Abstract
Nodaviruses are small bipartite RNA viruses which belong to the family of Nodaviridae. They are categorized into alpha-nodavirus, which infects insects, and beta-nodavirus, which infects fishes. Another distinct group of nodavirus infects shrimps and prawns, which has been proposed to be categorized as gamma-nodavirus. Our current review focuses mainly on recent studies performed on nodaviruses. Nodavirus can be transmitted vertically and horizontally. Recent outbreaks have been reported in China, Indonesia, Singapore and India, affecting the aquaculture industry. It also decreased mullet stock in the Caspian Sea. Histopathology and transmission electron microscopy (TEM) are used to examine the presence of nodaviruses in infected fishes and prawns. For classification, virus isolation followed by nucleotide sequencing are required. In contrast to partial sequence identification, profiling the whole transcriptome using next generation sequencing (NGS) offers a more comprehensive comparison and characterization of the virus. For rapid diagnosis of nodavirus, assays targeting the viral RNA based on reverse-transcription PCR (RT-PCR) such as microfluidic chips, reverse-transcription loop-mediated isothermal amplification (RT-LAMP) and RT-LAMP coupled with lateral flow dipstick (RT-LAMP-LFD) have been developed. Besides viral RNA detections, diagnosis based on immunological assays such as enzyme-linked immunosorbent assay (ELISA), immunodot and Western blotting have also been reported. In addition, immune responses of fish and prawn are also discussed. Overall, in fish, innate immunity, cellular type I interferon immunity and humoral immunity cooperatively prevent nodavirus infections, whereas prawns and shrimps adopt different immune mechanisms against nodavirus infections, through upregulation of superoxide anion, prophenoloxidase, superoxide dismutase (SOD), crustin, peroxinectin, anti-lipopolysaccharides and heat shock proteins (HSP). Potential vaccines for fishes and prawns based on inactivated viruses, recombinant proteins or DNA, either delivered through injection, oral feeding or immersion, are also discussed in detail. Lastly, a comprehensive review on nodavirus virus-like particles (VLPs) is presented. In recent years, studies on prawn nodavirus are mainly focused on Macrobrachium rosenbergii nodavirus (MrNV). Recombinant MrNV VLPs have been produced in prokaryotic and eukaryotic expression systems. Their roles as a nucleic acid delivery vehicle, a platform for vaccine development, a molecular tool for mechanism study and in solving the structures of MrNV are intensively discussed.
Keywords: Nodavirus, Diagnosis, Immunology, Vaccines, Virus-like particles
Introduction
The current review discusses recent studies related to nodaviruses. Recent reported outbreaks of nodaviruses, diagnostic assays, host immunological responses, vaccines, and virus-like particles (VLPs) are emphasized. To the best of our knowledge, there are only eight review articles related to nodavirus which had been published within the past five years: immunological-based detection of shrimp viruses (Chaivisuthangkura, Longyant & Sithigorngul, 2014); recombinant nodavirus-like particles as delivery system (Jariyapong, 2015); the life cycle of beta-nodaviruses (Low et al., in press); viral encephalopathy and retinopathy in aquaculture (Doan et al., 2017); interaction between beta-nodavirus and its host for development of prophylactic measures for viral encephalopathy and retinopathy (Costa & Thompson, 2016); reactive oxygen species-mediated cell death (Reshi, Su & Hong, 2014); mitochondrial disruption and necrotic cell death (Hong, 2013); and immunity to beta-nodavirus infections of marine fish (Chen, Wang & Chen, 2014). Another two review articles published within the past 10 years are about the biology and biomedical applications of Flock House virus (Venter & Schneemann, 2008), and white-tail-disease (WTD) in Macrobrachium rosenbergii (Bonami & Sri Widada, 2011). However, none of these articles review the recent advances in the study of nodaviruses as presented in the current review.
Survey Methodology
“PubMed” and “Scopus” were used to search for journal articles published within the last five years using the keyword “nodavirus”. These articles were screened and used as references for the current review. Additional information was obtained through the “Google” search engine with more specific keywords for older publications.
Nodavirus
Nodavirus belongs to the family of Nodaviridae. Generally, nodaviruses are classified into alpha-nodavirus and beta-nodavirus based on their hosts. Alpha-nodaviruses such as Nodamura virus (NoV), Flock House virus (FHV), black bettle virus (BBV), Pariacoto virus (PaV), and a recently discovered mosinovirus (MoNV) (Schuster et al., 2014) infect insects, whereas beta-nodaviruses such as striped jack nervous necrosis virus (SJNNV), barfin flounder nervous necrosis virus (BFNNV), redspotted grouper nervous necrosis virus (RGNNV), and tiger puffer nervous necrosis virus (TPNNV) infect fishes. Another type of nodavirus infects prawn, and is distinctive from alpha- and beta-nodaviruses (Naveen Kumar et al., 2013). This prawn nodavirus includes Macrobrachium rosenbergii nodavirus (MrNV) and Penaeus vannamei nodavirus (PvNV). Naveen Kumar et al. (2013) proposed that the MrNV and PvNV should be categorized into gamma-nodaviruses, based on their distinct genomic sequences compared with that of both the alpha- and beta-nodaviruses. More recent studies have identified another two prawn nodaviruses, namely the covert mortality nodavirus (CMNV) (Zhang et al., 2014; Zhang et al., in press) and Farfantepenaeus duorarum nodavirus (FdNV) (Ng et al., 2013), infecting Litopenaeus vannamei and F. duorarum, respectively. Although nodaviruses are usually named after their native hosts, nodaviruses are often capable of infecting multiple species. RGNNV has been reported to infect Asian seabass, Lates calcarifer (Banerjee et al., 2014); Nile tilapia, Oreochromis niloticus (Keawcharoen et al., 2015); and Amphiprion sebae Bleeker, a marine clownfish (Binesh et al., 2013), whereas MrNV has also been reported to infect Penaeus indicus, Penaeus monodon, and P. vannamei (Ravi et al., 2009; Senapin et al., 2012).
Fish nodavirus
The fish nodavirus, also known as Nervous Necrosis Virus (NNV), infects fishes and causes viral encephalopathy and retinopathy (VER). The first outbreak occurred in 1985 (Costa & Thompson, 2016). The infection was first described by Yoshikoshi & Inoue (1990) in the Japanese parrot fish Oplegathus fasciatus. Fishes infected by nodavirus suffer neurological disorders, which are characterized by intensive vacuolization of retina and central nervous systems, culminating in abnormal swimming pattern and darkening of fish color (Munday & Nakai, 1997). In fish, Nodavirus can be detected in many organs but central nervous system including the brain, spinal cord and retina are the main targets (Ghiasi et al., 2016). The fish nodavirus seriously affects aquaculture industry worldwide, resulting in great economic losses. Infection by this virus is often associated with high mortality rate, up to 100% in fish larvae and juveniles (Skliris et al., 2001). To date, nodavirus is known to affect over 120 fish species, particularly groupers and seabass such as the Asian seabass Lates calcarifer and European seabass Dicentrarchus labrax (Breuil et al., 1991; Costa & Thompson, 2016; Frerichs, Rodger & Peric, 1996; Munday, Kwang & Moody, 2002; Parameswaran et al., 2008). Although nodavirus mostly affects marine fishes, nodavirus infections in freshwater fishes such as European eels (Anguilla anguilla L.), yellow-wax pompano (Trachinotus falcatus), firespot snapper (Lutaanus erythropterus B.), cobia (Rachycentron canadum) and Chinese catfish (Parasilurus asotus) have been reported in Taiwan (Chi, Shieh & Lin, 2003). In addition, outbreaks in hybrid striped bass × white bass (Morone saxalitis × Morone chrysops) and largemouth bass (Micropterus salmoides) have also been reported in Italy (Bovo et al., 2011). Apart from horizontal transmission, fish nodavirus can be transmitted vertically through infections at the gonads, passing the virus to their progenies (Breuil et al., 2002; Kocan, Hershberger & Elder, 2001; Valero et al., 2015a).
Prawn nodavirus
Like fish nodavirus, prawn nodavirus has significant economic impact on the prawn aquaculture industry. Prawn nodavirus can be isolated from cephalothoraxes and whitish abdominal muscle (Zhang et al., 2014) of infected prawns. The most studied prawn nodavirus is the MrNV. It is a non-zoonotic nodavirus which infects M. rosenbergii, commonly known as the giant river prawn. MrNV was first isolated and reported in 1999 (Arcier et al., 1999) from M. rosenbergii. Infection by MrNV causes white tail disease (WTD) or white muscledisease (WMD), where infected cells undergo necrosis and turn whitish. The rate of mortality is extremely high (up to 100%) in larvae and post-larvae of M. rosenbergii (Qian et al., 2003; Ravi et al., 2009), causing great economic losses to M. rosenbergii hatchery and nursery farm industries. Despite the high mortality rate in larvae and post-larvae prawns, MrNV does not cause death in adult prawns. However, the adult prawns still serve as the virus carriers, transmitting the virus vertically to their offsprings (Sudhakaran et al., 2007), and horizontally to other prawns during cannibalization (Sahul Hameed et al., 2004). Another prawn virus, PvNV was first isolated in 2005 from a P. vannamei farm in Belize (Tang et al., 2007; Tang et al., 2011). Being a prawn nodavirus, PvNV shares 83% similarities with MrNV in its viral genome (Tang et al., 2011). It causes muscle necrosis, resulting in white, opaque lesions in the tail, similar to the symptoms of MrNV infection. However, the virulence of PvNV is not as high as MrNV, in which the former normally resulted in approximately 50% production loss in an infected farm (Tang et al., 2007). Apart from its native host, PvNV has also been demonstrated to be able to infect Penaeus monodon in an experimental infection (Tang et al., 2007).
Insect nodavirus
Unlike fish and prawn nodaviruses, insect nodaviruses do not have a direct impact on the global economy. Despite that, insect nodaviruses, especially the FHV and BBV, have served as excellent models to study the mechanisms of other positive-strand RNA viruses, such as those of Caliciviridae, Flaviviridae, Picornaviridae, and Togaviridae, due to their small genome size and high level of replication in compatible hosts (Ball & Johnson, 1998). FHV was originated from grass grub, Costelytra zealandica (Dearing et al., 1980). FHV has been demonstrated to be able to infect a wide variety of hosts, including insects, yeasts, plants, and mammalian cells. Apart from its original host C. zealandica, FHV also infects the common fruit fly, Drosophila melanogaster. Therefore, cell-lines derived from D. melanogaster such as Schneider Line 1 (DL1) have been established for the propagation of insect nodaviruses (Dearing et al., 1980; Miller, Schwartz & Ahlquist, 2001). When the yeast Saccharomyces cerevisiae was transfected with FHV, the viral genomic RNA induced the production of infectious virion capable of infecting Drosophila cells (Price, Rueckert & Ahlquist, 1996). In addition, FHV was also reported to infect the whole plants of barley, cowpea, chenopodium and tobacco (Selling, Allison & Kaesberg, 1990), as well as mammalian cells such as the baby hamster kidney cell (BHK21) (Ball, Amann & Garrett, 1992). Due to its wide host range, FHV has been an excellent model to study the mechanisms of other economically important RNA viruses. Another well-studied insect virus, BBV, was isolated from Heteronychus arator. BBV propagates well in Drosophila line 1 cells, but not in BHK21, mouse L-cell, mosquito cells (Aedes albopictus and A. aegypti), cabbage looper (Trichoplasia ni), fall armyworm (Spodoptera frugiperda) and line GM1 of D. melanogaster (Friesen et al., 1980). Selling & Rueckert (1984) established a plaque assay for nodaviruses using Drosophila cell-adapted BBV, which greatly facilitates the isolation and reassortant of nodaviruses (Kopek et al., 2010; Settles & Friesen, 2008). BBV’s structure has been studied intensively using electron microscopy and crystallization followed by small-angle x-ray scattering (Hosur et al., 1984). As in other nodaviruses, BBV appeared to form icosahedral structure with a triangulation number of T = 3. Furthermore, the RNA3 of nodavirus was identified to be a subgenomic mRNA of the viral RNA1 by studying BBV, and it can be isolated from cells infected by BBV (Friesen & Rueckert, 1982; Guarino et al., 1984). Another insect nodavirus, Boolarra virus was isolated from the ghost moth Oncopera intricoides (Reinganum, Bashiruddin & Cross, 1985). The viral morphogenesis was shown to be restricted to the cytoplasm of cultured Drosophila cell lines (Bashiruddin & Cross, 1987). A more recent Wuhan nodavirus was isolated from Pieris rapae larvae (Liu et al., 2006a). A study of its subgenomic RNA3 has provided an insight into the RNAi inhibitory property of the nodavirus B2 protein (Cai et al., 2010).
General features of nodavirus
In general, nodaviruses are non-enveloped zoonotic viruses with icosahedral structures. Their genomes comprise of two linear, positive-sense, single-stranded RNA. RNA 1 is approximately 3.1–3.2 kilobases (kb) in length, whereas RNA2 is approximately 1.2–1.4 kb. Both of which lack a poly-A tail at their 3′ ends (Comps, Pepin & Bonami, 1994; Mori et al., 1992). RNA 1 encodes for the RNA-dependent RNA polymerase (RdRP), which functions in replicating the viral RNA genome without involving an intermediate DNA. RNA 3, a subgenomic transcript of RNA 1, it encodes for a non-structural B2-like protein (Cai et al., 2010; Hayakijkosol & Owens, 2012; Lingel et al., 2005). B2 functions as a suppressor for the post-transcriptional gene silencing of host defense mechanisms through non-specific binding to double-stranded RNA generated during the virus replication (Fenner et al., 2006). RNA 2 encodes for the viral capsid protein, which forms the core of nodavirus. The nodavirus capsid protein assembles into virus particles with icosahedral structures, approximately 30 nm in diameter, with a triangulation number of 3 (T = 3) containing 180 capsid subunits. The virus particles package only the RNA 1 and RNA 2, forming simple but infectious virions.
Transmission of nodavirus
It has been confirmed that vertical transmission is the main mechanism of nodavirus spreading (Murwantoko et al., 2016; Zhang et al., in press). This vertical transmission in the aquaculture industry can be overcome by good biosecurity practices in hatchery-reared larvae and juveniles of some fish species. Besides vertical transmission, nodavirus may also infect the cultured fish even at the grow-out stages through horizontal transmission. Although nodaviruses detected in aquaculture farms are often with relatively low sequence variations, PCR based molecular analyses (including RT-PCR and nested PCR) have revealed different betanodaviruses with high numbers of sequence variations in wild fishes and even seawater samples. This implies that, nodavirus with different virulence may be shed by the less susceptible wild fish in water and consequently virulent forms of nodavirus in the seawater would infect the susceptible cultured fish (Nishi et al., 2016).
Recent incidence of nodavirus
Nodavirus infection has a great negative impact on the aquaculture industry. To date, more than 40 marine and freshwater fish species have been identified susceptible to nodavirus (particularly betanodavirus) infection (Nishi et al., 2016). It has been detected in freshwater prawn hatcheries in Indonesia (Murwantoko et al., 2016) and marine shrimp farms located in Fujian, Shandong and Hebei Provinces in China (Zhang et al., 2014). In addition, nodavirus caused mass mortality in cage-reared freshwater guppy Poicelia reticulate in Singapore (Hegde et al., 2003), larval rearing facility of marine clownfish, Amphiprion sebae in India (Binesh et al., 2013) and Asian seabass in India (Banerjee et al., 2014). Apart from affecting aquaculture industry, nodaviruses detected in wild golden grey mullet Liza aurata and sharpnose mullets Liza saliens were correlated to the dramatically decrease of mullets stock in the Caspian Sea (Zorriehzahra et al., 2014; Ghiasi et al., 2016).
Detection of nodavirus
General identification
Histopathology and Transmission Electron Microscopy (TEM) examinations were used to observe the presence of nodaviruses in fishes (Ghiasi et al., 2016) and shrimps (Zhang et al., 2014). In terms of histopathological analysis of nodavirus infected shrimps, necrotic epithelium and inclusions in the hepatopancreatic tubular epithelium are commonly observed in a nodavirus infected shrimp. In addition, viral inclusion and viral particles are commonly observed in the hepatopancreas using TEM (Zhang et al., 2014). Moreover, severe anemia associated with increase of neutrophil populations, decrease of lymphocyte populations, raise of liver enzyme profile and decline of total protein, albumin and total immunoglobulin levels were also observed in fishes infected with nodaviruses (Ghiasi et al., 2016).
Molecular identification
For the phylogenetic analysis, conventional and real-time reverse transcription PCR (RT-PCR) that amplify the RNA-dependent RNA polymerase (RdRp) of RNA 1 (Murwantoko et al., 2016) or the T4 region of RNA2 of nodavirus (Nishizawa et al., 1994; Hegde et al., 2003; Banerjee et al., 2014; Overgård et al., 2012), random shotgun metagenomic sequencing (Ng et al., 2013) and Illumina whole transcriptome metagenomic sequencing were able to detect the presence of nodavirus in infected organisms (Greninger & DeRisi, 2015) or even from seawater (Nishi et al., 2016). For example, tombunodavirus that shares nucleotide sequence similarity with that of nodavirus and tombuvirus family members was identified in the weekly metagenomic sequencing of organisms in San Francisco wastewater (Greninger & DeRisi, 2015). Nevertheless, whether this phenomenon was due to co-infection of nodavirus and tombuvirus or the real existence of tombunodavirus needs further validation. In another study by Conceição Neto et al. (2015), a putative novel member of nodavirus was detected in the fecal samples of otter (Lutra lutra) in Portugal based on the identification of RdRp in the metagenomic analysis. However, this nodavirus identified in the gut of the otter may have originated from a fish diet, which casts doubt on the report of a new host for nodavirus (Conceição Neto et al., 2015).
To improve molecular identification, virus isolation coupled with either the Sanger sequencing or next generation sequencing (NGS) allow specific characterization of a particular strain of nodavirus (Zhang et al., 2014). Based on the International Committee on Taxonomy of Viruses (ICTV), isolated nodaviruses can be classified according to the genetic diversity of the RNA2 segment by the simple and cost-effective Sanger sequencing method (Conceição Neto et al., 2015). Pairwise identity of the RNA2 with less than 80% at the nucleotide level and less than 87% at the amino acid level is classified as a novel species (Schuster et al., 2014). Compared to the partial sequence identity determined by the Sanger sequencing method, profiling the whole transcriptome of a nodavirus offers a more comprehensive comparison and characterization of the virus classification. For example, CMNV, an alphanodavirus that shares only 31-54% nucleotide sequence similarity with other nodaviruses in GenBank, was successfully characterized by sequencing the cDNA library using the Roche 454 sequencer (Zhang et al., 2014). In addition, fluorescence in situ hybridization (FISH) and nested RT-PCR assays that detect a specific nodavirus can be designed (Zhang et al., 2014). Another study by Schuster et al. (2014) reported that the identification of Mosinovirus (MoNV), a novel member of the family Nodaviridae, belongs neither to alpha- nor beta-nodaviruses. Without the isolation of the virus, recombination that was detected by the whole transcriptome 454 pyrosequencing in MoNV would not be accepted (Schuster et al., 2014). Although nodavirus can be detected in water samples, quantitative isolation of the nodavirus remains challenging with the current available protocols (Nishi et al., 2016).
Diagnosis of nodaviruses
Most of the diagnostic assays for nodaviruses are based on detection of the viral RNA through RT-PCR. In recent years, efforts have been focused to establish diagnostic assays which require minimal laboratory setup. A rapid and sensitive automated microfluidic chip system for the detection of piscine nodavirus in groupers has been developed (Kuo et al., 2012). The microfluidic chip contains an RT-PCR module capable of processing extracted RNA samples, and a capillary electrophoresis module. This microchip has been field-tested in an epidemiological investigation of NNV in Taiwan (Kuo et al., 2012).
Reverse-transcription loop-mediated isothermal amplification (RT-LAMP) is another potential point-of-care diagnostic assay, as a laboratory setup such as thermocycler and electrophoresis equipment can be omitted. Suebsing, Prombun & Kiatpathomchai (2013) developed an RT-LAMP with colorimetric gold nanoparticle probe assay for the detection of PvNV in P. vannamei and P. monodon. This assay is 10x more sensitive than the nested RT-PCR established by Tang et al. (2007). On the other hand, Zhang et al. (in press) used the RT-LAMP as a rapid and quantitative diagnostic assay for the detection of CMNV in P. vannamei. This assay is capable of detecting as little as 6.3 pg of total RNA from infected shrimps.
Assays based on lateral flow strips have also been deployed for diagnosis of nodavirus. Lin et al. (2014) combined RT-LAMP with a lateral flow dipstick (RT-LAMP-LFD) for the detection of MrNV, targeting six distinct regions of MrNV RNA2. The sensitivity of this RT-LAMP-LFD is 10x higher than that of the RT-LAMP. Toubanaki, Margaroni & Karagouni (2015b) also developed a lateral flow paper biosensor for the detection of NNV in European seabass. Instead of RT-LAMP, this lateral flow biosensor detects the viral RNA through RT-PCR using a 5′-biotin-tagged primer, a probe containing a poly-A tail, and gold nanoparticles conjugated to a poly-T oligonucleotide, with streptavidin forming the test line. This assay was reported to detect 270 pg of initial total RNA, which is less sensitive than the RT-LAMP based method (6.3 pg).
Apart from simply detecting the presence of nodavirus infection, it is also important to identify the genotype of the infecting virus, either for epidemiological study, or for specific strategy design to eliminate virus infection in an aquaculture farm. Toubanaki, Margaroni & Karagouni (2015a) developed a tetra-primer PCR which can amplify specifically RGNNV or SJNNV cDNA, thereby generating short PCR products of different sizes which can distinguish between RGNNV and SJNNV infections in European seabass.
Apart from detecting the virus at RNA level, the presence of nodavirus can also be evaluated by immunological methods, such as Western blotting, indirect florescent antibody, enzyme-linked immunosorbent assay (ELISA) and immunodot blot tests (Ghiasi et al., 2016; Hegde et al., 2003; Sri Widada et al., 2003). MrNV infection has been diagnosed with Western blotting, dot blot and ELISA using polyclonal antibodies against the recombinant MrNV capsid protein raised in rabbit (Farook et al., 2014a). Wang, Chang & Wen (2016) used an immunodot blot assay to detect MrNV with a polyclonal antibody raised against the recombinant viral capsid in a Wistar rat. In addition, Wangman et al. (2012) successfully produced monoclonal antibodies that bind specifically to MrNV capsid protein. These antibodies can be used to detect MrNV without cross-reaction with other common shrimp viruses. Although the immunological methods are less sensitive compared with the viral RNA-based detection methods, the former remains a viable alternative for many laboratories.
In vitro model for nodavirus studies
Cell lines are important models in virology, toxicology and gene expression studies. In virology, cell lines have been widely used to determine the infectivity, pathogenicity and infectious mechanisms of nodavirus (Abdul Majeed et al., 2013; Nishi et al., 2016). Currently, Channa striatus kidney (CSK) (kidney of Channa striatus), GB (brain of Epinephelus coioides), GF-1 (fin of Epinephelus coioides), SSN-1 (fry of Ophicephalus striatus), E-11 (clone of SSN-1), SISK (kidney of Lates calcarifer), SISS (spleen of Lates calcarifer), SIGE (eye of Epinephelus coioides), ICF (fin of Clarias batrachus), IEE (eye of Etroplus suratensis), IEG (gill of Etroplus suratensis), IEK (kidney of Etroplus suratensis), and IGK (kidney of Epinephelus coioides) fish cell lines were proven susceptible to nodavirus infections and thus suitable for in vitro propagation and studies of the viral infectious mechanisms (Abdul Majeed et al., 2013; Chi, Hu & Lo, 1999; Sarath Babu et al., 2013; Kai, Wu & Chi, 2014; Nishi et al., 2016). Among these cell lines, SISK, SISS and SIGE were found to be more susceptible to nodavirus infections and are thus suitable models for nodavirus propagation, diagnostic reagent and vaccine productions (Sarath Babu et al., 2013).
Immune response against nodavirus infection
Immunity plays an important role in the prevention and recovery of nodavirus infection in aquatic animals. Overall, activation of innate immunity (such as NK cells and antimicrobial peptides), cellular T cell type I interferon immunity, and humoral immunity (immunoglobulins antibodies) cooperatively prevent nodavirus infection (Chen, Wang & Chen, 2014; Costa & Thompson, 2016). Nodavirus mainly affects fishes at larval stage, which may be due to the lack of well-developed adaptive immune cells as present in adult fishes that restrict the viral replication, thus minimize the development of pathological and clinical signs (Overgård et al., 2012).
Pathogen-associated molecular patterns (PAMPs) on RNA viruses are first recognized by pattern-recognition receptors (PRRs). This process subsequently induces intracellular signals to activate defensive mechanisms (Chen et al., 2014). Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) are the two important classes of PRRs that sense the PAMPs of RNA viruses such as fish nodaviruses (Chen et al., 2014; Costa & Thompson, 2016). The RLR family consists of RIG-1, melanoma differentiation-associated gene 5 (MDA-5) and Laboratory of Genetics and Physiology 2 (LGP2). Activation of RLRs and TLRs subsequently promotes interferon type I antiviral immune response (Costa & Thompson, 2016). RLRs, MyD88-dependent TLRs (Chen et al., 2015) and TLR7 (Takano et al., 2011) were found upregulated and correlated with the production of type I interferon and pro-inflammation response. MDA5 is a member of RLRs that promotes transcription of interferon related immune factors, which include interferon regulatory factor 3 (IRF3), IRF7 (Yu et al., 2017), and interferon-stimulated response element (ISRE) such as interferon-stimulated gene 15 (ISG15) (Huang et al., 2013) and proinflammatory cytokines (Huang et al., 2016b). Unlike RIG-1 and MDA-5, the exact functions of LGP2 in different virus infections are controversial. LGP2 is generally reported as a positive regulator of RIG-1 and MDA-5 (Chen et al., 2014). However, a recent study showed a contrary function of LGP2, in which the overexpression of LGP2 suppressed the expression of MDA5, Mx promoter, ISRE, pro-inflammatory cytokines and type I interferon genes, thus resulting in a high viral load (Yu et al., 2016).
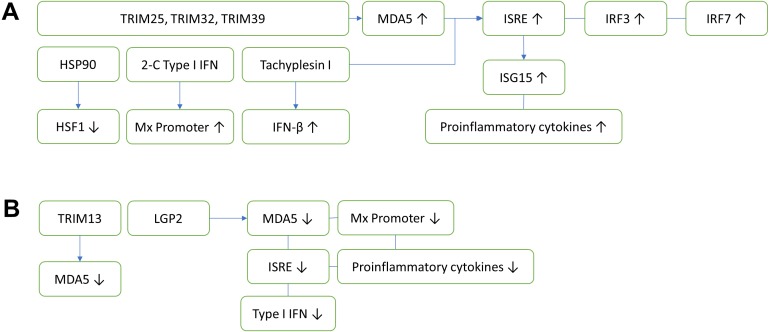
Tripartite motif-containing (TRIM) proteins are multi-domain proteins that exert important immune regulatory roles on TLR and RLRs mediated antiviral innate immunity (Huang et al., 2016a). The innate antiviral immunities of fish against nodavirus infection are summarized in Fig. 1. In fish, TRIM proteins play an important role in the recognition and initiation of protection against nodavirus infection. To date, fish TRIM25, TRIM32 and TRIM39 exerted positive antiviral responses via activation of MDA5 expression (Wang et al., 2016; Yang et al., 2016; Yu et al., 2017). On the other hand, TRIM13 exerted negative regulation on antiviral immunity against nodavirus infection through downregulation of MDA5 and the downstream IFN signaling pathway (Huang et al., 2016a).
Figure 1. Innate antiviral immunity of fish against nodavirus infection.
(A) Positive regulation which inhibits viral replication. TRIM25, TRIM32 and TRIM39 upregulate the expression of MDA5, which in turn induces ISRE, IRF3 and IRF7. The upregulation of ISRE also induces the expression of ISG15 and proinflammatory cytokines, cooperatively reducing the viral load. Other elements known to inhibit virus replication include HSP90, 2-C Type I IFN and Tachyplesin I, which downregulates HSF1, upregulates Mx promoter and IFN-β, respectively. (B) Negative regulation which promotes viral replication. TRIM13 and LGP2 downregulate MDA5, thereby reduce the ISRE. LGP2 also downregulates Mx promoter, proinflammatory cytokines and Type I IFN. TRIM, Tripartite motif-containing protein; MDA5, Melanoma differentiation-associated gene 5; IRF, Interferon regulatory factor; ISRE, Interferon-stimulated response element; HSF1, Heat shock transcription factor 1; HSP90, Heat shock protein 90; ISG, Interferon-stimulated gene.
Type I (α/β) and type II (γ) interferons play important roles in the innate immune responses against nodavirus infection in fish. Post detection by PRRs, IFN type I will be secreted and picked up by IFN receptor of neighboring cells, activating its Janus kinases 1 (JAK1)/signal transducer and activators of transcription (STAT) pathway which leads to the transcription of IFN-stimulated genes (ISGs) (Chen et al., 2014) and pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α (Costa & Thompson, 2016). Activation of ISGs via type I interferon subsequently promotes Mx promoter activity, which increases host resistance to nodavirus infection (Chen et al., 2014). Mx is one of the downstream antiviral effectors under type I interferon immunity (Sadler & Williams, 2008). The researchers demonstrated that the sevenband grouper Epinephelus septemfasciatusu pre-treated with non-lethal aquabirnavirus (ABV) developed a protection against the RGNNV nodavirus infection prior to the activation of type I interferon. This protection was attributed to the overexpression of the type I interferon downstream effector, the Mx gene in head kidney and brain of the fish (Pakingking Jr et al., 2005). Furthermore, inoculation of gilthead seabream Sparus aurata with lipopolysaccharides from Vibrio alginolyticus also stimulated the Mx gene expression in liver, which effectively reduced the load of nodavirus in the brain (Bravo et al., 2013).
Halibuts and groupers are known reservoirs of nodavirus but resistant to the virus and vertically transmit the disease (Chaves-Pozo et al., 2012; Overgård et al., 2012). Although the early proinflammatory type 1 interferon response helps to control the nodavirus infection in the juvenile Atlantic halibut Hippoglossus hippoglossus, the viral RNA was still detected in the brain, eye and head kidney of the fish even after 14 weeks post infection by the virus. This was accompanied by more drastic T cell mediated responses including upregulation of the T cell markers (CD4, CD8α and CD8β), ISG15, Mx and IFNγ genes expression (Overgård et al., 2012). However, elevation of proinflammatory cytokines without significant changes in CD4, CD8α and CD8β T cell markers was observed in the brain, eye and head kidney of Atlantic halibut at the early stage of nodavirus infection (Overgård et al., 2012). On the other hand, susceptible species such as European seabass were detected with delayed but stronger proinflammatory response, resulting in an irreparable brain damage (Poisa-Beiro et al., 2008; Valero et al., 2015b). In addition, a challenge study on sensitive seabass species with low titer of nodavirus induced early but short-term type I interferon response (Scapigliati et al., 2010). Moreover, Valero et al. (2015a) showed that nodavirus replicated more in the reservoir seabream’s testis than the susceptible seabass, through modulation of reproductive system that favor the transmission and shedding of the virus in the reservoir species. This result indicates that proinflammatory type I interferon did not involve in stimulating T cell proliferation at the early stage of the viral infection, and thus T cell type I interferon response is not sufficient to clear the nodavirus, resulting in vertical virus transmission in the reservoir species. The researchers proposed that antimicrobial peptides (AMPs) play an important role in vertical transmission of nodavirus in resistant fish species (Valero et al., 2015b). AMPs are a major component of the innate immune system in fish that activate antiviral effects upon nodavirus infection (Xie, Wei & Qin, 2016; Valero et al., 2015b). Grouper epinecidin-1 (CP643-1), complement factor 3 (c3), lysozyme (lyz), hepcidin (hamp), dicentracin (dic), piscidin (pis) or b-defensin (bdef) are AMPs found to be activated during nodavirus infection in both susceptible and resistant fish species (Valero et al., 2015b). CP643-1 was also reported to induce the Mx gene expression during nodavirus infection in fish (Chia et al., 2010). In addition, Tachyplesin I has been reported as an AMP found in resistant grouper strains which activates the antiviral activity through promotion of ISRE and IFN-β expression (Xie, Wei & Qin, 2016). Histones (H1 to H4) are another type of potential AMPs that may play some roles in the antiviral effects in fish. However, more studies are needed to investigate the function of histones in protecting fish against nodavirus infection (Valero et al., 2016a). Production of AMPs in resistant gilthead seabream and susceptible European seabass differs significantly, in which the AMPs were highly expressed in the brain but low in the gonad of gilthead seabream, whereas in European seabass it was highly expressed in the gonad but low in brain. These results indicate that vertical transmission of nodavirus by the resistant gilthead seabream could be attributed to the poor AMP response in the gonad. The European seabass containing a high level of AMPs in the gonad did not survive nodavirus infection as the AMP expression level in the brain was low (Valero et al., 2015b).
There are other immune factors contribute to the protection of fish against nodavirus infection. Esteban et al. (2013) reported that nodavirus strain 411/96 (RGNNV) induced early (day 1 post-challenged) expression of the peroxiredoxin natural killer enhancing factor A (NKEF-A), which involved in inflammation and innate immunity in both the brain and head kidney of gilthead seabream, but not in European seabass. This result shows that an early expression of NKEF-A which activates immune cells including the NK cells and macrophages is an important anti-nodavirus mechanism in resistant species. On the other hand, the involvement of CD83 gene in the immune response of fish during nodavirus infection was also evaluated. Downregulation of CD83-like molecule expression was observed in the head kidney of European seabass post-infected with nodavirus. Although CD83 was known as a marker for matured human dendritic cells, active thymic T cells and even B cells, the exact function of CD83 in fish lymphocytes is still unknown (Buonocore et al., 2012). Thus, more studies have to be performed to investigate the involvement of CD83 expression in immunity against nodavirus infection.
Nodavirus is a simple RNA virus with only three genes. However, it has developed some virus-host interaction properties, which include hijacking the host system and escaping host defense mechanism (Chen et al., 2014). Overexpression of heat shock transcription factor 1 (HSF1) promoted the replication of nodavirus at the initial stage of the viral infection, which could be due to an increase of fish body temperature as the expression of Mx protein was suppressed at high temperature condition (Wang, Chen & Chen, 2016). Suppression of HSF1 by the heat shock protein 90 (HSP90) thereby reduced the replication of nodavirus during the initial stage of the viral infection (Chen et al., 2010; Wang, Chen & Chen, 2016). Moreover, fish is more susceptible to nodavirus infection in the present of immunosuppressive agents. Lawrence, Reid & Whalen (2015) reported that an organotin compound, tributyltin, which is commonly used as antifouling paints for ships and fishing nets caused immune suppression in fishes. Exposure of Japanese medaka Oryzias latipes larvae to tributyltin increased their susceptibility towards SGWak97 nodavirus (RGNNV) infection, which resulted in a higher mortality in a dosage dependent manner. This phenomenon could be attributed to the immunosuppression caused by tributyltin on fish NK cell activity (Kitamura et al., in press).
Besides innate and cell mediated immunity, humoral immunity also plays an important role in protecting fish against nodavirus infection (Chen et al., 2014), especially the two immunoglobulins, IgM and IgT. Infection of susceptible seabass with low titer of nodavirus did not significantly alter the expression of IgM and IgT in the gills and spleen (Buonocore et al., 2017) but only induced a marginal increase of serum IgM (Scapigliati et al., 2010). On the other hand, activation of IgM+ and IgT+ B cells in the brain and overexpression of soluble IgT+ by B cells in the head kidney by early inflammatory response in the central nervous system reduced nodaviral replication in the resistant aquaculture-relevant fish species (Lopez-Munoz et al., 2012; Piazzon et al., 2016). Thus, activation of IgM and IgT expression by vaccination can protect the fish from nodavirus infection (Costa & Thompson, 2016).
Unlike vertebrates including fish, the understanding of prawn immunity against nodavirus infection is even limited. Based on current findings, prawns generally fight infections through non-specific innate immune responses including prophenoloxidase-activating system (Ourth & Renis, 1993; Popham et al., 2004) and over-accumulation of superoxide anion (Ravi et al., 2010), which were known to inactivate DNA and RNA viruses. However, the basic understanding on the prawn immunity against nodavirus infection has ignited a spark of interest among researchers to produce vaccines against the prawn nodavirus. Instead of the whole virus, Farook et al. (2014b) introduced a recombinant MrNV capsid protein (r-MCP) produced in E. coli into M. rosenbergii as a potential vaccine against the WTD. This r-MCP increased the level of prophenoloxidase, superoxide anion, and other anti-viral compounds such as crustin, peroxinectin, anti-lipopolysaccharides and heat shock proteins (HSP21, HSP70, HSP90), which protected the M. rosenbergii post-larvae from MrNV challenge up to 76%.
Advances in nodavirus vaccine development
Vaccination has been proposed as a solution to control and prevent nodavirus outbreaks in aquaculture industry (Pakingking Jr et al., 2010). Table 1 summarizes the studies on nodavirus vaccines. Among different types of vaccines, virus-like particles (VLPs) show the highest potential to induce a long lasting and protective humoral immunity (Liu et al., 2006b). Intramuscular administration of recombinant DGNNV VLPs produced in E. coli induced a high antibody titer which is capable to neutralize the virus in vitro. Even without an adjuvant, neutralizing antibodies induced by the DGNNV VLPs lasted over five months, further justifying the potential application of nodavirus VLPs as a vaccine. A recombinant betanodavirus capsid protein r-FNCP42 was generated by Vimal et al. (2014) based on the gene sequence of a fish nodavirus isolated from Asian seabass (L. calcarifer) larvae. Intramuscular injection of 50 µg r-FNCP42/fish resulted in 75% survival of juveniles of Asian seabass challenged with 1 × 106.5 TCID50 of nodavirus. As the genome sequence of r-FNCP42 shares more than 98–99% similarity with other fish nodaviruses including red spotted grouper nervous necrosis virus, Dicentrarchus labrax encephalitis virus, Asian seabass nervous necrosis virus, and Epinephelus tauvina nervous necrosis virus (ETNV), thus cross protectivity of r-FNCP42 against these nodaviruses should be tested. Naveen Kumar, Karunasagar & Karunasagar (2013) immunized M. rosenbergii through oral administration of inactivated bacteria encapsulated dsRNA of MrNV and XSV, where a post-feeding virus challenge showed promising results. The MrNV challenge at 24 h and 72 h post-feeding showed relative high percentage of survival at 80% and 75%, respectively, indicating a regulation via RNA interference. Ramya et al. (2014) used chitosan conjugated DNA vaccine, where XSV antisense (XSVAS) nucleotide sequence was cloned into the pcDNA plasmid vector. The presence of plasmid pcDNA-XSVAS was confirmed after 30 days of administration through oral feeding, where it provided approximately 50% protection to prawns challenged with crude extract of WTD-prawns. In addition, introduction of recombinant MrNV capsid protein through 24 h immersion followed by MrNV challenge boosted the relative percent survival of prawns by 76.03% (Farook et al., 2014b).
Table 1. Vaccines, route of administration and their protectivity.
| Type of vaccine | Route of vaccination | Protectivity | Remarks | References |
|---|---|---|---|---|
| Recombinant betanodavirus of RNA2 capsid protein r-FNCP42 | IM | 75% higher survival rate of juveniles of Asian seabass challenged with 1 × 106.5 TCID50 of nodavirus/fish | As the genome sequence analysis of r-FNCP42 has more than 98–99% of similarity with other fish nodavirus including red spotted grouper nervous necrosis virus, Dicentrarchus labrax encephalitis virus, Asian seabass nervous necrosis virus, and Epinephelus tauvina nervous necrosis virus (ETNV), thus cross protectivity of r-FNCP42 against other strains of nodavirus shall be tested. | Vimal et al. (2014);Vimal et al. (2016) |
| Recombinant r-FNCP42-DNA | IM | 77% higher survival rate of juveniles of Asian seabass challenged with 1 × 106.5 TCID50 of nodavirus/fish | Capsid protein was highly expressed in the heart, muscle and liver of the vaccinated fish. | Vimal et al. (2016) |
| Recombinant capsid protein MGNNV virus like particles (VLPs) | IM | ∼70% higher survival rate of juvenile European seabass (Dicentrarchus labrax) challenged with 105 TCID50/fish | MGNNV induced humoral immunity against nodavirus. | Thiery et al. (2006) |
| DNA vaccine pVHSV-G encoding glycoprotein of viral hemorrhagic septicaemia virus | IM | ∼54% higher survival rate of juvenile turbot (Scophthalmus maximus) challenged with 106.3 TCID50 | DNA vaccine induced inflammatory response that cross protect nodavirus infection. | Sommerset et al. (2003) |
| Synthetic peptides (N-terminal regions) of nodavirus DIEV RNA2 protein | IM | ∼27% higher survival rate of seabass challenged with 109 FCU/fish | Peptides induced humoral immunity. | Coeurdacier, Laporte & Pepin (2003) |
| Heat inactivated S1 and Sb2 nodavirus | IM | ∼33% and 26% higher survival rate of seabass challenged with 9 × 109 FCU/fish, respectively | Induced humoral immunity. | Coeurdacier, Laporte & Pepin (2003) |
| Virus-like particles (VLPs) of grouper nervous necrosis virus | IM | – | Induced humoral immunity. No challenge test was performed. | Liu et al. (2006b) |
| Recombinant RGNNV-CP | IM | ∼60% higher survival rate of humpback grouper challenged with 105.5 TCID50/fish, respectively | Induced humoral immunity. | Yuasa et al. (2002) |
| Recombinant ETNNV-CP (Epinephelus tauvina nervous necrosis virus-capsid protein) | IM | – | Induced stronger humoral immunity than formalin inactivated nodavirus. No challenge test was performed. | Hegde, Lam & Sin (2005) |
| Formalin inactivated nodavirus | IP | 60% higher survival rate of brown-marbled grouper challenged with 106.5 TCID50/fish of OSGBF1E | Induction of humoral immunity. | Pakingking Jr et al. (2009) |
| Recombinant capsid protein, recAHNV-C | IP | 29% higher survival rate of juvenile turbot (Scophthalmus maximus) challenged with 106 TCID50/ml AHNV | Fishes vaccinated with plasmid DNA expressing the recombinant capsid protein were not protected as the plasmid DNA only induced cellular but not humoral immunity. | Sommerset et al. (2005) |
| Formalin inactivated SGWak97 | IP | Not reported | Inactivated SGWak97 induced humoral immunity. | Pakingking Jr et al. (2009) |
| Recombinant rT2 SJNNV-CP (Scophthalmus maximus nervous necrosis virus-capsid protein) | IP | ∼36% higher survival rate of humpback grouper challenged with 6.3 × 107 TCID50/fish, respectively | Induced humoral immunity. | Húsgağ et al. (2001) |
| Chitosan-encapsulated DNA vaccine (CP-pNNV) | Oral | 55% higher survival rate of juvenile European seabass (Dicentrarchus labrax) challenged with 106 TCID50/fish | CP-pNNV failed to induce humoral immunity but activated interferon pathway and cell-medicated cytotoxicity. | Valero et al. (2016b) |
| Chitosan conjugated DNA vaccine pcDNA-XSVAS | Oral | Approximately 50% higher survival rate of prawn challenged with crude extract of prawn with WTD. | XSV with nodavirus caused white tail disease (WTD) in prawn. The challenge experiment shall consider using isolated virus instead of crude one. | Ramya et al. (2014) |
| Recombinant yeast expressing RGNNV-CP (red-spotted grouper necrosis virus capsid protein) | Oral | – | Induced humoral immunity in mice. No challenge test was performed. | Kim et al. (2013) |
| Artemia-encapsulated recombinant pET24a-NNV VP E. coli expressing nodavirus capsid protein | Oral | ∼34% higher survival rate of grouper larvae challenged with 105 TCID50/fish, respectively | Induced humoral immunity. | Lin et al. (2007) |
| Inactivated bacteria encapsulated dsRNA of MrNV and XSV | Oral | MrNV challenge 24 h and 72 h post-feeding showed relative percent survival of 80% and 75%, respectively | Protection through RNA interference with capsid and B2 genes of MrNV, and capsid gene of XSV. | Naveen Kumar, Karunasagar & Karunasagar (2013) |
| Solid lipid nanoparticles encapsulated binary ethylenimine inactivated nodavirus | Bath and Oral | 45% higher survival rate of grouper larvae challenged with 1 × 106 TCID50/ml HGNNV | Simple vaccination procedure that fit for larvae. Both routes of vaccinations induced pro-inflammatory cytokines expression, type I IFN response, humoral immunity and cellular immunity. | Kai & Chi (2008), Kai, Wu & Chi (2014) |
| Recombinant MrNV capsid protein | Bath | Immersion for 24 h followed by MrNV challenge showed 76.03% survival in 15 days post-challenge | Protection is believed to be through upregulation of prophenoloxidase, superoxide anion and SOD activity. | Farook et al. (2014b) |
Notes.
- IM
- intramuscular injection
- IP
- intraperitoneal injection
- Oral
- oral feeding
- Bath
- immersion
Virus-like particles
After decades since nodaviruses were first discovered, studies on their VLPs continue. DGNNV VLPs produced in E. coli were crystallized and studied with x-ray diffraction, revealing a T = 3 icosahedral structure approximately 38 nm in diameter, closely resembling the native virion (Luo et al., 2014). Recombinant FHV capsid protein produced in E. coli was also used in an in vitro assembly study (Bajaj & Banerjee, 2016). The capsid protein possesses additional N-terminal tag which hinders the assembly of the capsid protein into VLPs. Cleavage of this N-terminal region in vitro in the presence of Ca2+ ion allows the capsid protein to assemble into VLPs of different sizes. Despite the heterogenicity, these VLPs were capable of membrane disruption, a property required by the nodavirus to penetrate its host cells (Bajaj & Banerjee, 2016).
In addition, FHV VLPs have also been used to display foreign epitopes, such as that of hepatitis C virus (HCV) (Peng, Dai & Chen, 2005). Chen et al. (2006) used FHV VLPs to display the epitopes of HCV core protein and hepatitis B virus (HBV) surface antigen, where the displayed epitopes were shown to be immunogenic in guinea pigs. In another study, Manayani et al. (2007) fused the protective antigen-binding von Willebrand A domain of ANTXR2 cellular receptor to FHV VLPs. The researchers demonstrated that the fusion protein inhibited lethal anthrax toxin, and at the same time induced toxin-neutralizing antibody which protected rats from anthrax lethal toxin challenge. All of these studies demonstrated the potential of insect nodavirus VLPs as a foreign epitope presenting agent. In recent years, however, studies on nodavirus VLPs focused more on the prawn nodavirus, particularly MrNV.
The first prawn nodavirus VLPs were produced by Goh et al. (2011) via recombinant DNA technology. The recombinant MrNV capsid protein expressed in E. coli self assembles into VLPs of approximately 30 nm in diameter (Goh et al., 2011). Recently, they reported that 20–29 amino acids (a.a.) at the N-terminal region of MrNV capsid protein are responsible for RNA binding during the VLPs assembly through ionic interaction, where mutation of positively charged a.a. at this region to alanine abolished the RNA binding of the MrNV capsid protein (Goh et al., 2014). Despite the role of RNA binding, the N-terminal region (1–29 a.a.) is not required for the assembly of the VLPs, as demonstrated by Goh et al. (2014). Jariyapong et al. (2014) demonstrated the ability of the MrNV VLPs to encapsidate plasmid DNA in 0.035–0.042 mol ratio (DNA/ protein) through particle disassembly and reassembly, with the use of EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid) and Ca2+ ion, thereby opening a path for the MrNV VLPs to be used for the delivery of nucleic acid based therapeutic agents, such as DNA vaccine or siRNA.
VLPs have been widely used for displaying foreign epitopes, for instance the VLPs of human papilloma virus (Matic et al., 2011), HBV (Ibañez et al., 2013; Murray & Shiau, 1999; Yap et al., 2012), as well as bacteriophages (Hashemi et al., 2012; Kok et al., 2002; Tan et al., 2005; Wan et al., 2001). VLPs are known to enhance the immunogenicity of small epitopes displayed on the particles (Murata et al., 2003; Quan et al., 2008). We have displayed the immunodominant region of HBV on the surface of the MrNV VLPs through fusion at the C-terminal end of MrNV capsid protein and confirmed the fusion protein with immunogold TEM (Yong et al., 2015a). When introduced into BALB/c mice, this recombinant VLPs induced the production of anti-HBV antibodies, as well as the cellular immune responses including natural killer cells, cytotoxic T lymphocytes (CTL) and IFNγ. In addition, we have fused and displayed multiple copies of influenza A virus matrix 2 ectodomain (M2e) on the surface of the MrNV VLPs (Yong et al., 2015b). The displayed M2e epitopes were highly antigenic and immunogenic, where they correlated well with the copy number of M2e displayed on the surface of the VLPs. Most recently, Somrit et al. (2017) showed that the C-terminal region of MrNV capsid protein is exposed on the surface of the VLP, constituting the core of the viral capsid protrusion, through a homology-based modeling based on cucumber necrosis virus. When the C-terminal region was removed with chymotrypsin digestion, the internalization capability of the truncated VLPs into Sf9 cells reduced significantly, suggesting the importance of the C-terminal region in the viral infection.
In an attempt to discover the possible mechanism of MrNV infection pathway, we used the MrNV VLPs labeled with fluorescein as a model to study the MrNV entry and localization in Sf9 cells (Hanapi et al., 2017). Through the use of endosomal inhibitors coupled with laser confocal microscopy and live cell imaging, we demonstrated that the internalization of MrNV VLPs was facilitated by clathrin- and caveolae-mediated endocytosis. We have also identified a potential nuclear localization signal (NLS), which could aid in the localization of MrNV capsid protein to the nucleus based on the importin-α pathway (Hanapi et al., 2017).
Apart from the MrNV VLPs produced in E. coli, we have also produced the MrNV capsid protein in Sf9 cells through baculovirus expression system (Kueh et al., 2017). This eukaryotic produced MrNV capsid protein self-assembles into VLPs significantly larger than their prokaryotic counterparts. The Sf9 produced MrNV VLPs are structurally more homogenous as observed by TEM, representing a better candidate to be used in structural study. Subsequently, we used this MrNV VLPs produced in Sf9 for 3D structure reconstruction using the images obtained from cryogenic electron microscopy (Ho et al., 2017). The 3D structure of MrNV capsid at 7 Angstroms resolution reveals a T = 3 icosahedral structure distinctive to other insect and fish nodavirus capsids, characterized by large dimeric blade-like spikes exposed on the surface of the VLPs. This finding supports the assertion that prawn nodavirus should be classified into a new genus.
Conclusions
Prawn nodaviruses are relatively new compared to the typical alpha- and beta-nodaviruses. Despite their genomic and structural differences with the two established genera, prawn nodaviruses have yet been classified into a new genus. There are likely more prawn nodaviruses unknown to men, such as the recently discovered CMNV and FdNV. Isolation and characterization of these new prawn nodaviruses could contribute in creating a new genus of Nodaviridae, which is the gamma-nodavirus. In addition, there is not yet an effective mid- or long-term vaccine for shrimps and prawns against nodavirus infections. Due to the lack of adaptive immune response in crustacean, antigens that can induce protection against the infections have to be administrated from time to time, especially during the larval stage. Therefore, nodavirus vaccines based on recombinant proteins incorporated into feeds would be more relevant. Better yet, self-replicating DNA expression vector-based vaccines would be more cost-effective to be utilized in shrimp and prawn aquaculture industries.
Funding Statement
This study was supported by the Ministry of Science, Technology and Innovation, Malaysia (grant no: 02-01-04-SF2115) and UPM (grant no: GP-IPS/2016/9511000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Chean Yeah Yong and Swee Keong Yeap planned the contents of the manuscript, performed data searches, analyzed the data, wrote the paper, and prepared the figure and table.
Abdul Rahman Omar analyzed the data, contributed analysis tools, wrote the paper, and reviewed drafts of the paper.
Wen Siang Tan planned the contents of the manuscript, collect the data, analyzed the data, contributed analysis tools, and wrote the paper.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any data or code (literature review).
References
- Abdul Majeed et al. (2013).Abdul Majeed S, Nambi KS, Taju G, Sahul Hameed AS. Development, characterization and application of a new fibroblastic-like cell line from kidney of a freshwater air breathing fish Channa striatus (Bloch, 1793) Acta Tropica. 2013;127:25–32. doi: 10.1016/j.actatropica.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Arcier et al. (1999).Arcier JM, Herman F, Lightner DV, Redman RM, Mari J, Bonami JR. A viral disease associated with mortalities in hatchery-reared postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Diseases of Aquatic Organisms. 1999;38:177–181. doi: 10.1186/s40064-016-3127-z. [DOI] [Google Scholar]
- Bajaj & Banerjee (2016).Bajaj S, Banerjee M. In vitro assembly of polymorphic virus-like particles from the capsid protein of a nodavirus. Virology. 2016;496:106–115. doi: 10.1016/j.virol.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Ball, Amann & Garrett (1992).Ball LA, Amann JM, Garrett BK. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. Journal of Virology. 1992;66:2326–2334. doi: 10.1128/jvi.66.4.2326-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball & Johnson (1998).Ball LA, Johnson KL. Nodaviruses of insects. In: Miller LK, Ball LA, editors. The insect viruses. Plenum Publishing Corporation; New York: 1998. pp. 225–267. [Google Scholar]
- Banerjee et al. (2014).Banerjee D, Hamod MA, Suresh T, Karunasagar I. Isolation and characterization of a nodavirus associated with mass mortality in Asian seabass (Lates calcarifer) from the west coast of India. VirusDisease. 2014;25:425–429. doi: 10.1007/s13337-014-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiruddin & Cross (1987).Bashiruddin JB, Cross GF. Boolarra virus: ultrastructure of intracytoplasmic virus formation in cultured Drosophila cells. Journal of Invertebrate Pathology. 1987;49:303–315. doi: 10.1016/0022-2011(87)90062-0. [DOI] [Google Scholar]
- Binesh et al. (2013).Binesh CP, Renuka K, Malaichami N, Greeshma C. First report of viral nervous necrosis-induced mass mortality in hatchery-reared larvae of clownfish, Amphiprion sebae Bleeker. Journal of Fish Diseases. 2013;36:1017–1020. doi: 10.1111/jfd.12001. [DOI] [PubMed] [Google Scholar]
- Bonami & Sri Widada (2011).Bonami JR, Sri Widada J. Viral diseases of the giant fresh water prawn Macrobrachium rosenbergii: a review. Journal of Invertebrate Pathology. 2011;106:131–142. doi: 10.1016/j.jip.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Bovo et al. (2011).Bovo G, Gustinelli A, Quaglio F, Gobbo F, Panzarin V, Fusaro A, Mutinelli F, Caffara M, Fioravanti M. Viral encephalopathy and retinopathy outbreak in freshwater fish farmed in Italy. Diseases of Aquatic Organisms. 2011;96:45–54. doi: 10.3354/dao02367. [DOI] [PubMed] [Google Scholar]
- Bravo et al. (2013).Bravo J, Real F, Padilla D, Olveira JG, Grasso V, Roman L, Acosta F. Effect of lipopolysaccharides from Vibrio alginolyticus on the Mx gene expression and virus recovery from gilthead seabream (Sparus aurata L.) experimentally infected with Nodavirus. Fish & Shellfish Immunology. 2013;34:383–386. doi: 10.1016/j.fsi.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Breuil et al. (1991).Breuil G, Bonami JR, Pepin JF, Pichot Y. Viral infection (picorna-like virus) associated with mass mortalities in hatchery-reared sea-bass (Dicentrarchus labrax) larvae and juveniles. Aquaculture. 1991;97:109–116. doi: 10.1016/0044-8486(91)90258-9. [DOI] [Google Scholar]
- Breuil et al. (2002).Breuil G, Pepin JFP, Boscher S, Thiery R. Experimental vertical transmission of nodavirus from broodfish to eggs and larvae of the seabass, Dicentrarchus labrax (L.) Journal of Fish Diseases. 2002;25:697–702. doi: 10.1046/j.1365-2761.2002.00406.x. [DOI] [Google Scholar]
- Buonocore et al. (2012).Buonocore F, Randelli E, Tranfa P, Scapigliati G. A CD83-like molecule in seabass (Dicentrarchus labrax): molecular characterization and modulation by viral and bacterial infection. Fish & Shellfish Immunology. 2012;32:1179–1184. doi: 10.1016/j.fsi.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Buonocore et al. (2017).Buonocore F, Stocchi V, Nunez-Ortiz N, Randelli E, Gerdol M, Pallavicini A, Facchiano A, Bernini C, Guerra L, Scapigliati G, Picchietti S. Immunoglobulin T from seabass (Dicentrarchus labrax L.): molecular characterization, tissue localization and expression after nodavirus infection. BMC Molecular Biology. 2017;18:8. doi: 10.1186/s12867-017-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2010).Cai D, Qiu Y, Qi N, Yan R, Lin M, Nie D, Zhang J, Hu Y. Characterization of Wuhan nodavirus subgenomic RNA3 and the RNAi inhibition property of its encoded protein B2. Virus Research. 2010;151:153–161. doi: 10.1016/j.virusres.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Chaivisuthangkura, Longyant & Sithigorngul (2014).Chaivisuthangkura P, Longyant S, Sithigorngul P. Immunological-based assays for specific detection of shrimp viruses. World Journal of Virology. 2014;3:1–10. doi: 10.5501/wjv.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves-Pozo et al. (2012).Chaves-Pozo E, Guardiola FA, Mesequer J, Esteban MA, Cuesta A. Nodavirus infection induces a great innate cell-mediated cytotoxic activity in resistant, gilthead seabream, and susceptible, European seabass, teleost fish. Fish & Shellfish Immunology. 2012;33:1159–1166. doi: 10.1016/j.fsi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen HY, Liu W, Wu SY, Chiou PP, Li YH, Chen YC, Lin GH, Lu MW, Wu JL. RIG-I specifically mediates group II type I IFN activation in nervous necrosis virus infected zebrafish cells. Fish & Shellfish Immunology. 2015;43:427–435. doi: 10.1016/j.fsi.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2014).Chen YM, Kuo CE, Chen GR, Kao YT, Zou J, Secombes CJ, Chen TY. Functional analysis of an orange-spotted grouper (Epinephelus coioides) interferon gene and characterization of its expression in response to nodavirus infection. Developmental and Comparative Immunology. 2014;46:117–128. doi: 10.1016/j.dci.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2010).Chen YM, Kuo CE, Wang TY, Shie PS, Wang WC, Huang SL, Tsai TJ, Chen PP, Chen JC, Chen TY. Cloning of an orange-spotted grouper Epinephelus coioides heat shock protein 90AB (HSP90AB) and characterization of its expression in response to nodavirus. Fish & Shellfish Immunology. 2010;28:895–904. doi: 10.1016/j.fsi.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Chen, Wang & Chen (2014).Chen YM, Wang TY, Chen TY. Immunity to betanodavirus infections of marine fish. Developmental and Comparative Immunology. 2014;43:174–183. doi: 10.1016/j.dci.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2006).Chen Y, Xiong X, Liu X, Li J, Wen Y, Chen Y, Dai Q, Cao Z, Yu W. Immunoreactivity of HCV/HBV epitopes displayed in an epitope-presenting system. Molecular Immunology. 2006;43:436–442. doi: 10.1016/j.molimm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Chi, Hu & Lo (1999).Chi S, Hu W, Lo B. Establishment and characterization of a continuous cell line (GF-1) derived from grouper, Epinephelus coioides (Hamilton): a cell line susceptible to grouper nervous necrosis virus (GNNV) Journal of Fish Diseases. 1999;22:173–182. doi: 10.1046/j.1365-2761.1999.00152.x. [DOI] [Google Scholar]
- Chi, Shieh & Lin (2003).Chi SC, Shieh JR, Lin SJ. Genetic and antigenic analysis of betanodaviruses isolated from aquatic organisms in Taiwan. Diseases of Aquatic Organisms. 2003;55:221–228. doi: 10.3354/dao055221. [DOI] [PubMed] [Google Scholar]
- Chia et al. (2010).Chia TJ, Wu YC, Chen JY, Chi SC. Antimicrobial peptides (AMP) with antiviral activity against fish nodavirus. Fish & Shellfish Immunology. 2010;28:434–439. doi: 10.1016/j.fsi2009.11.020. [DOI] [PubMed] [Google Scholar]
- Coeurdacier, Laporte & Pepin (2003).Coeurdacier JL, Laporte F, Pepin JF. Preliminary approach to find synthetic peptides from nodavirus capsid potentially protective against seabass viral encephalopathy and retinopathy. Fish & Shellfish Immunology. 2003;14(5):435–447. doi: 10.1006/fsim.2002.0449. [DOI] [PubMed] [Google Scholar]
- Comps, Pepin & Bonami (1994).Comps M, Pepin JF, Bonami JR. Purification and characterization of two fish encephalitis viruses (FEV) infecting Lates calcarifer and Dicentrarchus labrax. Aquaculture. 1994;123:1–10. doi: 10.1016/0044-8486(94)90114-7. [DOI] [Google Scholar]
- Conceição Neto et al. (2015).Conceição-Neto N, Zeller M, Heylen E, Lefrère H, Mesquita JR, Matthijnssens J. Fecal virome analysis of three carnivores reveals a novel nodavirus and multiple gemycircularviruses. Virology Journal. 2015;12 doi: 10.1186/s12985-015-0305-5. Article 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa & Thompson (2016).Costa JZ, Thompson KD. Understanding the interaction between Betanodavirus and its host for the development of prophylactic measures for viral encephalopathy and retinopathy. Fish & Shellfish Immunology. 2016;53:35–49. doi: 10.1016/j.fsi.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Dearing et al. (1980).Dearing SC, Scotti PD, Wigley PJ, Dhana SD. A small RNA virus isolated from the grass grub, Costelytra zealandica (Coleoptera: Scarabaeidae) New Zealand Journal of Zoology. 1980;7:267–269. doi: 10.1080/03014223.1980.10423785. [DOI] [Google Scholar]
- Doan et al. (2017).Doan QK, Vandeputte M, Chatain B, Morin T, Allal F. Viral encephalopathy and retinopathy in aquaculture: a review. Journal of Fish Diseases. 2017;40:717–742. doi: 10.1111/jfd.12541. [DOI] [PubMed] [Google Scholar]
- Esteban et al. (2013).Esteban MA, Chaves-Pozo E, Arizcun M, Meseguer J, Cuesta A. Regulation of natural killer enhancing factor (NKEF) genes in teleost fish, gilthead seabream and European seabass. Molecular Immunology. 2013;55:275–282. doi: 10.1016/j.molimm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Farook et al. (2014a).Farook MA, Madan N, Taju G, Majeed SA, Nambi KS, Raj NS, Vimal S, Hameed AS. Production of recombinant capsid protein of Macrobrachium rosenbergii nodavirus (r-MCP43) of giant freshwater prawn, M. rosenbergii (de Man) for immunological diagnostic methods. Journal of Fish Diseases. 2014a;37:703–710. doi: 10.1111/jfd.12156. [DOI] [PubMed] [Google Scholar]
- Farook et al. (2014b).Farook MA, Sundar Raj N, Madan N, Vimal S, Abdul Majeed S, Taju G, Rajkumar T, Santhoshkumar S, Sivakumar S, Sahul Hameed AS. Immunomodulatory effect of recombinant Macrobrachium rosenbergii nodavirus capsid protein (r-MCP) against white tail disease of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879) Aquaculture. 2014b;433:395–403. doi: 10.1016/j.aquaculture.2014.07.004. [DOI] [Google Scholar]
- Fenner et al. (2006).Fenner BJ, Thiagarajan R, Chua HK, Kwang J. Betanodavirus B2 is an RNA interference antagonist that facilitates intracelluar viral RNA accumulation. Journal of Virology. 2006;80:85–94. doi: 10.1128/JVI.00296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs, Rodger & Peric (1996).Frerichs G, Rodger HD, Peric Z. Cell culture isolation of piscine neuropathy nodavirus from juvenile seabass, Dicentrarchus labrax. Journal of General Virology. 1996;77:2067–2071. doi: 10.1099/0022-1317-77-9-2067. [DOI] [PubMed] [Google Scholar]
- Friesen & Rueckert (1982).Friesen PD, Rueckert RR. Black beetle virus: messenger for protein B is a subgenomic viral RNA. Journal of Virology. 1982;42:986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen et al. (1980).Friesen P, Scotti P, Longworth J, Rueckert R. Black beetle virus: propagation in Drosophila line 1 cells and an infection-resistant subline carrying endogenous black beetle virus-related particles. Journal of Virology. 1980;35:741–747. doi: 10.1128/jvi.35.3.741-747.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi et al. (2016).Ghiasi M, Binaii M, Ghasemi M, Fazli H, Zorriehzahra MJ. Haemato-biochemical disorders associated with nodavirus like-agent in adult leaping mullet Liza saliens (Risso, 1810) in the Caspian Sea. VirusDisease. 2016;27:12–18. doi: 10.1007/s13337-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh et al. (2014).Goh ZH, Mohd NA, Tan SG, Bhassu S, Tan WS. RNA-binding region of Macrobrachium rosenbergii nodavirus capsid protein. Journal of General Virology. 2014;95:1919–1928. doi: 10.1099/vir.0.064014-0. [DOI] [PubMed] [Google Scholar]
- Goh et al. (2011).Goh ZH, Tan SG, Bhassu S, Tan WS. Virus-like particles of Macrobrachium rosenbergii nodavirus produced in bacteria. Journal of Virological Methods. 2011;175:74–79. doi: 10.1016/j.jviromet.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Greninger & DeRisi (2015).Greninger AL, DeRisi JL. Draft genome sequences of ciliovirus and brinovirus from San Francisco wastewater. Genome Announcements. 2015;3:e00651–15. doi: 10.1128/genomeA.00651-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino et al. (1984).Guarino LA, Ghosh A, Dasmahapatra B, Dasgupta R, Kaesberg P. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology. 1984;139:199–203. doi: 10.1016/0042-6822(84)90342-8. [DOI] [PubMed] [Google Scholar]
- Hanapi et al. (2017).Hanapi UF, Yong CY, Goh ZH, Alitheen NB, Yeap SK, Tan WS. Tracking the virus-like particles of Macrobrachium rosenbergii nodavirus in insect cells. PeerJ. 2017;5:e2947. doi: 10.7717/peerj.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi et al. (2012).Hashemi H, Pouyanfard S, Bandehpour M, Noroozbabaei Z, Kazemi B, Saelens X, Mokhtari-Azad T. Immunization with M2e-displaying T7 bacteriophage nanoparticles protects against influenza A virus challenge. PLOS ONE. 2012;7:e45765. doi: 10.1371/journal.pone.0045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakijkosol & Owens (2012).Hayakijkosol O, Owens L. B2 or not B2: RNA interference reduces Macrobrachium rosenbergii nodavirus replication in redclaw crayfish (cherax quadricarinatus) Aquaculture. 2012;326–329:40–45. doi: 10.1016/j.aquaculture.2011.11.023. [DOI] [Google Scholar]
- Hegde, Lam & Sin (2005).Hegde A, Lam TJ, Sin YM. Immune response of freshwater fish, guppy, Poicelia reticulate and gouramy, Trichogaster trichopterus to recombinant coat protein of Epinephelus tauvina nervous necrosis virus. Aquaculture. 2005;249:77–84. doi: 10.1016/j.aquaculture.2005.04.053. [DOI] [Google Scholar]
- Hegde et al. (2003).Hegde A, Teh HC, Lam TJ, Sin YM. Nodavirus infection in freshwater ornamental fish, guppy, Poicelia reticulate—comparative characterization and pathogenicity studies. Archives of Virology. 2003;148:575–586. doi: 10.1007/s00705-002-0936-x. [DOI] [PubMed] [Google Scholar]
- Ho et al. (2017).Ho KL, Kueh CL, Beh PL, Tan WS, Bhella D. Cryo-electron microscopy structure of the Macrobrachium rosenbergii nodavirus capsid at 7 Angstroms resolution. Scientific Reports. 2017;7:2083. doi: 10.1038/s41598-017-02292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong (2013).Hong JR. Betanodavirus: mitochondrial disruption and necrotic cell death. World Journal of Virology. 2013;2:1–5. doi: 10.5501/wjv.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosur et al. (1984).Hosur MV, Schmidt T, Tucker RC, Johnson JE, Selling BH, Rueckert RR. Black beetle virus—crystallization and particle symmetry. Virology. 1984;133:119–127. doi: 10.1016/0042-6822(84)90430-6. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2013).Huang Y, Huang X, Cai J, Wei S, Ouyang Z, Qin Q. Molecular cloning, expression and functional analysis of ISG15 in orange-spotted grouper, Epinephelus coioides. Fish & Shellfish Immunology. 2013;34:1094–1102. doi: 10.1016/j.fsi.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2016a).Huang Y, Yang M, Yu Y, Yang Y, Zhou L, Huang X, Qin Q. Grouper TRIM13 exerts negative regulation of antiviral immune response against nodavirus. Fish & Shellfish Immunology. 2016a;55:106–115. doi: 10.1016/j.fsi.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2016b).Huang Y, Yu Y, Yang Y, Yang M, Zhou L, Huang X, Qin Q. Antiviral function of grouper MDA5 against iridovirus and nodavirus. Fish & Shellfish Immunology. 2016b;54:188–196. doi: 10.1016/j.fsi.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Húsgağ et al. (2001).Húsgağ S, Grotmol S, Hjeltnes BK, Rødseth OM, Biering E. Immune response to a recombinant capsid protein of striped jack nervous necrosis virus (SJNNV) in turbot Scophthalmus maximus and Atlantic halibut Hippoglossus hippoglossus, and evalution of a vaccine against SJNNV. Diseases of Aquatic Organisms. 2001;45:33–44. doi: 10.3354/dao045033. [DOI] [PubMed] [Google Scholar]
- Ibañez et al. (2013).Ibañez LI, Roose K, De Filette M, Schotsaert M, De Sloovere J, Roels S, Pollard C, Schepens B, Grooten J, Fiers W, Saelens X. M2e-displaying virus-like particles with associated RNA promote T helper 1 type adaptive immunity against influenza A. PLOS ONE. 2013;8:e59081. doi: 10.1371/journal.pone.0059081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariyapong (2015).Jariyapong P. Nodavirus-based biological container for targeted delivery system. Artificial Cells, Nanomedicine, and Biotechnology. 2015;43:355–360. doi: 10.3109/21691401.2014.889702. [DOI] [PubMed] [Google Scholar]
- Jariyapong et al. (2014).Jariyapong P, Chotwiwatthanakun C, Somrit M, Jitrapakdee S, Xing L, Cheng HR, Weerachatyanukul W. Encapsulation and delivery of plasmid DNA by virus-like nanoparticles engineered from Macrobrachium rosenbergii nodavirus. Virus Research. 2014;179:140–146. doi: 10.1016/j.virusres.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Kai & Chi (2008).Kai YH, Chi SC. Efficacies of inactivated vaccines against betanodavirus in grouper larvae (Epinephelus coioides) by bath immunization. Vaccine. 2008;26:1450–1457. doi: 10.1016/j.vaccine.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Kai, Wu & Chi (2014).Kai YH, Wu YC, Chi SC. Immune gene expressions in grouper larvae (Epinephelus coioides) induced by bath and oral vaccinations with inactivated betanodavirus. Fish & Shellfish Immunology. 2014;40:563–569. doi: 10.1016/j.fsi.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Keawcharoen et al. (2015).Keawcharoen J, Techangamsuwan S, Ponpornpisit A, Lombardini ED, Patchimasiri T, Pirarat N. Genetic characterization of a betanodavirus isolated from a clinical disease outbreak in farm-raised tilapia Oreochromis niloticus (L.) in Thailand. Journal of Fish Diseases. 2015;38:49–54. doi: 10.1111/jfd.12200. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2013).Kim HJ, Lee JY, Kang HA, Lee Y, Park EJ, Kim HJ. Oral immunization with whole yeast producing viral capsid antigen provokes a stronger humoral immune response than purified viral capsid antigen. Letters in Applied Microbiology. 2013;58:285–291. doi: 10.1111/lam.12188. [DOI] [PubMed] [Google Scholar]
- Kitamura et al. (in press).Kitamura SI, Akizuki M, Song JY, Nakayama K. Tributyltin exposure increases mortality of nodavirus infected Japanese medaka Oryzias latipes larvae. Marine Pollution Bulletin. 2017 doi: 10.1016/j.marpolbul.2017.02.020. In Press. [DOI] [PubMed] [Google Scholar]
- Kocan, Hershberger & Elder (2001).Kocan RM, Hershberger PK, Elder NE. Survival of the North American strain of viral hemorrhagic septicemia virus (VHSV) in filtered seawater and seawater containing ovarian fluid, crude oil and serum-enriched culture medium. Diseases of Aquatic. 2001;44:75–78. doi: 10.3354/dao044075. [DOI] [PubMed] [Google Scholar]
- Kok et al. (2002).Kok WL, Yusoff K, Nathan S, Tan WS. Cloning, expression and display of the PreS domain of hepatitis B virus on filamentous bacteriophage M13. Journal of Biochemistry, Molecular Biology, and Biophysics. 2002;6:55–58. doi: 10.1080/10258140290010241. [DOI] [PubMed] [Google Scholar]
- Kopek et al. (2010).Kopek BG, Settles EW, Friesen PD, Ahlquist P. Nodavirus-induced membrane rearrangement in replication complex assembly requires replicase protein a, RNA templates, and polymerase activity. Journal of Virology. 2010;84:12492–12503. doi: 10.1128/JVI.01495-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh et al. (2017).Kueh CL, Yong CY, Masoomi Dezfooli S, Bhassu S, Tan SG, Tan WS. Virus-like particle of Macrobrachium rosenbergii nodavirus produced in Spodoptera frugiperda (Sf9) cells is distinctive from that produced in Escherichia coli. Biotechnology Progress. 2017;33:549–557. doi: 10.1002/btpr.2409. [DOI] [PubMed] [Google Scholar]
- Kuo et al. (2012).Kuo HC, Wang TY, Hsu HH, Lee SH, Chen YM, Tsai TJ, Ou MC, Ku HT, Lee GB, Chen TY. An automated microfluidic chip system for detection of piscine nodavirus and characterization of its potential carrier in grouper farms. PLOS ONE. 2012;7:e42203. doi: 10.1371/journal.pone.0042203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, Reid & Whalen (2015).Lawrence S, Reid J, Whalen M. Secretion of interferon gamma from human immune cells is altered by exposure to tributyltin and dibutyltin. Environmental Toxicology. 2015;30:559–571. doi: 10.1002/tox.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2007).Lin CC, Lin JHY, Chen MS, Yang HL. An oral nervous necrosis virus vaccine that induces protective immunity in larvae of grouper (Epinephelus coioides) Aquaculture. 2007;268:265–273. doi: 10.1016/j.aquaculture.2007.04.066. [DOI] [Google Scholar]
- Lin et al. (2014).Lin F, Liu L, Hao GJ, Cao Z, Sheng PC, Wu YL, Shen JY. Rapid detection of Macrobrachium rosenbergii nodavirus isolated in China by a reverse-transcription loop-mediated isothermal amplification assay combined with a lateral flow dipstick method. Bing Du Xue Bao. 2014;30:502–507. [PubMed] [Google Scholar]
- Lingel et al. (2005).Lingel A, Simon B, Izaurralde E, Sattler M. The structure of the flock house virus B2 protein, a viral suppressor of RNA interference shows a novel mode of double-stranded RNA recognition. EMBO Reports. 2005;6:1149–1155. doi: 10.1038/sj.embor.7400583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2006a).Liu C, Zhang J, Yi F, Wang J, Wang X, Jiang H, Xu J, Hu Y. Isolation and RNA1 nucleotide sequence determination of a new insect nodavirus from Pieris rapae larvae in Wuhan city, China. Virus Research. 2006a;120:28–35. doi: 10.1016/j.virusres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2006b).Liu W, Hsu CH, Chang CY, Chen HH, Lin CS. Immune response against grouper nervous necrosis virus by vaccination of virus-like particles. Vaccine. 2006b;24:6282–6287. doi: 10.1016/j.vaccine.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz et al. (2012).Lopez-Munoz A, Sepulcre MP, Garcia-Moreno D, Fuentes I, Bejar J, Manchado M, Alvarez MC, Meseguer J, Mulero V. Viral nervous necrosis virus persistently replicates in the central nervous system of asymptomatic gilthead seabream and promotes a transient inflammatory response followed by the infiltration of IgM+ B lymphocytes. Developmental and Comparative Immunology. 2012;37:429–437. doi: 10.1016/j.dci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Low et al. (in press).Low CF, Syarul Nataqain B, Chee HY, Rozaini MZH, Najiah M. Betanodavirus: dissection of the viral life cycle. Journal of Fish Diseases. 2017 doi: 10.1111/jfd.12638. In Press. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2014).Luo YC, Wang CH, Wu YM, Liu W, Lu MW, Lin CS. Crystallization and X-ray diffraction of virus-like particles from a piscine betanodavirus. Acta Crystallographica Section F Structural Biology and Crystallization Communications. 2014;70:1080–1086. doi: 10.1107/S2053230X14013703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manayani et al. (2007).Manayani DJ, Thomas D, Dryden KA, Reddy V, Siladi ME, Marlett JM, Rainey GJ, Pique ME, Scobie HM, Yeager M, Young JA, Manchester M, Schneemann A. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLOS Pathogens. 2007;3:1422–1431. doi: 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic et al. (2011).Matic S, Rinaldi R, Masenga V, Noris E. Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in Nicotiana benthamiana plants. BMC Biotechnology. 2011;11:106. doi: 10.1186/1472-6750-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Schwartz & Ahlquist (2001).Miller DJ, Schwartz MD, Ahlquist P. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. Journal of Virology. 2001;75:11664–11676. doi: 10.1128/JVI.75.23.11664-11676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori et al. (1992).Mori K, Nakai T, Muroga K, Arimoto M, Mushiake K, Furusawa I. Properties of a new virus belonging to Nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology. 1992;187:368–371. doi: 10.1016/0042-6822(92)90329-N. [DOI] [PubMed] [Google Scholar]
- Munday, Kwang & Moody (2002).Munday BL, Kwang J, Moody N. Betanodavirus infections of teleost fish: a review. Journal of Fish Diseases. 2002;25:127–142. doi: 10.1046/j.1365-2761.2002.00350.x. [DOI] [Google Scholar]
- Munday & Nakai (1997).Munday BL, Nakai T. Special topic review: nodaviruses as pathogens in larval and juvenile marine finfish. World Journal of Microbiology and Biotechnology. 1997;13:375–381. doi: 10.1023/A:1018516014782. [DOI] [Google Scholar]
- Murata et al. (2003).Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6753–6758. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray & Shiau (1999).Murray K, Shiau AL. The core antigen of hepatitis B virus as a carrier for immunogenic peptides. Biological Chemistry. 1999;380:277–283. doi: 10.1515/BC.1999.038. [DOI] [PubMed] [Google Scholar]
- Murwantoko et al. (2016).Murwantoko M, Bimantara A, Roosmanto R, Kawaichi M. Macrobrachium rosenbergii nodavirus infection in a giant freshwater prawn hatchery in Indonesia. SpringerPlus. 2016;5 doi: 10.1186/s40064-016-3127-z. Article 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveen Kumar, Karunasagar & Karunasagar (2013).Naveen Kumar S, Karunasagar I, Karunasagar I. Protection of Macrobrachium rosenbergii against white tail disease by oral administration of bacterial expressed and encapsulated double-stranded RNA. Fish & Shellfish Immunology. 2013;35:833–839. doi: 10.1016/j.fsi.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Naveen Kumar et al. (2013).Naveen Kumar S, Shekar M, Karunasagar I, Karunasagar I. Genetic analysis of RNA1 and RNA2 of Macrobrachium rosenbergii nodavirus (MrNV) isolated from India. Virus Research. 2013;173:377–385. doi: 10.1016/j.virusres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Ng et al. (2013).Ng TFF, Alvandi S, Varsani A, Burghart S, Breitbart M. Metagenomic identification of a nodavirus and a circular ssDNA virus in semi-purified viral nucleic acids from the hepatopancreas of healthy Farfantepenaeus duorarum shrimp. Diseases of Aquatic Organisms. 2013;105:237–242. doi: 10.3354/dao02628. [DOI] [PubMed] [Google Scholar]
- Nishi et al. (2016).Nishi S, Yamashita H, Kawato Y, Nakai T. Cell culture isolation of piscine nodavirus (betanodavirus) in fish-rearing seawater. Applied and Environmental Microbiology. 2016;82:2537–2544. doi: 10.1128/AEM.03834-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa et al. (1994).Nishizawa T, Mori K, Nakai T, Furusawa I, Muroga K. Polymerase chain reaction (PCR) amplification of RNA of striped jack nervous necrosis virus (SJNNV) Disease of Aquatic Organisms. 1994;18:103–107. doi: 10.3354/dao018103. [DOI] [Google Scholar]
- Ourth & Renis (1993).Ourth DD, Renis HE. Antiviral melanization reaction of Heliothis virescens hemolymph against DNA and RNA viruses in vitro. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1993;105:719–723. doi: 10.1016/0305-0491(93)90111-H. [DOI] [PubMed] [Google Scholar]
- Overgård et al. (2012).Overgård AC, Nerland AH, Fiksdal IU, Patel S. Atlantic halibut experimentally infected with nodavirus shows increased levels of T-cell marker and IFNγ transcripts. Developmental and Compartive Immunology. 2012;37:139–150. doi: 10.1016/j.dci.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Pakingking Jr et al. (2010).Pakingking Jr R, Bautista NB, Jesus-Ayson EG, Reyes O. Protective immunity against viral nervous necrosis (VNN) in brown-marbled grouper (Epinephelus fuscogutattus) following vaccination with inactivated betanodavirus. Fish & Shellfish Immunology. 2010;28:525–533. doi: 10.1016/j.fsi.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Pakingking Jr et al. (2005).Pakingking Jr R, Mori KI, Sugaya T, Oka M, Okinaka Y, Nakai T. Aquabirnavirus-induced protection of marine fish against piscine nodavirus infection. Fish Pathology. 2005;40:125–131. doi: 10.3147/jsfp.40.125. [DOI] [Google Scholar]
- Pakingking Jr et al. (2009).Pakingking Jr R, Seron R, Dela Peña L, Mori K, Yamashita H, Nakai T. Immune responses of Asian seabass, Lates calcarifer Bloch, against an inactivated betanodavirus vaccine. Journal of Fish Diseases. 2009;32:457–463. doi: 10.1111/j.1365-2761.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- Parameswaran et al. (2008).Parameswaran V, Kumar SR, Ahmed VPI, Sahul Hameed AS. A fish nodavirus associated with mass mortality in hatchery-reared Asian Seabass, Lates calcarifer. Aquaculture. 2008;275:366–369. doi: 10.1016/j.aquaculture.2008.01.023. [DOI] [Google Scholar]
- Peng, Dai & Chen (2005).Peng M, Dai CB, Chen YD. Expression and immunoreactivity of an epitope of HCV in a foreign epitope presenting system. World Journal of Gastroenterology. 2005;11:3363–3367. doi: 10.3748/wjg.v11.i22.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzon et al. (2016).Piazzon MC, Galindo-Villegas J, Pereiro P, Estensoro I, Calduch-Giner JA, Gomez-Casado E, Novoa B, Mulero V, Sitja-Bobadilla A, Perez-Sanchez J. Differential modulation of IgT and IgM upon parasitic, bacterial, viral, and dietary challenges in a perciform fish. Frontiers in Immunology. 2016;7 doi: 10.3389/fimmu.2016.00637. Article 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisa-Beiro et al. (2008).Poisa-Beiro L, Dios S, Montes A, Aranguren R, Figueras A, Novoa B. Nodavirus increases the expression of Mx and inflammatory cytokines in fish brain. Molecular Immunology. 2008;45:218–225. doi: 10.1016/j.molimm.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Popham et al. (2004).Popham HJ, Shelby KS, Brandt SL, Coudron TA. Potent virucidal activity in larval Heliothis virescens plasma against Helicoverpa zea single capsid nucleopolyhedrovirus. Journal of General Virology. 2004;85:2255–2261. doi: 10.1099/vir.0.79965-0. [DOI] [PubMed] [Google Scholar]
- Price, Rueckert & Ahlquist (1996).Price BD, Rueckert RR, Ahlquist P. Complete replication of an animal virus and maintenance of expression vectors derived from it in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9465–9470. doi: 10.1073/pnas.93.18.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian et al. (2003).Qian D, Shi Z, Zhang S, Cao Z, Liu W, Li L, Xie Y, Cambournac I, Bonami JR. Extra small virus-like particles (XSV) and nodavirus associated with whitish muscle disease in the giant freshwater prawn, Macrobrachium rosenbergii. Journal of Fish Diseases. 2003;26:521–527. doi: 10.1046/j.1365-2761.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- Quan et al. (2008).Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. Journal of Virology. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya et al. (2014).Ramya VL, Sharma R, Gireesh-Babu P, Patchala SR, Rather A, Nandanpawar PC, Eswaran S. Development of chitosan conjugated DNA vaccine against nodavirus in Macrobrachium rosenbergii (De Man, 1879) Journal of Fish Diseases. 2014;37:815–824. doi: 10.1111/jfd.12179. [DOI] [PubMed] [Google Scholar]
- Ravi et al. (2009).Ravi M, Nazeer Basha A, Sarathi M, Rosa Idalia HH, Sri Widada J, Bonami JR, Sahul Hameed AS. Studies on the occurrence of white tail disease (WTD) caused by MrNV and XSV in hatchery-reared post-larvae of Penaeus indicus and P. monodon. Aquaculture. 2009;292:117–120. doi: 10.1016/j.aquaculture.2009.03.051. [DOI] [Google Scholar]
- Ravi et al. (2010).Ravi M, Nazeer Basha A, Taju G, Ram Kumar R, Sahul Hameed AS. Clearance of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) and immunological changes in experimentally injected Macrobrachium rosenbergii. Fish & Shellfish Immunology. 2010;28:428–433. doi: 10.1016/j.fsi.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Reinganum, Bashiruddin & Cross (1985).Reinganum C, Bashiruddin JB, Cross GF. Boolarra virus: a member of the Nodaviridae isolated from Oncopera intricoides (Lepidoptera: Hepialidae) Intervirology. 1985;24:10–17. doi: 10.1159/000149613. [DOI] [PubMed] [Google Scholar]
- Reshi, Su & Hong (2014).Reshi ML, Su YC, Hong JR. RNA Viruses: ROS-mediated cell death. International Journal of Cell Biology. 2014;2014 doi: 10.1155/2014/467452. Article 467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler & Williams (2008).Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nature Reviews Immunology. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahul Hameed et al. (2004).Sahul Hameed AS, Yoganandhan K, Sri Widada J, Bonami JR. Experimental transmission and tissue tropism of Macrobrachium rosenbergii nodavirus (MrNV) and its associated extra small virus (XSV) Diseases of Aquatic Organisms. 2004;62:191–196. doi: 10.3354/dao062191. [DOI] [PubMed] [Google Scholar]
- Sarath Babu et al. (2013).Sarath Babu V, Abdul Majeed S, Nambi KS, Taju G, Madan N, Sundar Raj N, Sahul Hameed AS. Comparison of betanodavirus replication efficiency in ten Indian fish cell lines. Archieves of Virology. 2013;158:1367–1375. doi: 10.1007/s00705-013-1617-7. [DOI] [PubMed] [Google Scholar]
- Scapigliati et al. (2010).Scapigliati G, Buonocore F, Fandelli E, Casani D, Meloni S, Zarletti G, Tiberi M, Pietretti D, Boschi I, Manchado M, Martin-Antonio B, Jimenez-Cantizano R, Bobo G, Borghesan F, Lorenzen N, Einer-Jensen K, Adams S, Thompson K, Alonso C, Bejar J, Cano I, Borrego JJ, Alvarez MC. Cellular and molecular immune responses of the sea bass (Dicentrarchus labrax) experimentally infected with betanodavirus. Fish & Shellfish Immunology. 2010;28:303–311. doi: 10.1016/j.fsi.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Schuster et al. (2014).Schuster S, Zirkel F, Kurth A, Cleef KWR, Drosten C, Rij RP, Junglen S. A unique nodavirus with novel features: mosinovirus expresses two subgenomic RNAs, a capsid gene of unknown origin, and a suppressor of the antiviral RNA interference pathway. Journal of Virology. 2014;88:13447–13459. doi: 10.1128/JVI.02144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selling, Allison & Kaesberg (1990).Selling BH, Allison RF, Kaesberg P. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:434–438. doi: 10.1073/pnas.87.1.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selling & Rueckert (1984).Selling BH, Rueckert RR. Plaque assay for black beetle virus. Journal of Virology. 1984;51:251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapin et al. (2012).Senapin S, Jaengsanong C, Phiwsaiya K, Prasertsri S, Laisutisan K, Chuchird N, Limsuwan C, Flegel TW. Infections of MrNV (Macrobrachium rosenbergii nodavirus) in cultivated whiteleg shrimp Penaeus vannamei in Asia. Aquaculture. 2012;338–341:41–46. doi: 10.1016/j.aquaculture.2012.01.019. [DOI] [Google Scholar]
- Settles & Friesen (2008).Settles EW, Friesen PD. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. Journal of Virology. 2008;82:1378–1388. doi: 10.1128/JVI.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris et al. (2001).Skliris GP, Krondiris JV, Sideris DC, Shinn AP, Starkey WG, Richard RH. Phylogenetic and antigenic characterization of new fish nodavirus isolates from Europe and Asia. Virus Research. 2001;75:59–67. doi: 10.1016/S0168-1702(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Sommerset et al. (2003).Sommerset I, Lorenzen E, Lorenzen N, Bleie H. A DNA vaccine directed against a rainbow trout rhabdovirus induces early protection against a nodavirus challenge in turbot. Vaccine. 2003;21:4661–4667. doi: 10.1016/S0264-410X(03)00526-7. [DOI] [PubMed] [Google Scholar]
- Sommerset et al. (2005).Sommerset I, Skern R, Biering E, Bleie H, Fiksdal I, Grove S, Nerland AH. Protection against Atlantic halibut nodavirus in turbot is induced by recombinant capsid protein vaccination but not following DNA vaccination. Fish & Shellfish Immunology. 2005;18:13–29. doi: 10.1016/j.fsi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Somrit et al. (2017).Somrit M, Watthammawut A, Chotwiwatthanakun C, Ounjai P, Suntimanawong W, Weerachatyanukul W. C-terminal domain on the outer surface of the Macrobrachium rosenbergii nodavirus capsid is required for Sf9 cell binding and internalization. Virus Research. 2017;227:41–48. doi: 10.1016/j.virusres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Sri Widada et al. (2003).Sri Widada J, Durand S, Cambournac I, Qian D, Shi Z, Dejonghe E, Richard V, Bonami JR. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii dot-blot, in situ hybridization and RT-PCR. Journal of Fish Diseases. 2003;26:583–590. doi: 10.1046/j.1365-2761.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- Sudhakaran et al. (2007).Sudhakaran R, Ishaq Ahmed VP, Haribabu P, Mukherjee SC, Sri Widada J, Bonami JR, Sahul Hameed AS. Experimental vertical transmission of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) from brooders to progeny in Macrobrachium rosenbergii and artemia. Journal of Fish Diseases. 2007;30:27–35. doi: 10.1111/j.1365-2761.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Suebsing, Prombun & Kiatpathomchai (2013).Suebsing R, Prombun P, Kiatpathomchai W. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) combined with colorimetric gold nanoparticle (AuNP) probe assay for visual detection of Penaeus vannamei nodavirus (PvNV) Letters in Applied Microbiology. 2013;56:428–435. doi: 10.1111/lam.12065. [DOI] [PubMed] [Google Scholar]
- Takano et al. (2011).Takano T, Kondo H, Hirono I, Endo M, Saito-Taki T, Aoki T. Toll like receptors in teleosts. In: Bondad-Reantaso MG, Jones JB, Corsin F, Aoki T, editors. Diseases in asian aquaculture VII, vol. 2011. Selangor, Malaysia: Fish Health Section, Asian Fisheries Society; 2011. pp. 197–207. [Google Scholar]
- Tan et al. (2005).Tan GH, Yusoff K, Seow HF, Tan WS. Antigenicity and immunogenicity of the immunodominant region of hepatitis B surface antigen displayed on bacteriophage T7. Journal of Medical Virology. 2005;77:475–480. doi: 10.1002/jmv.20479. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2007).Tang KF, Pantoja CR, Redman RM, Lightner DV. Development of in situ hybridization and RT-PCR assay for the detection of a nodavirus (PvNV) that causes muscle necrosis in Penaeus vannamei. Diseases of Aquatic Organisms. 2007;75:183–190. doi: 10.3354/dao075183. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2011).Tang KF, Pantoja CR, Redman RM, Navarro SA, Lightner DV. Ultrastructural and sequence characterization of Penaeus vannamei nodavirus (PvNV) from Belize. Diseases of Aquatic Organisms. 2011;94:179–187. doi: 10.3354/dao02335. [DOI] [PubMed] [Google Scholar]
- Thiery et al. (2006).Thiery R, Cozien J, Cabon J, Lamour F, Baud M, Schneemann A. Induction of a protective immune response against viral nervous necrosis in the European seabass Dicentrarchus labrax by using betanodavirus virus-like particles. Journal of Virology. 2006;80:10201–10207. doi: 10.1128/JVI.01098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubanaki, Margaroni & Karagouni (2015a).Toubanaki DK, Margaroni M, Karagouni E. Development of a novel allele-specific PCR method for rapid assessment of nervous necrosis virus genotypes. Current Microbiology. 2015a;71:529–539. doi: 10.1007/s00284-015-0880-0. [DOI] [PubMed] [Google Scholar]
- Toubanaki, Margaroni & Karagouni (2015b).Toubanaki DK, Margaroni M, Karagouni E. Nanoparticle-based lateral flow biosensor for visual detection of fish nervous necrosis virus amplification products. Molecular and Cellular Probes. 2015b;29:158–166. doi: 10.1016/j.mcp.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Valero et al. (2015a).Valero Y, Arizcun M, Esteban MA, Bandin I, Olveira JG, Patel S, Cuesta A, Chaves-Pozo E. Nodavirus colonizes and replicates in the testis of gilthead seabream and European seabass modulating its immune and reproductive functions. PLOS ONE. 2015a;10:e0145131. doi: 10.1371/journal.pone.0145131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero et al. (2016a).Valero Y, Arizcun M, Esteban MA, Cuesta A, Chaves-Pozo E. Transcription of histones H1 and H2B in regulated by several immune stimuli in gilthead seabream and European seabass. Fish & Shellfish Immunology. 2016a;57:107–115. doi: 10.1016/j.fsi.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Valero et al. (2016b).Valero Y, Awad E, Buonocore F, Arizcun M, Esteban MA, Meseguer J, Chaves-Pozo E, Cuesta A. An oral chitosan DNA vaccine against nodavirus improves transcription of cell-mediated cytotoxicity and interferon genes in the European seabass juveniles gut and survival upon infection. Developmental and Comparative Immunology. 2016b;65:64–72. doi: 10.1016/j.dci.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Valero et al. (2015b).Valero Y, Garcia-Alcazar A, Esteban MA, Cuesta A, Chaves-Pozo E. Antimicrobial reponse is increased in the testis of European seabass, but not in gilthead seabream, upon nodavirus infection. Fish & Shellfish Immunology. 2015b;44:203–213. doi: 10.1016/j.fsi.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Venter & Schneemann (2008).Venter PA, Schneemann A. Recent insights into the biology and biomedical applications of Flock House virus. Cellular and Molecular Life Sciences. 2008;65:2675–2687. doi: 10.1007/s00018-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimal et al. (2016).Vimal S, Farook MA, Madan N, Abdul Majeed S, Nambi KSN, Taju G, Sundar raj N, Venu S, Subburaj R, Thirunavukkarasu AR, Sahul Hameed AS. Development, distribution and expression of a DNA vaccine against nodavirus in Asian Seabass, Lates calcarifier (Bloch, 1790) Aquaculture Research. 2016;47:1209–1220. doi: 10.1111/are.12578. [DOI] [Google Scholar]
- Vimal et al. (2014).Vimal S, Madan, Farook MA, Nambi KSN, Majeed SA, Rajkumar T, Venu S, Thirunavukkarasu AR, Hameed ASS. Production of recombinant vaccine using capsid gene of nodavirus to protect Asian seabass, Lates calcarifer (Bloch, 1790) Aquaculture. 2014;418-419:148–154. doi: 10.1016/j.aquaculture.2013.10.017. [DOI] [Google Scholar]
- Wan et al. (2001).Wan Y, Wu Y, Bian J, Wang XZ, Zhou W, Jia ZC, Tan Y, Zhou L. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine. 2001;19:2918–2923. doi: 10.1016/S0264-410X(00)00561-2. [DOI] [PubMed] [Google Scholar]
- Wang, Chang & Wen (2016).Wang CS, Chang CY, Wen CM. Developing immunological methods for detecting Macrobrachium rosenbergii nodavirus and extra small virus using a recombinant protein preparation. Journal of Fish Diseases. 2016;39:715–727. doi: 10.1111/jfd.12404. [DOI] [PubMed] [Google Scholar]
- Wang, Chen & Chen (2016).Wang TY, Chen YM, Chen TY. Molecular cloning of orange-spotted grouper (Epinephelus coioides) heat shock transcription factor 1 isoforms and characterization of their expressions in response to nodavirus. Fish & Shellfish Immunology. 2016;59:123–136. doi: 10.1016/j.fsi.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang W, Huang Y, Yu Y, Yang Y, Xu X, Chen X, Ni S, Qin Q, Huang X. Fish TRIM39 regulates cell cycle progression and exerts its antiviral function against iridovirus and nodavirus. Fish & Shellfish Immunology. 2016;50:1–10. doi: 10.1016/j.fsi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Wangman et al. (2012).Wangman P, Senapin S, Chaivisuthangkura P, Longyant S, Rukpratanporn S, Sithigorngul P. Production of monoclonal antibodies specific to Macrobrachium rosenbergii nodavirus using recombinant capsid protein. Diseases of Aquatic Organisms. 2012;98:121–131. doi: 10.3354/dao02431. [DOI] [PubMed] [Google Scholar]
- Xie, Wei & Qin (2016).Xie H, Wei J, Qin Q. Antiviral function of Tachyplesin I against iridovirus and nodavirus. Fish & Shellfish Immunology. 2016;58:96–102. doi: 10.1016/j.fsi.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang Y, Huang Y, Yu Y, Yang M, Zhou S, Qin Q, Huang X. RING domain is essential for the antiviral activity of TRIM25 from orange spotted grouper. Fish & Shellfish Immunology. 2016;55:304–314. doi: 10.1016/j.fsi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Yap et al. (2012).Yap WB, Tey BT, Alitheen NB, Tan WS. Display of the antigenic region of Nipah virus nucleocapsid protein on hepatitis B virus capsid. Journal of Bioscience and Bioengineering. 2012;113:26–29. doi: 10.1016/j.jbiosc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Yong et al. (2015a).Yong CY, Yeap SK, Goh ZH, Ho KL, Omar AR, Tan WS. Induction of humoral and cell-mediated immune responses by hepatitis B virus epitope displayed on the virus-like particles of prawn nodavirus. Applied and Environmental Microbiology. 2015a;81:882–889. doi: 10.1128/AEM.03695-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong et al. (2015b).Yong CY, Yeap SK, Ho KL, Omar AR, Tan WS. Potential recombinant vaccine against influenza A virus based on M2e displayed on nodaviral capsid nanoparticles. International Journal of Nanomedicine. 2015b;10:2751–2763. doi: 10.2147/IJN.S77405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikoshi & Inoue (1990).Yoshikoshi K, Inoue K. Viral nervous necrosis in hatchery-reared larvae and juveniles of Japanese parrotfish, Oplegathus fasciatus (Temminck & Schlegel) Journal of Fish Diseases. 1990;13:69–77. doi: 10.1111/j.1365-2761.1990.tb00758.x. [DOI] [Google Scholar]
- Yu et al. (2017).Yu Y, Huang X, Liu J, Zhang J, Hu Y, Yang Y, Huang Y, Qin Q. Fish TRIM32 functions as a critical antiviral molecule against iridovirus and nodavirus. Fish & Shellfish Immunology. 2017;60:33–43. doi: 10.1016/j.fsi.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2016).Yu Y, Huang Y, Yang Y, Wang S, Yang M, Huang X, Qin Q. Negative regulation of the antiviral response by grouper LGP2 against fish viruses. Fish & Shellfish Immunology. 2016;56:358–366. doi: 10.1016/j.fsi.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Yuasa et al. (2002).Yuasa K, Koesharyani I, Roza D, Mori K, Katata M, Nakai T. Immune response of humpback grouper, Cromileptes altivelis (Valenciennes) injected with the recombinant coat protein of betanodavirus. Journal of Fish Diseases. 2002;25:53–56. doi: 10.1046/j.1365-2761.2002.00325.x. [DOI] [Google Scholar]
- Zhang et al. (2014).Zhang Q, Liu Q, Liu S, Yang H, Liu S, Zhu L, Yang B, Jin J, Ding L, Wang X, Liang Y, Wang Q, Huang J. A new nodavirus is associated with covert mortality disease of shrimp. Journal of General Virology. 2014;95:2700–2709. doi: 10.1099/vir.0.070078-0. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (in press).Zhang Q, Liu S, Li J, Tian Y, Wang C, Li X, Xu T, Li J. Experimental vertical transmission of covert mortality nodavirus (CMNV) in Exopalaemon carinicauda. Journal of General Virology. 2017 doi: 10.1099/jgv.0.000731. In Press. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (in press).Zhang Q, Liu S, Yang H, Zhu L, Wan X, Li X, Huang J. Reverse transcription loop-mediated isothermal amplification for rapid and quantitative assay of covert mortality nodavirus in shrimp. Journal of Invertebrate Pathology. 2015 doi: 10.1016/j.jip.2015.09.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorriehzahra et al. (2014).Zorriehzahra MJ, Nazari A, Ghasemi M, Ghiasi M, Karsidani SH, Bovo G, Daud HHM. Vacuolating encephalopathy and retinopathy associated with a nodavirus-like agent: a probable cause of mass mortality of wild Golden grey mullet (Liza aurata) and Sharpnose grey mullet (Liza saliens) in Iranian waters of the Caspian Sea. VirusDisease. 2014;25:430–436. doi: 10.1007/s13337-014-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any data or code (literature review).