Abstract
Despite use of newer approaches, some patients being considered for autologous hematopoietic cell transplantation (HCT) may only mobilize limited numbers of hematopoietic progenitor cells (HPCs) into blood, precluding use of the procedure, or being placed at increased risk of complications due to slow hematopoietic reconstitution. Developing more efficacious HPC mobilization regimens and strategies may enhance the mobilization process and improve patient outcome. Although Notch signaling is not essential for homeostasis of adult hematopoietic stem cells (HSCs), Notch-ligand adhesive interaction maintains HSC quiescence and niche retention. Using Notch receptor blocking antibodies, we report that Notch2 blockade, but not Notch1 blockade, sensitizes hematopoietic stem cells and progenitors (HSPCs) to mobilization stimuli and leads to enhanced egress from marrow to the periphery. Notch2 blockade leads to transient myeloid progenitor expansion without affecting HSC homeostasis and self-renewal. We show that transient Notch2 blockade or Notch2-loss in mice lacking Notch2 receptor lead to decreased CXCR4 expression by HSC but increased cell cycling with CXCR4 transcription being directly regulated by the Notch transcriptional protein RBPJ. In addition, we found that Notch2-blocked or Notch2-deficient marrow HSPCs show an increased homing to the marrow, while mobilized Notch2-blocked, but not Notch2-deficient stem cells and progenitors, displayed a competitive repopulating advantage and enhanced hematopoietic reconstitution. These findings suggest that blocking Notch2 combined with the current clinical regimen may further enhance HPC mobilization and improve engraftment during HCT.
Introduction
Hematopoietic cell transplantation (HCT) is the only curative option for various neoplastic and a few non-neoplastic diseases.1 The vast majority of clinical autologous HCT procedures utilize hematopoietic progenitor cells (HPCs) mobilized into the blood. For a variety of reasons, some patients may not mobilize adequate numbers of HPCs and thus are not candidates for the autologous HCT procedure. In addition, in some subjects, less than an optimal number of HPCs may be obtained, resulting in slower hematopoietic reconstitution and increased risk of complications during the transplant.2–4 In recent years, the use of CXCR4 antagonizing molecules/peptides (i.e. AMD3100 or plerixafor) has enhanced HPC mobilization and overcome some of these limitations.5 Inadequate mobilization, however, still remains a problem for many patients and the development of more efficacious strategies may enhance patient outcome.6
The signaling molecule Notch is important for stem cell self-renewal and fate determination in many tissues, including the hematopoietic system. An important feature of Notch is its adhesive nature which was first described by cell aggregation assays in Drosophila studies.7,8 There are 4 Notch receptors (Notch1-4), and 2 families of Notch ligand: Jagged (JAG1-2), and delta-like (DLL1-4) ligand. Notch2 is the major isotype expressed on hematopoietic stem cells (HSC) and non-lymphoid progenitor cells.9–12 However, the precise role and the physiological significance of Notch receptors, either as adhesion and/or signaling molecules, in HSC homeostasis and functional support are still not completely understood.
Notch signaling transactivation is consequent to a functional engagement of the Notch receptor with the Notch ligand. We previously reported that hematopoietic stem cell and progenitors (HSPCs) with faulty Notch-ligand interaction due to the loss of O-fucose modification of Notch display increased cell cycling and decreased adhesion to marrow osteoblastic lineage cells.11 These HSPCs exhibit enhanced egress from the marrow. However, the significance and the mechanism of Notch downstream signaling in the maintenance of HSC quiescence are not clear. Here we report that prior treatment with Notch2 blocking antibody sensitizes HSPC to the mobilizing stimuli of G-CSF and AMD3100 with a 3–4-fold increase in mobilization without affecting the overall bone marrow HSC homeostasis and self-renewal. Moreover, we demonstrate that Notch signaling directly regulates CXCR4 expression, and hence transient Notch2 blockade decreases CXCR4 concentration and increases cell cycling. Consistent with these findings, transient Notch2 blockade leads to greater HSPC homing to the marrow and a competitive repopulating advantage of the progenitors with enhanced recovery of hematopoietic elements.
Methods
Mice
The Institutional Animal Care and Use Committee of Case Western Reserve University approved all aspects of the animal research described in this study. C57Bl/6 (Ly5.2) and B6.SJL-Ptrca Pep3b/BoyJ (B6.BoyJ:Ly5.1) mice were maintained in the lab. Vav-Cre/Notch2F/F mice were generated by crossing Vav-Cre mice (008610; Jackson Laboratory, Bar Harbor, ME, USA) with Notch2F/F mice (010525; Jackson Laboratory, Bar Harbor, ME, USA).
In vivo Notch receptor blockade
Humanized anti-Notch1 (anti-NRR1, Genentech), anti-Notch2 (anti-NRR2, Genentech) or control antibody (anti-ragweed, Genentech, South San Francisco, CA, USA) have been described previously.11 Antibodies were injected i.p. either at 15 mg/mL for anti-NRR1 and anti-ragweed, or at 25 mg/mL for anti-NRR2 as a single dose or twice weekly three days apart for a total of 4 doses.
HSPC mobilization assays
Hematopoietic stem cell and progenitor mobilization was performed as described.13 Briefly, mice were injected subcutaneously with 2.5 mg G-CSF (Amgen, Thousand Oaks, CA, USA), twice daily for two days, followed by subcutaneous injection of 5 mg/kg AMD3100 (Sigma-Aldrich, St. Louis, MO, USA). Blood (250 mL) and hematopoietic tissues were collected 1 hour (h) later for the determination of circulating, splenic and marrow HSPC frequencies.
Bone marrow analysis, transplantation, qRT-PCR, cell cycle analysis, chromatin immunoprecipitation, luciferase reporter analysis, and multi-photon intravital imaging
See details in the Online Supplementary Appendix.
Statistical analysis
Data are presented as mean±Standard Deviation (S.D.) unless otherwise stated. Statistical significance was assessed by Student t-test, Pearson χ2 test, and Wilcoxon rank sum test.
Results
Transient Notch2 signaling blockade promotes HSPC egress in response to mobilizing reagents
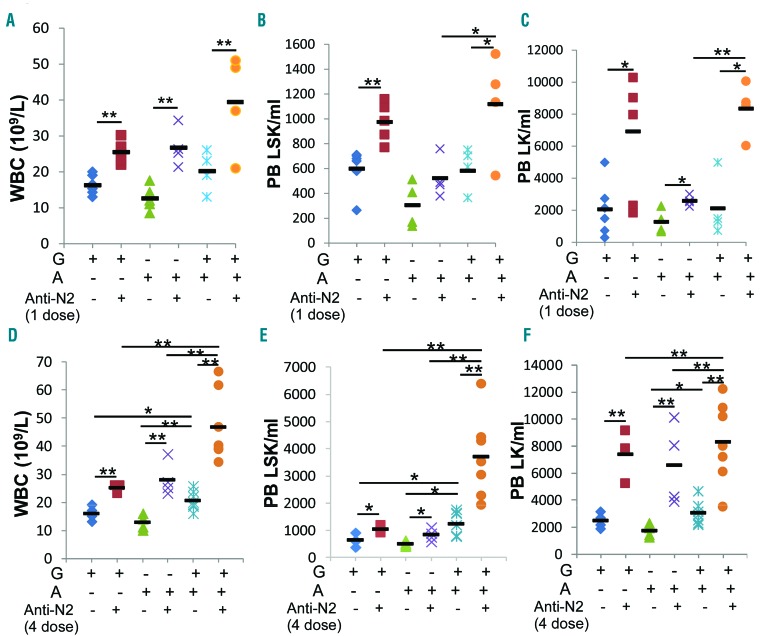
To examine the effects of Notch signaling blockade in HSPC egress, we applied Notch blocking antibody or iso-type control antibody to wild-type (WT) mice. The Notch receptor-specific antibody does not interfere with receptor-ligand interaction but blocks the cleavage of Notch receptor and hence the downstream signaling activation. Because a single dose of Notch1 or Notch2 blocking antibody did not increase HSPC circulation in the periphery (data not shown), we applied a single dose of Notch blocking antibody followed by treatment with mobilizing reagents, using a commonly adopted regimen in mouse studies [4 doses of granulocyte-colony stimulating factor (G-CSF) or one dose of AMD3100, either alone or combined].13 We found that a single dose of anti-Notch2 followed by G-CSF or AMD3100 resulted in a 56% and 111% increase in white blood cell (WBC) counts compared with either reagent alone. Anti-Notch2 plus G-CSF increased circulating LSK and LK cells by 63% and 2.4-fold more, respectively, than G-CSF alone, while anti-Notch2 plus AMD3100 had a milder effect. When anti-Notch2 was followed by combined G-CSF and AMD3100 stimulation, it further increased WBC count and mobilized more LSKs and LKs (Figure 1A–C). We did not observe any significant change in circulating HSPCs in mice receiving anti-Notch1 (data not shown).
Figure 1.
Notch2 blocking antibody sensitizes hematopoietic stem cell and progenitors (HSPC) egress in response to granulocyte-colony stimulating factor (G-CSF) or/and AMD3100. Mice were given 4 doses of G-CSF in two days, or a single dose of AMD3100, or 4 doses of G-CSF followed by AMD3100 the next day, two days after a single dose (A–C) or 4 doses of Notch2-blocking or control antibody (D–F) (see details of treatment scheme in Online Supplementary Figure S1). Twenty-four hours (h) after the last dose of G-CSF, or 1 h after AMD3100, peripheral blood (PB) was analyzed for white blood cell (WBC) counts (A and D) and the presence of LSK (B and E) and LK (C and F) cells by FACS in PB (n=4–7/group). Results are pooled from 3 independent experiments and presented as mean±Standard Deviation (S.D.). Student t-test *P<0.05, **P<0.01.
Previous studies showed that up to 4 doses of Notch2 blocking antibody were required to achieve the complete on-target biological effects of Notch2 inhibition.14 We tested this observation by applying 4 doses of antibodies to mice (Online Supplementary Figure S1A). We observed a 68% increase in WBC counts in mice receiving 4 doses of anti-Notch2 alone. We also observed a moderate increase in circulating LK cells in mice receiving either anti-Notch1 or anti-Notch2 (Online Supplementary Figure S1B and C). Spleen-residing LSK and LK cells, however, increased after Notch2 blockade but not after Notch1 blockade (Online Supplementary Figure S1D).
We then compared a single dose versus 4 doses of Notch2 antibody combined with G-CSF or/and AMD3100 (Online Supplementary Figure S1E). Four doses of anti-Notch2 combined with G-CSF or AMD3100 alone resulted in an 87% and 120% increase in WBC counts, increased circulating LKs by 2.0- and 2.8-fold more, and increased LSKs by 61% and 68% more, respectively, than either reagent alone (Figure 1D–F). When 4 doses of anti-Notch2 were combined with both reagents, WBC counts further increased to 46.6×109/L, and LSKs and LKs increased by 2.0-fold and 1.7-fold more in the periphery compared to controls that were stimulated with both reagents but without anti-Notch2 (Figure 1D–F). In comparison, mice receiving Notch1 antibody had no significant increase in HSPCs in the circulation or in the spleen (Online Supplementary Figure S1F–H). Both splenic LSK and LK frequencies were also increased by combining anti-Notch2 with either G-CSF or AMD3100, and further increased by combining both reagents (Online Supplementary Figure S1I). We concluded from these observations that: 1) blocking Notch2 but not Notch1 induces enhanced HSPC egress; 2) although Notch2 blockade synergizes with either G-CSF or AMD3100 in promoting HSPC egress, a stronger stimulating effect on WBC counts and mobilized LSKs is achieved when anti-Notch2 is combined with both reagents; while 3) a single dose anti-Notch2 in the combined regimen showed a similar effect on increasing WBC and LK numbers as the 4-dose regimen, it induced a lower increment in circulating LSK than the 4-dose regimen.
Notch2 deficiency causes increased HSPC egress
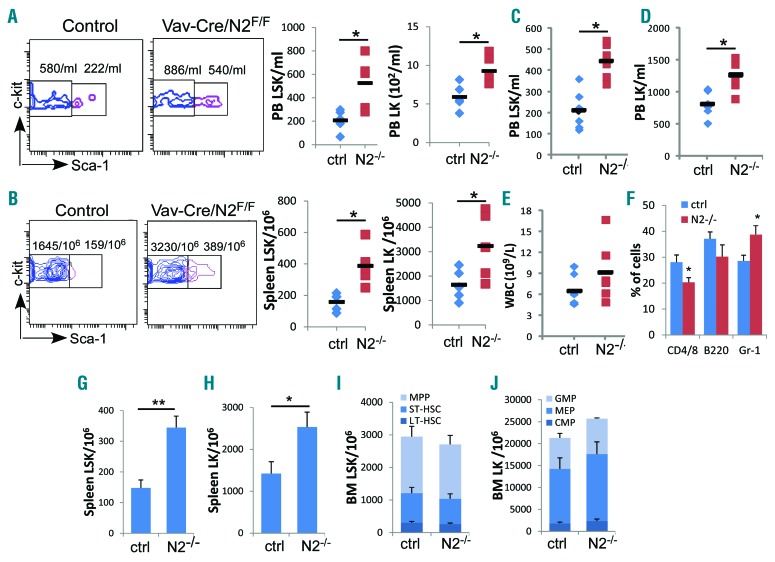
To confirm that the observed HSPC egress after Notch2 blockade is indeed caused by the loss of Notch2 signaling, we analyzed mice with Notch2 deficiency in the hematopoietic system using the Vav-Cre/Notch2F/F mice that had efficient deletion of Notch2 expression on HSPCs (Online Supplementary Figure S2). We found that circulating LSK and LK cells increased from 208/mL and 591/mL in control mice to 526/mL and 927/mL in Notch2-deficient mice (Figure 2A), respectively. Spleen-residing LSKs and LKs also increased by 2.4- and 2.0-fold more compared to the control mice (Figure 2B). Moreover, the increased HSPC egress persisted after Notch2-deficient bone marrow cells were transferred into lethally irradiated WT mice (Figure 2C and D). The greater HSPC egress was accompanied by a 46% increase in WBC counts (Figure 2E) and 30% increase in circulating granulocytes, but a 28% relative reduction in circulating T cells at 12 weeks after transplantation (Figure 2F). In addition, there were 1.3-fold and 78% increases in LSKs and LKs in the spleen (Figure 2G and H). In comparison, consistent with other reports, the bone marrow HSPC homeostasis of the de novo Vav-Cre/Notch2F/F mice or of the recipients receiving Notch2-deficient cells remained largely unaffected (Figure 2I and J).12,15 These findings are consistent with the increased circulating and spleen-residing HSPCs in mice receiving Notch2 blocking antibody, and suggest that Notch2 signaling loss is responsible for increased HSPC egress.
Figure 2.
Notch2 deficiency causes increased hematopoietic stem cell and progenitors (HSPC) egress. (A and B) HSPCs present in the periphery (A) and in the spleen (B) of 8-week old Vav-Cre/Notch2F/F mice and control mice (Vav-Cre/Notch2+/+) were determined by FACS. Representative FACS profile (gated on Lin−c-kit+ cells) and bar graphs of total numbers of LSK cells and LK cells in the blood (A) and in the spleen (B) pooled from 3 similar experiments in which Vav-Cre/Notch2F/F and control mice (n=5) were examined. (C–F) Total numbers of LSK cells (C), LK cells (D), white blood cell counts (E), and frequencies of T cells (CD4/CD/8), B cells (B220) and granulocytes (Gr-1) (F) present in the peripheral blood (PB) of recipient mice 12 weeks after receiving bone marrow (BM) transplantation from control (n=6) or Vav-Cre/Notch2F/F mice (n=6). (G–J) Spleen-residing LSK (G) and LK (H) frequencies, as well as BM HSC subpopulations, including LT-HSC (Flt3−CD34−LSK), ST-HSC (Flt3−CD34+LSK) and MPP (Flt3+CD34+LSK) (I), and LK subsets including CMP (Lin−c-kit+Sca–1−CD34+FcγRII/III−), MEP (Lin−c-kit+Sca-1−CD34−FcγRII/III−) and GMP (Lin−c-kit+Sca-1−CD34+FcγRII/III+) (J) were determined by FACS in the same group of transplanted mice. Results are pooled from 2 experiments and presented as mean±Standard Deviation (S.D.). Student t-test *P<0.05, **P<0.01.
Notch2 blockade mildly alters bone marrow HSPC homeostasis but does not affect HSC self-renewal
It is important to determine if Notch2 blockade would adversely affect HSPC homeostasis and HSC self-renewal and differentiation. We therefore analyzed marrow HSPC populations after 4 doses of Notch2 antibody treatment. The numbers of marrow total LSK, LK, and common lymphoid progenitor (CLP) cells were decreased modestly in Notch2 antibody-treated mice compared to control-treated mice (Online Supplementary Figure S3A–C). A more detailed analysis of LSK and LK subpopulations revealed a mild reduction of multi-potential progenitors (MPPs), common myeloid progenitors (CMPs), and megakaryocyte-erythroid progenitors (MEPs) in Notch2 antibody-treated mice compared to control mice (Online Supplementary Figure S3D and E). While it is possible that Notch2 blockade caused a transient reduction of the marrow HSPC generation,12 these alterations also are likely reflective of HSPC redistribution by Notch2 blockade, considering that HSPC numbers were increased in the periphery and in the spleen (Figure 1) and there is no change in HSPC apoptosis (Online Supplementary Figure S3F). On the other hand, we did not observe any significant alterations in any of the HSPC subsets in mice that received Notch2 antibody combined with G-CSF and AMD3100 (Online Supplementary Figure S3G–I). These data suggest that mild HSPC alterations caused by Notch2 blockade can be compensated by the proliferative stimuli through G-CSF and/or AMD3100. We also noted that mice receiving Notch1 antibody had a 32% reduction in CLP cells (Online Supplementary Figure S3C), a finding consistent with other reports that Notch1 is involved in the CLP development in the marrow.9,16
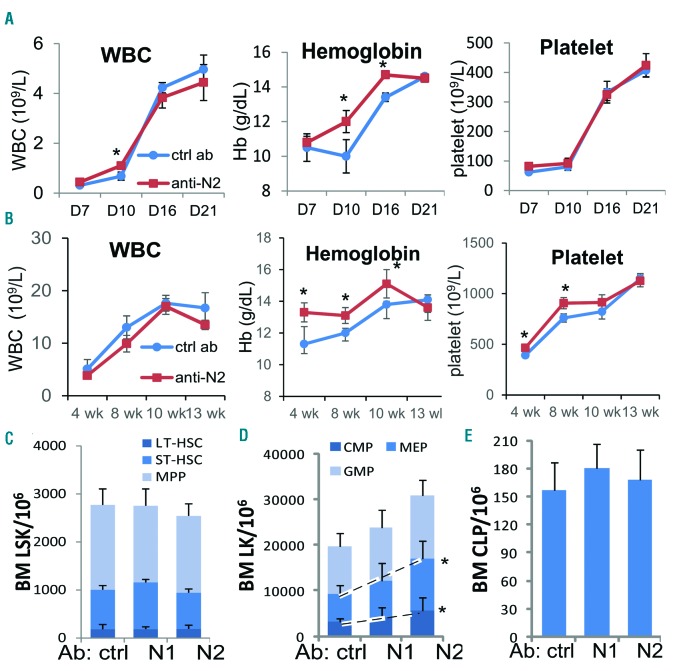
We then assessed the effects of Notch blockade on marrow HSC differentiation and self-renewal by transplanting Notch1- or Notch2-blocked bone marrow cells into lethally irradiated WT mice. On day 10, we observed a transient high WBC count and a higher hemoglobin concentration which continued until ten weeks after transplantation, and higher platelets at four and eight weeks in mice receiving Notch2-blocked marrow cells than in mice receiving control antibody-treated cells (Figure 3A and B) or Notch1-blocked donor cells (Online Supplementary Figure S4). We did not find any significant alteration in the numbers of LSK cells, stem cell subpopulations, or CLP in mice receiving Notch2-blocked marrow cells (Figure 3C–E). However, the MEPs and CMPs derived from Notch2-blocked donors, but not from Notch1-blocked donors, were expanded by approximately 92% and 75%, respectively, compared to those derived from control antibody-treated donors (Figure 3D).
Figure 3.
Faster hematopoietic recovery from Notch2-blocked marrow progenitors after transplantation. (A and B) Platelet counts, white blood cell (WBC) counts, and hemoglobin levels on days 7, 10, 16 and 21 (A) (n=4–5/group), and at 4, 8, 10, and 13 weeks (B) after transplantation were determined (n=6/group, pooled from 2 experiments). (C–E) Bone marrow frequencies of LSK subsets (C), CMP/MEP/GMP cells (D), and CLP cells (E) were determined in the marrow of mice three months after receiving transplantation (n=6–7/group, pooled from 2 experiments). Results are presented as mean±Standard Deviation (S.D.). Student t-test *P<0.05, **P<0.01.
We then took marrow cells from the primary recipients and performed secondary transplantation (Online Supplementary Figure S5). We did not observe a significant difference in the frequency of stem cells or progenitors from Notch2-blocked (Online Supplementary Figure S5A and B) or Notch2-deficient marrow in the secondary transplant recipients than from controls (Online Supplementary Figure S5C and D). As expected, Notch1 blockade had no effect on the HSPC homeostasis after primary or secondary transplantation either (Figure 3C–E and Online Supplementary Figure S5B). These findings suggest that transient Notch2 blockade induces a short-term expansion of myeloid progenitors during stress hematopoiesis, such as bone marrow transplantation, resulting in faster reconstitution of platelet and hemoglobin, whereas the more primitive bone marrow stem cells and lymphoid progenitors are unaffected by Notch2 blockade.
Notch2 blockade induces enhanced homing and reconstitution of the stem cells and myeloid progenitors from mobilized HSPCs
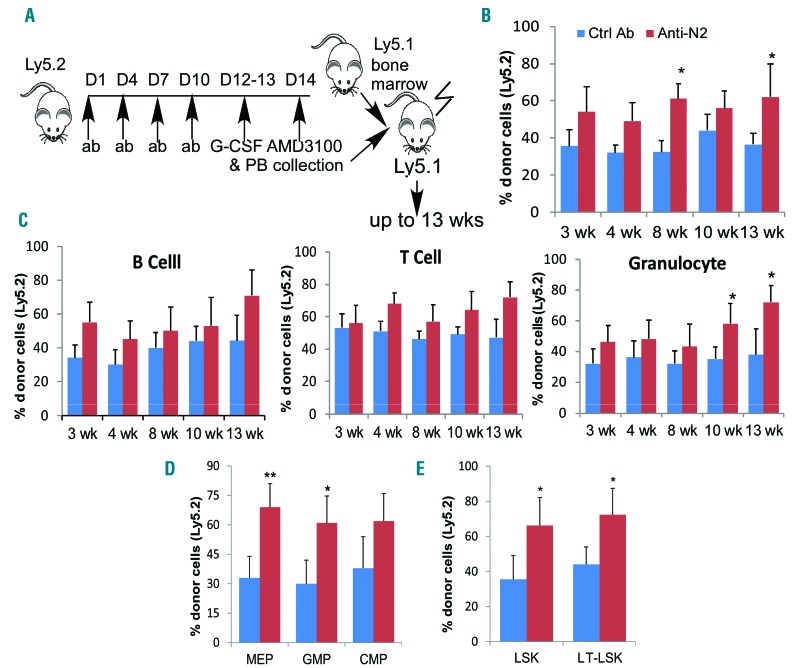
To determine the reconstitution potential of mobilized and Notch2-blocked HSPC, we used a competitive transplantation assay in which circulating HSPCs (Ly5.2) mobilized by Notch2 blockade (combined with G-CSF and AMD3100) compete with congenic bone marrow cells (Ly5.1) for engraftment in lethally irradiated recipients (Ly5.1) (Figure 4A). Evaluation of donor reconstitution revealed a trend toward a higher chimerism at three weeks and significantly higher chimerism sustained by HSPCs mobilized by Notch2 blockade at eight and 12 weeks after transplantation (Figure 4B). Granulocyte numbers derived from mobilized and Notch2-blocked HSPCs were significantly higher than those derived from control cells by ten and 13 weeks after transplantation, while lymphocytes contributed by Notch2-blocked HSPCs also showed a trend of increase (Figure 4C). Accordingly, bone marrow GMPs and MEPs derived from Notch2-blocked HSPCs increased compared to those derived from control donors (Figure 4D). Similarly, proportions of LSKs and long-term HSCs (LT-HSCs) derived from Notch2-blocked HSPCs were much higher than those derived from control donors (Figure 4E). These findings suggest a competitive advantage of Notch2-blocked stem cells and myeloid progenitors over controls after transplantation.
Figure 4.
Notch2 blockade induces enhanced reconstitution of the stem cells and myeloid progenitors from mobilized hematopoietic stem cell and progenitors (HSPC). (A) Scheme of competitive reconstitution by mobilized circulating HSPCs in which mononuclear cells from 200 mL blood collected from control antibody or Notch2 antibody-treated mice (Ly5.2) were mixed with 0.4×106 competitor marrow cells (Ly5.1), and transplanted into lethally irradiated recipient mice (Ly5.1). (B) The percentage of donor Ly5.2+ cell chimerism in the peripheral blood (PB) mononuclear cells of recipient mice (n=5–7/group) at various time points after transplantation. (C–E) The percentage of peripheral B cells, T cells and granulocytes (C), bone marrow megakaryocyte-erythroid progenitors (MEPs) and granulocyte-macrophage progenitors (GMPs) (D), and bone marrow total LSK and LT-hematopoietic stem cells (LT-HSCs) (E) derived from Ly5.2+ mobilized HSPCs in recipient mice at various time points after transplantation. Results are presented as mean±Standard Deviation (S.D.). Student t-test *P<0.05, **P<0.01. wk: weeks.
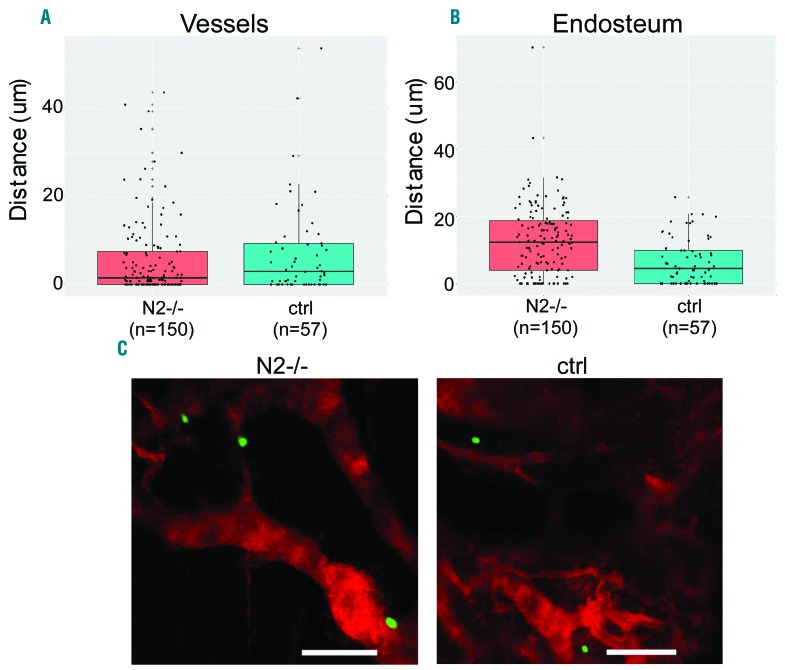
The enhanced chimerism from Notch2-blocked mobilized HSPC suggests that Notch2 blockade may enhance HSPC homing and/or engraftment. Because the number of mobilized HSPC was not sufficient to allow a direct imaging analysis for homing, we assessed the homing and niche locations of adoptively transferred bone marrow HSPCs from Notch2-deficient or from Notch2 antibody-treated mice. We observed that 1.6-fold more of Notch2-deficient (Figure 5A) and 1.4-fold more Notch2-blocked progenitors (Online Supplementary Figure S6A) homed to the bone marrow compared to their corresponding controls (P<0.001 by 2-sample Pearson χ2 test). Notch2-deficient (Figure 5A and C) or Notch2-blocked progenitors (Online Supplementary Figure S6A and C) showed similar spatial locations to the blood vessels as their controls. In comparison, Notch2-deficient progenitors were positioned more distal from the endosteum than control progenitors, with a median distance of 12.6 and 6.7 μm, respectively (P<0.001) (Figure 5B). However, there was no great difference between Notch2-blocked progenitors and the controls regarding their mean distance to the endosteum progenitors (Online Supplementary Figure S6B).
Figure 5.
Notch2 loss enhances hematopoietic stem cell and progenitors (HSPC) homing and leads to altered localizations relative to the endosteum. Isolated bone marrow LK cells (0.2×106) from Vav-Cre/Notch2F/F mice and control mice were labeled with CFSE and transferred into lethally-irradiated wild-type (WT) mice. Twenty-four hours later, 2-photon imaging was performed to locate CFSE+ cells in the calvarium. The endosteum is highlighted by the blue second harmonic signal, while the vessel was labeled by TRITC-Dextran dye. (A) The shortest 3D distances between the LK cells and the blood vessel or the endosteum (μm) were compared for control and Notch2-deficient cells. Wilcoxon rank sum test was performed. Data shown were pooled from 3 mice (3 experiments) in each group. Cell numbers counted in the entire calvarium of each recipient were 30, 63, and 57 (total n=150) derived from Vav-Cre/Notch2F/F mice, and 9, 26, and 22 (total n=57) derived from control (ctrl) mice, in experiments 1, 2 and 3, respectively. (B) Representative 2D images show the locations of control versus Notch2-deficient LK cells relative to the blood vessel. Bar size=100 μm.
Notch2 signaling blockade increases HSPC cell cycling and down-regulates CXCR4 expression
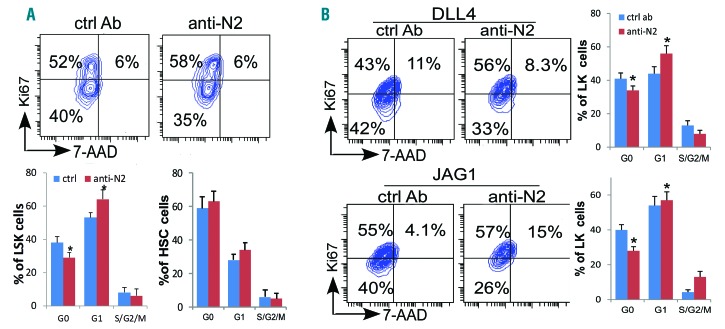
To understand the mechanism underlying the enhanced sensitization to mobilizing reagents and an expansion of myeloid progenitors from Notch2-blocked donors during transplantation, we assessed the cell-cycling status of the marrow HSPCs by the proliferation marker Ki67 in conjunction with the DNA-specific dye 7-AAD as a measure of quiescence. Compared to the HSPCs from mice receiving control antibody, Notch2-blocked LSKs but not HSCs had decreased quiescent cells in G0 fraction but increased proliferative cells in G1 fraction (Figure 6A). A similar reduction of G0 and an increase of G1 LSK cells were observed in Notch2-deficient LSK cells (data not shown). These findings suggest that Notch2 helps maintain HSPC quiescence. To confirm that the observed reduction in HSPC quiescence following Notch2 blockade is a result of direct effect on the HSPC cells, we applied the antibody in an in vitro co-culture system where isolated LSK cells were co-cultured with Notch ligand DLL4 or JAG1-expressing OP9 cells. In this co-culture system, Notch receptor interaction with Notch ligand in vitro promotes HSPC adhesion to ligand-expressing osteoblasts and inhibits HSPC cycling, an effect that could be blocked by ligand-neutralizing antibody.11 Here we found that treating cells with anti-Notch2 caused a similar reduction in quiescent LSKs but an increase in cycling LSKs in G1 and S/G2/M fractions (Figure 6B).
Figure 6.
Notch2 signaling blockade increases hematopoietic stem cell and progenitor (HSPC) cell cycling. (A) Cell-cycling status of the marrow LSK cells and CD150+CD48−LSKs hematopoietic stem cells (HSCs) was determined by the proliferation marker Ki67 in conjunction with the DNA-specific dye 7-AAD in mice receiving 4 doses of control or Notch2 blocking antibody. One representative FACS profile of LSK cells, and the relative proportion of cells in G0, G1 and S-G2/M phase of the cell cycle in LSK and HSC (bottom). Results are pooled from 2 experiments, and are presented as average±Standard Deviation (S.D.) (n=5/each group). (B) 1.5×105 LK cells were co-cultured with confluent OP9-DLL4 or OP9-JAG1 cells in the presence of control (ctrl) or Notch2 blocking antibody (400 ng/mL) for four days before cell-cycling analysis on gated LK cells. One representative FACS profile of 3 similar experiments. Student t-test *P<0.05.
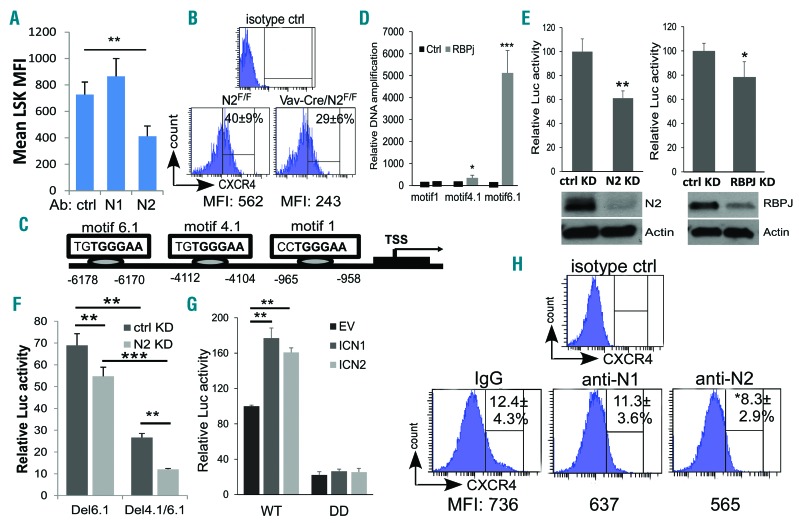
Because the cell surface chemokine receptor CXCR4 is essential for the colonization of bone marrow by HSPC as well as for the maintenance of stem cell quiescence,17–19 we investigated the expression of CXCR4 on LSK cells from Notch2-blocked bone marrow or Notch2-deficient mice. Cell surface CXCR4 expressed by Notch2-blocked LSKs decreased by approximately 50% when compared to the controls (Figure 7A). Similarly, CXCR4 expression by Notch2-deficient LSKs was also decreased by approximately 50% when compared to control LSKs (Figure 7B). These observations raised the possibility that Notch2 signaling directly regulates CXCR4 expression. Indeed, analysis of CXCR4 promoter identified several RBPJ/CSL binding sequences (TGGGAA) (Figure 7C). CHIP analysis revealed that RBPJ binds strongly to motif −6.1kb and weakly to motif −4.1kb but not to motif −1kb (Figure 7D). In addition, co-transfection of the CXCR4 promoter construct harboring the two RBPJ binding motifs together with NOTCH2 siRNA or RBPJ siRNA resulted in an approximately 40% and approximately 20% decrease in CXCR4 reporter activity, respectively, compared to the control siRNA treatment (Figure 7E). The reporter activity decreased by 32% and 75% when the promoter construct was transfected with either motif 6.1 (Del6.1) or both motifs 4.1 and 6.1 were deleted (Del4.1/6.1) in the luciferase reporter assay. Furthermore, when cells were transfected with the Del6.1 or the Del4.1/6.1 construct, the reporter activity showed even greater reduction after Notch2 was down-regulated by siRNA (Figure 7F). In comparison, Notch1 or Notch2 overexpression resulted in increased CXCR4 reporter activity; this increased activity was also dependent on the two essential RBPJ binding motifs (Figure 7G). Finally, when bone marrow LK cells were co-cultured with OP9-DLL4 cells, blocking Notch2, but not Notch1, there was a reduction in LK cell surface expression of CXCR4 (Figure 7H). Together, these observations suggest that CXCR4 expression is directly regulated by Notch signaling, and that CXCR4 on marrow HSPC is down-regulated by Notch2 blockade.
Figure 7.
Notch2 signaling regulates hematopoietic stem cell and progenitor (HSPC) CXCR4 expression. (A and B) Cell surface expression of CXCR4 in mean fluorescence intensity (MFI) was assessed by FACS analysis of bone marrow LSK cells from mice receiving 4 doses of control (ctrl) or Notch1/2 blocking antibody (n=5/each group) (A), or from Vav-Cre/Notch2F/F mice and control mice (Vav-Cre/Notch2+/+) (n=4/each group) (B). (C) CXCR4 promoter region has 3 potential RBP-J binding sites. (D) CHIP assay of wild-type LK bone marrow cells. Immunoprecipitation was conducted with control antibody, or anti-RBPJ followed by PCR of the CXCR4 promoter. Results shown are mean±Standard Deviation (S.D.) of 3 biological replicates. Student t-test *P<0.05, ***P<0.001. (E) CXCR4-Luc report construct with 2.0 kb CXCR4 promoter sequence containing motif 4.1 and motif 6.1 was transfected into 293T cells expressing RBPJ (RBPJ KD), Notch2 (N2 KD), or control siRNA (ctrl KD). (F) The dependence of the CXCR4-Luc reporter activity on motif 4.1 and 6.1 was assessed by transfecting the CXCR4 reporter construct with single motif 6.1 deleted (Del6.1) or both motif 4.1 and 6.1 deleted (Ddel4.1/6.1) into 293T cells that expressed control siRNA or Notch2 siRNA. (G) CXCR4-Luc reporter activity was determined by transfecting the wild-type CXCR4-Luc reporter construct (WT) or the construct with both 4.1- and 6.1-motif deleted (DD) into 293T cells expressing ICN1-expression plasmid (ICN1 OE), ICN2-expression plasmid (ICN2 OE), or control plasmid (empty vector; EV). (H) Bone marrow WT LK cells were isolated and co-cultured with OP9-DLL4 cells for 96 hours in the presence of Notch1, Notch2, or control antibody (400 ng/mL). CXCR4 expression was assessed by FACS. (E-H) Mean±S.D of 3 biological replicates. Student t-test *P<0.05, **P<0.01, ***P<0.001.
Discussion
In this study, we demonstrated that blocking Notch2 combined with the current clinical regimen further enhances HSPC mobilization and homing without affecting HSC homeostasis. We found that Notch2 blockade leads to increased HSPC cell cycling and down-regulated CXCR4 expression. We showed that CXCR4 is directly regulated by the Notch transcriptional protein RBPJ. In addition, we showed that Notch2 blockade leads to a competitive repopulating advantage of mobilized HSPC after transplantation. These results suggest that Notch2 may serve as a new target for promoting HSPC mobilization and HSPC engraftment during transplantation.
Notch is a signaling molecule important for stem cell self-renewal and fate determination in many tissues, including the hematopoietic system. Despite in vitro evidence that activation of Notch stimulates HSC self-renewal,20–23 in vivo studies do not support the concept that Notch has an essential role in adult HSC steady state homeostasis.15,24 Nevertheless, Notch2 was found to be responsible for the rate of generation of repopulating stem cells during stress hematopoiesis in the early phase of hematopoietic recovery.12 Consistently, we found that Notch2 blockade alone is associated with a transient reduction in the bone marrow progenitors and HSCs, likely caused by the decreased immediate replenishment of the HSPCs in the absence of Notch2. However, since blocking Notch2 decreases HSC quiescence and niche retention, Notch2 blockade leads to increased HSPC cell cycling and egress from the marrow. In comparison, blocking Notch1 neither affects HSC quiescence, nor causes HSPC exit from the marrow. Therefore, Notch2 but not Notch1 is important for HSC quiescence maintenance and proper niche localization. There is no significant effect on HSPC homeostasis when Notch2 blockade is employed in conjunction with G-CSF and AMD3100, and no apparent negative effect on short-term or long-term HSC reconstitution when these bone marrow cells are used in the transplantation procedures.
Long-term hematopoiesis is maintained by a small pool of HSCs that ensure balanced proliferation, differentiation, and quiescence. One of the major mechanisms that retains HSPCs in their bone marrow niches and directs their migration and homing from blood to bone marrow involves interaction of the CXCR4 receptor with α-chemokine stromal-derived factor 1 (SDF-1). CXCR4 promotes HSC quiescence and blocking CXCR4/SDF-1 signaling hampers HSC retention.18,19,25 The importance of the CXCR4/SDF-1 as a retention mechanism for HSPC is underscored by the extensive efforts to develop mobilization reagents by targeting this axis, e.g. by antagonizing CXCR4,13,26 by downregulation of SDF-1 expression or suppression of SDF-1-producing cells,27,28 or by proteolytic cleavage of CXCR4 and SDF-1.29,30 Other potential agents and novel strategies to promote HPC mobilization have been tested.31 These include, but are not limited to, S1P agonists,32 VCAM/VLA-4 inhibitors,33 parathyroid hormone,34 proteosome inhibitors,35 Groβ,36,37 CD26,38 and heparan sulphate.39 Here, we report that blocking Notch2 combined with G-CSF and AMD3100 induces enhanced HSPC mobilization, and that Notch signaling directly regulates CXCR4 expression. Because we did not find any significant alterations in the expression of other major cell surface adhesion molecules caused by the lack of Notch2 expression (Online Supplementary Figure S7), presumably this down-regulated CXCR4 is responsible, at least partially, for the increased cell cycling and egress of HSPCs in Notch2-deficient mice or after Notch2 blockade. Other possible mechanisms that should not be excluded may involve a direct adhesive effect by Notch2-ligand interaction with stromal niche cells independent of CXCR4 in promoting HSC niche retention, as Notch ligand neutralizing antibodies are still able to induce HSPC egress even in mice deficient in RBPJ-mediated canonical Notch signaling.11
Interestingly, we find that Notch2-blocked or Notch2-deficient HSPCs display enhanced homing to the bone marrow. This is accompanied with increased repopulating ability in peripheral HSPCs mobilized by Notch2 blockade, enhanced hematopoietic recovery, and expansion of the GMPs and MEPs derived from Notch2-blocked cells. These properties are likely due to the increased proliferative capacity and rapid differentiation associated with Notch2 deficiency itself12 or with downregulation of CXCR4 by Notch2-blockade, as a similar observation was found in CXCR4 haplo-insufficient HSPCs.40 Alternatively, a transient downregulation of CXCR4 by Notch2 antibody during HSPC egress is followed by a compensatory increase in CXCR4 that mediates the increased homing after HSPCs leave the bone marrow. However, the insufficient numbers of mobilized HSCs mean a precise assessment of CXCR4 expression cannot be made. In addition, this argument cannot be applied to Notch2 deficient mice. We do not exclude the possibility that the enhanced homing of Notch2-blocked/deficient HSPCs is mediated by other molecules that are directly or indirectly regulated by Notch2. Consistent with other reports,12 we found that, unlike Notch2-blocked HSPCs, Notch2-deficient HSPCs showed similar or a mild reduction in chimerism in the competitive setting when compared to the control HSPCs (Online Supplementary Figure S8), and displayed a trend of reduction in frequency in the secondary transplant recipients (Online Supplementary Figure S5E and F). This indicates that HSC function related to stress hematopoiesis is somewhat impaired by Notch2 deficiency despite increased homing. This paradoxical finding may be explained by a distal spatial location of Notch2-deficient HSPC relative to the endosteum; however, the underlying cause needs further investigation. Endothelial cells41–43 and perivascular stromal cells19,44 are important components of HSC niches. A direct contact of HSCs with endothelial cells through Notch-ligand interactions has been shown to prevent HSC exhaustion.42 On the other hand, we showed that HSPCs with faulty Notch-ligand interaction display a similar distal spatial location relative to the endosteum.11 Whether this reflects a disrupted interaction between HSPCs and the Notch ligand-expressing supporting cells in the quiescent niche or in the vicinity of endosteum, or is caused by altered cell metabolism, or some other still unidentified mechanism, needs further investigation. Importantly, transient Notch2 blockade does not affect HSC self-renewal, hence it is unlikely that transplant of Notch2-blocked HSPCs will adversely affect HSC long-term reconstitution in recipients.
The more effective HSPC mobilization and the enhanced engraftment related to Notch2 blockade may be in general useful as a strategy to achieve satisfactory engraftment and improve patient outcome in HCT. Nearly all autologous HCT procedures are performed using mobilized HPCs. Successful outcome is dependent on infusing an adequate number of functionally active HPCs. Until recently, a mobilization strategy of G-CSF, either alone or in combination with chemotherapy, failed to mobilize an ‘optimal’ CD34 cell dose in up to 40% of patients.45 AMD3100, in combination with G-CSF, increases total CD34+ cells mobilized, and is often used for HPC mobilization in myeloma and non-Hodgkin lymphoma patients.46 Unfortunately, this approach does not always lead to adequate HPC collection in patients who have previously been exposed to extensive cytotoxic therapy; furthermore, in some instances, multiple HCT procedures may be contemplated that require greater numbers of HPCs.5,47,48
Since findings from this study were performed using a ‘standard’ animal model, an optimal regimen needs to be better defined in order to achieve the maximum mobilization in humans. Nevertheless, our findings from animal studies suggest that Notch2 is a potential target to be considered for the mobilizing regimen in conjunction with AMD3100 and G-CSF for patients thought to be potentially at risk of not being able to mobilize sufficient numbers of CD34 cells. This strategy, as the data suggest, could improve collection efficacy and reduce procedure-related costs and the risks associated with large volume or multiple collections.49,50 Potentially, this approach could accelerate hematopoietic recovery, considering the enhanced homing and engraftment of the HSPCs and increased granulocyte recovery which could potentially reduce the risk of infection, and ultimately improve both short- and long-term patient outcome.
Supplementary Material
Acknowledgments
We thank Dr. Hal Broxmeyer for valuable suggestions about our experimental design.
Footnotes
Funding
This study was supported in part by research funding from NIH HL103827 and American Cancer Society LIB-125064 to LZ, and by the Department of Pathology Case Western Reserve University faculty startup fund to WX and LZ.
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/10/1785
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354 (17):1813–1826. [DOI] [PubMed] [Google Scholar]
- 2.Moog R. Management strategies for poor peripheral blood stem cell mobilization. Transfus Apher Sci. 2008;38(3):229–236. [DOI] [PubMed] [Google Scholar]
- 3.Perseghin P, Terruzzi E, Dassi M, et al. Management of poor peripheral blood stem cell mobilization: incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41(1):33–37. [DOI] [PubMed] [Google Scholar]
- 4.Jantunen E, Kvalheim G. Mobilization strategies in hard-to-mobilize patients with lymphoid malignancies. Eur J Haematol. 2010;85(6):463–471. [DOI] [PubMed] [Google Scholar]
- 5.Moreb JS, Salmasinia D, Hsu J, et al. Long-Term Outcome after Autologous Stem Cell Transplantation with Adequate Peripheral Blood Stem Cell Mobilization Using Plerixafor and G-CSF in Poor Mobilizer Lymphoma and Myeloma Patients. Adv Hematol. 011;2011:517561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiff PJ, Micallef I, Nademanee AP, et al. Transplanted CD34(+) cell dose is associated with long-term platelet count recovery following autologous peripheral blood stem cell transplant in patients with non-Hodgkin lymphoma or multiple myeloma. Biol Blood Marrow Transplant. 2011;17 (8):1146–1153. [DOI] [PubMed] [Google Scholar]
- 7.Fehon RG, Kooh PJ, Rebay I, et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61(3):523–534. [DOI] [PubMed] [Google Scholar]
- 8.Rebay I, Fleming RJ, Fehon RG, et al. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: implications for Notch as a multifunctional receptor. Cell. 1991;67(4):687–699. [DOI] [PubMed] [Google Scholar]
- 9.Oh P, Lobry C, Gao J, et al. In vivo mapping of notch pathway activity in normal and stress hematopoiesis. Cell Stem Cell. 2013; 13(2):190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao D, Huang Y, Huang X, et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. 2011;117(21):5652–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Yu S, Zimmerman G, et al. Notch Receptor-Ligand Engagement Maintains Hematopoietic Stem Cell Quiescence and Niche Retention. Stem Cells. 2015;33 (7):2280–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varnum-Finney B, Halasz LM, Sun M, et al. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. 2011;121(3):1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010; 464(7291):1052–1057. [DOI] [PubMed] [Google Scholar]
- 15.Maillard I, Koch U, Dumortier A, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2(4):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu VW, Saez B, Cook C, et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J Exp Med. 2015;212(5):759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73(5):630–638. [DOI] [PubMed] [Google Scholar]
- 18.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205(4):777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. [DOI] [PubMed] [Google Scholar]
- 20.Duncan AW, Rattis FM, DiMascio LN, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6(3):314–322. [DOI] [PubMed] [Google Scholar]
- 21.Karanu FN, Murdoch B, Gallacher L, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. 2000; 192(9):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stier S, Cheng T, Dombkowski D, et al. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99(7):2369–2378. [DOI] [PubMed] [Google Scholar]
- 23.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–1281. [DOI] [PubMed] [Google Scholar]
- 24.Mancini SJ, Mantei N, Dumortier A, et al. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005; 105(6):2340–2342. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng YS, Li H, Kang YL, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelo-suppression. Blood. 2011;117(2):429–439. [DOI] [PubMed] [Google Scholar]
- 26.Abraham M, Biyder K, Begin M, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25(9):2158–2166. [DOI] [PubMed] [Google Scholar]
- 27.Christopher MJ, Liu F, Hilton MJ, et al. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114(7):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler IG, Barbier V, Wadley R, et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(23):375–385. [DOI] [PubMed] [Google Scholar]
- 29.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011; 25(2):211–217. [DOI] [PubMed] [Google Scholar]
- 30.Levesque JP, Hendy J, Takamatsu Y, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003; 111(2):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24(5):976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993; 90(20):9374–93748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner S, Zaruba MM, Huber B, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008;36(9):1157–1166. [DOI] [PubMed] [Google Scholar]
- 35.Niesvizky R, Mark TM, Ward M, et al. Overcoming the response plateau in multiple myeloma: a novel bortezomib-based strategy for secondary induction and high-yield CD34+ stem cell mobilization. Clin Cancer Res. 2013;19(6):1534–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King AG, Horowitz D, Dillon SB, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001; 97(6):1534–1542. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda S, Bian H, King AG, et al. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110(3):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broxmeyer HE, Hangoc G, Cooper S, et al. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. [DOI] [PubMed] [Google Scholar]
- 39.Saez B, Ferraro F, Yusuf RZ, et al. Inhibiting stromal cell heparan sulfate synthesis improves stem cell mobilization and enables engraftment without cytotoxic conditioning. Blood. 2014;124(19):2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott DH, Gao JL, Liu Q, et al. Chromothriptic cure of WHIM syndrome. Cell. 2015;160(4):686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. [DOI] [PubMed] [Google Scholar]
- 42.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481(7382):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. [DOI] [PubMed] [Google Scholar]
- 45.Pusic I, Jiang SY, Landua S, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045–1056. [DOI] [PubMed] [Google Scholar]
- 46.Mohty M, Hubel K, Kroger N, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(7):865–872. [DOI] [PubMed] [Google Scholar]
- 47.Moskowitz CH, Glassman JR, Wuest D, et al. Factors affecting mobilization of peripheral blood progenitor cells in patients with lymphoma. Clin Cancer Res. 1998; 4(2):311–316. [PubMed] [Google Scholar]
- 48.Keating GM. Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs. 2011;71(12):1623–1647. [DOI] [PubMed] [Google Scholar]
- 49.Michon B, Moghrabi A, Winikoff R, et al. Complications of apheresis in children. Transfusion. 2007;47(10):1837–1842. [DOI] [PubMed] [Google Scholar]
- 50.Bojanic I, Dubravcic K, Batinic D, et al. Large volume leukapheresis: Efficacy and safety of processing patient's total blood volume six times. Transfus Apher Sci. 2011; 44(2):139–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.