Abstract
Polycystic ovary syndrome (PCOS) is a common endocrinopathy among women associated with reproductive, metabolic and psychological features. While weight management is recommended as first-line treatment, it is unclear if women with PCOS achieve similar benefits as women without PCOS. This systematic review thus aimed to compare the efficacy of weight management interventions in women with and without PCOS. Databases were searched until May 2017. The primary outcome was weight and anthropometric, reproductive, metabolic and psychological measures were secondary outcomes. Of 3264 articles identified, 14 studies involving n = 933 (n = 9 high and n = 5 moderate risk of bias) met the inclusion criteria. No statistically significant differences in weight or weight loss following the intervention were found between women with and without PCOS in five studies, with the remaining studies not comparing the difference in weight or weight loss between these groups. Secondary outcomes did not differ significantly between the two groups. This review identified that there is a paucity of high quality research in this area and that more rigorous research is needed.
Keywords: polycystic ovary syndrome, obesity, insulin resistance, weight loss, systematic review
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies in women of reproductive age, with a prevalence of 6–18%, depending on the diagnostic criteria used and the population studied [1,2,3]. The condition is underpinned by insulin resistance (IR) and hyperandrogenism. These hormonal abnormalities both contribute to clinical features including hirsutism or acne, oligo-/anovulation and polycystic ovarian morphology, type 2 diabetes (DM2), metabolic syndrome and cardiovascular disease [4,5,6,7]. PCOS also accounts for the majority of cases of anovulatory infertility [8], and women with PCOS have an increased risk of pregnancy and neonatal complications [9]. Women with PCOS also have a higher risk of psychological complications, including depression and anxiety [10].
The etiology of PCOS is complex and poorly understood. Both genetic and environment factors contribute to the syndrome [11], and both gonadotropin hypersecretion and IR increase androgen secretion [12,13,14]. Women with PCOS appear to have a higher rate of weight gain and a higher prevalence of overweight, obesity and central obesity, compared to women without PCOS [15,16,17]. Obesity, especially central obesity, worsens the clinical and biochemical presentation of the syndrome, contributing to IR, hyperandrogenism, reproductive disorders, diabetes and cardiovascular disease [18,19,20]. Weight loss, in turn, improves all the features of PCOS, and lifestyle (diet, physical activity and behavior) changes and weight management are recommended as first line treatment for PCOS [21,22] to improve hormonal disturbances and to prevent future reproductive and metabolic complications. Preconception lifestyle interventions and weight loss are also recommended before infertility treatment is initiated [23], and lead to higher ovulation rates compared to oral contraceptive pretreatment [24].
It has been proposed that weight management interventions may be less effective in women with PCOS compared to those without PCOS given the higher rate of longitudinal weight gain in PCOS [17]. This may be related to the hormonal aberrations of PCOS such as hyperandrogenemia or IR, contributing to abnormalities in energy homeostasis and dietary intake including gut hormone regulation [25,26], or an altered metabolism due to reduced postprandial thermogenesis [27]. However, the literature is limited and contradictory. The aim of this systematic review is thus to assess the effect of lifestyle (dietary and non-dietary) weight management interventions on anthropometric, reproductive and metabolic outcomes in women with PCOS, compared to women without PCOS.
2. Materials and Methods
2.1. Selection Criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement checklist [28]. The detailed PICO (Population, Intervention, Comparison, Outcome) framework in Supplementary Table S1, established a priori, was used to include and exclude articles for this systematic review. Briefly, articles were included if they reported a study that compared women with PCOS to women without PCOS; following a weight management intervention; and investigated anthropometric, fertility, reproductive non-fertility, metabolic, quality of life or emotional wellbeing outcomes. The primary outcome was weight management defined as either weight loss, weight maintenance or prevention of weight gain. Secondary anthropometric, reproductive, metabolic and psychological outcomes are listed in Table S1 and were analysed based on the most complete data, which were body mass index (BMI), waist circumference for fat distribution, computed tomography for fat and lean mass, number of ovulations, total testosterone, sex hormone-binding globulin (SHBG), free androgen index (FAI), fasting glucose, oral glucose tolerance test (OGTT), fasting insulin, total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides, and blood pressure.
2.2. Systematic Search for Evidence
A systematic search (Supplementary Table S2) was developed incorporating terms related to weight management (including lifestyle, behaviour, pharmacological, surgical and complementary and alternative interventions) and combined with terms related to PCOS. The search strategy was limited to English language articles and there were no limits on year of publication. The following electronic databases were searched via the OVID platform to identify relevant literature up to May 2017: Ovid MEDLINE(R) 1946 to Present with Daily Update; Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations; Evidence-Based Medicine (EBM) Reviews incorporating Cochrane Database of Systematic Reviews, EBM Reviews—ACP Journal Club, EBM Reviews—Database of Abstracts of Reviews of Effects, EBM Reviews—Cochrane Central Register of Controlled Trials, EBM Reviews—Cochrane Methodology Register, EBM Reviews—Health Technology Assessment, EBM Reviews—National Health Service (NHS) Economic Evaluation Database; PsycINFO; and EMBASE. CINAHL Plus was searched separately on the same date. Bibliographies of relevant articles were also searched for identification of additional studies.
2.3. Inclusion of Studies
To determine the literature to be assessed further, one trained reviewer (J.K. for search to 23 September 2015 and E.C.T for search to 11 May 2017) screened the titles, abstract sections and keywords of every article retrieved by the search strategy using the selection criteria described in Table S1. Full articles were retrieved for further assessment if the information given suggested that the article met the selection criteria, or if it was unclear. Three additional reviewers were consulted throughout the screening process.
2.4. Quality Appraisal of the Evidence
Methodological quality, in terms of risk of bias, of the included studies was assessed by two reviewers (J.K. and E.C.T.) in consultation with experienced reviewers (M.M. and L.J.M.) using criteria developed a priori [29], designed for cohort studies. Individual quality items were investigated using a descriptive component approach that assessed selection bias, performance bias, attrition bias, reporting bias, potential confounding, and appropriateness of statistical analysis. Any disagreement or uncertainty was resolved by discussion among authors to reach a consensus. Using this approach, each study was allocated a risk of bias rating. Where there was more than one article describing a study, all articles were used to complete one risk of bias assessment on the study.
2.5. Data Extraction
Double data extraction, according to the selection criteria described in Table S1 was conducted by two independent reviewers for the original search (J.K. and A.J.) and by two independent reviewers for the updated search (A.J. and E.C.T.). Information was collected on relevant outcome data and included point estimates, measures of variability and number of participants. Where there was more than one article describing a study, data from the most current and comprehensive article was extracted, and any additional outcome data reported in additional articles were subsequently extracted.
2.6. Data Synthesis
Due to the heterogeneity in interventions, a meta-analysis was not performed, results are thus presented narratively and in tabular form. Publication bias could not be assessed due to the lack of meta-analysis as this requires a meta-analysis to be performed on 10 or more studies. All studies reported baseline and endpoint values, except for two studies that reported the difference from baseline to post-intervention. Outcome data has been excluded in instances where only the mean was presented with no corresponding variance data (standard deviation (SD), standard error (SE), or confidence interval (CI)). Where necessary, unit conversions were performed so that results are presented in SI units; in some instances, where conversion factors could not be obtained, the unit from the original manuscript has been retained.
3. Results
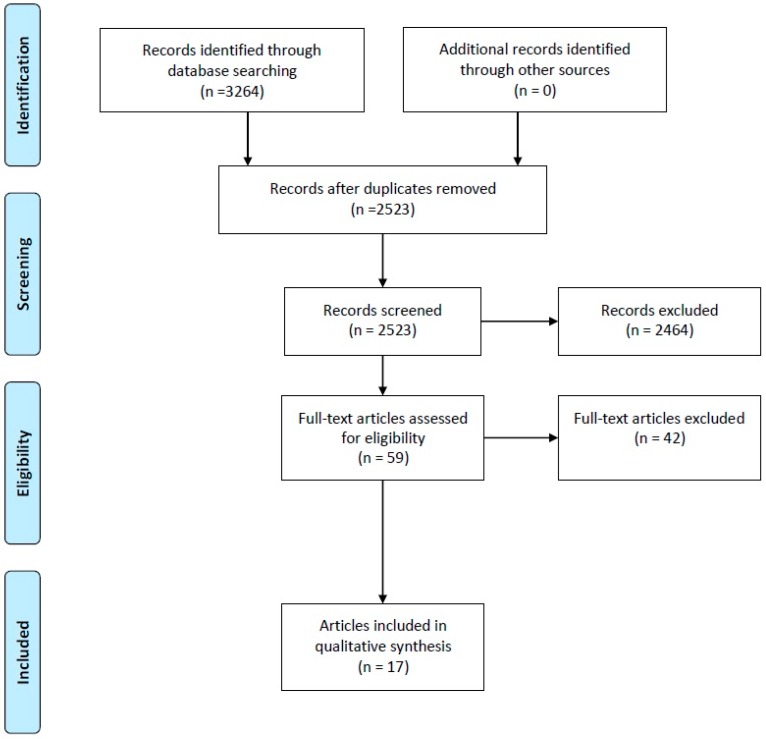
The search returned 3264 articles (Figure 1). Fifty-nine full text articles were retrieved for further evaluation and 17 articles [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] reporting 14 studies met the inclusion criteria. Of these 17 articles, one study was reported across two articles [31,32] and one study [35] reported data across different time points [36,37]. Two studies reported data from two intervention groups, respectively [38,39]. A table of studies, excluded based on full text, is found in Supplementary Table S3: Table of Excluded Studies. All between group comparisons presented here are presented as the authors reported that data and is per protocol for all analyses.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
3.1. Characteristics and Quality of Included Studies
The characteristics of included studies are reported in Table 1. All studies were comparative/parallel design. Two were designed as randomized controlled studies (RCTs) but comprised two intervention groups, respectively, and were used as comparative studies [38,39]. Five studies had a moderate risk of bias, whilst nine studies had a high risk of bias (Supplementary Table S4). Interventions varied across the included studies (diet, diet + behavior change program, diet + Metformin, diet + anti-obesity drug, anti-obesity drug, diet + anti-obesity drug + exercise, bariatric surgery, and various exercise training programs), as did the duration of interventions. Nine studies were designed with the specific aim of weight loss [34,37,38,39,41,42,43,45,46], while the remaining five studies did not state if the aim was weight loss [30,31,33,40,44]. The sample size of the studies varied from 31 to 1016.
Table 1.
Characteristics of included studies.
| Study | Design | Country | PCOS Details at Baseline N (Completers) |
Non-PCOS Details at Baseline N (Completers) |
Attrition Rate | Current Medication | Specific Exclusion Criteria | Intervention | Outcomes | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Diamanti-Kandarakis 2007 [30] | Comparative study | Greece | Age 27.5 ± 5.77 BMI 35.4 ± 5.31 ESHRE/ASRM n = 29 |
Age 32.1 ± 5.64 BMI 36.4 ± 6.47 n = 18 |
Not reported | Not reported | Galactorrhea | Duration: 24 weeks Normal protein diet, 600 kcal/day energy deficit, Orlistat (Xenical®, Roche) 120 mg × 3 times daily Aim of weight loss: not stated |
Anthropo-metric: BMI Reproductive: T, SHBG Metabolic: FG, 2HRG, FI |
Moderate |
| Moran 2007 [34] | Comparative study | Australia | Age 31.7 ± 6.2 BMI 35.7 ± 5.8 ESHRE/ASRM n = 15 |
Age 37.1 ± 4.7 BMI 35.5 ± 5.1 n = 17 |
PCOS 17% Non-PCOS 11% |
No hormonal or insulin-sensitising drugs pre study | Pregnancy, breastfeeding | Duration: 8 weeks Energy restricted diet (two meals daily replaced with commercially available meal replacements) Aim of weight loss: Yes |
Anthropo-metric: Weight, WC Reproductive: T, SHBG, FAI Metabolic: FI, lipids Others: IL-6, TNF-α |
Moderate |
| Hutchison 2011 [31] Harrison 2012 [32] |
Comparative study | Australia | BMI > 27 (37.4 ± 1.5) Age 20–40 (29.5 ± 1.4) NIH n = 13 |
BMI > 27 (35.7 ± 1.3) Age 20-40 (35.0 ± 1.1) n = 8 |
PCOS 35% Non-PCOS 43% |
No hormonal or insulin-sensitising drugs pre study | Diabetes, adrenal disorders, recent weight change, regular physical activity, pregnancy, breastfeeding, smoking | Duration: 12 weeks supervised intensified exercise training 60 min three times weekly Aim of weight loss: not stated |
Anthropo-metric: WC, Weight, BMI, VF, SCFAT Reproductive: T, SHBG, FAI Metabolic: FG, FI, lipids, BP |
Moderate |
| Cheang 2016 [43] | Comparative study | USA | Age 26.9 ± 4.6 BMI 36.6 ± 5.1 Modified ESHRE/ASRM n = 16 |
Age 27.5 ± 5.7 BMI 35.8 ± 4.8 n = 15 |
PCOS 53% Non-PCOS 44% |
No unstable medication use for 6 months for disorders such as hypertension or dyslipidemia | Weight loss attempts in 3 months pre study, diabetes, pulmonary, cardiac, renal, neurologic, hepatic, psychiatric, infectious, neoplastic, malignant disease, pregnancy Non-PCOS: history gestational diabetes, family history abnormal glucose tolerance, hypertension, dyslipidemia |
Duration: 8 weeks Standardized hypocaloric diet (50% carbohydrate, 20% protein, 30% fat) with 500–1000 kcal/day deficit. No modification of physical activity or other weight loss methods. Aim of weight loss: Yes |
Anthropometric: Weight, BMI Metabolic: FG, FI | Moderate |
| Kogure 2016 [44] | Comparative study | Brazil | Age 28.1 ± 5.4 BMI 28.4 ± 6.0 ESHRE/ASRM n = 45 |
Age 29.6 ± 5.2 BMI 26.2 ± 5.7 n = 52 |
PCOS 38% Non-PCOS 46% |
No hormonal contraceptive us drugs pre or during study | Systemic diseases, smoking, pregnancy | Duration: 4 months Progressive resistance training (PRT) for 1 h/day three times per week. Aim of weight loss: not stated |
Anthropometric: Weight, BMI, WC, TFFM, % body fat Reproductive: T, SHBG, FAI Metabolic: FG, FI, HOMA-IR |
Moderate |
| Villa 1999 [40] | Comparative study | Italy | Age 26.3 ± 5 BMI 27.5 ± 6.8 NIH n = 22 |
Age not reported BMI 27.4 ± 6.8 n = 14 |
Not reported | Not reported | Not reported | Duration: 4–5 weeks 50 mg Naltrexone daily Aim of weight loss: not stated |
Anthropo-metric: BMI Reproductive: T, SHBG, FAI Metabolic: FG, FI |
High |
| Kowalska 2001 [33] | Comparative study | Poland | Age 25.3 ± 4.8 BMI 34.7 ± 6.0 NIH n = 11 |
Age 27.9 ± 7.3 BMI 36.2 ± 6.0 n = 19 |
PCOS 27% Non-PCOS 40% |
Not reported | No additional | Duration: 4–5 months Hypocaloric diet (1200–1400 kcal/day), Metformin 500 mg three times daily Aim of weight loss: not stated |
Anthropo-metric: BMI, WC Reproductive: T, FAI, SHBG Metabolic: FI |
High |
| Panidis 2014 [35] Vosnakis 2013 [36] Panidis 2008 [37] |
Comparative study | Greece | Age 26.1 ± 6.4 BMI 34.5 ± 5.9 ESHRE/ASRM n = 101 |
Age 31.5 ± 4.7 BMI 34.9 ± 5.4 n = 29 |
Not reported | No hormonal or insulin-sensitising drugs pre or during study | Galactorrea | Duration: 6 months Normal protein, energy-restricted diet (600 kcal/day energy deficit, moderate intensity aerobic exercise, 1 h × 3 times/week, Orlistat 120 mg before each meal) Aim of weight loss: Yes |
Anthropo-metric: WC, BMI Reproductive: T, FAI, SHBG Metabolic: FG, lipids |
High |
| Kahal 2015 [41] | Comparative study | UK | Age 33.9 ± 6.7 BMI 37.9 ± 5.0 ESHRE/ASRM n = 13 |
Age 33.5 ± 7.1 BMI 36.5 ± 4.6 n = 12 |
PCOS 32% Non-PCOS 29% |
No medication | Alcohol intake >14 units/week Non-PCOS: history of hirsutism or menstrual irregularities |
Duration: 6 months Liraglutide 0.6 mg o.d subcutaneous injection for 1 week, 1.2 mg o.d for 1 week, and 1.8 mg o.d thereafter for 6 months. No diet or exercise advice given. Aim of weight loss: Yes |
Anthropometric: Weight, BMI | High |
| Nikokavora 2015 [42] | Comparative study | UK | Age 35.7 ± 8.9 BMI 40.0 ± 6.3 Diagnostic criteria not reported n = 137 |
Age 35.8 ± 8.9 BMI 40.0 ± 6.3 n = 137 |
PCOS 73% Non-PCOS 73% |
Not reported | Type 1 diabetes, porphyria, lactose intolerance, major cardio- or cerebrovascular disease, history of renal or hepatic disease, cancer, epilepsy, major psychological or eating disorders, breastfeeding, pregnant, birth or miscarriage prior 3 months | Duration: 12 weeks Commercial weight management program (LighterLife Total), 600 kcal (36% protein, 36% carbohydrate, 28% fat) as food packs alongside behavior change program Aim of weight loss: Yes |
Anthropometric: Weight, BMI | High |
| Bhandari 2016 [45] | Comparative study | India | Age 27.8 ± 4.50 BMI 42.5 ± 5.71 ESHRE/ASRM n = 43 |
Age 29.3 ± 4.96 BMI 45.0 ± 6.11 n = 32 |
Not reported | No hormonal, fertility or insulin-sensitising drugs pre or during study | Systemic diseases like hypothyroidism or hyperprolactinaemia, surgical complications intra or post operatively | Duration: 6 months post-surgery Sleeve gastrectomy (bariatric surgery) Aim of weight loss: Yes |
Anthropometric: Weight, BMI Other: Abnormal menstrual cycles |
High |
| Al-Eisa 2017 [46] | Comparative study | Egypt | Age 27.9 ± 4.1 BMI 33.5 ± 2.75 ESHRE/ASRM n = 30 |
Age 27.6 ± 5.7 BMI 31.7 ± 3.8 n = 30 |
Not reported | No hormonal drugs pre or during study | Normal BMI, other diseases such as diabetes or viral infections | Duration: 12 weeks treadmill walking, 45 min three times per week for 12 weeks. Aim of weight loss: Yes |
Anthropometric: Weight, BMI, WC Metabolic: FG, FI |
High |
| Pasquali 2000 [38] | RCT | Italy | BMI > 28 WHR > 0.80 NIH n = 18 |
BMI > 28 WHR > 0.80 n = 17 |
Diet: PCOS 0% Non-PCOS 25% Metform-in: PCOS 17% Non-PCOS 0% |
No hormonal or insulin-sensitising drugs pre study | Diabetes, renal or liver dysfunction | Duration: 6 months One month hypocaloric diet (1200–1400 kcal daily), Metformin 850 mg twice daily. Aim of weight loss: Yes |
Anthropo-metric: Weight, BMI, SAT, VAT Reproductive: T, SHBG Metabolic: FG, FI |
Moderate |
| Toscani 2011 [39] | RCT | Brazil | Age 22.7 + 5.68 Most participants BMI ≥ 25 NIH n = 18 |
Age 29.4 + 5.74 Most participants BMI ≥ 25 n = 22 |
Not reported | No hormonal drugs pre study | Diabetes, renal dysfunction | Duration: 2 months Diet 1: HP (30% protein, 40% carbohydrate, 30% fat) Diet 2: NP (15% protein, 55% carbohydrate, 30% fat) Aim of weight loss: Yes |
Anthropo-metric: Weight, WC, BMI Reproductive: T, SHBG, FAI Metabolic: BP, FG, 2HRG, FI, 2HRI, lipids |
High |
2HRG: 2 h glucose; 2HRI: 2 h insulin; BMI, body mass index; BMR, basal metabolic rate; BP, blood pressure; FAI, free androgen index; FG, fasting glucose; FI, fasting insulin; IL-6, interleukin-6; PCOS, polycystic ovary syndrome; SAT, subcutaneous abdominal tissue; SCFAT, subcutaneous fat; SHBG, sexual-hormone binding globulin; T, total testosterone; TFFM, total fat free mass; TFM, total fat mass; TNF-α, tumor necrosis factor-α; VAT, visceral abdominal tissue; VF, visceral fat; WC, waist circumference.
3.2. Outcomes: Anthropometric
3.2.1. Weight
Six studies including eight intervention groups reported weight as endpoint data [31,38,39,42,45,46], whilst a further three studies reported change in weight [34,41,43], and one study reported the estimated difference in weight from baseline to post-intervention [44] (Table 2). Five of the studies reported that the difference in weight post-intervention or the change in weight from baseline between the PCOS and non-PCOS groups was not statistically significantly different, whilst the remainder of studies did not report a p-value.
Table 2.
Anthropometric outcomes.
| Outcome | Reference | Intervention | Baseline PCOS: Mean ± SD | Baseline Non-PCOS: Mean ± SD | p-Value * | Post-Intervention PCOS: Mean ± SD | Post-Intervention Non-PCOS: Mean ± SD | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Pasquali 2000 [38] | Hypocaloric diet | 102 ± 19 | 106 ± 13 | NR | 97 ± 18 | 100 ± 13 | NR |
| Cheang 2016 [43] | Hypocaloric diet | 99.2 ± 13.3 | 97.6 ± 15.4 | 0.7508 | −4.08 ± 3.65 | −4.69 ± 2.98 | 0.6281 | |
| Moran 2007 [34] | Hypocaloric diet | 95.1 ± 19.3 | 95.5 ± 16.5 | NS | −3.9 ± 3.6 | −4.5 ± 4.1 | 0.642 | |
| Toscani 2011 [39] | High protein diet | 74.62 ± 18.8 | 75.89 ± 13.49 | NR | 71.4 ± 15.45 | 74.54 ± 13.71 | NR | |
| Toscani 2011 [39] | Normal protein diet | 82.85 ± 15.18 | 77.51 ± 13.31 | NR | 79.82 ± 16.51 | 74.31 ± 13.88 | NR | |
| Nikokavoura 2015 [42] | VLCD + behaviour change | 108.3 ± 18.1 | 107.4 ± 19.8 | 0.713 | 89.8 ± 16.7 | 88.0 ± 17.6 | 0.19 | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 103 ± 18 | 101 ± 8 | NR | 94 ± 17 | 88 ± 7 | NR | |
| Kahal 2015 [41] | AO drug (Liraglutide) | 102.1 ± 17.1 | 100.4 ± 15.1 | NS | −3.0 ± 4.2 | −3.8 ± 3.4 | 0.56 | |
| Bhandari 2016 [45] | Bariatric surgery | 106.89 ± 17.79 | 117.03 ± 19.89 | NR | 77.27 ± 10.72 | 84.89 ± 13.18 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 100.5 ± 4.5 ! | 96.2 ± 3.5 ! | 0.42 | 95.3 ± 4.8 ! | 96.9 ± 4.5 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 73.1 ± 15.6 | 68.1 ± 15.4 | NS | 0.52 [−0.31, 1.36] ^ | 0.13 [−0.64, 0.90] ^ | 0.14 | |
| Al−Eisa 2017 [46] | Aerobic training | 89.8 ± 6.95 | 84.9 ± 7.2 | NR | 84.8 ± 6.42 | 82.2 ± 5.72 | NR | |
| BMI (kg/m2) | Pasquali 2000 [38] | Hypocaloric diet | 39.6 ± 6.9 | 40.1 ± 6.2 | NR | 38 ± 6.2 | 37.8 ± 5.7 | NR |
| Cheang 2016 [43] | Hypocaloric diet | 36.6 ± 5.1 | 35.8 ± 4.8 | 0.6507 | −1.46 [−0.72, −2.20] # | −1.80 [−1.14, −2.45] # | 0.4829 | |
| Nikokavoura 2015 [42] | VLCD + behaviour change | 40.0 ± 6.3 | 40.0 ± 6.3 | 0.955 | 33.2 ± 6.0 | 32.8 ± 5.7 | NR | |
| Kowalska 2001 [33] | Hypocaloric diet + Metformin | 34.7 ± 6.0 | 36.2 ± 6.0 | NS | 31.4 ± 4.8 | 35.8 ± 7.9 | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 39.8 ± 7.9 | 37.4 ± 3.0 | NR | 36.4 ± 7.4 | 32.9 ± 3.4 | NR | |
| Diamanti−Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 35·43 ± 5·31 | 36·39 ± 6·47 | 0.58 | 29.7 ± 4.57 | 30.15 ± 4.13 | NR | |
| Vosnakis 2013 [36] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 34.83 ± 6.39 | 36.79 ± 6.98 | NR | 30.21 ± 5.78 | 31.01 ± 4.93 | NR | |
| Kahal 2015 [41] | AO drug (Liraglutide) | 37.9 ± 5.0 | 36.5 ± 4.6 | NS | −1.0 ± 1.5 | −1.4 ± 1.2 | 0.43 | |
| Villa 1999 [40] | AO drug (Naltrexone) | 27.5 ± 6.8 | 27.4 ± 6.8 | NS | 26.8 ± 6.7 | 27 ± 6.8 | NR | |
| Bhandari 2016 [45] | Bariatric surgery | 42.52 ± 5.66 | 45.03 ± 6.3 | 0.0717 | 30.76 ± 2.93 | 32.67 ± 3.51 | 0.013 | |
| Hutchison 2001 [31] | Intensified exercise training | 37.4 ± 1.5 ! | 35.7 ± 1.3 ! | 0.43 | 35 ± 1.6 ! | 35.9 ± 1.8 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 28.4 ± 6.0 | 26.2 ± 5.7 | NS | 0.21 [−0.11, 0.54] ^ | 0.05 [−0.25, 0.35] ^ | 0.08 | |
| Al−Eisa 2017 [46] | Aerobic training | 33.45 ± 2.75 | 31.7 ± 3.8 | NR | 28.5 ± 2.25 | 26.8 ± 2.54 | NR | |
| WC (cm) | Pasquali 2000 [38] | Hypocaloric diet | 109 ± 19 | 109 ± 11 | NR | 104 ± 13 | 105 ± 12 | NR |
| Toscani 2011 [39] | High protein diet | 87.74 ± 14.08 | 83.92 ± 9.13 | <0.05 | 86 ± 12.92 | 81.83 ± 9.13 | NR | |
| Toscani 2011 [39] | Normal protein diet | 93.32 ± 8.05 | 84.02 ± 9.03 | <0.05 | 90.03 ± 10.41 | 81.7 ± 11.72 | NR | |
| Kowalska 2001 [33] | Hypocaloric diet + Metformin | 98.1 ± 14.8 | 102.3 ± 13.0 | NS | 93.4 ± 11.8 | 100 ± 19.5 | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 107 ± 16 | 102 ± 6 | NR | 100 ± 15 | 94 ± 6 | NR | |
| Panidis 2008 [37] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 101.52 ± 2.67 | 100.53 ± 3.94 | NS | 87.86 ± 2.29 | 87.67 ± 2.82 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 106.8 ± 3.4 ! | 102.8 ± 2.6 ! | 0.39 | 103.1 ± 4 ! | 99.9 ± 4.1 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 81.7 ± 12.8 | 76.2 ± 11.3 | <0.05 | 0.86 [0.32, 1.40] ^ | 0.27 [−0.21, 0.75] | 0.21 | |
| Al−Eisa 2017 [46] | Aerobic training | 96.2 ± 3.52 | 94.2 ± 3.82 | NR | 93.8 ± 3.26 | 72.7 ± 2.6 | NR | |
| VAT/VF (cm2) | Pasquali 2000 [38] | Hypocaloric diet | 121 ± 48 | 181 ± 94 | NR | 108 ± 36 | 159 ± 83 | NR |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 151 ± 91 | 133 ± 38 | NR | 113 ± 59 | 100 ± 37 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 129.2 ± 12.8 ! | 121.5 ± 9.4 ! | 0.65 | 107.6 ± 15.1 ! | 132.7 ± 18.1 ! | NR | |
| SAT/SCFAT (cm2) | Pasquali 2000 [38] | Hypocaloric diet | 589 ± 127 | 554 ± 118 | NR | 574 ± 111 | 508 ± 107 | NR |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 535 ± 147 | 554 ± 79 | NR | 485 ± 170 | 462 ± 81 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 590.2 ± 35.2 ! | 550.3 ± 45.2 ! | 0.49 | 538.4 ± 40.2 ! | 558.5 ± 74.5 ! | NR |
* Between-group difference; ! mean ± SEM; ^ estimated difference [95% confidence interval]; # mean [95% confidence interval]; AO, anti-obesity; BMI, body mass index; NR, not reported; NS, not significant; PCOS, polycystic ovary syndrome; SAT, subcutaneous adipose tissue area; SCFAT, subcutaneous fat; VAT, visceral adipose tissue area; VF, visceral fat; VLCD, very low calorie diet; WC, waist circumference.
3.2.2. Body Mass Index (BMI)
Thirteen intervention groups from 12 studies [30,31,33,36,38,40,41,42,43,44,45,46] reported BMI (Table 2). One study reported a statistically significant difference in BMI following bariatric surgery (p = 0.013), with women with PCOS having a lower BMI post-surgery than women without PCOS [45]. This study, however, had a high risk of bias, with a number of key elements of the study protocol not reported and should therefore be interpreted with caution. Three studies reported a non-statistically significant difference in BMI between the two groups, whilst eight studies did not report a p-value.
3.2.3. Waist Circumference (WC)
Seven studies including nine intervention groups [31,33,37,38,39,44,46] reported WC (Table 2). The majority of studies did not compare between-group differences after the interventions, with only one study reporting a non-statistically significant difference in WC between groups following progressive resistance training [44].
3.2.4. Body Composition
Three intervention groups from two studies [31,38] measured body composition with computed tomography (CT). One study with an exercise intervention reported abdominal visceral fat (VF) and subcutaneous fat (SCFAT). Two intervention groups from one study with the interventions diet + Metformin and diet + placebo reported visceral adipose tissue area (VAT) and subcutaneous adipose tissue area (SAT). None of the studies reported whether there was between-group statistical significance (Table 2).
3.3. Outcomes: Fertility
Ovulation
One study with a diet intervention [34] reported double the number of ovulatory events in women without PCOS compared to women with PCOS, which was statistically significant (Table 3).
Table 3.
Reproductive outcomes.
| Outcome | References | Intervention | Baseline PCOS: Mean ± SD | Baseline Non-PCOS: Mean ± SD | p-Value * | Post-intervention PCOS: Mean ± SD | Post-intervention Non-PCOS: Mean ± SD | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Number of ovulations | Moran 2007 [34] | Hypocaloric diet | NR | NR | NR | 1.9 ovulations | 1.0 ovulations | <0.001 |
| Total testosterone (nmol/L) | Pasquali 2000 [38] | Hypocaloric diet | 1.77 ± 0.59 | 1.32 ± 0.42 | <0.05 | 1.63 ± 0.45 | 1.14 ± 0.35 | NR |
| Kowalska 2001 [33] | Hypocaloric diet + Metformin | 3.57 ± 1.01 | 1.91 ± 0.42 | <0.05 | 2.39 ± 1.11 | 1.7 ± 0.59 | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 2.36 ± 1.21 | 1.46 ± 0.38 | <0.01 | 1.7 ± 0.87 | 1.25 ± 0.38 | NR | |
| Diamanti-Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 3.01 ± 0.94 | 1.50 ± 0.43 | <0.001 | 2.28 ± 0.65 | 1.49 ± 0.36 | <0.05 | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 2.56 ± 1.00 | 1.33 ± 0.45 | <0.001 | 2.1 ± 0.78 | 1.41 ± 0.74 | 0.006 | |
| Villa 1999 [40] | AO drug (Naltrexone) | 1.7 ± 0.5 | 1.4 ± 0.5 | NS | 1.9 ± 1.76 | 1.5 ± 1.2 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 2.9 ± 0.2! | 1.6 ± 0.2 ! | <0.01 | 2.8 ± 0.3 ! | 1.8 ± 0.3 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 3.12 ± 1.22 | 2.58 ± 1.02 | <0.05 | 0.59 [0.30, 0.89] ^ | 0.42 [0.16, 0.68] ^ | 0.15 # | |
| SHBG (nmol/L) | Pasquali 2000 [38] | Hypocaloric diet | 16.0 ± 7.04 | 20.2 ± 10.7 | NS | 13.8 ± 2.1 | 28.1 ± 14.7 | NR |
| Kowalska 2001 [33] | Hypocaloric diet + Metformin | 32.0 ± 18.3 | 32.5 ± 16.5 | NS | 38.6 ± 19.3 | 36.5 ± 13.3 | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 18.7 ± 15.0 | 23.4 ± 22.7 | NS | 16.7 ± 8.1 | 28.9 ± 16.5 | NR | |
| Diamanti-Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 28.72 ± 12.48 | 40.92 ± 19.54 | 0.01 | 37.21 ± 17.59 | 58.6 ± 27.02 | <0.05 | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 30.3 ± 13.2 | 47.8 ± 34.7 | 0.012 | 40.3 ± 20.4 | 62.2 ± 35.5 | NS | |
| Villa 1999 [40] | AO drug (Naltrexone) | 30.2 ± 20.4 | 38.2 ± 16.4 | NS | 32.5 ± 20.9 | 39.2 ± 15.7 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 29.0 ± 1.8! | 43.6 ± 7.8 ! | 0.04 | 30.7 ± 2.8 ! | 54.3 ± 10.6 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 54.9 ± 37.8 | 63.0 ± 35.7 | NS | 0.12 [0.02, 0.23] ^ | 0.09 [−0.01, 0.18] ^ | 0.37 # | |
| FAI | Kowalska 2001 [33] | Hypocaloric diet + Metformin | 14.49 ± 8.49 | 9.97 ± 7.95 | <0.05 | 9.31 ± 9.95 | 5.49 ± 3.9 | NR |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 10.25 ± 6.31 | 3_71 ± 2.11 | <0.001 | 6.76 ± 4.32 | 2.71 ± 1.74 | 0.021 | |
| Villa 1999 [40] | AO drug (Naltrexone) | 9.3 ± 6.5 | 4.9 ± 3.1 | <0.05 | 8.9 ± 5.7 | 5.2 ± 3.3 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 10.7 ± 1.1 ! | 4.6 ± 0.9 ! | <0.01 | 10.1 ± 1.6 ! | 4.1 ± 1.1 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 8.3 ± 6.3 | 5.6 ± 4.6 | <0.05 | 0.98 [−0.03, 1.99] ^ | 0.37 [−0.50, 1.24] ^ | 0.25 # |
* Between-group difference; ! mean ± SEM; ^ estimated difference [95% confidence interval]; # p value adjusted for age, BMI, and HOMA-IR; AO, anti-obesity; FAI, free androgen index; NR, not reported; NS, not significant; PCOS, polycystic ovary syndrome; SHBG, sex hormone-binding globulin.
3.4. Outcomes: Reproductive Non-Fertility
3.4.1. Total Testosterone
Eight intervention groups from seven studies reported total testosterone [30,31,33,35,38,40,44]. One study with a hypocaloric diet + an anti-obesity drug intervention (Orlistat) [30], and another study with an anti-obesity drug intervention (Orlistat) + a hypocaloric diet + exercise intervention [35] reported a statistically significant difference in total testosterone following intervention between the two study groups, with the non-PCOS group having lower values in both studies (Table 3). However, as expected in both studies, the women with PCOS had statistically significantly higher levels of testosterone at baseline than women without PCOS.
3.4.2. Sex Hormone-Binding Globulin (SHBG)
Eight intervention groups from seven studies reported SHBG [30,31,33,35,38,40,44], with the majority of studies not reporting whether there were between-group statistical significance (Table 3). One study with a hypocaloric diet + anti-obesity drug (Orlistat) intervention [30] reported a statistically significant difference between groups (p < 0.05) following the intervention; however, there was also a statistically significant difference in SHBG at baseline between the groups (p < 0.05).
3.4.3. Free Androgen Index (FAI)
Five studies reported FAI [31,33,35,40,44], with one study with an anti-obesity drug intervention (Orlistat) + a hypocaloric diet + exercise intervention [35] reporting a statistically significant difference in values post intervention between groups (p = 0.021), with women in the non-PCOS group having a lower mean value (Table 3). However, as expected, women with PCOS in this study had a higher mean FAI at baseline (p < 0.001).
3.5. Outcomes: Metabolic
3.5.1. Fasting Glucose
Eleven intervention groups from nine studies reported fasting glucose [30,31,35,38,39,40,43,44,46]. The majority of studies did not report whether there were between-group statistical significance, and three studies [35,43,44] reported that there was no statistically significant difference in fasting glucose post-intervention between the two groups (Table 4).
Table 4.
Glucose and insulin homeostasis.
| Outcome | References | Intervention | Baseline PCOS: Mean ± SD | Baseline Non-PCOS: Mean ± SD | p-Value * | Post-Intervention PCOS: Mean ± SD | Post-Intervention Non-PCOS: Mean ± SD | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Fasting glucose (mmol/L) | Pasquali 2000 [38] | Hypocaloric diet | 5.61 ± 1.0 | 5.11 ± 0.56 | NS | 5.27 ± 0.61 | 5.16 ± 0.94 | NR |
| Cheang 2016 [43] | Hypocaloric diet | 4.75 ± 0.45 | 4.67 ± 0.21 | 0.60 | −0.06 (−0.25, 0.12) % | 0.01 (−0.13, 0.15) % | 0.5041 | |
| Toscani 2011 [39] | High protein diet | 4.97 ± 0.34 | 4.95 ± 0.45 | NS | 5.02 ± 0.40 | 5.05 ± 0.54 | NR | |
| Toscani 2011 [39] | Normal protein diet | 4.90 ± 0.36 | 4.98 ± 0.41 | NS | 4.98 ± 0.35 | 5.02 ± 0.35 | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 5.49 ± 1.61 | 4.94 ± 0.56 | NS | 5 ± 0.94 | 4.94 ± 0.72 | NR | |
| Diamanti-Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 5.72 ± 0.51 | 5.77 ± 0.61 | 0.92 | 5.52 ± 0.47 | 5.69 ± 0.52 | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 5.58 ± 0.57 | 5.81 ± 0.66 | NS | 5.28 ± 0.56 | 5.58 ± 0.51 | NS | |
| Villa 1999 [40] | AO drug (Naltrexone) | 4.65 ± 0.33 | 4.55 ± 0.22 | NS | 4.33 ± 0.56 | 4.48 ± 0.37 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 5.0 ± 0.1 ! | 4.8 ± 0.1 ! | 0.57 | 4.9 ± 0.1 ! | 4.9 ± 0.1 ! | NR | |
| Kogure 2016 [44] | Progressive resistance training | 5.34 ± 0.91 | 5.31 ± 0.97 | NS | 0.37 [0.08, 0.66] $ | 0.30 [0.04, 0.56] $ | 0.12 ** | |
| Al-Eisa 2017 [46] | Aerobic training | 4.50 ± 2.80 | 5.59 ± 1.57 | NR | 4.50 ± 2.80 | 5.59 ± 1.57 | NR | |
| OGTT-glucose (mmol/L) | Toscani 2011 [39] | High protein diet | 6.27 ± 1.60 | 5.02 ± 0.99 | NR | 6.92 ± 2.0 | 5.63 ± 1.56 | NR |
| Toscani 2011 [39] | Normal protein diet | 6.55 ± 1.50 | 5.41 ± 1.02 | NR | 6.64 ± 2.16 | 5.18 ± 1.02 | NR | |
| Diamanti-Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 6.35 ± 1.35 | 6.54 ± 1.54 | 0.65 | 6.17 ± 0.85 | 6.21 ± 1.77 | NR | |
| Fasting insulin (pmol/L) | Pasquali 2000 [38] | Hypocaloric diet | 240.36 ± 214.53 | 149.24 ± 79.64 | NS | 136.33 ± 103.32 | 103.32 ± 76.06 | NR |
| Toscani 2011 [39] | High protein diet | 76.05 (60.00–123.41) ^ | 57.85 (28.20–84.94) ^ | <0.05 | 61.67 (52.71–108.83) ^ | 56.74 (33.20–97.92) ^ | NR | |
| Toscani 2011 [39] | Normal protein diet | 128.83 (93.06–213.28) ^ | 59.59 (41.04–84.52) ^ | <0.05 | 129.15 (76.99–233.04) ^ | 48.00 (31.14–80.14) ^ | NR | |
| Pasquali 2000 [38] | Hypocaloric diet + Metformin | 308.53 ± 218.12 | 217.40 ± 58.84 | NS | 154.98 ± 223.86 | 102.6 ± 60.99 | NR | |
| Kowalska 2001 [33] | Hypocaloric diet + Metformin | 26.2 ± 13.9 # | 18.3 ± 14.2 # | <0.05 | 16.9 ± 9.1 # | 20.0 ± 13.5 # | NR | |
| Diamanti-Kandarakis 2007 [30] | Hypocaloric diet + AO drug (Orlistat) | 127.37 ± 61.12 | 125.5 ± 87.09 | 0.45 | 76.4 ± 34.93 | 77.02 ± 47.3 | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 129.87 ± 75.01 | 133.34 ± 161.82 | NS | 81.08 ± 50.94 | 68.16 ± 40.18 | NS | |
| Villa 1999 [40] | AO drug (Naltrexone) | 99.7 ± 75 | 104.3 ± 63.1 | NS | 90.4 ± 61 | 85.3 ± 39.4 | NR | |
| Hutchison 2011 [31] | Intensified exercise training | 141.6 (100.8–181.2) ^ | 72.6 (58.8–115.8) ^ | 0.02 | 97.8 (66.6–231.0) ^ | 115.2 (76.2–177.6) ^ | NR | |
| Kogure 2016 [44] | Progressive resistance training | 64.59 ± 47.92 | 36.11 ± 31.25 | <0.05 | 0.90 [−0.21, 1.94] $ | −0.90 [−1.88, 0.07] $ | 0.58 ** | |
| Al-Eisa 2017 [46] | Aerobic training | 17.8 ± 4.20 @ | 20.6 ± 8.2 @ | NR | 14.8 ± 2.9 @ | 16.1 ± 5.1 @ | NR |
* Between-group difference; ! mean ± SEM; ^ median (IQ range); # IU/L; @ data reported as mU/mL (as per original study); % mean (95% confidence interval); $ estimated difference [95% confidence interval]; ** p value adjusted for age, BMI, and HOMA-IR; AO, anti-obesity; f-glucose, fasting glucose; f-insulin, fasting insulin; NR, not reported; NS, not significant; OGTT-glucose, oral glucose tolerance test glucose; PCOS, polycystic ovary syndrome.
3.5.2. Oral Glucose Tolerance Test (OGTT-Glucose)
Three intervention groups from two studies reported OGTT-glucose [30,39]. None of these studies reported whether there were between-group statistical significance following the interventions (Table 4).
3.5.3. Fasting Insulin
Eleven intervention groups from nine studies reported fasting insulin [30,31,33,35,38,39,40,44,46]. Only one of these studies reported a p-value for the between-group difference following the interventions (p = 0.58) [44] (Table 4).
3.5.4. Lipids
Four intervention groups from three studies reported results for blood lipids [31,35,39]. One study with an anti-obesity drug intervention (Orlistat) + a hypocaloric diet + exercise intervention [35] reported a statistically significant difference in total, LDL and HDL cholesterol post-intervention between groups, with women in the PCOS group having lower mean values (Table 5).
Table 5.
Lipids and blood pressure.
| Outcome | References | Intervention | Baseline PCOS: Mean ± SD | Baseline Non-PCOS: Mean ± SD | p-Value * | Post-intervention PCOS: Mean ± SD | Post-intervention Non-PCOS: Mean ± SD | p-Value * |
|---|---|---|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | Toscani 2011 [39] | High protein diet | 4.60 ± 1.15 | 4.24 ± 0.71 | NR | 4.32 ± 1.12 | 4.19 ± 0.82 | NR |
| Toscani 2011 [39] | Normal protein diet | 4.31 ± 1.04 | 4.05 ± 1.13 | NR | 4.02 ± 0.82 | 3.86 ± 1.36 | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 5.04 ± 1.00 | 5.48 ± 0.93 | NS | 4.52 ± 0.82 | 4.55 ± 0.87 | <0.001 | |
| Hutchison 2011 [31] | Intensified exercise training | 5.0 ± 0.3 ! | 4.7 ± 0.2 ! | 0.81 | 4.4 ± 0.2 ! | 4.8 ± 0.4 ! | NR | |
| LDL cholesterol (mmol/L) | Toscani 2011 [39] | High protein diet | 3.91 ± 1.09 | 3.66 ± 0.61 | NR | 3.78 ± 1.1 | 3.54 ± 0.76 | NR |
| Toscani 2011 [39] | Normal protein diet | 3.62 ± 0.95 | 3.30 ± 1.11 | NR | 3.33 ± 0.75 | 3.13 ± 1.34 | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 3.30 ± 0.90 | 3.65 ± 0.68 | NS | 2.87 ± 0.84 | 2.98 ± 0.47 | 0.001 | |
| Hutchison 2011 [31] | Intensified exercise training | 3.3 ± 0.2 ! | 3.0 ± 0.2 ! | 0.48 | 3 ± 0.2 ! | 3.1 ± 0.4 ! | NR | |
| HDL cholesterol (mmol/L) | Toscani 2011 [39] | High protein diet | 1.30 ± 0.19 | 1.35 ± 0.38 | NR | 1.27 ± 0.2 | 1.34 ± 0.39 | NR |
| Toscani 2011 [39] | Normal protein diet | 1.19 ± 0.32 | 1.39 ± 0.26 | NR | 1.18 ± 0.32 | 1.5 ± 0.25 | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic exercise | 1.20 ± 0.22 | 1.32 ± 0.31 | NS | 1.21 ± 0.23 | 1.21 ± 0.26 | 0.006 | |
| Hutchison 2011 [31] | Intensified exercise training | 1.0 ± 0.1 ! | 1.2 ± 0.1 ! | 0.04 | 1 ± 0.1 ! | 1.2 ± 0.1 ! | NR | |
| Triglycerides (mmol/L) | Toscani 2011 [39] | High protein diet | 0.86 (0.47–1.47) ^ | 0.68 (0.47–0.76) ^ | NR | 0.55 (0.49–0.71) ^ | 0.92 (0.55–1.02) ^ | NR |
| Toscani 2011 [39] | Normal protein diet | 0.97 (0.67–1.30) ^ | 0.71 (0.41–1.62) ^ | NR | 0.94 (0.81–1.15) ^ | 0.88 (0.64–1.21) ^ | NR | |
| Panidis 2014 [35] | Hypocaloric diet + AO drug (Orlistat) + moderate intensity aerobic training | 1.17 ± 0.56 | 1.28 ± 0.62 | NS | 0.99 ± 0.44 | 0.87 ± 0.25 | NS | |
| Hutchison 2011 [31] | Intensified exercise training | 1.4 ± 0.2 ! | 1.2 ± 0.2 ! | 0.46 | 0.9 ± 0.1 ! | 1.3 ± 0.1 ! | NR | |
| BP systolic (mmHg) | Toscani 2011 [39] | High protein diet | 125.7 ± 19.0 | 116.1 ± 10.41 | NR | 126 ± 23.1 | 117.85 ± 10.18 | NR |
| Toscani 2011 [39] | Normal protein diet | 119.1 ± 16.4 | 116.43 ± 10.3 | NR | 119.36 ± 15.38 | 110.71 ± 7.32 | NR | |
| Harrison 2012 [32] | Intensified exercise training | 108 ± 14.6 ! | 118 ± 16.7 ! | NS | 109 ± 10.4 ! | 116 ± 16.2 ! | NR | |
| BP diastolic (mmHg) | Toscani 2011 [39] | High protein diet | 77.9 ± 10.75 | 74.6 ± 8.46 | NR | 80 ± 11.2 | 74 ± 8.83 | NR |
| Toscani 2011 [39] | Normal protein diet | 78 ± 11.83 | 75.14 ± 9.6 | NR | 77.82 ± 12.02 | 72.57 ± 7.72 | NR | |
| Harrison 2012 [32] | Intensified exercise training | 72 ± 10.2 ! | 75 ± 8.8 ! | NS | 69 ± 7.4 ! | 73 ± 10.5 ! | NR |
* Between-group difference; ! mean ± SEM; ^ median (IQ range); AO, anti-obesity; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NR, not reported; NS, not significant; PCOS, polycystic ovary syndrome.
3.5.5. Blood Pressure (BP)
Three intervention groups from two studies reported results for BP [32,39]. None of the studies reported whether there were between-group statistical significance (Table 5).
4. Discussion
In this systematic review, we evaluated for the first time the effect of weight management interventions in women with PCOS compared to women without PCOS. We identified 14 studies in 933 women with considerable clinical heterogeneity across a range of lifestyle (dietary and non-dietary) and non-lifestyle weight management interventions including diet, diet + behavior change program, diet + Metformin, diet + anti-obesity drug, anti-obesity drug, diet + anti-obesity drug + exercise, bariatric surgery, and various exercise training programs. Overall, there were no statistically significant differences in weight at the end of an intervention or in change in weight, between groups for five of the interventions included, with the remainder of studies not reporting whether there was a difference between groups post-intervention. For the majority of secondary endpoints, there was little difference between groups. Furthermore, five of the included studies had a moderate risk of bias, whilst nine studies had a high risk of bias.
The included studies are set in a research environment, with structured interventions, isocaloric intake and frequent contact and interaction with trained professionals. This mirrors many structured lifestyle interventions, and like all research in lifestyle interventions, occurred in a motivated research population.
As with all lifestyle intervention studies, our results may not reflect the outcomes of community-based studies or self-induced lifestyle change. Our previous research has shown a greater 10-year weight gain (2.6 kg (95% CI: 1.2–4.0)) in community-based cohort studies [17], and an elevated prevalence of overweight (risk ratio (RR) (95% CI): 1.95 (1.52, 2.50)), obesity (2.77 (1.88, 4.10)), central obesity (1.73 (1.31, 2.30)) and BMI (2.5 kg/m2 (95% CI: 1.9–3.1)) in systematic reviews [15] of women with PCOS compared to women without PCOS. This may relate to hyperinsulinemia, reduced postprandial thermogenesis [27], altered metabolic rate [47], impaired regulation of gut hormones or appetite regulation or limited self-regulation of food intake [48,49,50]. While the evidence here is not reliable enough to state conclusively if there is no difference in the effect of weight management interventions in women with and without PCOS, if this was indeed the case, this could indicate that appetite and self-regulation may be more prominent contributors to weight in PCOS. These mechanisms are more likely to selectively manifest in a free-living environment with ad libitum food intake. Our past longitudinal community cohort studies also show unselected community dwelling women with PCOS report a higher caloric intake, which corresponds closely to higher rates of weight gain [51]. More research into appetite and self-regulation around food intake are needed in PCOS.
The majority of secondary endpoints were similar between women with and without PCOS following the specified interventions. Similar to weight, a large number of the included studies did not report whether there was a statistically significant difference between groups for secondary endpoints following the intervention. While for a limited number of outcomes, there was a statistically significant difference in these measures at study completion, this difference was also present at baseline indicating that examining the within-group change from baseline to the end of the intervention would be more appropriate. We also report changes in secondary outcomes including total testosterone and SHBG at study completion between women with and without PCOS. However, women with PCOS generally have hyperandrogenism and lower SHBG [52] and differences at the end of the intervention are again likely related to baseline differences.
Here, it was not possible to determine the relative effectiveness of specific dietary or non-dietary interventions. Based on our findings reported elsewhere [21,22], recommendations in PCOS for lifestyle or pharmacological intervention and weight management should mirror those for the general population. These include a diet with reduced energy intake and a weight loss maintenance program for maintaining weight. Pharmacological treatment can increase efficacy of lifestyle interventions [53,54]. A recent meta-analysis reported that a metformin plus lifestyle intervention results in more reduction in fat and improved menstrual cyclicity, compared to lifestyle alone in PCOS [53]. In general populations, a large meta-analysis (n = 80 studies, n = 26,455 participants) reported that diet interventions in isolation are as effective for short-term (six months) weight loss compared to anti-obesity drugs. Long-term (24 months) weight-loss, however, was greater with anti-obesity drugs [55]. Overall, bariatric surgery is an option for those who have not responded to these interventions with sufficient weight loss or for those with morbid obesity and co-morbidities [56,57]. Overall, the relative efficacy of different weight management interventions in women with and without PCOS warrants further investigation.
The strengths of this study include the comprehensive range of lifestyle and weight management interventions and of obesity-related outcomes studied. We also included studies where weight loss was not the specific goal, broadening applicability of our findings to weight gain prevention, weight maintenance and use in lean women with PCOS. The weaknesses of this review include the inability to statistically combine study results due to clinical heterogeneity. Additionally, this systematic review was limited to studies in English. Many of the included studies did not report numeric differences between groups and relevant data could not be extracted. The identified studies also had moderate to high risk of bias and were generally small with significant drop-outs. Many did not strictly exclude PCOS status in their controls. Future research should address these methodological weaknesses to improve study quality. We also did not prospectively register this review on PROSPERO; however, it was designed in 2012 at which time PROSPERO had only been active for a relatively short amount of time, and it was not yet standard practice for all systematic reviews to be prospectively registered. Additional biases may also have occurred through identifying articles only in English and through not contacting authors for missing data. Detailed reporting of inclusion and exclusion criteria, increased participant numbers, attrition rates, anthropometric measurement protocols, and publishing study protocols are suggested to improve the reliability and validity of future research. Endpoint reporting could also be improved across the range of obesity-related health implications in PCOS, and through the use of a pre-specified list of outcomes similar to that suggested for infertility treatment [58].
5. Conclusions
We report on the first systematic review comparing the effectiveness of weight management strategies in women with and without PCOS. Our findings are that there is insufficient evidence to indicate significant differences in weight loss in women with PCOS compared to women without PCOS with lifestyle or weight management strategies. We cannot determine the relative efficacy of different types of weight management interventions and note considerable limitations with the current studies. Further research with larger samples is warranted in controlled clinical trials and free-living environments assessing a full range of defined PCOS and obesity-related health implications. At the current time, recommendations for weight management for the general population should be applied for women with PCOS with some degree of confidence that similar efficacy of structured interventions is likely.
Acknowledgments
L.J.M. is supported by a National Heart Foundation Future Leader Fellowship. H.T. holds an National Health and Medical Research Council (NHMRC) Practitioner fellowship.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6643/9/9/996/s1, Table S1: Selection criteria, Table S2: Search terms, Table S3: Excluded studies, Table S4: Critical appraisals of included studies.
Author Contributions
J.K. contributed to the conception and design of the study; acquisition, analysis and interpretation of data; prepared, drafted and revised the article critically for important intellectual content and approved the final draft for publication. M.M. contributed substantially to the design of the study; analysis and interpretation of data; prepared, drafted and revised the article for important intellectual content and approved the final draft for publication. A.E.J. contributed to acquisition of data; interpretation of data; and revised the article for important intellectual content and approved the final draft for publication. E.S.-V. contributed to the conception and design of the study; and revised the article critically for important intellectual content and approved the final draft for publication. H.T. contributed to the conception and design of the study; interpretation of data; and revised the article critically for important intellectual content and approved the final draft for publication. L.J.M. contributed to the conception and design of the study; analysis and interpretation of data; and prepared, drafted and revised the article critically for important intellectual content and approved the final draft for publication. E.C.T. contributed to the acquisition, analysis and interpretation of data in the updated searches; updated and revised the article critically for important intellectual content and approved the final draft for publication.
Conflicts of Interest
The authors declare no conflict of interest. None of the funders had any role in the design, analysis or writing of this article.
References
- 1.March W.A., Moore V.M., Willson K.J., Phillips D.I., Norman R.J., Davies M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion M., Calvo R.M., San Millan J.L., Sancho J., Avila S., Escobar-Morreale H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected caucasian women from Spain. J. Clin. Endocrinol. Metab. 2000;85:2434–2438. doi: 10.1210/jc.85.7.2434. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E., Kouli C.R., Bergiele A.T., Filandra F.A., Tsianateli T.C., Spina G.G., Zapanti E.D., Bartzis M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 4.Stepto N.K., Cassar S., Joham A.E., Hutchison S.K., Harrison C.L., Goldstein R.F., Teede H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013;28:777–784. doi: 10.1093/humrep/des463. [DOI] [PubMed] [Google Scholar]
- 5.Moran L.J., Misso M.L., Wild R.A., Norman R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update. 2010;16:347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 6.Teede H., Deeks A., Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:1–10. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild R.A., Carmina E., Diamanti-Kandarakis E., Dokras A., Escobar-Morreale H.F., Futterweit W., Lobo R., Norman R.J., Talbott E., Dumesic D.A. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 8.Broekmans F.J., Knauff E.A.H., Valkenburg O., Laven J.S., Eijkemans M.J., Fauser B. PCOS according to the Rotterdam consensus criteria: Change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- 9.Boomsma C.M., Eijkemans M.J., Hughes E.G., Visser G.H., Fauser B.C., Macklon N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 10.Barry J.A., Kuczmierczyk A.R., Hardiman P.J. Anxiety and depression in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2011;26:2442–2451. doi: 10.1093/humrep/der197. [DOI] [PubMed] [Google Scholar]
- 11.Vink J.M., Sadrzadeh S., Lambalk C.B., Boomsma D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 12.Dunaif A. Insulin resistance in women with polycystic ovary syndrome. Fertil. Steril. 2006;86(Suppl. 1):S13–S14. doi: 10.1016/j.fertnstert.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Gilling-Smith C., Story H., Rogers V., Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin. Endocrinol. 1997;47:93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 14.Moret M., Stettler R., Rodieux F., Gaillard R.C., Waeber G., Wirthner D., Giusti V., Tappy L., Pralong F.P. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology. 2009;89:131–139. doi: 10.1159/000160911. [DOI] [PubMed] [Google Scholar]
- 15.Lim S.S., Davies M.J., Norman R.J., Moran L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update. 2012;18:618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 16.Yildiz B.O., Bozdag G., Yapici Z., Esinler I., Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 2012;27:3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 17.Teede H.J., Joham A.E., Paul E., Moran L.J., Loxton D., Jolley D., Lombard C. Longitudinal weight gain in women identified with polycystic ovary syndrome: Results of an observational study in young women. Obesity. 2013;21:1526–1532. doi: 10.1002/oby.20213. [DOI] [PubMed] [Google Scholar]
- 18.Balen A.H., Conway G.S., Kaltsas G., Techatrasak K., Manning P.J., West C., Jacobs H.S. Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum. Reprod. 1995;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 19.Kiddy D.S., Sharp P.S., White D.M., Scanlon M.F., Mason H.D., Bray C.S., Polson D.W., Reed M.J., Franks S. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: An analysis of 263 consecutive cases. Clin. Endocrinol. (Oxf.) 1990;32:213–220. doi: 10.1111/j.1365-2265.1990.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 20.Hart R., Doherty D.A. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J. Clin. Endocrinol. Metab. 2015;100:911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 21.Moran L.J., Pasquali R., Teede H.J., Hoeger K.M., Norman R.J. Treatment of obesity in polycystic ovary syndrome: A position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil. Steril. 2009;92:1966–1982. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Teede H.J., Misso M.L., Deeks A.A., Moran L.J., Stuckey B.G., Wong J.L., Norman R.J., Costello M.F. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med. J. Aust. 2011;195:S65–S112. doi: 10.5694/mja11.10915. [DOI] [PubMed] [Google Scholar]
- 23.European Society of Human Reproduction and Embryology. The Thessaloniki, and ASRM-Sponsored PCOS Consensus Workshop Group Consensus on infertility treatment related to polycystic ovary syndrome. Hum. Reprod. 2008;23:462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 24.Legro R.S., Dodson W.C., Kris-Etherton P.M., Kunselman A.R., Stetter C.M., Williams N.I., Gnatuk C.L., Estes S.J., Fleming J., Allison K.C., et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2015;100:4048–4058. doi: 10.1210/jc.2015-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran L.J., Noakes M., Clifton P.M., Tomlinson L., Galletly C., Norman R.J. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003;88:812–819. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 26.Moran L.J., Noakes M., Clifton P.M., Wittert G.A., Le Roux C.W., Ghatei M.A., Bloom S.R., Norman R.J. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am. J. Clin. Nutr. 2007;86:1603–1610. doi: 10.1093/ajcn/86.5.1603. [DOI] [PubMed] [Google Scholar]
- 27.Robinson S., Chan S.-P., Spacey S., Anyaoku V., Johnston D.G., Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin. Endocrinol. 1992;36:537–543. doi: 10.1111/j.1365-2265.1992.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [(accessed on 7 September 2017)]; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Diamanti-Kandarakis E., Katsikis I., Piperi C., Alexandraki K., Panidis D. Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2007;66:103–109. doi: 10.1111/j.1365-2265.2006.02693.x. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison S.K., Stepto N.K., Harrison C.L., Moran L.J., Strauss B.J., Teede H.J. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011;96:E48–E56. doi: 10.1210/jc.2010-0828. [DOI] [PubMed] [Google Scholar]
- 32.Harrison C.L., Stepto N.K., Hutchison S.K., Teede H.J. The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndrome. Clin. Endocrinol. (Oxf.) 2012;76:351–357. doi: 10.1111/j.1365-2265.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- 33.Kowalska I., Kinalski M., Straczkowski M., Wolczyski S., Kinalska I. Insulin, leptin, IGF-I and insulin-dependent protein concentrations after insulin-sensitizing therapy in obese women with polycystic ovary syndrome. Eur. J. Endocrinol. 2001;144:509–515. doi: 10.1530/eje.0.1440509. [DOI] [PubMed] [Google Scholar]
- 34.Moran L.J., Noakes M., Clifton P.M., Wittert G.A., Belobrajdic D.P., Norman R.J. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2007;92:2944–2951. doi: 10.1210/jc.2006-2336. [DOI] [PubMed] [Google Scholar]
- 35.Panidis D., Tziomalos K., Papadakis E., Chatzis P., Kandaraki E.A., Tsourdi E.A., Katsikis I. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin. Endocrinol. 2014;80:432–438. doi: 10.1111/cen.12305. [DOI] [PubMed] [Google Scholar]
- 36.Vosnakis C., Georgopoulos N.A., Rousso D., Mavromatidis G., Katsikis I., Roupas N.D., Mamali I., Panidis D. Diet, physical exercise and Orlistat administration increase serum anti-Mullerian hormone (AMH) levels in women with polycystic ovary syndrome (PCOS) Gynecol. Endocrinol. 2013;29:242–245. doi: 10.3109/09513590.2012.736557. [DOI] [PubMed] [Google Scholar]
- 37.Panidis D., Farmakiotis D., Rousso D., Kourtis A., Katsikis I., Krassas G. Obesity, weight loss, and the polycystic ovary syndrome: Effect of treatment with diet and orlistat for 24 weeks on insulin resistance and androgen levels. Fertil. Steril. 2008;89:899–906. doi: 10.1016/j.fertnstert.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 38.Pasquali R., Gambineri A., Biscotti D., Vicennati V., Gagliardi L., Colitta D., Fiorini S., Cognigni G.E., Filicori M., Morselli-Labate A.M. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2000;85:2767–2774. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 39.Toscani M.K., Mario F.M., Radavelli-Bagatini S., Wiltgen D., Matos M.C., Spritzer P.M. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: A randomized study. Gynecol. Endocrinol. 2011;27:925–930. doi: 10.3109/09513590.2011.564686. [DOI] [PubMed] [Google Scholar]
- 40.Villa P., Valle D., Mancini A., De Marinis L., Pavone V., Fulghesu A.M., Mancuso S., Lanzone A. Effect of opioid blockade on insulin and growth hormone (GH) secretion in patients with polycystic ovary syndrome: The heterogeneity of impaired GH secretion is related to both obesity and hyperinsulinism. Fertil. Steril. 1999;71:115–121. doi: 10.1016/S0015-0282(98)00405-1. [DOI] [PubMed] [Google Scholar]
- 41.Kahal H., Aburima A., Ungvari T., Rigby A.S., Coady A.M., Vince R.V., Ajjan R.A., Kilpatrick E.S., Naseem K.M., Atkin S.L. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr. Disord. 2015;15:14. doi: 10.1186/s12902-015-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikokavoura E.A., Johnston K.L., Broom J., Wrieden W.L., Rolland C. Weight loss for women with and without polycystic ovary syndrome following a very low-calorie diet in a community-based setting with trained facilitators for 12 weeks. Diabetes Metab. Syndr. Obes. Targets Ther. 2015;8:495. doi: 10.2147/DMSO.S85134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheang K.I., Sistrun S.N., Morel K.S., Nestler J.E. Effect on insulin-stimulated release of D-chiro-inositol-containing inositolphosphoglycan mediator during weight loss in obese women with and without polycystic ovary syndrome. Int. J. Endocrinol. 2016;2016:7631804. doi: 10.1155/2016/7631804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kogure G.S., Miranda-Furtado C.L., Silva R.C., Melo A.S., Ferriani R.A., De Sá M., Dos Reis R.M. Resistance exercise impacts lean muscle mass in women with polycystic ovary syndrome. Med. Sci. Sports Exerc. 2016;48:589–598. doi: 10.1249/MSS.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 45.Bhandari S., Ganguly I., Bhandari M., Agarwal P., Singh A., Gupta N., Mishra A. Effect of sleeve gastrectomy bariatric surgery-induced weight loss on serum AMH levels in reproductive aged women. Gynecol. Endocrinol. 2016;32:799–802. doi: 10.3109/09513590.2016.1169267. [DOI] [PubMed] [Google Scholar]
- 46.Al-Eisa E., Gabr S.A., Alghadir A.H. Effects of supervised aerobic training on the levels of anti-Mullerian hormone and adiposity measures in women with normo-ovulatory and polycystic ovary syndrome. J. Pak. Med. Assoc. 2017;67:499. [PubMed] [Google Scholar]
- 47.Georgopoulos N.A., Saltamavros A.D., Vervita V., Karkoulias K., Adonakis G., Decavalas G., Kourounis G., Markou K.B., Kyriazopoulou V. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertil. Steril. 2009;92:250–255. doi: 10.1016/j.fertnstert.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 48.Hirschberg A.L., Naessen S., Stridsberg M., Bystrom B., Holtet J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2004;19:79–87. doi: 10.1080/09513590400002300. [DOI] [PubMed] [Google Scholar]
- 49.Moran L.J., Noakes M., Clifton P.M., Wittert G.A., Tomlinson L., Galletly C., Luscombe N.D., Norman R.J. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J. Clin. Endocrinol. Metab. 2004;89:3337–3344. doi: 10.1210/jc.2003-031583. [DOI] [PubMed] [Google Scholar]
- 50.Moran L.J., Noakes M., Clifton P.M., Wittert G.A., Williams G., Norman R.J. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am. J. Clin. Nutr. 2006;84:77–87. doi: 10.1093/ajcn/84.1.77. [DOI] [PubMed] [Google Scholar]
- 51.Moran L.J., Ranasinha S., Zoungas S., McNaughton S.A., Brown W.J., Teede H.J. The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum. Reprod. 2013;28:2276–2283. doi: 10.1093/humrep/det256. [DOI] [PubMed] [Google Scholar]
- 52.Legro R.S., Kunselman A.R., Dodson W.C., Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 1999:84. doi: 10.1097/00006254-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Naderpoor N., Shorakae S., de Courten B., Misso M.L., Moran L.J., Teede H.J. Metformin and lifestyle modification in polycystic ovary syndrome: Systematic review and meta-analysis. Hum. Reprod. Update. 2015;21:560–574. doi: 10.1093/humupd/dmv025. [DOI] [PubMed] [Google Scholar]
- 54.Apovian C.M., Aronne L.J., Bessesen D.H., McDonnell M.E., Murad M.H., Pagotto U., Ryan D.H., Still C.D. Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 55.Franz M.J., VanWormer J.J., Crain A.L., Boucher J.L., Histon T., Caplan W., Bowman J.D., Pronk N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., Hu F.B., Hubbard V.S., Jakicic J.M., Kushner R.F., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yumuk V., Tsigos C., Fried M., Schindler K., Busetto L., Micic D., Toplak H. European guidelines for obesity management in adults. Obes. Facts. 2015;8:402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harbin Consensus Conference Workshop Group Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT): Modifying the CONSORT statement. Fertil. Steril. 2014;102:952–959. doi: 10.1016/j.fertnstert.2014.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.