Abstract
Irritable bowel syndrome (IBS) affects 7–15% of the general population. A recently devised dietary approach consists of restricting foods with highly fermentable oligo-, di-, and monosaccharides, and polyols (FODMAPs), which can trigger and/or exacerbate IBS symptoms. The aim of this study is to use meta-analysis to provide an update on the randomised control trials (RCTs) and cohort studies, and examine them separately in relation to diet type. Papers were selected using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. Cohen’s d and odds ratios were used as a measure of effect size for RCTs. A random effects model was used to account for different sources of variation among studies. Heterogeneity was assessed using Q statistics, I2, Tau, and Tau2. Publication bias was analysed and represented by a funnel plot, and funnel plot symmetry was assessed with Egger’s test. The results showed that in the RCTs, the patients receiving a low-FODMAP diet experienced a statistically significant pain and bloating reduction compared with those receiving a traditional diet; as regards to stool consistency, there was no significant difference between treatments. A significant reduction in abdominal pain and bloating were described by patients receiving a low-FODMAP diet compared with those receiving a high-FODMAP diet. In cohort studies, pain and bloating were significantly reduced after treatment compared with the baseline diet. We conclude that there is evidence that a low-FODMAP diet could have a favourable impact on IBS symptoms, especially abdominal pain and bloating. However, it remains to be demonstrated whether a low-FODMAP diet is superior to conventional IBS diets, especially in the long term.
Keywords: irritable bowel syndrome, nutrition, meta-analysis, epidemiology
1. Introduction
Irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and colorectal cancer (CRC) are chronic intestinal conditions whose high incidence and prevalence make them major healthcare problems [1,2,3,4,5]. IBS affects 7–15% of the general population [4,5]. It is twice as frequent in women [6] and is diagnosed more often in patients less than 50 years of age [7]. It is characterised by recurrent episodes of functional gastrointestinal symptoms whose pathophysiological mechanisms are not completely clear [8]. The most common symptoms include abdominal pain, bloating, constipation, and/or diarrhoea [8]. IBS negatively impacts quality of life and causes a substantial burden on healthcare resources [9,10]. Like the clinical phenotypes, the pathophysiological mechanisms underlying the syndrome are heterogeneous and not fully understood [11]. However, there is evidence that IBS may result from a combination of gastrointestinal motility changes, visceral hypersensitivity, low-grade inflammation, altered microbiota, and food components [12,13,14,15]. Due to the diversity of IBS symptoms and their considerable variability over time, a wide range of pharmacological treatments are employed which often only target the primary symptom; thus, when multiple symptoms are present, the treatments administered are often inadequate. This has led to the investigation of use of dietary therapies as a treatment option. Food is therefore a central and constant issue for patients with IBS. Up to 70% of IBS patients associate symptom onset or exacerbation with certain foods [16,17,18,19]. However, avoiding foods such as dairy products, wheat, citrus fruit, caffeine, and alcohol often results in negligible symptom improvement [4,18,20]. Current dietary advice for IBS patients includes regularly scheduled meals, a reduction in fibre intake, elimination of lactose-containing foods, avoidance of trigger foods, which are most commonly dairy products, wheat, and fructose, avoidance of gas-producing foods such as beans, cabbage, and onions, and limitations on caffeine, alcohol, and fatty foods [18,21]. Elimination of lactose-containing foods is still highly controversial, as this is not required by all patients. Some IBS patients have shown good lactose tolerance. A recently devised dietary approach consists of restricting foods with highly fermentable oligo-, di-, and monosaccharides, as well as polyols (FODMAPs), which can trigger and/or exacerbate IBS symptoms [22,23]. FODMAPs are osmotically active short-chain carbohydrates (SCCs) that are poorly absorbed and rapidly fermented by gut bacteria [24,25,26]. Increased intraluminal water volume, due to osmotic activity and gas production from their fermentation, causes intestinal luminal distension and induces gastrointestinal symptoms in susceptible individuals [27]. Furthermore, FODMAPs also appear to be involved in symptom generation through direct and indirect effects on gut microbiota, gut barrier, immune response, and visceral sensation [28]. It has been reported that a low-FODMAP diet can have a positive impact on IBS symptoms [26,29,30,31,32].
The main mechanism of action of low-FODMAP diets is thought to be a reduction in small intestinal absorption of osmotically active SCCs, resulting in diminished intestinal water content and downstream effects on colonic fermentation and gas production [33,34]. Recent studies have reported that, compared to baseline, low-FODMAP diets reduce the serum levels of proinflammatory interleukins (ILs) IL-6 and IL-8, the levels of faecal bacteria (Actinobacteria, Bifidobacterium and Faecalibacterium prausnitzii), faecal total short-chain fatty acids (SCFAs), and n-butyric acid [35,36,37,38].
The response to a low-FODMAP diet may be associated with factors related to patient demographics, microbiome composition and metabolism, and IBS subtype; however, there are no large-scale studies of its predictors [27,39]. The ability to predict responses would not only enable the ability to streamline resources and improve clinical results, but also provide insights into pathogenic mechanisms.
A recent meta-analysis, including data up to March 2015 [40], completed randomised control trials (RCTs) stratified by outcome, but the study did not divide diets by FODMAP type. The meta-analysis in this study provides an update on the RCTs and cohort studies that have been published in the intervening period and examines them separately in relation to diet type. In particular, it compares: (i) low-FODMAP diets and traditional IBS diets in RCTs; (ii) low- and high-FODMAP diets in RCTs; and (iii) baseline and post-treatment data in cohort studies of patients receiving a low-FODMAP diet.
2. Materials and Methods
The papers to be included in the meta-analysis were sought in the MEDLINE, EMBASE, Scopus, Clinicaltrials.gov, Web of Science, and Cochrane Library databases in March 2017. The search terms used were: FODMAP OR FODMAPS OR fermentable, poorly absorbed, short-chain carbohydrates, OR fermentable oligosaccharides, disaccharides, monosaccharides and polyols and (FODMAP OR FODMAPs OR fermentable, poorly absorbed, short-chain carbohydrates, OR fermentable oligosaccharides, disaccharides, monosaccharides and polyols) AND (Irritable Bowel Syndromes OR Syndrome, Irritable Bowel OR Syndromes, Irritable Bowel) OR (Colon, Irritable OR Irritable Colon) OR (Colitis, Mucous OR Colitides, Mucous OR Mucous Colitides OR Mucous Colitis). Papers were selected using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1) and the PRISMA checklist (Table S1) [41].
Figure 1.
Flow chart search strategy.
A manual search of possible references of interest was also performed. Only studies published in English over the previous 10 years were considered. The papers were selected by three independent reviewers (P.M.A., V.D.N., and G.L.); a methodologist (E.A.) resolved any disagreements. The study included clinical investigations involving the effect of a FODMAP diet on IBS patients. In particular, we assessed RCTs comparing a low-FODMAP diet with a traditional IBS diet, and a low-FODMAP diet with a high-FODMAP diet; cohort studies examining the effect of a low-FODMAP diet, comparing baseline with the follow-up, were also included. Outcomes evaluated were abdominal pain and bloating, which were assessed in all three study types. Since stool consistency and frequency were evaluated in all RCTs comparing FODMAP and traditional diets, these outcomes were also included.
Bias was assessed using the Cochrane Collaboration tool for assessing risk of bias [42] and the Newcastle-Ottawa scale for cohort studies (Tables S2 and S3) [43].
Statistical Analysis
Cohen’s d, with 95% confidence interval (CI) and p-value, was used as a measure of effect size. Odds ratios (ORs), with 95% CI and p-value, were used as a measure of effect size for the RCTs.
Effect sizes were pooled across studies to obtain an overall effect size. A random effects model was used to account for different sources of variation among studies. Heterogeneity was assessed using Q statistics, I2, Tau, and Tau2. The stability of study findings was checked with moderator analysis.
Publication bias was analyzed and represented by a funnel plot, and funnel plot symmetry was assessed with Egger’s test [44]. Finally, publication bias was checked using the trim and fill procedure; we used Rosenthal’s estimator and fail-safe number to analyze publication bias [45]. PROMETA 3 software (IDo Statistics-Internovi, Cesena, Italy) was used.
3. Results
The search found 362 records in the databases and eight records through the manual search. After the removal of 132 duplicates there remained 238 papers; of these, 215 were excluded for different reasons (Figure 1). In the second phase of the PRISMA flow-chart, full-text articles were identified for eligibility; of these, 11 were excluded for the following reasons: three compared the FODMAP to a placebo [35], lactobacillus [46], or hypnotherapy [47], one considered healthy controls versus IBD patients [48]; one involved a paediatric population [39]; one administered the FODMAP to non-celiac gluten-sensitive patients [49]; one included interventions that only regarded two different types of rye bread (normal versus low-FODMAP rye bread) [31]; one study reviewed two different types of educational training [50]; one was a retrospective study [51]; one regarded fructose restriction [27]; and finally one did not include any outcomes of interest for our study [52]. This left six RCTs, of which three compared the traditional IBS diet to the low-FODMAP diet [29,30,36] and three compared the low- and high-FODMAP diets [25,26,53] (Table 1). Six cohort studies [54,55,56,57,58] compared patients’ conditions at baseline and after administration of the low-FODMAP diet (Table 2). In each meta-analysis, sensitivity analysis indicated that the meta-analytical findings were stable.
Table 1.
Characteristics of the included randomised control trial (RCT) studies in the meta-analysis.
| Patient Population | Year (Mean ± SD or Median) | % Female | Symptoms and Stool Characteristics | |||||||||||||
| Study, Year, Country | Duration of Follow-up |
Assessed
for Eligibility |
Randomised | Intervention/Control | Drop Outs | Low- FODMAP group |
Traditional IBS group |
p | Low- FODMAP group |
Traditional IBS group |
p | Abdom Pain * | Bloating | Stool Consistency | Stool Frequency | |
| Study group | Control group | |||||||||||||||
| RCT Low-FODMAP vs. Traditional IBS Diets | ||||||||||||||||
| Eswaran, 2016, USA [30] | 4 weeks | 171 | 92 | 50/42 | Study group n = 5 | Control group n = 3 | 41.6 ± 41.7 Low- FODMAP group |
43.8 ± 15.2 Traditional IBS group |
(p = 0.49) | 66.0 Low- FODMAP group |
76.2 Traditional IBS group |
(p = 0.35) | X | X | X | X |
| Böhn, 2015, Sweden [29] | 4 weeks | 84 | 75 | 38/37 | Study group n = 5 | Control group n = 3 | 44.0 Low- FODMAP group |
41.0 Traditional IBS group |
(p = 0.35) | 79.0 Low- FODMAP group |
84.0 Traditional IBS group |
(p = 0.59) | X | X | X | X |
| Staudacher, 2012, UK [36] | 4 weeks | 99 | 41 | 19/22 | Study group n = 3 | Control group n = 3 | 35.2 Low- FODMAP group |
35.0 Traditional IBS group |
(p = 0.94) | 63.0 Low- FODMAP group |
68.0 Traditional IBS group |
(p = 0.74) | X | X | X | X |
| RCT low-FODMAP vs. Medium/High FODMAP Diets | ||||||||||||||||
| McIntosh, 2016, Canada [53] | 3 weeks | 37 | 40 | 20/20 | Study group n = 2 |
Control group n = 1 | 50.2 Low- FODMAP group |
51.4 High- FODMAP |
(p = NS) ** | 83.3 Low- FODMAP group |
89.4 High- FODMAP |
(p = NS) * | X | X | - | - |
| Halmos, 2014, Australia [26] | 3 weeks | 45 | 30 | 15/15 | IBS group n = 7 | Healthy subject group n = 8 | 41.0 IBS group |
31.0 Healthy subject group | (p = NS) ** | 70.0 IBS group |
75.0 Healthy subject group |
(p = NS) ** | X | X | - | - |
| Ong, 2010, Australia [25] | 11 days | 15 | 15 | 15/15 | Not Reported | 50.2 Low- FODMAP group |
51.4 High- FODMAP |
(p = NS) ** | 83.3 Low- FODMAP group |
89.4 High- FODMAP |
(p = NS) ** | X | X | - | - | |
SD: Standard deviation, RTC: Randomized Controlled Trials, X = symptoms assessed; - = symptoms not assessed, FODMAP: Food with Highly Fermentable Oligo, Di- and Monosaccharides and Polyols IBS: Irritable Bowel Syndrome * Abdom. Pain = abdominal pain; ** NS = not significant (as reported in the included studies).
Table 2.
Characteristics of the included cohort studies in the meta-analysis.
| Study, Year, Country | Duration of Follow-Up | Assessed for Eligibility | Completed Study (No. of Patients) | Lost at Follow-Up | Years (Mean) | % Female | Symptoms and Stool Characteristics | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal Pain | Bloating | Stool Consistency | Stool Frequency | |||||||
| Valeur, 2016, Norway [34] | 4 weeks | 97 | 63 | 34 | 38.4 | 88.9 | X | X | - | - |
| De Roest, 2013, New Zeland [54] | 15 months | 192 | 90 | 102 | 47.0 | 84.4 | X | X | - | - |
| Huaman, 2015, Spain [55] | 2 months | 30 | 24 | 6 | 40.0 | 79.0 | X | X | - | - |
| Pérez y López, 2015, Mexico [56] | 3 weeks | Not reported | 31 | 0 | 46.4 | 87.0 | X | X | - | - |
| Mazzawi, 2013, Norway [57] | 3–9 months | Not reported | 46 | 0 | 35.0 | 76.0 | X | - | - | - |
| Staudacher, 2011, UK [58] | 9 months | Not reported | 43 | 0 | 37.8 | 65.0 | X | X | - | - |
X = symptoms assessed; - = symptoms not assessed.
3.1. Low-FODMAP Diet Versus Traditional IBS Diet
The primary studies (k = 3 RCTs) by Bohn [29], Eswaran [30], and Staudacher [36] compared groups of IBS patients receiving a low-FODMAP diet to those receiving a traditional diet. These studies examined four outcomes: reduction of abdominal pain, reduction of abdominal bloating, increase of stool consistency, and reduction of stool frequency. Their main features are reported in Table 1.
3.1.1. Abdominal Pain
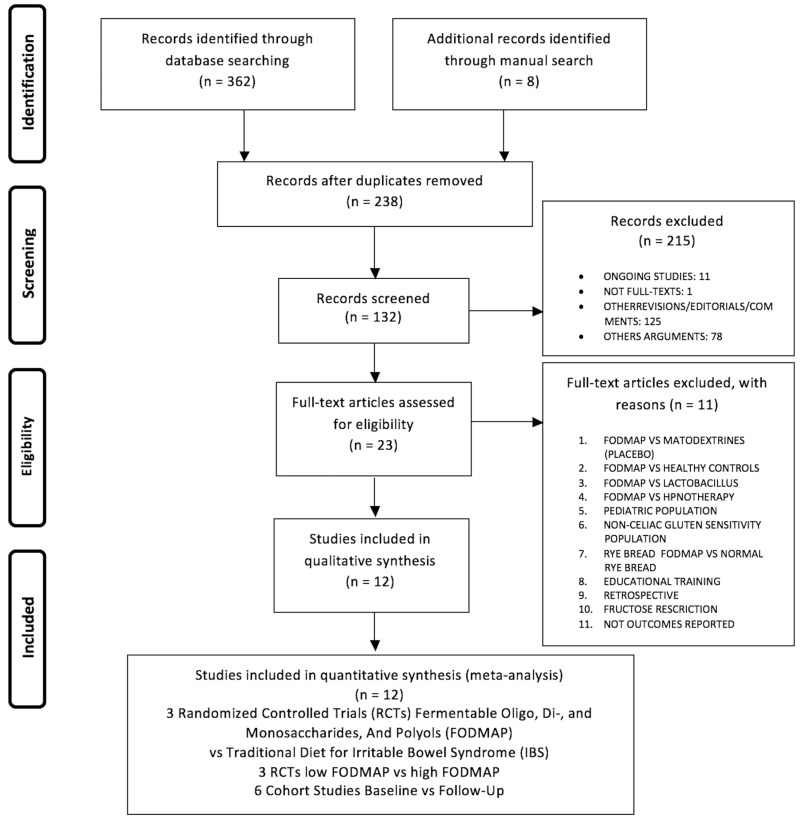
The present meta-analysis demonstrates that the patients receiving a low-FODMAP diet experienced a statistically significant pain reduction compared to those receiving a traditional diet. The overall effect size was odds ratio (OR) = 0.44 (Table 3); there was no statistical heterogeneity (Table 3, Figure 2A). Publication bias analysis did not highlight any differences between observed and estimated values (zero trimmed studies) (Figure 2B). Egger’s test was not statistically significant (Table 3).
Table 3.
Meta-analysis results.
| Pooled Analysis | Heterogeneity | Publication Bias | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Effect Size | CI | p Value | Q | I2 | p Value | T2 | T | Egger’s | Begg and Mazdumdar’s | Fail-Safe | Rosenthal | ||
| T | p Value | Z | p Value | No. | No. | |||||||||
| RCTs Low-FODMAP vs. Traditional IBS Diet (k = 3) [29,30,36] | ||||||||||||||
| Abdominal Pain | 0.44 (OR) | (0.26; 0.79) | 0.006 | 2.43 | 17.81 | 0.296 | 0.05 | 0.23 | −0.19 | 0.877 | 0.52 | 0.602 | 4 | 25 |
| Bloating | 0.32 (OR) | (0.15; 0.66) | <0.0001 | 1.97 | 0.00 | 0.374 | 0.00 | 0.00 | −1.21 | 0.439 | −0.52 | 0.602 | 11 | 25 |
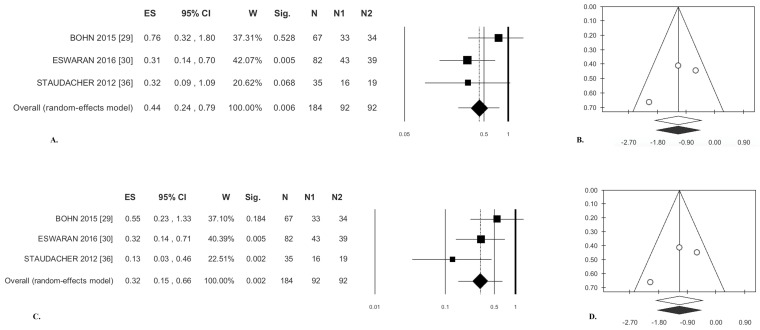
| Stool Consistency | 0.24 * | (−0.13; 0.61) | 0.209 | 3.07 | 34.84 | 0.216 | 0.04 | 0.19 | −0.02 | 0.989 | −0.52 | 0.602 | 0 | 25 |
| Stool Frequency | −0.54 * | (−0.83; −0.24) | <0.0001 | 1.67 | 0.00 | 0.434 | 0.00 | 0.00 | −5.74 | 0.110 | −1.57 | 0.117 | 8 | 25 |
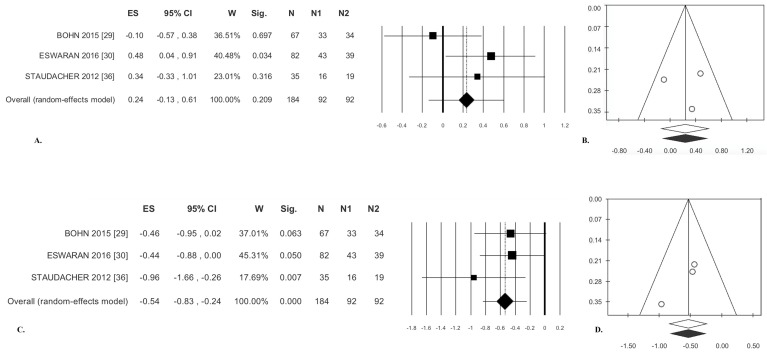
| RCTs Low-FODMAP vs. Medium/High FODMAP (k = 3) [25,26,53] | ||||||||||||||
| Abdominal Pain | 0.17 (OR) | (0.08; 0.34) | <0.0001 | 1.14 | 0.00 | 0.567 | 0.00 | 0.00 | −4.69 | 0.150 | −1.54 | 0.018 | 17 | 25 |
| Bloating | 0.13 (OR) | (0.04; 0.40) | <0.0001 | 4.11 | 51.37 | 0.128 | 0.51 | 0.72 | −8.89 | 0.071 | −0.57 | 0.017 | 66 | 40 |
| Cohort Studies (k = 6) [34,54,55,56,57,58] | ||||||||||||||
| Abdominal Pain | −0.59 * | (−0.76; −0.42) | <0.0001 | 2.85 | 0.00 | 0.723 | 0.00 | 0.00 | −2.45 | 0.070 | −1.69 | 0.091 | 66 | 40 |
| Bloating | −0.64 * | (0.82; −0.46) | <0.0001 | 1.20 | 0.00 | 0.878 | 0.00 | 0.00 | −1.13 | 0.342 | −0.98 | 0.327 | 59 | 40 |
CI: Confidence Interval; OR: Odds Ratio; * Cohen’s d.
Figure 2.
Low-FODMAP diet versus traditional IBS diet. Abdominal pain: (A) forest plot and (B) funnel plot. Bloating: (C) forest plot and (D) funnel plot.
3.1.2. Bloating
Patients managed with a low-FODMAP diet experienced significant bloating reduction compared with those receiving a traditional diet, OR = 0.32 (Table 3), and there was no significant heterogeneity (Table 3, Figure 2C). Analysis of publication bias by the trim and fill method did not lead to the exclusion of any paper (Figure 2D). Egger’s test was not significant (Table 3).
3.1.3. Stool Consistency
There was no significant difference between treatments (effect size (ES) = 0.24, Table 3); statistical heterogeneity was moderate but not significant (Figure 3A). Analysis of publication bias with the trim and fill method failed to exclude any paper (Figure 3B). Egger’s test was not significant (Table 3).
Figure 3.
Low-FODMAP diet versus traditional IBS diet. Stool consistency: (A) forest plot and (B) funnel plot. Stool frequency: (C) forest plot and (D) funnel plot.
3.1.4. Stool Frequency
There was a significant difference between treatments for this outcome (ES = −0.54; p < 0.001). There was no statistical heterogeneity (Table 3, Figure 3C). Analysis of publication bias with the trim and fill method failed to exclude any paper (Figure 3D). Egger’s test was not significant (Table 3).
3.2. Low-FODMAP Diet vs. Medium/High-FODMAP Diet
The primary studies (k = 3 RCTs) compared patients managed with a low-FODMAP diet and patients receiving a high/medium-FODMAP diet [25,26,53]. Their main characteristics are listed in Table 1. This set of studies examined two outcomes: reduction of abdominal pain and of bloating.
3.2.1. Abdominal Pain
Significantly reduced abdominal pain was described by patients receiving a low-FODMAP diet compared with those receiving a high-FODMAP diet (OR = 0.17). There was no statistical heterogeneity (Table 3). Analysis of publication bias with the trim and fill method failed to exclude any paper (Figure 4B). Finally, Egger’s test was not significant (Table 3).
Figure 4.
Low-FODMAP diet versus medium/high-FODMAP. Abdominal pain: (A) forest plot and (B) funnel plot. Bloating: (C) forest plot and (D) funnel plot.
3.2.2. Bloating
The patients receiving a low-FODMAP diet reported a significant reduction of bloating compared with those given a high-FODMAP diet (OR = 0.13); statistical heterogeneity was moderate but not significant (Table 3, Figure 4C). Analysis of publication bias by the trim and fill method did not lead to the exclusion of any paper (Figure 4D). Egger’s test was not significant (Table 3).
3.3. Cohort Studies
The primary studies (k = 6) compared baseline versus post-treatment data in patients treated with a low-FODMAP diet [34,54,55,56,57,58] (Table 2). Two outcomes were assessed in this set: reduction of abdominal pain and reduction of bloating. Meta-regressions were performed for both outcomes using gender, age, and year of publication.
3.3.1. Abdominal Pain
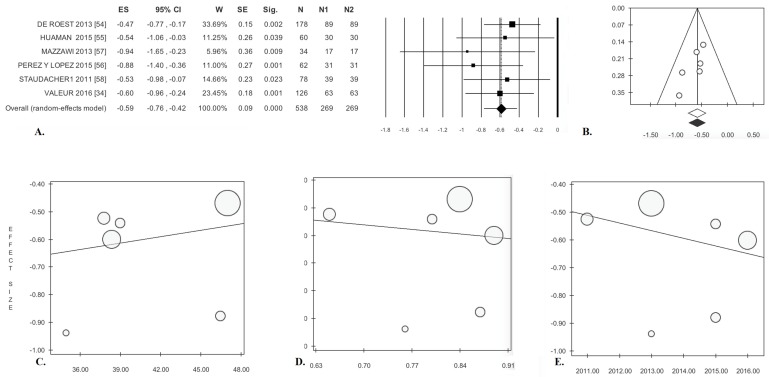
Pain after treatment was significantly reduced compared with baseline in these patients (ES = −0.59). There was no statistical heterogeneity (Table 3, Figure 5A). Analysis of publication bias by the trim and fill method did not result in the exclusion of any paper (Figure 5B). Egger’s test was not significant (Table 3). The meta-regression lines for age (p = 0.652), gender (p = 0.817), and year of publication (p = 0.543) were not significant (Figure 5C–E).
Figure 5.
Low-FODMAP diet in cohort studies. Abdominal pain: (A) forest plot and (B) funnel plot. Meta-regression: (C) mean age, (D) gender, and (E) publication year.
3.3.2. Bloating
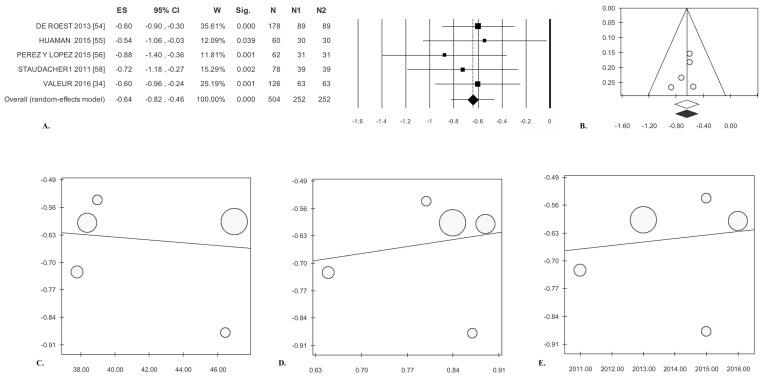
Significantly reduced bloating was reported by patients after treatment (ES = −0.64). There was no statistical heterogeneity (Table 3, Figure 6A). Analysis of publication bias by the trim and fill method did not lead to the exclusion of any paper (Figure 6B). Finally, Egger’s test was not significant (Table 3). The meta-regression lines for age (p = 0.808), gender (p = 0.747), and year of publication (p = 0.804) were not significant (Figure 6C–E).
Figure 6.
Low-FODMAP diet in cohort studies. Bloating: (A) forest plot and (B) funnel plot. Meta-regression: (C) mean age, (D) gender, and (E) publication year.
4. Discussion
Several clinical trials have reported that reducing high-FODMAP foods achieves adequate symptom relief in approximately 70% of IBS patients [32,59,60]. In a recent meta-analysis, Marsh et al. [40] reported the efficacy of a low-FODMAP diet on the functional gastrointestinal symptoms associated with IBS and IBD, and found a significant improvement in symptom severity and quality of life scores compared to patients receiving a normal Western diet.
The meta-analysis in this study provides an update on the RCTs and cohort studies that have been published since then, and examines them separately in relation to diet type. Our results showed that a low-FODMAP diet versus a traditional IBS diet created a statistically significant reduction in abdominal pain, bloating, and stool frequency.
Significant reductions in abdominal pain and bloating were also found in patients administered a low-FODMAP compared to those receiving a medium or a high-FODMAP diet. Similarly, analysis of the six cohort studies demonstrated a significant reduction in abdominal pain and bloating, from baseline to follow-up, in patients treated with a low-FODMAP diet. The meta-regression lines for age and gender were not significant. Overall, the results of this meta-analysis confirm those reported in the meta-analysis by Marsh et al. [40]. The first limitation of this study lies in the relatively small number of primary studies. Moreover, given the low number of primary studies, to be able to provide quality evidence, we used random effect’s model as suggested by Liberati et al. [61]. A second limitation is the lack of blinding. However, if IBS patients have a good knowledge of food and its FODMAP content, the food in their dietary treatment, or the food choices taught to them, cannot be blinded. A third limitation is the inadequate treatment duration, which does not allow for a long-term assessment. In fact, studies involving long-term follow-ups are few. In a recent retrospective study, only one third of IBS patients receiving a low-FODMAP diet were still adherent to their treatment after a median follow-up of 18 months, even though they reported reasonable symptom relief [51]. Nevertheless, a recent prospective study in the UK [62] showed that a low-FODMAP diet can be effective and nutritionally adequate up to 18 months after initial dietitian-led education. In this study, 82% of patients who concluded the short-term FODMAP restriction phase (six weeks), continued to follow an adapted FODMAP diet in which FODMAPs were gradually reintroduced, and 70% of them maintained adequate long-term symptom relief. However, it should also be highlighted that the results of the present study were not affected by statistically significant heterogeneity and publication bias is not present. A fourth limitation is the fact that, in the studies analysed in this meta-analysis, the FODMAP diet is never compared with the current standard dietary advice for IBS, as reported by the British National Institute for Health and Care Excellence (NICE) [63].
It should be emphasised that even though a low-FODMAP diet improves IBS symptoms compared with a normal diet, this does not in fact demonstrate that the low-FODMAP treatment is superior to the conventional IBS dietary intervention. Studies comparing the efficacy of a low-FODMAP diet versus proper dietary advice for IBS (NICE diet) did not show a clear-cut advantage over the low-FODMAP diet [30,50]. Furthermore, a high-FODMAP comparator diet has the potential to exaggerate symptoms in the control [32].
A recent placebo-controlled clinical trial conducted by Staudacher et al. [64] confirmed that the low-FODMAP diet reduces the relative abundance of bifidobacterial, but the co-administration of a probiotic (VLS#3) reduces the loss of the bacterial stain. The effects of IBD treatment with a low-FODMAP diet combined with probiotics need to be clarified by further clinical trials [64].
It has also been hypothesized that patients who follow this diet may be at risk of reduced intake of fiber and some micronutrients, such as calcium, iron, zinc, folate B, D vitamins, and natural antioxidants, especially in subjects with limited access to the expensive alternative items for this diet [65]. However, a prospective study [62] showed that a dietitian-led low-FODMAP diet can be nutritionally adequate for up to 18 months. Excluding the first restriction phase of six weeks, following an adapted FODMAP diet was nutritionally adequate in macronutrients, micronutrients, and energy intake, despite having a lower FODMAP content (20.6 ± 14.9 g/day) when compared to the habitual diet (29.4 ± 22.9 g/day). Another study showed that this diet does not seem to cause vitamin D and folic acid deficiencies, even in the restriction phase [66]. Although there are few studies that evaluated the nutritional adequacy of the low-FODMAP diet, it is reasonable to think that, where properly supported by an experienced dietitian, this diet can be nutritionally adequate in the long term.
5. Conclusions
There is evidence that a low-FODMAP diet can have a favorable impact on IBS symptoms, especially abdominal pain, bloating, and diarrhoea. However, it remains to be demonstrated whether a low-FODMAP diet is superior to conventional IBS diets, especially in the long term. In addition, further studies are needed to demonstrate whether the low-FODMAP diet is superior to the traditional IBS diet following the NICE guidelines in the long term. Long-term FODMAP depletion may entail physiological consequences on the intestinal microbiome, colonocyte metabolism, and nutritional status that should not be underestimated, and needs further investigation.
Finally, the purpose of this meta-analysis was to provide propositions to help drive future research on this topic of growing interest among researchers, and assist with designing the epidemiological studies with comparability features in order to achieve better outcomes in clinical practice.
Acknowledgments
We have not received funds in support of research work or for covering the costs to public in open access.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/9/9/940/s1, Figure S1: Sensitivity analysis of RCTs with low-FODMAP vs. traditional IBS diet, Table S1: PRISMA check list, Table S2: Cochrane tool for assessing risk of bias, Table S3: Newcastle-Ottawa scale assessment for cohort studies.
Author Contributions
E.A.: Guarantor of the article, study concept and design, literature search, data analysis, and manuscript writing. V.D.N.: literature search. P.M.A.: literature search, data abstraction, and graphic processing. G.L.: literature search, study concept, and manuscript writing. All authors have approved the final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Altobelli E., Lattanzi A., Paduano R., Varrassi G., di Orio F. Colorectal cancer prevention in Europe: Burden of disease and status of screening programs. Prev. Med. 2014;62:132–141. doi: 10.1016/j.ypmed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Altobelli E., D’Aloisio F., Angeletti P.M. Colorectal cancer screening in countries of European Council outside of the EU-28. World J. Gastroenterol. 2016;22:4946–4957. doi: 10.3748/wjg.v22.i20.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burisch J., Jess T., Martinato M., Lakatos P.L. ECCO-EpiCom. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis. 2013;7:322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.American College of Gastroenterology Task Force on Irritable Bowel Syndrome. Brandt L.J., Chey W.D., Foxx-Orenstein A.E., Schiller L.R., Schoenfeld P.S., Spiegel B.M., Talley N.J., Quigley E.M. An evidence-based position statement on the management of irritable bowel syndrome. Am. J. Gastroenterol. 2009;104:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 5.Andrews E.B., Eaton S.C., Hollis K.A., Hopkins J.S., Ameen V., Hamm L.R., Cook S.F., Tennis P., Mangel A.W. Prevalence and demographics of irritable bowel syndrome: Results from a large web-based survey. Aliment. Pharmacol. Ther. 2005;22:935–942. doi: 10.1111/j.1365-2036.2005.02671.x. [DOI] [PubMed] [Google Scholar]
- 6.Müller-Lissner S.A., Bollani S., Brummer R.J., Coremans G., Dapoigny M., Marshall J.K., Muris J.W., Oberndorff-Klein W.A., Pace F., Rodrigo L., et al. Epidemiological aspects of irritable bowel syndrome in Europe and North America. Digestion. 2001;64:200–204. doi: 10.1159/000048862. [DOI] [PubMed] [Google Scholar]
- 7.El-Salhy M., Gundersen D., Gilja O.H., Hatlebakk J.G., Hausken T. Is irritable bowel syndrome an organic disorder? World J. Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz B.J., Fisher R.S. The irritable bowel syndrome. N. Engl. J. Med. 2001;344:1846–1850. doi: 10.1056/NEJM200106143442407. [DOI] [PubMed] [Google Scholar]
- 9.Chang L. Review article: Epidemiology and quality of life in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2004;20:31–39. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 10.Maxion-Bergemann S., Thielecke F., Abel F., Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chey W.D., Kurlander J., Eswaran S. Irritable bowel syndrome: A clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 12.Anastasi J.K., Capili B., Chang M. Managing irritable bowel syndrome. Am. J. Nurs. 2013;113:42–52. doi: 10.1097/01.NAJ.0000431911.65473.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer E.A. Clinical practice. Irritable bowel syndrome. N. Engl. J. Med. 2008;358:1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer E.A., Labus J.S., Tillisch K., Cole S.W., Baldi P. Towards a system view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajilić-Stojanović M., Jonkers D.M., Salonen A., Hanevik K., Raes J., Jalanka J., de Vos W.M., Manichanh C., Golic N., Enck P., et al. Intestinal microbiota and diet in IBS: Causes, consequences, or epiphenomena? Am. J. Gastroenterol. 2015;110:278–287. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Böhn L., Störsrud S., Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol. Motil. 2013;25:23–30. doi: 10.1111/nmo.12001. [DOI] [PubMed] [Google Scholar]
- 17.Hayes P.A., Fraher M.H., Quigley E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014;10:164–174. [PMC free article] [PubMed] [Google Scholar]
- 18.Eswaran S., Tack J., Chey W.D. Food: The forgotten factor in the irritable bowel syndrome. Gastroenterol. Clin. N. Am. 2011;40:141–162. doi: 10.1016/j.gtc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Farré R., Tack J. Food and symptom generation in functional gastrointestinal disorders: Physiological aspects. Am. J. Gastroenterol. 2013;108:698–706. doi: 10.1038/ajg.2013.24. [DOI] [PubMed] [Google Scholar]
- 20.Moayyedi P., Ford A.C., Quigley E.M., Lembo A.J., Saito Y.A., Schiller L.R., Soffer E.E., Spiegel B.M., Quigley E.M. Task Force on the Management of Functional Bowel Disorders. The American College of Gastroenterology irritable bowel syndrome monograph: Translating systematic review data to clinical practice. Gastroenterology. 2010;138:789–791. doi: 10.1053/j.gastro.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 21.Dalrymple J., Bullock I. Diagnosis and management of irritable bowel syndrome in adults in primary care: Summary of NICE guidance. BMJ. 2008;336:556–568. doi: 10.1136/bmj.39484.712616.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuck C.J., Muir J.G., Barrett J.S., Gibson P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: Role in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2014;8:819–834. doi: 10.1586/17474124.2014.917956. [DOI] [PubMed] [Google Scholar]
- 23.Gibson P.R., Varney J., Malakar S., Muir J.G. Food components and irritable bowel syndrome. Gastroenterology. 2015;148:1158–1574. doi: 10.1053/j.gastro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Barrett J.S., Gearry R.B., Muir J.G., Irving P.M., Rose R., Rosella O., Haines M.L., Shepherd S.J., Gibson P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 25.Ong D.K., Mitchell S.B., Barrett J.S., Shepherd S.J., Irving P.M., Biesiekierski J.R., Smith S., Gibson P.R., Muir J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 26.Halmos E.P., Power V.A., Shepherd S.J., Gibson P.R., Muir J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd S.J., Lomer M.C., Gibson P.R. Short-chain carbohydrates and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013;108:707–717. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M., Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut. 2017;66:966–974. doi: 10.1136/gutjnl-2016-313425. [DOI] [PubMed] [Google Scholar]
- 29.Böhn L., Störsrud S., Liljebo T., Collin L., Lindfors P., Törnblom H., Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology. 2015;149:1399–1407. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 30.Eswaran S.L., Chey W.D., Han-Markey T., Ball S., Jackson K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am J Gastroenterol. 2016;111:1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 31.Laatikainen R., Koskenpato J., Hongisto S.M., Loponen J., Poussa T., Hillilä M., Korpela R. Randomised clinical trial: Low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016;44:460–470. doi: 10.1111/apt.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson P.R. The evidence base for efficacy of the low FODMAP diet in irritable bowel syndrome: Is it ready for prime time as a first-line therapy? J. Gastroenterol. Hepatol. 2017;32:32–35. doi: 10.1111/jgh.13693. [DOI] [PubMed] [Google Scholar]
- 33.Gibson P.R., Shepherd S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 34.Valeur J., Røseth A.G., Knudsen T., Malmstrøm G.H., Fiennes J.T., Midtvedt T., Berstad A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion. 2016;94:50–56. doi: 10.1159/000448280. [DOI] [PubMed] [Google Scholar]
- 35.Hustoft T.N., Hausken T., Ystad S.O., Valeur J., Brokstad K., Hatlebakk J.G., Lied G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017 doi: 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- 36.Staudacher H.M., Lomer M.C., Anderson J.L., Barrett J.S., Muir J.G., Irving P.M., Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 37.Staudacher H.M., Irving P.M., Lomer M.C., Whelan K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014;11:256–266. doi: 10.1038/nrgastro.2013.259. [DOI] [PubMed] [Google Scholar]
- 38.Staudacher H.M., Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016;75:306–318. doi: 10.1017/S0029665116000021. [DOI] [PubMed] [Google Scholar]
- 39.Chumpitazi B.P., Cope J.L., Hollister E.B., Tsai C.M., McMeans A.R., Luna R.A., Versalovic J., Shulman R.J. Randomised clinical trial: Gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015;42:418–427. doi: 10.1111/apt.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh A., Eslick E.M., Eslick G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016;55:897–906. doi: 10.1007/s00394-015-0922-1. [DOI] [PubMed] [Google Scholar]
- 41.Moher D., Altman D.G., Liberati A., Tetzlaff J. PRISMA statement. Epidemiology. 2011;22:128. doi: 10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P.T., Green S., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series Chapter 8. WILEY; Hoboken, NJ, USA: 2008. Assessing Risk of Bias in Included Studies Published Online. [Google Scholar]
- 43.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 31 March 2017)];2001 Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 44.Rothstein H.R., Sutton A.J., Borenstein M. Publication Bias in Meta-Analysis. Wiley; Chichester, UK: 2005. [Google Scholar]
- 45.Fragkos K.C., Tsagris M., Frangos C.C. Publication bias in meta-analysis: Confidence intervals for Rosenthal’s fail-safe number. Int. Sch. Res. Not. 2014;2014:825383. doi: 10.1155/2014/825383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen N., Andersen N.N., Végh Z., Jensen L., Ankersen D.V., Felding M., Simonsen M.H., Burisch J., Munkholm P. Ehealth: Low FODMAP diet vs. Lactobacillus rhamnosus GG in irritable bowel syndrome. World J. Gastroenterol. 2014;20:16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters S.L., Yao C.K., Philpott H., Yelland G.W., Muir J.G., Gibson P.R. Randomized clinical trial: The efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016;44:447–459. doi: 10.1111/apt.13706. [DOI] [PubMed] [Google Scholar]
- 48.Major G., Pritchard S., Murray K., Yelland G.W., Muir J.G., Gibson P.R. Colon Hypersensitivity to Distension, Rather than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology. 2017;152:124–133. doi: 10.1053/j.gastro.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 49.Biesiekierski J.R., Peters S.L., Newnham E.D., Tsai C.M., McMeans A.R., Luna R.A., Versalovic J., Shulman R.J. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 50.Whigham L., Joyce T., Harper G., Irving P.M., Staudacher H.M., Whelan K., Lomer M.C. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J. Hum. Nutr. Diet. 2015;28:687–696. doi: 10.1111/jhn.12318. [DOI] [PubMed] [Google Scholar]
- 51.Maagaard L., Ankersen D.V., Végh Z., Burisch J., Jensen L., Pedersen N., Munkholm P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016;22:4009–4019. doi: 10.3748/wjg.v22.i15.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen N. EHealth: Self-management in inflammatory bowel disease and in irritable bowel syndrome using novel constant-care web applications. EHealth by constant-care in IBD and IBS. Dan. Med. J. 2015;62:B5168. doi: 10.1097/MIB.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 53.McIntosh K., Reed D.E., Schneider T., Dang F., Keshteli A.H., De Palma G., Madsen K., Bercik P., Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut. 2016;66:1241–1251. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 54.De Roest R.H., Dobbs B.R., Chapman B.A., Batman B., O’Brien L.A., Leeper J.A., Hebblethwaite C.R., Gearry R.B. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: A prospective study. Int. J. Clin. Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 55.Huamán J.W., Felip A., Guedea E., Jansana M., Videla S., Saperas E. The diet low in fermentable carbohydrates short chain and polyols improves symptoms in patients with functional gastrointestinal disorders in Spain. Gastroenterol. Hepatol. 2015;38:113–122. doi: 10.1016/j.gastrohep.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 56.López N.P.Y., Torres-López E., Zamarripa-Dorsey F. Clinical response in Mexican patients with irritable bowel syndrome treated with a low diet low in fermentable carbohydrates (FODMAP) Rev. Gastroenterol. Mex. 2015;80:180–185. doi: 10.1016/j.rgmx.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Mazzawi T., Hausken T., Gundersen D., El-Salhy M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur. J. Clin. Nutr. 2016;70:175–181. doi: 10.1038/ejcn.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staudacher H.M., Whelan K., Irving P.M., Lomer M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 59.Gibson P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017;32:40–42. doi: 10.1111/jgh.13695. [DOI] [PubMed] [Google Scholar]
- 60.Nanayakkara W.S., Skidmore P.M., O’Brien L., Wilkinson T.J., Gearry R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016;9:131–142. doi: 10.2147/CEG.S86798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 62.O’Keeffe M., Jansen C., Martin L., Williams M., Seamark L., Staudacher H.M., Irving P.M., Whelan K., Lomer M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2017 doi: 10.1111/nmo.13154. [DOI] [PubMed] [Google Scholar]
- 63.Irritable Bowel Syndrome in Adults: Diagnosis and Management. [(accessed on 1 July 2017)]; Available online: https://www.nice.org.uk/guidance/cg61.
- 64.Staudacher H.M., Lomer M.C.E., Farquharson F.M., Louis P., Fava F., Franciosi E., Scholz M., Tuohy K.M., Lindsay J.O., Irving P.M., et al. Diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Catassi G., Lionetti E., Gatti S., Catassi C. The low FODMAP diet: Many question marks for a catchy acronym. Nutrients. 2017;9:E292. doi: 10.3390/nu9030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincenzi M., Del Ciondolo I., Pasquini E., Gennai K., Paolini B. Effects of a low FODMAP diet and specific carbohydrate diet on symptoms and nutritional adequacy of patients with irritable bowel syndrome: Preliminary results of a single-blinded randomized trial. J. Transl. Int. Med. 2017;30:120–126. doi: 10.1515/jtim-2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.