We write to share our experience with serological failure of standard influenza vaccine in patients treated with the novel Bruton tyrosine kinase (BTK)-inhibitor ibrutinib. Influenza causes significant morbidity and mortality amongst cancer patients,1 as illustrated by the recent H1N1 outbreak.2 Routine vaccination schedules recommend yearly influenza vaccination in immunocompromised hosts.3
Ibrutinib is the first-in-class BTK inhibitor and is now approved for the treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma and Waldenstrom macroglobulinemia (WM).4 Although deficiency of BTK leads to severe hypogammaglobulinemia in the pediatric disease X-linked agammaglobulinemia,5 current studies of ibrutinib therapy in adult patients have not shown significant falls in immunoglobulin levels despite continuous exposure.6,7 This may be related to the fact that adults carry a mature repertoire of long-lived plasma cells from previous infections and vaccinations. However, the critical dependency of B-cell maturation on functional BTK7 raises the possibility that patients on long-term ibrutinib therapy may not be able to respond to new challenges such as vaccinations and infections. Indeed, patients receiving other anti-B-cell agents, such as rituximab, have demonstrated poor serological responses to influenza vaccination during the first year after exposure,8 with recovery of vaccine responsiveness to 50% at 6 months, and normal responsiveness 12 months after exposure.9 Sun et al. recently published their experience of influenza vaccination responsiveness in 19 ibrutinib-treated patients, finding seroconversion to at least one vaccine strain in 26%.10 However, neither the extent of prior treatment in their population nor the proportion of patients who received high-dose influenza vaccine (Fluzone) was clear and there was no control arm. We sought to prospectively test serological responses in patients vaccinated with standard-dose influenza vaccine at our institution for the 2015 influenza season.
Between the 21st of April and 18th of May 2015, 14 consecutive patients with CLL (n=13) or WM (n=1) treated with ibrutinib received a single intramuscular dose of influenza trivalent vaccination (Fluvax® or Fluarix®) and were prospectively tested for serological responses before and after vaccination. Serum samples were collected at baseline and at 30 days (convalescent sample) from patients and from 50 community-living, healthy elderly controls (median age 67 years; range, 60–88 years). Serum hemagglutination inhibition (HI) titers against the vaccine viruses were determined using a standardized method.11 The viruses tested represented the viruses contained in the 2015 Australian influenza vaccine: A/California/7/2009 [A(H1N1)pdm09], A/Switzerland/9715293/2013 [A(H3N2)], and B/Phuket/3073/2013 (B/Yamagata lineage).
Serum samples with HI titers below the detection limit (<10) were assigned a titer of 5 and those with titers >1280 were assigned a titer of 1280. A post-vaccination titer of ≥40 was considered to indicate seropositivity, while a 4-fold increase in post-vaccination titer was considered to indicate seroconversion. Titers were transformed on the log scale and reported as crude geometric mean titers (GMT), a meaningful immunological parameter representing adequate antibody response after vaccination. A Pearson chi-squared test with Yates continuity correction was used to compare proportions of seropositive and seroconverted subjects between groups. Crude GMT were compared using the Welch two sample t-test. All statistical analyses were performed using R version 3.1.3.
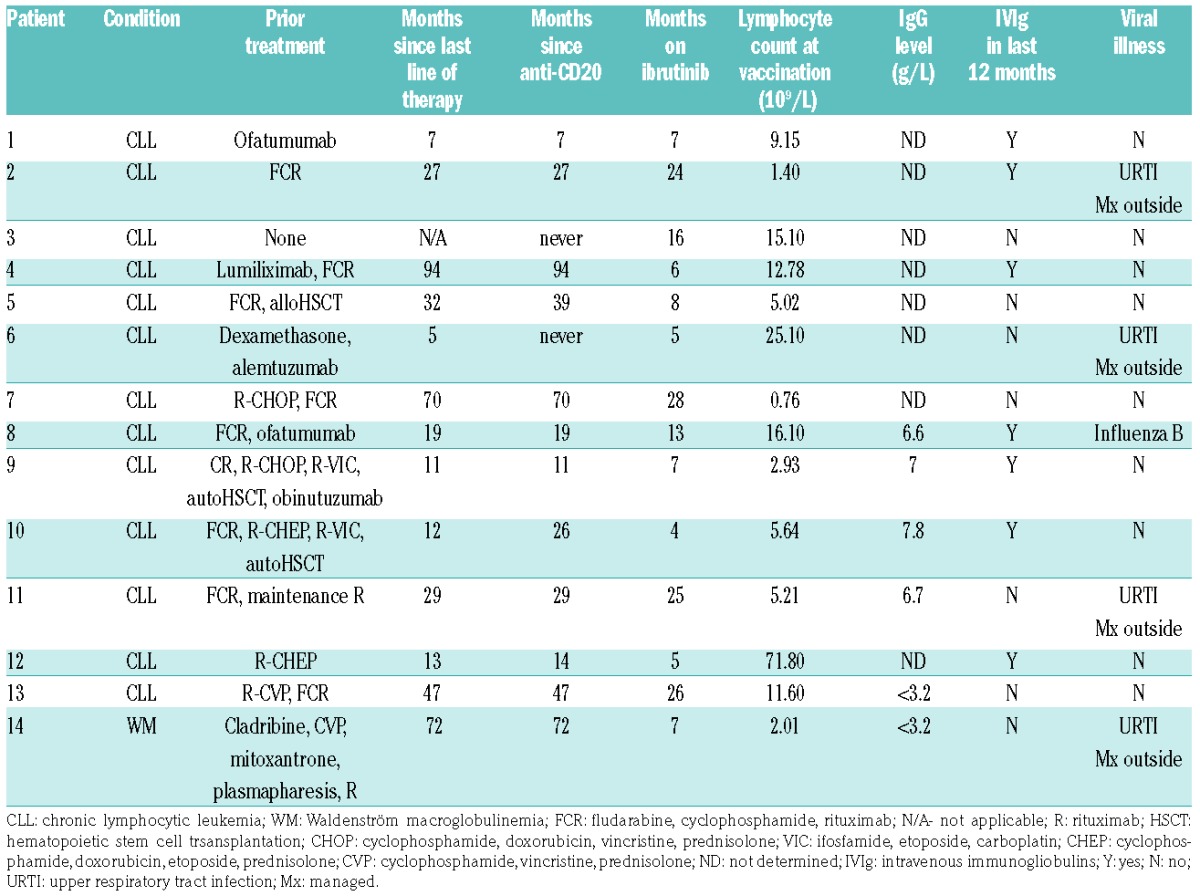
The median duration of ibrutinib therapy in the 14 patients was 7.5 months (range, 4–28 months). The median age of the patients was 68 years (range, 51–84 years). Ninety-three percent had received prior therapy for CLL or WM, although most (86%) had not received anti-CD20 monoclonal antibodies in the preceding 12 months, with a median time from last anti-CD20 of 28 months (range, 7–94 months) (Table 1). All patients were in stable partial remission on ibrutinib during this study.
Table 1.
Patients’ condition, prior treatment and immunological parameters.
The serological responses of the ibrutinib-treated patients and those of elderly controls are shown in Table 2. Notably, of the 14 ibrutinib-treated patients, only one (7%) demonstrated evidence of seroconversion (P=0.003, P=0.007 and P=0.16 for A/California/7/2009 (H1N1), A/Switzerland/9715293/2013 (H3N2) and B/Phuket/3073/2013 (B/Yamagata lineage), respectively, as compared against elderly matched controls). Accordingly, the post-vaccination GMT (post-GMT) was significantly reduced in ibrutinib-treated patients for all trivalent influenza antigen components (Table 2). Clinically, one patient developed confirmed influenza B and four others developed upper respiratory tract infections of unconfirmed etiology, managed externally. No patient received prophylactic oseltamivir and none required hospital admission. The patient who did respond serologically was the patient with WM (patient 14 in Table 1), who had not had previous lines of therapy for 72 months, and had a low baseline IgG.
Table 2.
Ibrutinib-treated patients (n=14) versus elderly controls (n=50).
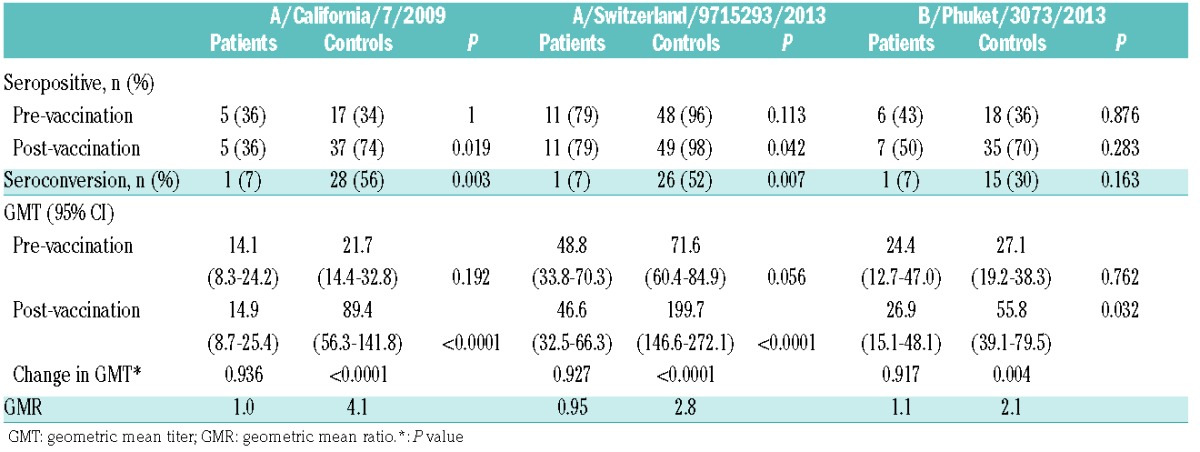
Our results demonstrate an absence of serological response to influenza vaccination in patients currently on treatment with the BTK inhibitor ibrutinib. This is in contrast to the findings of a recent study by Sun et al.10 One possible explanation for this difference may be the extent of prior treatment received. Patients in our study had received a median of two lines of prior therapy, whereas the prior treatment histories of patients in the study by Sun et al. were not reported.10 Seroconversion rates correlate with prior therapy: 10–50% of patients with untreated CLL seroconvert following influenza vaccination,9,12,13 and among patients who had received prior treatment, the duration of time since last chemotherapy was longer for those who gained seroprotection from influenza vaccination than for those who were not seroprotected (17.5 versus 4.7 months, respectively, P=0.001).9 An alternative explanation could be that the use of high-dose influenza vaccination may have contributed to higher seroconversion rates, as an undisclosed proportion of patients in the study by Sun et al. received the high-dose influenza vaccine Fluzone. Thirdly, although it is convention to assess vaccination response at 4 weeks, as in our study, it has been noted previously that vaccine responsiveness may be delayed in patients with hematologic malignancies,14 and assessing response at 12 weeks could potentially have detected cases of late seroconversion. Finally, although serological responses are the recognized standard of vaccination response assessment, it may be that measuring immunoglobulin responses to vaccination is problematic in ibrutinib-treated patients, and other methods to assess response, such as flow cytometric antigen-specific T-cell assays, may be necessary.15
Our data were collected from a real world cohort of patients who had received prior treatment and raise important questions about the response to vaccines against other pathogens (e.g. pneumococcus and hepatitis B), and the ability of these patients to mount adequate serological responses and immunological memory against new infectious challenges. This effect is likely attributable to ibrutinib as only 14% our cohort had received anti-CD20 therapy within the preceding year. Although it could be argued that immunoparesis from CLL itself could explain this result, it is unlikely to entirely explain the complete lack of response seen here, as higher rates of seroconversion in untreated CLL patients have been reported. To clarify this question of the attributable effect of ibrutinib to poor vaccine response in CLL patients, a study with three arms, namely healthy controls, ibrutinib-naïve CLL patients and those on ibrutinib, would be required. Larger studies of alternative vaccination strategies in patients receiving BTK-inhibitor therapy are also warranted.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding: the Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health
References
- 1.Elting LS, Whimbey E, Lo W, Couch R, Andreeff M, Bodey GP. Epidemiology of influenza A virus infection in patients with acute or chronic leukemia. Support Care Cancer. 1995;3(3):198–202. [DOI] [PubMed] [Google Scholar]
- 2.Denholm JT, Gordon CL, Johnson PD, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010;192(2):84–86. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–318. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine (Baltimore). 1996;75(6):287–299. [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomised study. Br J Haematol. 2005;130(1):96–98. [DOI] [PubMed] [Google Scholar]
- 9.de Lavallade H, Garland P, Sekine T, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96(2):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun C, Gao J, Couzens L, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network WGIS. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Vol. 2016 Available from: http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf?ua=1; 2011. [Google Scholar]
- 12.Van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, Van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12(5):420–424. [DOI] [PubMed] [Google Scholar]
- 13.Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1994;91(3):115–118. [DOI] [PubMed] [Google Scholar]
- 14.Mazza JJ, Yale SH, Arrowood JR, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005; 3(4):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haining WN, Evans JW, Seth NP, et al. Measuring T cell immunity to influenza vaccination in children after haemopoietic stem cell transplantation. Br J Haematol. 2004;127(3):322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


