transfusion dependent and not dependent at week 24 (P = 0.4547; Fig. 2 c, g), whereas there was a statistically significant difference in the control subgroups (P = 0.0323; Fig. 2 d, h). Among patients who were transfusion dependent at week 24, ruxolitinib versus control treatment prolonged OS in those with baseline anemia (191 vs 127 weeks) and those without baseline anemia (not reached vs 90 weeks; overall P = 0.0014).
Discussion
This exploratory pooled analysis of the COMFORT trials demonstrated that long-term treatment with ruxolitinib prolonged survival compared with best available treatment or placebo in patients with int-2 or high-risk MF. Importantly, ruxolitinib treatment was associated with statistically significant improvements in OS irrespective of baseline anemia status or transfusion requirements at week 24. These findings agree with previous reports from the COMFORT trials [8, 9, 11].
Anemia and the resulting dependence on RBC transfusions have been associated with reduced OS in patients with MF [4, 5, 11, 14, 15]. Ruxolitinib treatment may cause an initial reduction in hemoglobin levels in some patients with MF; however, the levels typically stabilize within 24 to 36 weeks [9, 12]. Furthermore, a previous report demonstrated that ruxolitinib was associated with prolonged survival regardless of baseline anemia status [11]. The current analysis expanded on these findings by demonstrating that patients’ week 24 transfusion status did not significantly affect OS in the ruxolitinib group but was associated with reduced OS in the control group.
Although the survival benefit associated with ruxolitinib treatment in patients with int-2 or high-risk MF is well established, further improvements in patient outcomes may be achieved by limiting the cytopenias experienced by some patients during treatment initiation. A recent phase 2 study of patients with int-2 or high-risk MF assessed combination treatment with ruxolitinib and danazol to obviate ruxolitinib-related anemia and thrombocytopenia [16]. Hematologic stabilization was achieved in most patients; however, the trial was halted due to modest efficacy per International Working Group-Myeloproliferative Neoplasms Research and Treatment criteria, and results from only 14 patients were reported. Further research is necessary to identify strategies that may be able to limit the initial cytopenias associated with ruxolitinib treatment in some patients.
Conclusions
Taken together, these findings indicate that anemia and transfusion status at week 24 do not affect the survival advantage of patients with int-2 or high-risk MF treated with ruxolitinib. Moreover, they underscore the importance of monitoring for cytopenias, which are generally manageable with ruxolitinib dose modifications [8, 10] and adjuvant therapy. Given these data, there is a rationale for exploring ruxolitinib earlier in the disease course to assess the effect on the natural history of MF.
Acknowledgements
Statistical programming was performed by Jessy Gao, MA (Incyte Corporation). Medical writing assistance was provided by Brett D. Mahon, PhD (Complete Healthcare Communications, LLC [West Chester, PA], a CHC Group company) and was funded by Incyte Corporation.
Funding
The COMFORT-I trial was sponsored by Incyte Corporation; COMFORT-II was sponsored by Novartis Pharmaceuticals and designed by Incyte Corporation. The exploratory analyses included in the current report were funded by Incyte Corporation.
Availability of data and materials
All pertinent data generated for this exploratory analysis are included in this published article; further information is available from the corresponding author on reasonable request.
Abbreviations
- HR
Hazard ratio
- int-2
Intermediate-2
- IPSS
International Prognostic Scoring System
- ITT
Intent to treat
- JAK
Janus kinase
- MF
Myelofibrosis
- OS
Overall survival
- PET-MF
Post–essential thrombocythemia myelofibrosis
- PMF
Primary myelofibrosis
- PPV-MF
Post–polycythemia vera myelofibrosis
- RBC
Red blood cell
- RPSFT
Rank-preserving structural failure time
Authors’ contributions
All authors contributed to writing the manuscript and approved the final draft for submission. SV, JG, RAM, AMV, J-JK, FC, CNH, RP, and VG participated in the collection of data and data interpretation. WS, AN, PL, TD, and PG contributed to the data analysis and interpretation.
Ethics approval and consent to participate
Both trials (COMFORT-I and -II) were approved by the institutional review boards at each participating site and conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
SV has served on advisory boards for Incyte Corporation and received research funding for the conduct of clinical studies from Incyte Corporation, Roche, AstraZeneca, Lilly Oncology, Geron, NS Pharma, Bristol-Myers Squibb, Celgene, Gilead, Seattle Genetics, Promedior, CTI BioPharma Corp, Galena BioPharma, Pfizer, and Genentech. JG has received honoraria and research funding from Incyte Corporation and served on an advisory committee for Incyte Corporation. RAM has received research funding from Incyte Corporation, Gilead, CTI BioPharma Corp, Promedior, and Celgene; he has served as a consultant for Novartis, Ariad, and Galena. AMV has received research funding from and served on advisory committees and speakers bureaus for Novartis. J-JK has received research funding from Novartis and AOP Orphan. FC has served on advisory committees for Novartis, Baxalta, and AOP Orphan and on speakers bureaus for Novartis and Baxalta. CNH has received honoraria from and served on speakers bureaus for Novartis, Shire, CTI BioPharma Corp, Gilead, Baxalta, and Incyte Corporation; served as a consultant for Novartis, CTI BioPharma, and Baxalta; and received research funding and reimbursement for travel accommodations or expenses from Novartis. RP served on speakers bureaus for and received research funding from Ariad, Bristol-Myers Squibb, and Novartis. WS, AN, and PL are employees of and have equity ownership in Incyte Corporation. TD is an employee of and has equity ownership in Novartis Pharmaceutical Corporation. PG is an employee of and has equity ownership in Novartis Pharma AG. VG has served as a consultant for and received research funding from Incyte Corporation and Novartis and has received honoraria from Novartis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Srdan Verstovsek, Phone: (713) 745-3429, Email: sverstov@mdanderson.org.
Jason Gotlib, Email: gotlib@stanford.edu.
Ruben A. Mesa, Email: mesar@uthscsa.edu
Alessandro M. Vannucchi, Email: amvannucchi@unifi.it
Jean-Jacques Kiladjian, Email: jean-jacques.kiladjian@sls.aphp.fr.
Francisco Cervantes, Email: fcervan@clinic.ub.es.
Claire N. Harrison, Email: Claire.Harrison@gstt.nhs.uk
Ronald Paquette, Email: RPaquette@mednet.ucla.edu.
William Sun, Email: wsun@incyte.com.
Ahmad Naim, Email: anaim@incyte.com.
Peter Langmuir, Email: plangmuir@incyte.com.
Tuochuan Dong, Email: tuochuan.dong@novartis.com.
Prashanth Gopalakrishna, Email: prashanth.gopalakrishna@novartis.com.
Vikas Gupta, Email: Vikas.Gupta@uhn.ca.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Mesa R, Miller CB, Thyne M, Mangan J, Goldberger S, Fazal S, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167. doi: 10.1186/s12885-016-2208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30:2995–3001. doi: 10.1200/JCO.2012.42.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 5.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 6.JAKAVI® (ruxolitinib) Full Prescribing Information, Novartis Europharm Limited, Horsham, UK. 2015. [Google Scholar]
- 7.JAKAFI® (ruxolitinib) Full Prescribing Information, Incyte Corporation, Wilmington, DE, USA. 2016. [Google Scholar]
- 8.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 10.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 11.Gupta V, Harrison C, Hexner EO, Al-Ali HK, Foltz L, Montgomery M, et al. The impact of anemia on overall survival in patients with myelofibrosis treated with ruxolitinib in the COMFORT studies. Haematologica. 2016;101:e482–e4e4. doi: 10.3324/haematol.2016.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100:479–488. doi: 10.3324/haematol.2014.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verstovsek S, Gupta V, Gotlib JR, Mesa RA, Vannucchi AM, Kiladijan JJ, et al. A pooled overall survival analysis of 5-year data from the COMFORT-I and COMFORT-II trials of ruxolitinib for the treatment of myelofibrosis. Presented at: American Society of Hematology 58th Annual Meeting, December 3-6, 2016; San Diego, CA.
- 14.Al-Ali HK, Stalbovskaya V, Gopalakrishna P, Perez-Ronco J, Foltz L. Impact of ruxolitinib treatment on the hemoglobin dynamics and the negative prognosis of anemia in patients with myelofibrosis. Leuk Lymphoma. 2016;57:2464–2467. doi: 10.3109/10428194.2016.1146950. [DOI] [PubMed] [Google Scholar]
- 15.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 16.Gowin K, Kosiorek H, Dueck A, Mascarenhas J, Hoffman R, Reeder C, et al. Multicenter phase 2 study of combination therapy with ruxolitinib and danazol in patients with myelofibrosis. Leuk Res. 2017;60:31–35. doi: 10.1016/j.leukres.2017.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All pertinent data generated for this exploratory analysis are included in this published article; further information is available from the corresponding author on reasonable request.


