Abstract
The first-in-class Bruton’s tyrosine kinase inhibitor ibrutinib has proven clinical benefit in B-cell malignancies; however, atrial fibrillation (AF) has been reported in 6–16% of ibrutinib patients. We pooled data from 1505 chronic lymphocytic leukemia and mantle cell lymphoma patients enrolled in four large, randomized, controlled studies to characterize AF with ibrutinib and its management. AF incidence was 6.5% [95% Confidence Interval (CI): 4.8, 8.5] for ibrutinib at 16.6-months versus 1.6% (95%CI: 0.8, 2.8) for comparator and 10.4% (95%CI: 8.4, 12.9) at the 36-month follow up; estimated cumulative incidence: 13.8% (95%CI: 11.2, 16.8). Ibrutinib treatment, prior history of AF and age 65 years or over were independent risk factors for AF. Multiple AF events were more common with ibrutinib (44.9%; comparator, 16.7%) among patients with AF. Most (85.7%) patients with AF did not discontinue ibrutinib, and more than half received common anticoagulant/antiplatelet medications on study. Low-grade bleeds were more frequent with ibrutinib, but serious bleeds were uncommon (ibrutinib, 2.9%; comparator, 2.0%). Although the AF rate among older non-trial patients with comorbidities is likely underestimated by this dataset, these results suggest that AF among clinical trial patients is generally manageable without ibrutinib discontinuation (clinicaltrials.gov identifier: 01578707, 01722487, 01611090, 01646021).
Introduction
Clinical trials of ibrutinib have demonstrated consistent benefits and improvements in overall survival (OS) and progression-free survival (PFS) among patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and relapsed or refractory mantle cell lymphoma (MCL), including those with high-risk disease features.1–4 Ibrutinib therapy is generally well tolerated, but has been associated with atrial fibrillation (AF), with an overall clinical trial incidence of 6–16%.1–6 In a phase II study that enrolled 86 patients with CLL/SLL, incidence of AF reached 16% in longer-term follow up.5 In a meta-analysis,7 the pooled rate of AF was 3.3 [95% Confidence Interval (CI): 2.5, 4.1] per 100 person-years in ibrutinib-treated patients versus 0.84 (95%CI: 0.32, 1.6) per 100 person-years in non-ibrutinib-treated patients. However, risk factors, natural history, and management strategies of AF associated with ibrutinib treatment are largely unknown. Because continuous treatment is required to maintain benefit from single-agent ibrutinib therapy, understanding a patient’s natural history and optimizing AF management should improve the safe use of ibrutinib in B-cell malignancies. Management of AF typically relies on rate and/or rhythm control, depending on underlying structural cardiovascular disease (CVD).8–10
Systemic thromboembolic events (specifically stroke) are the most frequent major complication of AF, along with other cardiovascular (CV) complications and increased mortality.11,12 Anticoagulation, commonly with vitamin K antagonists, reduces the risk of stroke by approximately two-thirds, while increasing bleeding risks. Thus, risk calculators (i.e. CHA2DS2-VASC) have been developed to weigh benefits against risks of anticoagulation in patients without an underlying malignancy.
Most patients with CLL/SLL and non-Hodgkin lymphomas are diagnosed at 65 years or over and have multiple medical comorbidities.5,13,14 It has been reported that 6% of patients aged 65 years or over and diagnosed with CLL/SLL had AF at baseline (higher than the 1.0–1.8% of an age-matched general population), and 6% more developed AF over a 5-year treatment period,13–15 suggesting that patients with CLL/SLL may have a higher risk of developing AF than the normal population.
Data regarding management of AF in ibrutinib-treated patients are limited, and the association between ibrutinib therapy and increased rates of bleeding needs to be considered in the context of AF management in these patients. To further characterize ibrutinib-associated AF and describe management thereof, we report here a pooled analysis of all cases of AF across four randomized controlled trials (RCTs).
Methods
Study populations from initial data reports of the four RCTs [PCYC-1112 (RESONATE, clinicaltrials.gov identifier: 01578707), PCYC-1115 (RESONATE-2, clinicaltrials.gov identifier: 01722487), CLL3001 (HELIOS, clinicaltrials.gov identifier: 01611090), and MCL3001 (RAY, clinicaltrials.gov identifier: 01646021)] were pooled, including patients randomized to receive ibrutinib [alone or with bendamustine plus rituximab (BR)] and patients receiving comparator therapy (ofatumumab, chlorambucil, placebo plus BR, or temsirolimus). The studies were approved by the institutional review board or independent ethics committee at each institution. Data from the initial study reports were used for the detailed pooled analysis. Limited data on incidence of AF were available in the extended follow-up period and were included for patients randomized to ibrutinib. Patients were subject to similar eligibility criteria; specifically, patients requiring vitamin K antagonists, such as warfarin, or strong CYP3A4/5 inhibitors were excluded, although other anticoagulants and antiplatelet agents were permitted. Patients were censored at crossover. Patients were excluded if they had uncontrolled or clinically significant CVD, including uncontrolled arrhythmia or class III or IV congestive heart failure or a history of myocardial infarction, unstable angina, or acute coronary syndrome within six months prior to randomization. Complete study methodologies are detailed elsewhere (Online Supplementary Appendix 1).1–4
The incidences of AF and atrial flutter events were referred to collectively as AF. All treatment-emergent AF events (defined as events occurring after first dose of study drug until 30 days after last dose) are reported. CV events captured using Standardised Medical Dictionary for Regulatory Activities (MedDRA) Queries (SMQ) were grouped into five CVD categories: arrhythmia, congestive heart failure, ischemic heart disease, hypertension, and ischemic CNS vascular conditions (Online Supplementary Appendix 2). Bleeding events listed were captured using SMQ.
CHA2DS2-VASc scores estimating risk of stroke in patients with AF were evaluated using patients’ characteristics at baseline. Cox regression models were used to perform univariate and multivariate analyses of risk factors for developing AF. These analyses evaluated age increase, gender (male), AF risk increase (prior history of AF/flutter), increase in body mass index, Rai stage and prior history of AF/abnormal heart rhythm, coronary artery disease, diabetes, hyperlipidemia, hypertension, and valvular heart disease.13 The univariate model included each single factor plus treatment group. Cumulative incidence of AF was estimated in a Cox regression model accounting for deaths and disease progression without prior AF as competing risk events.5,16 The AF risk score for each CLL patient with no prior history of AF was computed using the Shanafelt predictive model.13
Results
Patients’ characteristics and incidence of AF
In total, 1505 patients were included, with 756 randomized to ibrutinib (alone or with BR) and 749 to comparator (Table 1). One hundred thirty-nine patients had previously treated MCL and the remainder had newly diagnosed or previously treated CLL. At the time of the initial study reports, the median follow up in the pooled analysis was 16.6 months; median duration of exposure was 13.3 months for the ibrutinib group and 5.8 months for the comparator (Online Supplementary Table S1). With a median follow up of 16.6 months, 6.5% (95%CI: 4.8, 8.5) of patients receiving ibrutinib and 1.6% (95%CI: 0.8, 2.8) receiving the comparator [relative risk 4.1 (95%CI: 2.2, 7.5)] reported AF while on treatment (Figure 1). Most AF events developed de novo in patients without a history of AF. The incidence of AF was 7.0% (95%CI: 5.1, 9.3) in CLL patients and 4.3% (95%CI: 1.6, 9.2) in MCL patients treated with ibrutinib. Patients treated with ibrutinib combination therapy (HELIOS study) had a 7.7% (95%CI: 4.9, 11.4) incidence of AF, compared with 5.8% (95%CI: 3.8, 8.3) in ibrutinib monotherapy patients. The exposure-adjusted incidence rates of AF per 100 patient-months were 0.503 for the ibrutinib group and 0.199 for the comparator. The estimated cumulative incidence of AF was higher in patients treated with ibrutinib versus comparators [7.4% (95%CI: 5.6, 9.6) vs. 1.9% (95%CI:1.0, 3.4)] (Figure 2A and B). Median age of patients developing AF was 71 years for both groups, which is older than the overall median age of 67 years. History of prior AF/abnormal heart rhythm was more common in patients who had AF on study (ibrutinib, 26.5%; comparator, 25.0%) than in patients who did not (ibrutinib, 10.6%; comparator, 10.4%). Patients with a history of hypertension were more likely to develop AF than those without [31 of 328 (9.5%) vs. 18 of 428 (4.2%)] in the ibrutinib group. The majority of patients with prior hypertension did not develop clinically evident AF on ibrutinib (ibrutinib, 90.5%; comparator, 96.9%) during the observation period. In patients without a history of hypertension, 38 developed de novo hypertension; only one patient developed de novo hypertension and AF.
Table 1.
Baseline demographic and clinical characteristics of patients in the pooled analysis.
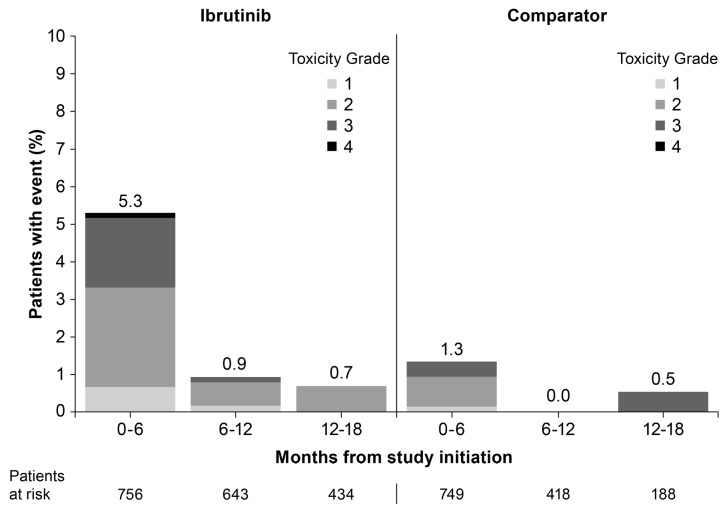
Figure 1.
Onset of first atrial fibrillation event by treatment.
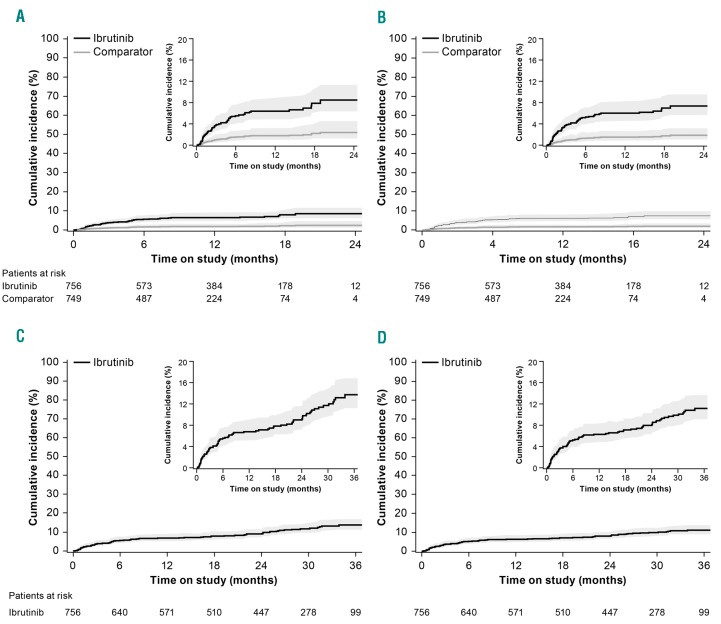
Figure 2.
Cumulative incidence (95% CI) of atrial fibrillation with ibrutinib. (A) unadjusted for competing risks (death and progressive disease) and (B) adjusted. With extended follow up: unadjusted (C) and adjusted (D).
Longer-term follow up in patients randomized to ibrutinib provided an additional 8467 patient-months for analysis. During this period, 29 additional patients experienced AF. Newly reported cases of AF occurred at a continuous low rate over time. With extended follow up, 78 ibrutinib-treated patients [10.4% (95%CI: 8.4, 12.9)] experienced AF. Estimated cumulative incidence rate of AF at 36 months was 13.8% (95%CI: 11.2, 16.8) (Figure 2C). After adjusting for competing risks of progressive disease and death, estimated cumulative incidence rate of AF was 11.2% (95%CI: 9.0, 13.8) (Figure 2D).
Clinical features of treatment-emergent AF
In the first six months, 5.3% of ibrutinib patients developed AF with a continued low rate over time. The median time to onset of AF was 2.8 months (range 0.3–17.5) for the ibrutinib group and 2.0 months (range 0.6–18.9) for the comparator, with a median follow up of 16.6 months. In 2 patients in the ibrutinib group and 4 in the comparator, an AF event occurred after the patient had permanently discontinued study drug (within 30 days) for other reasons. Overall, median duration of AF episodes was three days for both groups; however, the range varied widely. The mean (SD) duration of AF episodes was 12.6 (29.5) days for the ibrutinib group and 5.1 (5.5) days for comparator. The majority of patients experiencing AF had only one episode [27 of 49 (55.1%) for ibrutinib; 10 of 12 (83.3%) for comparator] (Online Supplementary Table S2); 22 patients (44.9%) in the ibrutinib group had multiple episodes and 2 patients (16.7%) in the comparator had two episodes (Online Supplementary Tables S2 and S3). Among patients who had two or more AF episodes in the ibrutinib group, the median time between events was 1.1 months.
Common Toxicity Criteria grade 1 or 2 AF occurred in 27 (3.6%) patients in the ibrutinib group and 8 (1.1%) patients in the comparator group, accounting for more than half of the AF events that occurred in either group (Online Supplementary Appendix 3). AF events leading to hospitalization (including grade 3 and 4 events) were reported as serious adverse events (SAEs) in 23 (3.7%) patients receiving ibrutinib and 6 (1.0%) receiving comparator. Among these SAEs, 17 patients in the ibrutinib group and 3 patients in the comparator group reported grade 3 events. Only one grade 4 event was reported, which was in the ibrutinib group. No deaths were attributed to AF in either group.
With extended follow up, the median time to onset of AF in patients randomized to ibrutinib was 5.7 (range 0.3–40.2) months. Of the 78 patients with AF, almost two-thirds [49 (62.8%)] had only one episode of AF and more than half [43 (55.1%)] had AF events of grade 2 or lower (Online Supplementary Table S4).
Predictors of AF in trial patients
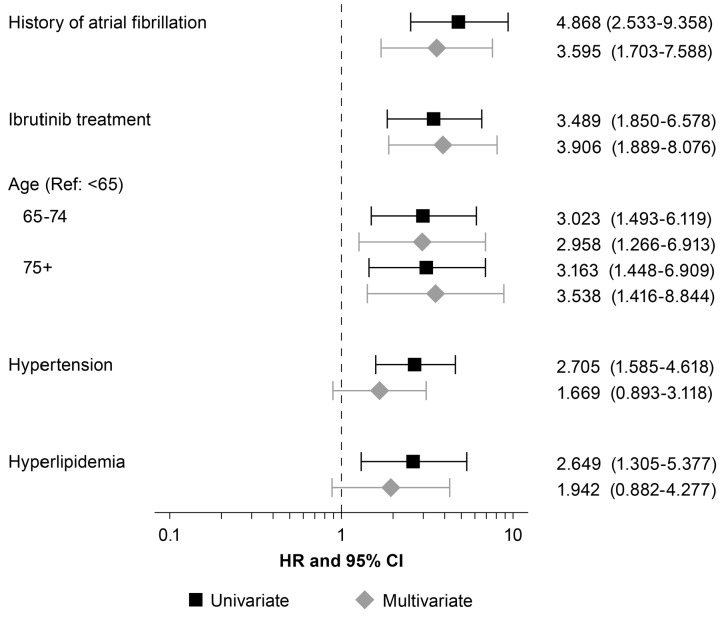
Univariate analyses identified prior history of AF, ibrutinib therapy, age over 65 years, hypertension, and hyperlipidemia as significant risk factors for developing AF. Multivariate analyses showed prior history of AF, ibrutinib therapy, and age over 65 years as independent predictors of AF (Figure 3). The influence of prior coronary artery disease, valvular heart disease, and diabetes were also evaluated and not identified as significant risk factors for developing AF while on ibrutinib.
Figure 3.
Significant factors for development of atrial fibrillation using univariate and multivariate Cox regression. HR: Hazards Ratio; CI: Confidence Interval.
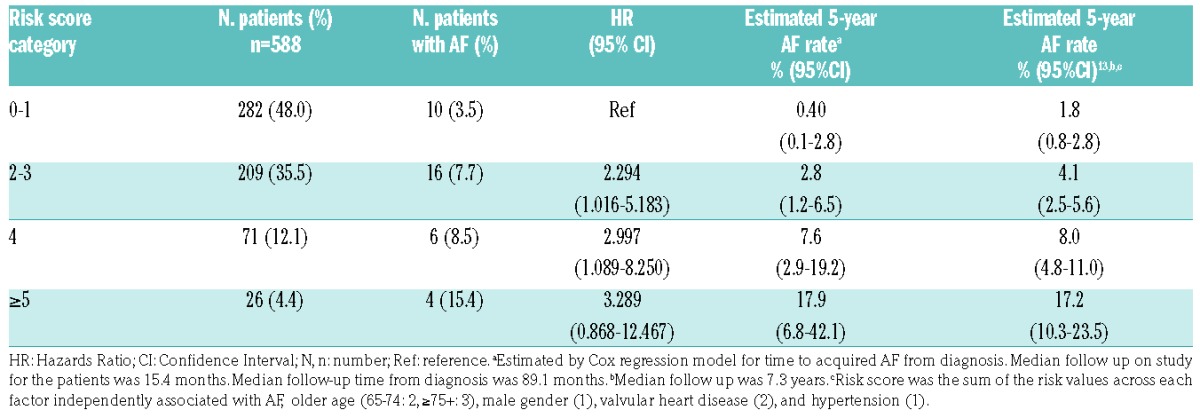
In CLL patients without a history of AF who were treated with ibrutinib, the incidence and risk of de novo AF increased with Shanafelt risk score category (Table 2 and Online Supplementary Figure S1). Estimated 5-year de novo AF rates were 0.4% in category 0–1, 2.8% in category 2–3, 7.6% in category 4, and 17.9% in category ≥5.
Table 2.
Incidence of de novo atrial fibrillation (AF) by Shanafelt risk score category13 in chronic lymphocytic leukemia patients with no history of AF treated with ibrutinib.
Management of study therapy concurrent with AF events
Twenty-four of 49 patients with AF in the ibrutinib (49.0%) and 4 of 12 in the comparator (33.3%) group were managed without any interruption or modification of study drug. Patients who required dose modification or interruption were slightly older; all other baseline demographic and clinical characteristics were similar between the two groups (Online Supplementary Table S5).
No patient in either group had a dose reduction attributed to AF; however, a similar proportion in each group had dose interruptions due to AF [16 of 49 (32.7%) for ibrutinib; 4 of 12 (33.3%) for comparator]. The median duration of interruption was 11 days for ibrutinib and 17 days for comparator. With the caveat of small numbers, there was no statistical difference between the 18-month PFS rate in 6 patients with AF who had ibrutinib dose interruption for seven days or more versus those with dose interruption for fewer than seven days [66.7% (95%CI: 19.5, 90.4) vs. 71.4% (95%CI: 54.0, 83.2)]. Seven of 49 (14.3%) patients in the ibrutinib group and no patients in the comparator group discontinued study treatment due to AF. Approximately one-half of patients with multiple AF events had dose interruptions (Online Supplementary Figure S2), and 5 of 22 (22.7%) discontinued. Plots of AF events, dose interruptions, and concomitant therapy for individual patients with AF are found in the Online Supplementary Figure S2. Of ibrutinib patients with AF and extended follow up, approximately half [41 of 78 (52.6%)] were managed without dose reduction or interruption of study treatment (Online Supplementary Table S4).
Medical management of AF
Atrial fibrillation was primarily managed with treatment commonly used for rate and rhythm control, with the most frequently used agents digoxin [11 of 49 (22.4%)], bisoprolol [10 of 49 (20.4%)], and amiodarone [8 of 49 (16.3%)] in the ibrutinib group, and amiodarone [4 of 12 (33.3%)] and diltiazem [3 of 12 (25.0%)] in the comparator group (Online Supplementary Table S6A). In the ibrutinib group, 2 patients were managed using cardioversion and one had a pacemaker inserted due to concomitant bradycardia. Patients did not have serial electrocardiographic monitoring to evaluate return to normal sinus rhythm; however, 36 of 49 patients receiving ibrutinib (73.5%) and 9 of 12 receiving comparator (75.0%) had their AF events reported as recovered or resolved.
More than one-third [17 of 49 (34.7%)] of ibrutinib patients who had an AF event on study were taking antiplatelet medications at study entry, and 4 (8.2%) were taking an anticoagulant. In the comparator group, 2 patients each were on antiplatelet and anticoagulant medications (Table 1). Many patients who experienced AF received concomitant anticoagulant and/or antiplatelet medication; the most commonly used antiplatelet agent was aspirin, and the most commonly used anticoagulant was low-molecular-weight heparin (Online Supplementary Table S6A and B). Among ibrutinib-treated patients who received anticoagulant/antiplatelet medications at any time on study, median treatment duration was longest for aspirin and novel oral anticoagulants (Online Supplementary Table S6A and B and Online Supplementary Figure S2).
Patients with AF tended to have higher CHA2DS2-VASc scores regardless of study treatment, with 35 of 49 ibrutinib patients and 10 of 12 comparator patients having scores ≥2 (Online Supplementary Table S7). All 35 ibrutinib patients received anticoagulation: 23 (65.7%) received an anticoagulant alone and 12 (34.3%) received both anticoagulant and antiplatelet medications. All 10 patients in the comparator group received an anticoagulant and 20.0% of those also received an antiplatelet agent.
Bleeding events with the use of anticoagulant and antiplatelet medications
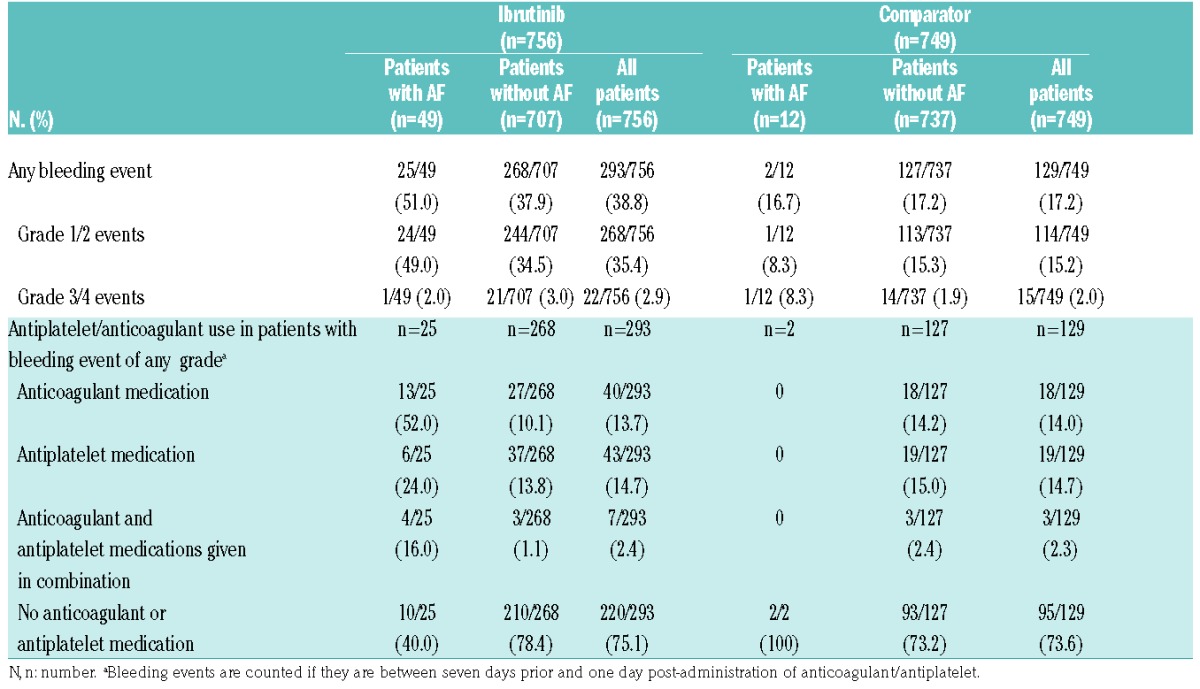
Overall, 38.8% (293 of 756) of patients in the ibrutinib group and 17.2% (129 of 749) of patients in the comparator experienced a bleeding event; most in each group were grade 1 or 2 (91.5% and 88.3%, respectively). Bleeding occurred irrespective of whether patients experienced AF (Table 3). Of the patients with AF, 25 of 49 (51.0%) in the ibrutinib group had bleeding events; timing of the bleeding events relative to onset of AF was not evaluated. Bleeding events were grade 1 or 2 in severity in all patients in the ibrutinib group (Online Supplementary Figure S3). In the ibrutinib group, 10 of 25 (40.0%) patients had not received anticoagulant/antiplatelet medication. Nine (36.0%) patients were receiving a single anticoagulant/antiplatelet medication at the time of the bleeding event (aspirin, n=1; novel oral anticoagulants, n=5; low-molecular-weight heparin, n=3), and 6 (24%) were receiving at least two medications (2 of whom received three). All 35 patients with CHA2D22-VASc scores ≥2 who developed AF on ibrutinib received anticoagulant/antiplatelet medication.
Table 3.
Rates of bleeding in ibrutinib and comparator patient groups in terms of the presence of atrial fibrillation (AF) and use of antiplatelet and/or anticoagulant agents.
During the study, 12 (34.3%) of these patients had a grade 1 or 2 bleeding event, 7 of whom were on a single anticoagulant/antiplatelet medication and 5 of whom were on two or more medications.
Three bleeding events resulted in death, all in the ibrutinib group (ruptured abdominal aortic aneurism, subdural hematoma, post-procedural hemorrhage); one patient was on aspirin at the time of the fatal bleed, the other 2 patients had not received concomitant anticoagulants/antiplatelets, while none of the patients had AF while on treatment (Online Supplementary Table S8).
Clinical sequelae of AF
Cardiovascular disease clinical sequelae were captured using MedDRA SMQ and grouped into five CVD categories: arrhythmia, congestive heart failure, ischemic heart disease, hypertension, and ischemic CNS vascular conditions (Online Supplementary Appendixn 2). Among patients who had a single AF episode, these CVD clinical sequelae were seen with similar frequency in both groups: 5 of 27 (18.5%) of patients in the ibrutinib group compared with 2 of 10 (20.0%) patients in the comparator (Online Supplementary Table S9). Thirteen patients on ibrutinib who had multiple AF episodes, and both patients on comparator, developed clinical CVD sequelae; 9 (69.2%) and 2 (100%) patients, respectively, had a history of one of these conditions. One comparator-treated patient with an AF event had an ischemic CNS vascular condition within the observation time; no ibrutinib-treated patients had an ischemic CNS vascular condition.
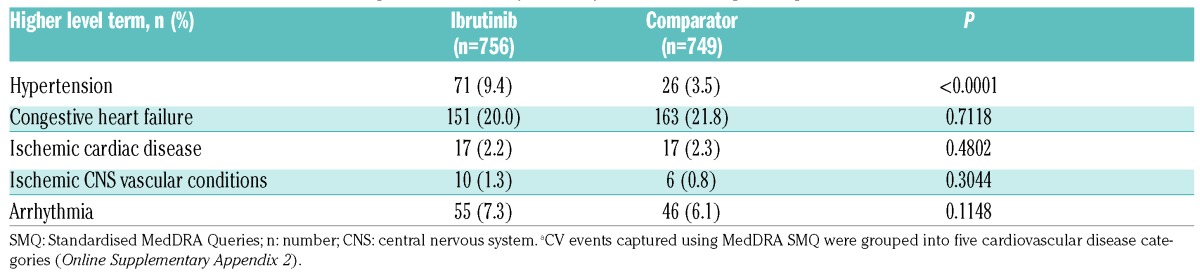
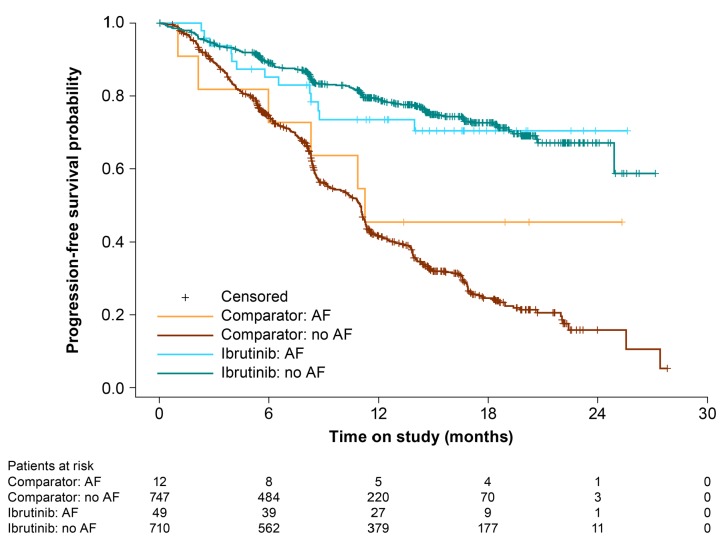
Given that clinical complications of AF can occur in the absence of clinically symptomatic AF, incidences of cardiovascular events (as defined above) were also evaluated in the full cohort. Hypertension was the only group term that occurred on study at a significantly higher rate in the ibrutinib group compared with the comparator (Table 4). Furthermore, CLL patients experiencing AF on ibrutinib had similar PFS duration as patients who did not (Figure 4).
Table 4.
Cardiovascular (CV) events occurring while on therapy listed by MedDRA SMQ grouping.a
Figure 4.
Progression-free survival in patients with and without atrial fibrillation (AF).
Discussion
Ibrutinib has shown a highly favorable benefit-risk ratio for patients with CLL/SLL and relapsed or refractory MCL, albeit with certain side effects including AF. To date, the risk factors, natural history, therapeutic management, and outcomes of ibrutinib-related AF have not been well characterized. In this pooled analysis of four RCTs, with a median follow up of 16.6 months, the incidence of AF in patients treated with ibrutinib was 6.5% (95%CI: 4.8, 8.5%). The incidence of AF was 10.4% (95%CI: 8.4, 12.9) with additional follow up, which is relatively consistent with prior clinical studies and independent reports.5,13,17–20 The incidence of AF was highest in the first six months, and then continued at a low rate. Multivariate analysis showed that use of ibrutinib, prior history of AF, and age over 65 years were associated with a higher risk of AF. Older patients, in general, have a higher propensity for CVD including AF, so it is not surprising that the patients developing AF in this pooled analysis were older than the overall study population. In addition, although history of AF was a predictor of AF in this pooled analysis, we noted that 85.2% of patients with a history of AF did not have a recurrence while being treated with ibrutinib at a median follow up of 16.6 months. However, the small number of patients with AF and the exclusion of patients with significant cardiac disease from the clinical trials may limit the interpretation of the findings in other patient populations. Given the rate of AF is highest in the first six months of ibrutinib therapy and may be higher in older, non-trial populations, patients should be carefully monitored for signs of AF, particularly if they are older or have a preexisting history.
In a separate analysis investigating only those patients on ibrutinib without a prior history of AF, we investigated the utility of the Shanafelt risk score in estimating the likelihood of developing AF among our CLL study population on ibrutinib, using a similar methodology as the original report.13 In the Shanafelt predictive model, older age, male gender, valvular heart disease, and hypertension were identified as risk factors independently associated with de novo AF at the time of CLL diagnosis.13 We observed a similar increased risk of de novo AF among patients with higher score categories, which may be useful for counseling patients. The estimated 5-year AF rates were similar to those observed in the original report.13
Current recommendations for management of AF in patients on ibrutinib indicate that therapy should be interrupted for any new onset or worsening grade ≥3 non-hematologic toxicity, including AF. Once symptoms have resolved to grade 1 or baseline, treatment may be reinitiated at the starting dose. For patients who develop AF, physicians should follow the appropriate guidelines for clinical management of AF. In this pooled analysis, the approach to AF management was heterogeneous and included practices in accordance with the ibrutinib protocol recommendations and prescribing information as well as CV standard of care for AF.21,22 Approximately half of patients developing AF on ibrutinib were able to be managed without dose interruption or modificiation. In a recent report by Thompson et al., patients with CLL who had ibrutinib interrupted at the the onset of AF had an inferior PFS compared with that seen in patients who continued ibrutinib or had dose reductions.23
In this analysis, interrupting ibrutinib therapy for seven days or more in the context of AF did not appear to significantly impact 18-month PFS; given the limited sample size, however, these findings should be interpreted with caution. The majority of the patients did not discontinue ibrutinib due to AF so limited data were available on patients receiving an alternative therapy due to AF in our study. Among 7 patients who discontinued ibrutinib, one patient received subsequent chemoimunotherapy with BR. However, there are a number of agents in the CLL landscape that provide alternative options for CLL patients who may need to discontinue ibrutinib treatment due to AF, including novel agents like idelalisib and venetoclax. A few reports have been published on patient outcomes after ibrutinib discontinuation due to AEs, and suggest that outcomes are better than those seen in the setting of disease progression.24
The approach to medical management of AF in patients receiving ibrutinib should take into account the potential risk of pharmacokinetic interactions with commonly used anticoagulant/antiplatelet medications. US prescribing information for ibrutinib currently recommends a dose reduction to 140 mg daily for co-administration with moderate CYP3A/4 inhibitors.21,22 At the time the studies in this pooled analysis were conducted, however, dose interruption or modification was at the physician’s discretion, and many patients continued on full-dose ibrutinib while receiving moderate CYP3A/4 inhibitors to manage AF, specifically amiodarone and diltiazem. It was beyond the scope of the current analysis to determine whether increased toxicity resulted from this approach; however, current practice and prudence would dictate avoidance of CYP3A/4 inhibitors if possible or dose reduction of ibrutinib consistent with the prescribing information.21,22
In vitro studies suggest that ibrutinib induces platelet aggregation defects due to the inhibition of Bruton’s tyrosine kinase and TEC in the glycoprotein VI collagen-activated pathway,25,26 and therefore concomitant use of anticoagulants or aspirin with ibrutinib could enhance bleeding risk. In the current analysis, serious bleeding events occurred in 2.9% of patients treated with ibrutinib overall and in 2.0% of patients with AF. Due to the heterogeneous approach to AF management, it was difficult to characterize the impact of specific medication combinations on the risk of bleeding; however, the very low incidence of grade ≥3 bleeding events, even among patients receiving more than one anticoagulant/antiplatelet agent, is reassuring. These results, however, are in contrast to a recent report of real-world experience with 56 patients who developed AF on ibrutinib, in whom a 14% rate of major bleeding was seen.6 These results suggest that more data are needed on the specific risk factors for major bleeds in ibrutinib-treated patients, and until those are available, additional caution and monitoring are warranted in clinical practice, particularly for older patients with comorbidities who are likely to have a higher bleeding risk.6 Notably, in this analysis, no thromboembolic events were observed among ibrutinib-treated patients developing AF. It will be of interest for future studies to determine whether ibrutinib use itself has sufficient antiplatelet activity to confer some of the benefits of aspirin or other antiplatelet agents.
In this pooled analysis of relatively young and healthy clinical trial subjects, we did not find an increased rate of congestive heart failure, ischemic cardiac disease, stroke, or other arrhythmias among patients with AF in the ibrutinib group relative to the comparator. This finding should be interpreted with caution given the small sample size and limited follow up; however, it is notable given that patients with AF generally had higher rates of comorbidities like hypertension and hyperlipidemia at study entry. When we evaluated the study population as a whole (regardless of the presence of symptomatic AF), we noted an increase in the incidence of de novo and recurrent or ongoing hypertension in patients treated with ibrutinib. Patients with a history of hypertension were more likely to develop AF; however, in patients without a history of hypertension, 38 of 428 patients developed de novo hypertension but only one patient developed de novo hypertension and AF, suggesting that at least so far, these events are not highly correlated. Ibrutinib therapy has been associated with hypertension in clinical trials, making it difficult to discern if the signal we saw was a direct treatment effect or clinical sequelae related to subclinical AF in a subset of patients.
This analysis has inherent limitations, particularly in its post hoc assessment of completed studies, which focused primarily on oncological rather than CV outcomes. Patient numbers in certain subgroups were low, despite having access to data from four large RCTs, and the inclusion of patients with MCL who received a different dose of ibrutinib and have different disease biology, as well as the inclusion of patients treated with both ibrutinib alone or in combination with BR, may impact on the interpretation of the findings. The method of capturing AEs and concomitant medications limited our ability to evaluate dose intensity, sequencing of anticoagulation in patients with AF, and the temporal relationship between AF and bleeding events. Furthermore, due to study exclusion criteria related to certain serious comorbidities, patients on the clinical trials were undoubtedly healthier than most treated in general practice. Given these limitations, this study may underestimate the incidence of AF among older patients treated outside a clinical trial setting with ibrutinib. A recently published retrospective study of CLL patients treated at several cancer centers found that AF persisted in 62% of 56 ibrutinib-treated patients despite AF-directed therapy.6 Three episodes of cardiac failure, one stroke, and major bleeding events in 14% of patients were observed in that study.6 Algorithm-based guidelines have been proposed to manage ibrutinib-associated AF but have not yet been validated.27 Additional larger datasets, perhaps population-based, will be required to determine representative rates of AF with ibrutinib in different patient groups, to better characterize the incidence of AF-related complications, and to evaluate the value of proposed guidelines outside of a clinical trial setting.
Prudence dictates that clinicians consider the benefit-risk profile of ibrutinib therapy in patients with a history of AF or other predisposing risk factors. Results of this pooled analysis of more than 1500 patients in four RCTs suggest that, with appropriate vigilance and monitoring, the majority of patients with known risk factors for AF can be safely treated with ibrutinib. Alternative treatment options are available for those who discontinued ibrutinib due to AF. However, most patients who develop AF on treatment will not require treatment discontinuation and many can be managed safely with commonly used anticoagulant/antiplatelet medications. Prospective clinical studies focusing on detailed evaluation of the cardiac effects of ibrutinib are warranted to further elucidate the potential mechanisms of AF.28,29
Supplementary Material
Acknowledgments
The authors thank the patients, families, caregivers, research nurses, study co-ordinators and support staff who contributed to all of the studies.
Footnotes
Funding
This analysis was sponsored by Janssen Research & Development, LLC and Pharmacyclics, LLC. Medical writing and editorial assistance was provided by PAREXEL International and was funded by Janssen Global Services, LLC.
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/10/1796
References
- 1.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqui M, Valdez J, Soto S, Bray A, Tian X, Wiestner A. Atrial fibrillation in CLL/SLL patients on ibrutinib. Presented at: American Society of Hematology (ASH) 57th Annual Meeting; December 5–8, 2015; Orlando, FL. [Google Scholar]
- 6.Thompson PA, Levy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–466. [DOI] [PubMed] [Google Scholar]
- 7.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138–140. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–2429. [DOI] [PubMed] [Google Scholar]
- 9.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 11.Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34(14):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong DP, Eikelboom JW, Healey JS, Connolly SJ. Atrial fibrillation is associated with increased mortality: causation or association? Eur Heart J. 2013;34(14):1027–1030. [DOI] [PubMed] [Google Scholar]
- 13.Shanafelt TD, Parikh SA, Noseworthy PA, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2017;58(7):1630–1639. [DOI] [PubMed] [Google Scholar]
- 14.Barrientos JC, Meyer N, Song X, Rai KR. Characterization of atrial fibrillation and bleeding risk factors in patients with chronic lymphocytic leukemia (CLL): a population-based retrospective cohort study of administrative medical claims data in the United States (US). Presented at: American Society of Hematology (ASH) 57th Annual Meeting; December 5–8, 2015; Orlando, FL. [Google Scholar]
- 15.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. Proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assoc. 1999;94:496–509. [Google Scholar]
- 17.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. [DOI] [PubMed] [Google Scholar]
- 19.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015; 126(6):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56. [DOI] [PubMed] [Google Scholar]
- 21.IMBRUVICA (ibrutinib) [US prescribing information]. Horsham, PA: Janssen Biotech, Inc.; 2016. [Google Scholar]
- 22.IMBRUVICA (ibrutinib) [summary of product characteristics]. Beerse, Belgium: Janssen Pharmaceutica NV; 2016. [Google Scholar]
- 23.Thompson PA, Levy V, Tam CS, et al. The impact of atrial fibrillation on subsequent survival of patients receiving ibrutinib as treatment of chronic lymphocytic leukemia (CLL): an international study. Blood. 2015;126(23)3301. [Google Scholar]
- 24.Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016; 128(18):2199–2205. [DOI] [PubMed] [Google Scholar]
- 25.Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783–787. [DOI] [PubMed] [Google Scholar]
- 26.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991–3995. [DOI] [PubMed] [Google Scholar]
- 27.Vrontikis A, Carey J, Gilreath JA, Halwani A, Stephens DM, Sweetenham JW. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30(11):pii:219802. [PubMed] [Google Scholar]
- 28.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–3830. [DOI] [PubMed] [Google Scholar]
- 29.Yang T, Moslehi JJ, Roden DM. Proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton’s tyrosine kinase (BTK) used in cancer therapy [abstract]. Circulation. 2015;132(Suppl 3):A14587. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.