Abstract
Background
Avian host species have different roles in the amplification and maintenance of West Nile virus (WNV), therefore identifying key taxa is vital in understanding WNV epidemics. Here, we present a comprehensive case study conducted on red-footed falcons, where host-vector, vector-virus and host-virus interactions were simultaneously studied to evaluate host species contribution to WNV circulation qualitatively.
Results
Mosquitoes were trapped inside red-footed falcon nest-boxes by a method originally developed for the capture of blackflies and midges. We showed that this approach is also efficient for trapping mosquitoes and that the number of trapped vectors is a function of host attraction. Brood size and nestling age had a positive effect on the number of attracted Culex pipiens individuals while the blood-feeding success rate of both dominant Culex species (Culex pipiens and Culex modestus) markedly decreased after the nestlings reached 14 days of age. Using RT-PCR, we showed that WNV was present in these mosquitoes with 4.2% (CI: 0.9–7.5%) prevalence. We did not detect WNV in any of the nestling blood samples. However, a relatively high seroprevalence (25.4% CI: 18.8–33.2%) was detected with an enzyme-linked immunoabsorbent assay (ELISA). Using the ELISA OD ratios as a proxy to antibody titers, we showed that older seropositive nestlings have lower antibody levels than their younger conspecifics and that hatching order negatively influences antibody levels in broods with seropositive nestlings.
Conclusions
Red-footed falcons in the studied system are exposed to a local sylvatic WNV circulation, and the risk of infection is higher for younger nestlings. However, the lack of individuals with viremia and the high WNV seroprevalence, indicate that either host has a very short viremic period or that a large percentage of nestlings in the population receive maternal antibodies. This latter assumption is supported by the age and hatching order dependence of antibody levels found for seropositive nestlings. Considering the temporal pattern in mosquito feeding success, maternal immunity may be effective in protecting progeny against WNV infection despite the short antibody half-life measured in various other species. We conclude that red-footed falcons seem to have low WNV host competence and are unlikely to be effective virus reservoirs in the studied region.
Keywords: Culicidae, Transmission ecology, Mosquito trap, Arthropod vector, Passive immunity, Host competence, Falco vespertinus, Lineage 2, Antibody
Background
West Nile virus (WNV) is the most widespread member of the arthropod-borne group of the genus Flavivirus, family Flaviviridae [1]. Virus strains belonging to genetic lineages 1 and 2 have been causing an increasing number of epidemics in North America [2–4] and Europe [5–12]. Today, WNV is considered one of the most important pathogens causing viral neurological disease in humans [13].
The virus is maintained in an enzootic cycle between vectors and avian hosts, while humans [14], equines [15] and other vertebrate taxa are predominantly dead-end hosts [16]. Therefore, to assess human infection risks and predict the spatio-temporal patterns of disease outbreaks it is vital to better understand the complex avian host-mosquito vector transmission ecology of WNV [17, 18]. A wide array of bird species have been identified as potential virus amplifying hosts [19]. Competent arthropod vectors also belong to a range of taxa [20, 21];, however, ornithophilic mosquitoes (Diptera: Culicidae) are established to be the group predominantly responsible for maintaining the sylvatic cycle of the virus.
Pathogenicity in birds seems to be rather species-specific, and the effect of infection ranges from subclinical to rapid development of fatal neuropathy [22]. WNV can also have a substantial negative impact on an avian population and may even demand attention in the conservation management of high priority species [22, 23]. However, morbidity and mortality rates do not necessarily reflect the epidemiological role of a host species [24]. A more sophisticated approach is to evaluate or quantify host competence, i.e. the ability of a host to generate infection in another susceptible host [25, 26]. Recent studies focusing on WNV host competence were able to pinpoint avian “superspreader” and “supersuppressor” species in North America, and through these, they were able to explain the geographical variation in human spillover rates [26]. In the complex WNV host-vector system, host competence is a function of the magnitude and length of viremia, vector contact rates and host mortality rates [25]. Estimating these parameters for individual species, however, requires a combination of laboratory experiments and field studies which may not be feasible for endangered species. Here, we present a case study where we implemented a comprehensive study design using various methods simultaneously to evaluate host competence of a high conservation value species in WNV circulation under natural conditions.
The studied avian host was the red-footed falcon (Falco vespertinus), a species of high international conservation concern [27, 28]. WNV has been reported to cause central nervous disease and mortality in a few sporadic cases for nestlings, but not in adult birds in Hungary [29]. Red-footed falcons are long-range trans-Saharan migrants [30] and may therefore be amongst the candidate species responsible for large spatial scale dispersal of WNV. These raptors are also facultative colonial breeders [31–33] and therefore potentially more vulnerable to rapidly spreading infections [34] compared to territorially breeding birds. Nestlings and juvenile birds are thought to be important in viral amplification [35, 36] as they are localized in the nest and presumably have a less effective immune response against infections. We, therefore, concentrated on red-footed falcon broods and their relationship with vectors and WNV.
Despite its importance, host-vector interaction in WNV transmission ecology studies is often neglected as there are no widely accepted methods currently available to quantify it. Instead, vectors collected with traps that attract mosquitoes through visual and/or olfactory cues (e.g. CDC traps) are used to assess virus presence, quantify virus prevalence in vectors and as a proxy to potential vector loads on hosts [37]. Albeit these methods are cheap and easy to implement they only account for vector abundance, or rather the availability of mosquitoes reacting to the attractant, but completely fail to provide information on actual host-vector contact rates. A more promising method is to use live birds as baits to attract mosquitoes [38]. However, these are limited in the possible number of bait-species that can be used and also fail to account for avoidance strategies of hosts. Tomás et al. [39] suggested and used an easy yet effective method to quantify biting midges (Ceratopogonidae) and blackflies (Simuliidae) in Passerine nest-boxes with the help of a non-invasive adhesive. If also applicable to trap ornithophilic mosquitoes, this method may revolutionize in situ WNV transmission ecology studies as it allows to directly measure the vector species composition and the effects of nestling characteristics on attracted and blood-fed vectors.
Here, we initially aimed to evaluate whether WNV vectors can be trapped directly in the vicinity of red-footed falcon broods and whether we can quantify attraction patterns, virus prevalence, and blood-feeding success of vectors attracted by the studied hosts. Simultaneously, we aimed to estimate WNV status of falcon broods through estimating seroprevalence and frequency of nestlings in viremia, to assess the population level effects of WNV on the host species, and to evaluate the potential virus reservoir role of red-footed falcons.
Methods
Study area
Field work was carried out during June and July each year from 2010 to 2012, at the Vásárhelyi-plains (46°28'16"N, 20°36'17"E) protected an area of the Körös-Maros National Park Directorate in southern Hungary. The study site holds 4 artificial nest-box colonies where over 100 pairs of red-footed falcons breed each year [40], along with numerous kestrels (Falco tinnunculus), jackdaws (Corvus monedula) and long-eared owls (Asio otus) [41]. Breeding parameters of birds using the nest-boxes was collected within the scope of an ongoing long-term research program [40–43]. Therefore, detailed information such as egg laying dates, clutch size, brood size, and fledging success was readily available. All broods selected for the study were breeding in the same type of standard nest-box. The area is renowned for a large saline lake and other wetlands that are important stop-over and wintering sites of various wader and geese species and common cranes (Grus grus). The surrounding habitat is characterized by a mosaic of grasslands and arable fields, with an extensive network of channels and drainage ditches, providing ample possibilities for blood sucking dipterans to breed.
Mosquito sampling
We used a modified version of the methods described by Tomás et al. [39] to estimate the number of vectors attracted by red-footed falcon broods. Initially, we applied 5 ml gel adhesive (Johnson’s Baby Oil Gel with Chamomile; Johnson & Johnson, Dusseldorf, Germany) on one side of 10 × 15 cm transparent (0.2 mm) plastic sheets. We then secured these sheets (with the gel facing upwards) on the inner side of the nest-boxes’ roofs for 24 h. The sheets were secured with board pins in a fashion to create an arc, thus allowing ample space for flying arthropods to get trapped (Fig. 1). As opposed to other adhesives, the advantage of the gel is that it is easily dissolved with petrol, leaving trapped arthropods unharmed. This allows trapped Culicidae specimens to be reliably identified, sexed and it is also possible to evaluate their feeding status. All collected animals were stored in 70% ethanol after the gel was dissolved. The species, sex and feeding status (blood-fed or not) was identified under a stereomicroscope (Olympus SZ-50, Olympus Co. Tokyo, Japan).
Fig. 1.

The device used to trap mosquitoes attracted by falcon broods. The upper side of the plastic sheet is covered with an adhesive gel that traps insects landing on the surface. The sheets were left in this position for 24 h
Sampling was carried out in three consecutive breeding seasons from 2010 to 2012. Each year we first randomly selected empty nest-boxes within each colony. These were used to test whether hematophagous vectors are attracted to either the nest-box or the adhesive gel alone (56 samples collected) by comparing the results obtained here with that in active nests. At the same time, we used a stratified random sampling approach to select broods where mosquito traps were placed. Strata considered were colony and breeding stage, the latter classified into five groups; before hatching (incubation), 1st week, 2nd week, 3rd week and 4th week after hatching. Red-footed falcons incubate throughout the night and also brood young nestlings at night. Presumably, mosquitoes react to the incubating parent rather than the eggs themselves. Thus our analyses may be interpreted as a comparison between a single adult (incubation), an adult with small nestlings (1st week) and older nestlings alone.
Weather is a key factor controlling mosquito activity [44, 45] and consequently WNV infection rates [46]. Therefore, we only selected three days each year for sampling to minimize variation caused by changes in ambient temperature and humidity. On each date broods in various breeding stages were sampled, thus creating a cross-section of the host population. Altogether a total of 309 samples collected in 166 clutches were used in the analyses (Table 1).
Table 1.
Number of mosquito sampling events in nest-boxes according to year and breeding stage. Control samples derive from boxes where no breeding occurred in the given year. WNV prevalence estimates in mosquitoes and seroprevalence estimates in nestlings was carried out in 2011
| Year | Breeding stage | ||||||
|---|---|---|---|---|---|---|---|
| Incubation period | 1st week | 2nd week | 3th week | 4th week | No. of controls | Total no. of sampling events | |
| 2010 | 1 | 7 | 13 | 15 | 1 | 6 | 37 |
| 2011 | 34 | 10 | 16 | 27 | 20 | 19 | 107 |
| 2012 | 58 | 34 | 23 | 36 | 14 | 31 | 165 |
| total | 93 | 51 | 52 | 78 | 35 | 56 | 309 |
Assessing WNV prevalence in vectors
WNV prevalence has been shown to cumulate in vertebrate hosts late in the summer [47], therefore to minimize laboratory costs and to maximize the probability of detecting WNV presence we only used mosquitoes trapped in the late breeding stage in 2011. We first grouped the samples by species, sex and according to the nest-box, the animals were trapped in. Specimens that were blood filled were excluded from the analyses to avoid inconclusive results. A total of 779 mosquitoes of three species (Culex pipiens, Culex modestus and Coquillettidia richiardii) were investigated, the majority being Cx. pipiens (725 individuals). We then selected every 6th specimen from these subsets for individual WNV identification. The remaining animals were pooled (5 × 4 = 20 individuals/pool) and also analysed [Cx. pipiens: 145 individuals, 29 pools; Cx. modestus: 3 pools (20 + 20 + 7 individuals); Cq. richiardii: 1 pool (7 individuals)]. Samples were tested for the presence of WNV nucleic acid (RNA) by a reverse-transcriptase - polymerase chain reaction (RT-PCR) using the primer pair FL1-f and FL1-r as described earlier [48]. Specific PCR products were sequenced directly from both ends with the same primers [48].
WNV seroprevalence in red-footed falcon nestlings
We used 42 broods that were also selected for mosquito abundance sampling to assess WNV seroprevalence in the studied population. The samples were chosen to represent both the spatial heterogeneity of breeding pairs (colonies) and temporal heterogeneity of the egg laying dates. Blood samples of 0.8–1.0 ml were taken by basilic venipuncture from fledgelings (n = 139) reaching the second half of the breeding stage (17–24 days) in 2011. Sera were separated from coagulated blood samples and stored at -20 °C until processing. The remaining serum and cellular elements were stored at -80 °C until being further processed by simultaneous RNA and DNA extraction using the Roche High Pure Viral Nucleic Acid kit (Lewes, United Kingdom). Serum samples were tested for the presence of anti-WNV antibodies using the ID Screen® West Nile Competition ELISA kit (ID VET, Montpellier, France), according to the manufacturers’ instructions. This diagnostic kit detects IgY antibodies by competitive ELISA directed against the Pr-E envelope protein of the West Nile virus. We used 2 ELISA plates for the analyses. Obtained optical density (OD) values were transformed into OD ratios (i.e. Competition Rate = (OD sample / OD Negative control) × 100). These ratios were then handled and analysed with two different approaches. First, we elaborated the recommendations of the manufacturer and defined cut off levels of the test as positive (competition rate ≤ 40%); doubtful (40% < competition rate ≤ 50%); and negative (competition rate > 50%). Secondly, we considered OD ratio as a proxy for antibody levels (e.g. [49, 50]) and analysed these values on a continuous scale (see Statistical analyses). Blood samples were also tested for the presence of WNV nucleic acid (RNA) with the same technique described above.
Statistical analyses
To understand the relationship between blood-sucking dipteran abundance and host traits we used generalized linear mixed effects models (GLMM) with Poisson distribution and log link function [51–53]. In the next step, we used GLMMs with binomial distribution and logit link function to assess how the ratio of blood filled parasites is affected by these variables. Random factors were the date of sampling, Nest ID and Colony ID for all aforementioned models. All dipteran species were analysed in separate models.
To estimate the WNV seroprevalence in red-footed falcon nestlings, we applied the methods described in Messam et al. [54]. We used the same procedure to estimate WNV prevalence in the vector species, using only the non-pooled samples for the analysis.
We used linear mixed effects (LME) models [51, 55] to estimate the relationship between WNV ELISA OD ratios and nestling characteristics. First, we selected ELISA-positive nestlings and modelled their OD ratios as a function of nestling age measured in days. We then selected all nestlings in broods with at least one WNV-seropositive nestling and assessed their hatching order based on weight (measured with 300 g spring scale to the nearest 2 g), the length of central tail feathers (measured with a ruler to the nearest 1 mm), wing chord (measured with a ruler to the nearest 1 mm) and wing bone (measured with a caliper to the nearest 0.1 mm) lengths recorded at the time of sampling. This hatching order was subsequently used as a predictor for nestling OD ratios. The Nest ID and the ELISA Plate ID were used as random factors in case of both LME models. We also ran the models with the full set of nestlings and obtained numerically similar results.
We used the decrease of deviance and the likelihood ratio test (LR) to select non-significant variables in case of all models described above. All analyses were carried out in R, version 3.2.3 [56] using the following packages; dplyr [57], epiR [58], epitools [59], nlme [60], lme4 [53], car [61], effects [62] lmeans [63].
Results
Host-dependent mosquito attraction and blood-feeding success patterns
We trapped a total of 11,592 mosquitoes belonging to 4 species from red-footed falcon nest-boxes, namely Culex pipiens Linnaeus, 1758 (n = 10,203), Culex modestus Ficalbi, 1889 (n = 1332), Coquillettidia richiardii Ficalbi, 1889 (n = 56), Ochlerotatus dorsalis (Meigen, 1830) (n = 1). All trapped individuals were females. As the latter two species had orders of magnitude lower abundance compared to Cx. pipiens and Cx. modestus we excluded them from further analyses. We did not trap any mosquitoes in control nest-boxes; however, other arthropods like canopy dwelling or non-parasitic nest substrate feeding species were trapped in small numbers.
Brood size and the breeding stage had a significant effect (Poisson GLMM, LR χ 2 test; brood size χ 2 = 132.06, df = 1, P < 0.001, breeding stage χ 2 = 181.39, df = 1, P < 0.001) on the number of Cx. pipens in nest-boxes. Considering the latter variable, the number of individuals significantly increased after hatching, peaked at the 2nd week, and from here on it significantly decreased until fledging. (Table 2, Fig. 2a). Meanwhile, the ratio of blood-fed Cx. pipiens individuals was also significantly affected by breeding stage (Binomial GLMM, LR χ 2 test, breeding stage χ 2 = 29.83, df = 1, P < 0.001; brood size χ 2 = 0.05, df = 1, P = 0.82); the probability of finding a blood-engorged mosquito was highest in the 1st and 2nd week after hatching, and later significantly decreased for the second half of the nestling stage (Table 3, Fig. 2b). In the case of Cx. modestus, brood size significantly increased while breeding stage did not affect (Poisson GLMM, LR χ 2 test; brood size χ 2 = 24.6, df = 1, P < 0.001; breeding stage χ 2 = 2.17, df = 1, P = 0.71) the number of attracted mosquitoes (Table 2, Fig. 3a). The pattern of the ratio of blood-fed Cx. modestus (Fig. 3b) individuals resemble the pattern of Cx. pipiens (Fig. 2b). However, the data did not allow to completely replicate the same analysis as only 2 blood-fed Cx. modestus individuals were trapped in the incubation period (Table 3, Fig. 3b).
Table 2.
Comparisons of the effect of breeding stage on overall mosquito abundance using consecutive contrasts for Poisson GLMMs. The reported values show the mean differences in abundance between the corresponding breeding stage pairs (see also Figs. 2a, 3a)
| Species | Variable | Contrast levels | Difference in abundance | SE | P-value |
|---|---|---|---|---|---|
| Cx. pipiens | Breeding stage | 1st week vs incubation | 2.92 | 1.8 | 0.106 |
| 2nd week vs 1st week | 12.76 | 4.75 | 0.007 | ||
| 3rd week vs 2nd week | -6.31 | 2.55 | 0.013 | ||
| 4th week vs 3rd week | -4.64 | 1.98 | 0.02 | ||
| Cx. modestus | Breeding stage | 1st week vs incubation | -0.25 | 0.322 | 0.43 |
| 2nd week vs 1st week | 0.11 | 0.21 | 0.58 | ||
| 3rd week vs 2nd week | -0.05 | 0.18 | 0.77 | ||
| 4th week vs 3rd week | 0.22 | 0.19 | 0.25 |
Abbreviation: SE standard error
Fig. 2.
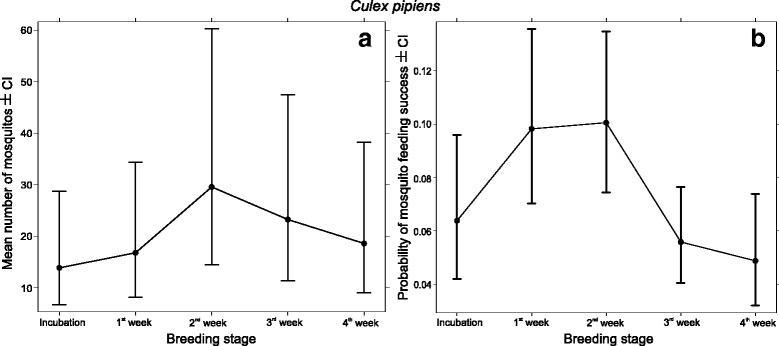
Model estimates (± 95% CI) of the effects of the breeding stage on the number of attracted (a) and proportion of blood filled mosquitoes (b) of Cx. pipiens individuals (see also Tables 2, 3). The values presented here were transformed for the corresponding response scale and calculated for mean brood size. The highest number of attracted mosquitoes was in the second week after hatching, and this also coincided with the peak of blood-feeding success
Table 3.
Comparisons of the effect of breeding stage on overall mosquito blood-feeding success using consecutive contrasts for Binomial GLMMs. The reported values show the differences in mean probabilities of blood-feeding success between the corresponding breeding stage pairs (see also Figs. 2b, 3b)
| Species | Variable | Contrast levels | Difference in blood-feeding success | SE | P-value |
|---|---|---|---|---|---|
| Cx. pipiens | Breeding stage | 1st week vs incubation | 0.034 | 0.01 | 0.031 |
| 2nd week vs 1st week | 0.002 | 0.01 | 0.86 | ||
| 3rd week vs 2nd week | -0.044 | 0.01 | < 0.001 | ||
| 4th week vs 3rd week | 0.007 | 0.01 | 0.447 | ||
| Cx. modestus a | Breeding stage | 2nd week vs 1st week | 0.021 | 0.02 | 0.48 |
| 3rd week vs 2nd week | -0.065 | 0.02 | 0.001 | ||
| 4th week vs 3rd week | 0.011 | 0.02 | 0.53 |
ablood-feeding success was not estimated during incubation due to sample size constraints
Abbreviation: SE standard error
Fig. 3.
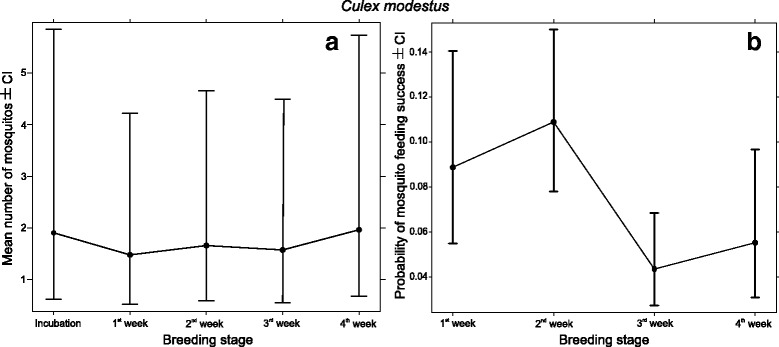
Model estimates (± 95% CI) of the effects of the nestling age on the number of attracted (a) and proportion of blood filled mosquitoes (b) of Cx. modestus individuals (see also Tables 2,3). The values presented here were transformed for the corresponding response scale and calculated for mean brood size. Nestling age did not have a significant effect on the total number of attracted mosquitoes. However, blood-feeding success patterns peaked in the second week after hatching
WNV prevalence in vectors
We found 1 pooled and 6 individual Cx. pipiens (n = 145) samples to be WNV-positive, corresponding to a 4.2% (95% CI: 0.9–7.5%) virus prevalence assuming perfect test detection (calculated only for individual samples). The positive samples were found in three nest-boxes, each in a different colony, indicating WNV carrying mosquito presence throughout the study site. One nest-box had 5 WNV-positive individual Cx. pipiens samples while the remaining 1 individual and 1 pooled sample were from different nest-boxes and different colonies. Sequence analysis determined that the detected virus belongs to the genetic lineage 2 of WNV and it is closely related to the WNV isolates from the study year and previous years [8].
WNV seropositivity in red-footed falcons
Our results showed relatively high seroprevalence among red-footed falcon nestlings; 35 of the sampled 134 individuals were ELISA-positive, while 10 were classified as doubtful (25.4% ± 3.7% SE, CI: 18.8–33.2%). However, we did not detect WNV by RT-PCR in any of the nestling blood samples, suggesting that none of the individuals were viraemic at the time of sampling. Of the 42 broods, 16 had at least one seropositive nestling. We found no evidence of large scale spatial pattern of the observed seropositivity, as the ratio of seropositive broods did not differ significantly between colonies (Fischer’s exact test: P = 0.94). Moreover, we could not detect any temporal pattern either, as there was no significant difference in mean egg laying date between broods without seropositive and broods with at least one seropositive nestling (Welch t-test: t = -0.1095, df = 34.601, P = 0.91). However, the OD ratio significantly decreased with increasing nestling age among ELISA-positive nestlings (LME LR χ 2 test; nestling age χ 2 = 4.97, df = 1, P = 0.02) indicating that older seropositive nestlings have lower antibody levels. We also found a significant decrease in OD ratios corresponding with within brood hatching order among broods with at least a single ELISA-positive nestling (LME LR χ 2 test; hatching order χ 2 = 5.36, df = 1, P = 0.02) (Fig. 4). This shows that nestlings hatching later in a brood had higher antibody levels.
Fig. 4.
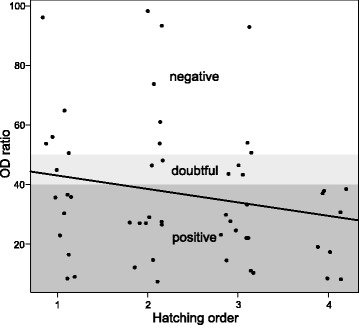
ELISA OD ratios according to hatching order in broods where at least one ELISA-seropositive nestling was found. The black line shows a significant mean decrease in OD ratios (i.e. increase in antibody levels) according to LME model parameter estimate. The grey areas along the y axis depict ELISA classification categories as recommended by the manufacturer. Note that the OD ratio values were jittered along the x-axis. The results show that nestlings that hatched later (i.e. closer to the time of sampling) may have higher antibody levels
Discussion
Here, we comprehensively investigated vector attraction patterns, blood-feeding success rate, WNV prevalence and host serum seroprevalence under natural conditions in a colonial raptor. Initially, we verified that our modified version of the trap described by Tomás et al. [39] was effective in collecting Culicidae species and that the trapped individuals were attracted by the hosts. The two most common mosquito species (Cx. pipens and Cx. modestus) attracted by red-footed falcon broods are well known WNV vectors [38, 64]. The number of these vector individuals showed a positive linear relationship with the number of nestlings in a brood, indicating that each nestling may receive similar vector loads regardless of brood size. Mosquitoes use multiple olfactory cues and skin emanations to locate vertebrate hosts [65]. Presumably, larger broods produce increased host stimuli attracting the insects from a larger area, hence the observed pattern.
We also demonstrated nestling age dependent vector attraction and blood-feeding success rate. First, our results indicated that Cx. pipiens is disproportionately attracted to nestling age categories where the adult birds are absent compared to age categories where adult presence is presumed (incubation and the 1st week after hatching). It is possible that albeit an incubating adult is larger than the nestlings, a single bird with a complete plumage may be emitting less intensive cues compared to a brood of semi-grown nestlings Secondly, blood-feeding success rates are considerably higher for both Culex species in the first two weeks after hatching, and subsequently drop in later breeding stages. This decrease in both attraction and blood-feeding success coincides with the development of body and flight feathers. The gradual shift from downy feathers to juvenile plumage presumably decreases the body surface where mosquitoes may feed [66] and by acting as a better insulator may also decrease host attractiveness. Furthermore, it may also be adaptive for mosquitoes to select for young nestlings as the probability of successful blood-feeding may be higher compared to that on fledglings.
It has to be emphasized that our method to quantify mosquito attraction and blood-feeding patterns hinder the estimation of true host-vector contact rates. Nonetheless, it allows us to speculate that younger red-footed falcon nestlings are at higher risk of infection by vector-borne pathogens.
We also showed that the very mosquitoes attracted to the nests harbour WNV. Although the virus was only present in Cx. pipiens, this is likely due to the fact that this species was an order of magnitude more abundant than Cx. modestus. The obtained prevalence estimate (~4%, 95% CI: 0.9–7.5%) is in the range of that found in North America for Lineage 1 strains [9, 67–70] but somewhat higher than estimated for Hungary in general [71] and the Czech Republic [12]. However, this estimate does not indicate large scale amplification of the virus, despite the fact that the attributes of the studied host-vector system (coloniality, a large number of potential vectors and hosts) would, in theory, allow for effective virus circulation and accumulation. Nonetheless, it is likely that this prevalence estimate indicates a stable WNV sylvatic cycle at the study site.
Although we confirmed WNV presence in vectors directly attracted by red-footed falcon nestlings, the virus was not present in detectable amounts in blood samples of the exposed birds. Despite the lack of viremia, we found that a considerable proportion (~25%) of these nestlings was WNV-seropositive. These seemingly contradicting observations may arise if either all seropositive nestlings were infected soon after hatching and/or the duration of detectable viremia was remarkably short. This scenario is however unlikely based on estimates in viremia magnitude and length of large falcons [72] and a comparative study on multiple species [73]. More probable is that the majority, if not all, seropositive nestlings had maternally derived antibodies against WNV [37, 74]. This is also corroborated by two additional results. First, age dependent increase in ELISA OD ratios, indicate that Ig levels decay with the days elapsed from hatching as opposed to increasing and/or stagnation in infected birds [72]. Secondly, ELISA OD ratios decreased with hatching order, showing that younger nestlings have higher Ig values in nests with at least one seropositive nestling. Red-footed falcons typically hatch 1–2 days apart in a clutch, therefore, both results show that the time-scale of maternally derived antibody decay can be measured in days [75–77]. However, antibody decrease in free ranging birds with the active immune response is detectable over months [78]. Nemeth et al. [79] argued that the rapid decay of maternal antibodies in nestling house sparrows is unlikely to offer effective protection to offspring [79].
If successful blood-feeding probability is nestling age-dependent, it is possible that the efficiency of passive immunity is not exclusively linked to the half-life of maternal antibodies.
Conclusions
Understanding vector-borne arboviral infection systems in avian hosts requires a comprehensive approach that entails studying host-vector and vector-pathogen interactions. Using a specific trap placed in the vicinity of the brood, culicid vectors directly attracted by the studied hosts can be quantified in natural conditions, and these samples can also be used to estimate viral loads of vectors. WNV has an established sylvatic cycle and poses a direct threat to red-footed falcon nestlings. The array of soft evidence presented here points to the prediction that either nestlings have very short viremia or that a large proportion of red-footed falcon females breeding in the studied population allocates WNV antibodies to their eggs. This also leads to the prediction that the breeding adults have been infected by the virus either at the breeding site or during their wintering period in Africa. Considering the host-vector contact rate patterns, yolk transfer of antibodies may be an efficient measure to protect nestlings with naive immune systems from the clinical effects of a WNV infection. The highest risk of contact with a potential WNV vector is in the first two weeks after hatching when maternal antibodies are expected to be sufficiently high based on estimated decay patterns in other birds. In any case, our results do not allow us to explicitly rule out either of the following two competing theories, i.e. that the observed patterns are either caused by very short viremia or by maternal transfer of antibodies. However, red-footed falcon nestlings are likely to have low WNV host competence under both scenarios and are less likely to be key hosts maintaining the sylvatic cycle of the virus, at least during their breeding season.
Acknowledgements
Field work was greatly assisted by Zsalkin Emese Széles and Dorottya Molnár for which we are grateful. We also thank Professor Paul Reiter for his most helpful comments on earlier versions of the manuscript.
Funding
This study was partially funded by the grant NKFIH-K120118, EU grant FP7–261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext019 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission. Further funding was provided by the HU-SRB IPA CBC project (HU-SRB 0901/122/120), NKB 15950, LIFE+ (LIFE11/NAT/HU/000926) and the OTKA K67900 grant.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Raw data used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI
Confidence interval
- ELISA
Enzyme-linked immunoabsorbent assay
- GLMM
Generalized linear mixed effects models
- LME
Linear mixed effects
- SE
Standard error
- WNV
West Nile virus
Authors’ contributions
ZS, PF and KE had designed the study. TB, KE, MB, KSz and ÁD performed the laboratory experiments and molecular analyses. TB, KE, AH and LP contributed to the design of the study. ZS, PF, LK, PP, ÉH and SzS carried out the field work. ZS identified collected mosquito specimens. PF and AH performed the statistical analyses. All authors contributed to drafting the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal handling and sampling protocols were approved by the Ethical Regulation of MME/BirdLife Hungary (Professional and ethical policies of bird ringing - 2009) and by the relevant Research Licence Decision of National Environmental and Nature Conservation Authority (14/3710–2/2009 and 14/2583–5/2011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zoltán Soltész, Email: soltesz@entomologia.hu, Email: soltesz.zoltan@okologia.mta.hu.
Károly Erdélyi, Email: erdelyik@nebih.gov.hu.
Tamás Bakonyi, Email: Bakonyi.Tamas@univet.hu, Email: Tamas.Bakonyi@vetmeduni.ac.at.
Mónika Barna, Email: Barna.Monika@univet.hu.
Katalin Szentpáli-Gavallér, Email: szentpali-gavallerk@nebih.gov.hu.
Szabolcs Solt, Email: solt.szabolcs@gmail.com.
Éva Horváth, Email: horvatheva86@gmail.com.
Péter Palatitz, Email: palatitz.peter@gmail.com.
László Kotymán, Email: laszlo.kotyman@kmnp.hu.
Ádám Dán, Email: dana@nebih.gov.hu.
László Papp, Email: flyer.papp@gmail.com.
Andrea Harnos, Email: harnos.andrea@univet.hu.
Péter Fehérvári, Email: fehervari.peter@univet.hu, Email: peter.fehervari@nhmus.hu.
References
- 1.Weissenböck H, Hubálek Z, Bakonyi T, Nowotny N. Zoonotic mosquito-borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet Microbiol. 2010;140:271–280. doi: 10.1016/j.vetmic.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 3.Hayes EB, Gubler DJ. West Nile Virus: Epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 5.Hubalek Z. European experience with the West Nile virus ecology and epidemiology: could it be relevant for the New World? Viral Immunol. 2000;13:415–426. doi: 10.1089/vim.2000.13.415. [DOI] [PubMed] [Google Scholar]
- 6.Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, et al. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 2002;8:1372–8. [DOI] [PMC free article] [PubMed]
- 7.Zeller HG, Schuffenecker I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis. 2004;23:147–156. doi: 10.1007/s10096-003-1085-1. [DOI] [PubMed] [Google Scholar]
- 8.Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenbock H, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakonyi T, Ferenczi E, Erdélyi K, Kutasi O, Csörgő T, Seidel B, et al. Explosive spread of a neuroinvasive lineage 2 West Nile virus in central Europe, 2008/2009. Vet Microbiol. 2013;165:61–70. [DOI] [PubMed]
- 10.Cernescu C, Ruta S. Romanian experience in post-event environment surveillance for West Nile virus infections. Int J Environ Studies. 2008;65:529–538. doi: 10.1080/00207230802259691. [DOI] [Google Scholar]
- 11.Balança G, Gaidet N, Savini G, Vollot B, Foucart A, Reiter P, et al. Low West Nile virus circulation in wild birds in an area of recurring outbreaks in southern France. Vector Borne Zoonotic Dis. 2009;9:737–41. [DOI] [PubMed]
- 12.Rudolf I, Bakonyi T, Sebesta O, Mendel J, Peško J, Betášová L, et al. West Nile virus lineage 2 isolated from Culex modestus mosquitoes in the Czech Republic, 2013: expansion of the European WNV endemic area to the North. Euro Surveill. 2014;19:2–5. doi: 10.2807/1560-7917.es2014.19.31.20867. [DOI] [PubMed] [Google Scholar]
- 13.Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015:e376230. [DOI] [PMC free article] [PubMed]
- 14.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/S1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 15.Trock SC, Meade BJ, Glaser AL, Ostlund EN, Lanciotti RS, Cropp BC, et al. West Nile virus outbreak among horses in New York State, 1999 and 2000. Emerg Infect Dis. 2001;7:745–747. doi: 10.3201/eid0704.017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der MKM, Pensaert MB, Nauwynck HJ. West Nile virus in the vertebrate world. Arch Virol. 2005;150:637–657. doi: 10.1007/s00705-004-0463-z. [DOI] [PubMed] [Google Scholar]
- 17.Marra PP, Griffing S, Caffrey C, Kilpatrick MA, McLean R, Brand C, et al. West Nile virus and wildlife. Bioscience. 2004;54:393–402.
- 18.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. [DOI] [PubMed]
- 19.Pérez-Ramírez E, Llorente F, Jiménez-Clavero MÁ. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6:752–81. [DOI] [PMC free article] [PubMed]
- 20.Hubálek Z, Halouzka J. West Nile fever - a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643–50. [DOI] [PMC free article] [PubMed]
- 21.Johnson G, Panella N, Hale K, Komar N. Detection of West Nile virus in stable flies (Diptera: Muscidae) parasitizing juvenile American white pelicans. J Med Entomol. 2010;47:1205–11. [DOI] [PubMed]
- 22.Nemeth NM, Oesterle PT. West Nile virus from an avian conservation perspective. Int Zoo Yb. 2014;48:101–115. doi: 10.1111/izy.12031. [DOI] [Google Scholar]
- 23.LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc R Soc London B. 2006;273:2327–33. [DOI] [PMC free article] [PubMed]
- 25.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–78. [PubMed]
- 26.Levine RS, Mead DG, Hamer GL, Brosi BJ, Hedeen DL, Hedeen MW, et al. Supersuppression: Reservoir competency and timing of mosquito host shifts combine to reduce spillover of West Nile virus. Am J Trop Med Hyg. 2016;95:1174–84. [DOI] [PMC free article] [PubMed]
- 27.Palatitz P, Fehérvári P, Solt S, Barov B. European species action plan for the red-footed falcon Falco vespertinus. BirdLife International; 2009. p. 56. Available from: http://ec.europa.eu/environment/nature/conservation/wildbirds/action_plans/docs/neophron_percnopterus.pdf
- 28.Palatitz P, Fehérvári P, Solt S, Horváth É. Breeding population trends and pre-migration roost site survey of the red-footed falcon in Hungary. Ornis Hungarica. 2015;23:77–93.
- 29.Erdélyi K, Bakonyi T, Dán Á, Fehérvári P, Gyuranecz M, Juhász T. Disease and mortality in red-footed falcon (Falco vespertinus) nestlings - ordeals facing a long distance migrant species at it’s breeding habitat. Rovinj, Croatia; 2008.
- 30.Fehérvári P, Lázár B, Palatitz P, Solt S, Nagy A, Prommer M, et al. Pre-migration roost site use and timing of post-nuptial migration of red-footed falcons (Falco vespertinus) revealed by satellite tracking. Ornis Hungarica. 2014;22:36–47.
- 31.Purger JJ, Tepavcevic A. Pattern analysis of red-footed falcon (Falco vespertinus) nests in the rook (Corvus frugilegus) colony near Torda (Voivodina, Yugoslavia), using fuzzy correspondences and entropy. Ecol Model. 1999;117:91–97. doi: 10.1016/S0304-3800(99)00012-5. [DOI] [Google Scholar]
- 32.Fehérvári P, Harnos A, Solt S, Palatitz P. Modeling habitat selection of the red-footed falcon (Falco vespertinus): a possible explanation of recent changes in breeding range within Hungary. AEER. 2009;7:59–69.
- 33.Fehérvári P, Solt S, Palatitz P, Barna K, Ágoston A, Gergely J, et al. Allocating active conservation measures using species distribution models: a case study of red-footed falcon breeding site management in the Carpathian Basin. Anim Conserv. 2012;15:648–657. doi: 10.1111/j.1469-1795.2012.00559.x. [DOI] [Google Scholar]
- 34.Sovada MA, Pietz PJ, Converse KA, Tommy King D, Hofmeister EK, Scherr P, et al. Impact of West Nile virus and other mortality factors on American white pelicans at breeding colonies in the northern plains of North America. Biol Conserv. 2008;141:1021–1031. doi: 10.1016/j.biocon.2008.01.019. [DOI] [Google Scholar]
- 35.Kilpatrick AM, LaDeau SL, Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. doi: 10.1642/0004-8038(2007)124[1121:EOWNVT]2.0.CO;2. [DOI] [Google Scholar]
- 36.Loss SR, Hamer GL, Goldberg TL, Ruiz MO, Kitron UD, Walker ED, et al. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector Borne Zoonotic Dis. 2009;9:13. [DOI] [PMC free article] [PubMed]
- 37.Sovada MA, Pietz PJ, Hofmeister EK, Bartos AJ. West Nile virus in American white pelican chicks: transmission, immunity, and survival. Am J Trop Med Hyg. 2013;88:1152–8. [DOI] [PMC free article] [PubMed]
- 38.Victoriano Llopis I, Tomassone L, Grego E, Serrano E, Mosca A, Vaschetti G, et al. Evaluating the feeding preferences of West Nile virus mosquito vectors using bird-baited traps. Parasit Vectors. 2016;9:479. doi: 10.1186/s13071-016-1744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomás G, Merino S, Martínez-De La Puente J, Moreno J, Morales J, Lobato E. A simple trapping method to estimate abundances of blood-sucking flying insects in avian nests. Anim Behav. 2008;75:723–729. doi: 10.1016/j.anbehav.2007.08.018. [DOI] [Google Scholar]
- 40.Kotymán L, Solt S, Horváth É, Palatitz P, Fehérvári P. Demography, breeding success and effects of nest type in artificial colonies of red-footed falcons and allies. Ornis Hungarica. 2015;23:1–21.
- 41.Fehérvári P, Piross IS, Kotymán L, Solt S, Horváth É, Palatitz P. Species specific effect of nest-box cleaning on settlement selection decisions in an artificial colony system. Ornis Hungarica. 2015;23:66–76. doi: 10.1515/orhu-2015-0006. [DOI] [Google Scholar]
- 42.Palatitz P, Fehérvári P, Solt S, Kotymán L, Neidert D, Harnos A. Exploratory analyses of foraging habitat selection of the red-footed falcon (Falco vespertinus). Acta Zool Acad Sci H. 2011;57:255–68.
- 43.Palatitz P, Fehérvári P, Solt S, Horváth É, Kotymán L. Hunting efficiency of red-footed falcons in different habitats. Ornis Hungarica. 2015;23:32–47.
- 44.Lebl K, Brugger K, Rubel F. Predicting Culex pipiens/restuans population dynamics by interval lagged weather data. Parasit Vectors. 2013;6:129. doi: 10.1186/1756-3305-6-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaskopoulou A, L’Ambert G, Petric D, Bellini R, Zgomba M, Groen TA, et al. Ecology of West Nile virus across four European countries: review of weather profiles, vector population dynamics and vector control response. Parasit Vectors. 2016;9:482. doi: 10.1186/s13071-016-1736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 2010;3:19. [DOI] [PMC free article] [PubMed]
- 47.Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, et al. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoonotic Dis. 2008;8:57–68. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- 48.Erdélyi K, Ursu K, Ferenczi E, Szeredi L, Rátz F, Skáre J, et al. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector Borne Zoonotic Dis. 2007;7:181–188. doi: 10.1089/vbz.2006.0586. [DOI] [PubMed] [Google Scholar]
- 49.Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257–264. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer-Verlag; 2000. [Google Scholar]
- 52.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evolut. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–51. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 54.Messam LLM, Branscum AJ, Collins MT, Gardner IA. Frequentist and Bayesian approaches to prevalence estimation using examples from Johne’s disease. Anim Health Res Rev. 2008;9:1. doi: 10.1017/S1466252307001314. [DOI] [PubMed] [Google Scholar]
- 55.Faraway JJ. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. Boca Raton, Florida: Chapman & Hall/CRC; 2006. [Google Scholar]
- 56.R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/. Accessed 15 Jan 2017.
- 57.Wickham H, Francois R. dplyr: A grammar of data manipulation. R package version 04. 2015;1:20. [Google Scholar]
- 58.Stevenson M, Nunes T, Heuer C, Marshall J, Sanchez J, Thornton R, et al. epiR: Tools for the analysis of epidemiological data. 2016. https://CRAN.R-project.org/package=epiR Accessed 15 Jan 2017.
- 59.Aragon T. epitools: Epidemiology Tools. 2012. https://CRAN.R-project.org/package=epitools. Accessed 15 Jan 2017.
- 60.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: linear and nonlinear mixed effects models. 2016. http://CRAN.R-project.org/package=nlme. Accessed 15 Jan 2017.
- 61.Fox J, Weisberg S. An R companion to applied regression. Second. Thousand Oaks CA: Sage; 2011. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. Accessed 15 Jan 2017.
- 62.Fox J. Effect displays in R for generalised linear models. J Stat Softw. 2003;8:1–27. doi: 10.18637/jss.v008.i15. [DOI] [Google Scholar]
- 63.Lenth RV. Least-Squares Means. The R Package lsmeans. J Stat Softw. 2016;69(1):1–33.
- 64.Balenghien T, Vazeille M, Grandadam M, Schaffner F, Zeller H, Reiter P, et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008;8:589–96. [DOI] [PubMed]
- 65.Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 66.Burkett-Cadena ND, Ligon RA, Liu M, Hassan HK, Hill GE, Eubanks MD, et al. Vector-host interactions in avian nests: Do mosquitoes prefer nestlings over adults? Am J Trop Med Hyg. 2010;83:395–9. [DOI] [PMC free article] [PubMed]
- 67.Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.017413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernard KA, Maffei JG, Jones SA, Kauffman EB, Ebel G, Dupuis AP, et al. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679. doi: 10.3201/eid0704.017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dimenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, et al. Emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Grande Valley. J Med Entomol. 2006;43:594–599. doi: 10.1093/jmedent/43.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007;7:365–86. [DOI] [PMC free article] [PubMed]
- 71.Szentpáli-Gavallér K, Antal L, Tóth M, Kemenesi G, Soltész Z, Dán Á, et al. Monitoring of West Nile virus in mosquitoes between 2011–2012 in Hungary. Vector Borne Zoonotic Dis. 2014;14:648–55. [DOI] [PMC free article] [PubMed]
- 72.Ziegler U, Angenvoort J, Fischer D, Fast C, Eiden M, Rodriguez AV, et al. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Vet Microbiol. 2013;161:263–273. doi: 10.1016/j.vetmic.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 73.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stout WE, Cassini AG, Meece JK, Papp JM, Rosenfield RN, Reed KD. Serologic evidence of West Nile virus infection in three wild raptor populations. Avian Dis. 2005;49:371–375. doi: 10.1637/7335-012805R1.1. [DOI] [PubMed] [Google Scholar]
- 75.Gibbs SE, Hoffman DM, Stark LM, Marlenee NL, Blitvich BJ, Beaty BJ, et al. Persistence of antibodies to West Nile virus in naturally infected rock pigeons (Columba livia) Clin Diagn Lab Immunol. 2005;12:665–667. doi: 10.1128/CDLI.12.5.665-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hahn DC, Nemeth NM, Edwards E, Bright PR, Komar N. Passive West Nile virus antibody transfer from maternal eastern screech-owls (Megascops asio) to progeny. Avian Dis. 2006;50:454–5. [DOI] [PubMed]
- 77.Nemeth NM, Bowen RA. Dynamics of passive immunity to West Nile virus in domestic chickens (Gallus gallus domesticus) Am J Trop Med Hyg. 2007;76:310–317. [PubMed] [Google Scholar]
- 78.McKee EM, Walker ED, Anderson TK, Kitron UD, Brawn JD, Krebs BL, et al. West Nile virus antibody decay rate in free-ranging birds. J Wild Dis. 2015;51:601–608. doi: 10.7589/2014-07-175. [DOI] [PubMed] [Google Scholar]
- 79.Nemeth NM, Oesterle PT, Bowen RA. Passive immunity to West Nile virus provides limited protection in a common passerine species. Am J Trop Med Hyg. 2008;79:283–90. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. Raw data used and/or analysed during the current study are available from the corresponding author on reasonable request.


