Acalabrutinib is a new, irreversible Bruton tyrosine kinase (BTK) inhibitor, reported to be more selective than ibrutinib.1–3 BTK inhibitors are often combined with anti-CD20 antibodies in the clinic, but ibrutinib interferes with some of the cell-mediated mechanisms of action of anti-CD20 antibodies, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent phagocytosis, perhaps due to inhibition of other BTK family kinases such as ITK or TEC.4–6 The aim of this work was to verify whether acalabrutinib differs from ibrutinib with respect to inhibition of ADCC and antibody-dependent phagocytosis, induced by anti-CD20 rituximab and obinutuzumab. Unlike ibrutinib, acalabrutinib did not interfere with any of the anti-tumor, immune-mediated mechanisms of anti-CD20 antibodies.
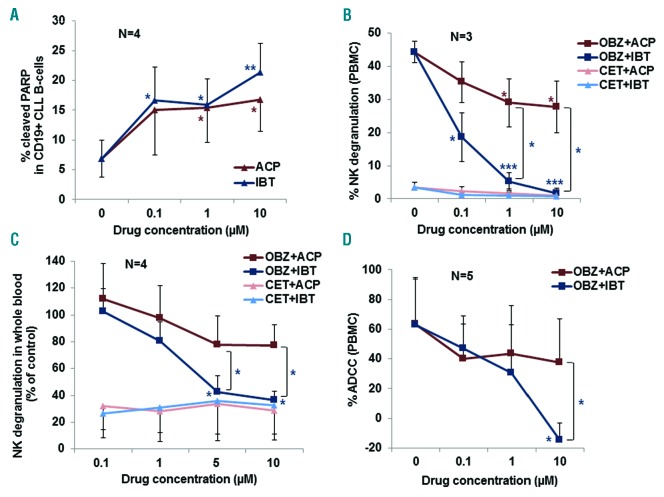
We first wished to confirm that acalabrutinib is an active drug and induces apoptosis of chronic lymphocytic leukemia (CLL) B cells.3 To do this, we performed assays using CLL B cells plated for 48 h with increasing concentrations of acalabrutinib or ibrutinib as a control. Apoptosis was then measured by flow cytometry using antibodies specific for cleaved PARP and CD19. The characteristics of the CLL patients used are presented in Online Supplementary Table S1. As shown in Figure 1A, both acalabrutinib and ibrutinib induced apoptosis of CLL B cells at concentrations ≥1 μM (P<0.05) (n=4). These data are in agreement with those of previous studies, reporting significant induction of apoptosis at 48–72 h with 1 μM acalabrutinib or ibrutinib, a concentration that should be reached in vivo.3 Of note, the peak plasma concentrations of ibrutinib are somewhat lower (340–540 nM) than those of acalabrutinib (1–2 μM).1 Both acalabrutinib and ibrutinib have previously been shown to inhibit BTK and downstream signaling (in particular ERK and S6 phosphorylation), leading to Mcl-1 down-modulation and PARP/caspase 3 cleavage, suggesting that these mechanisms may be at least in part responsible for the apoptosis induction by these drugs.3 Nonetheless further mechanistic studies are warranted.
Figure 1.
Effects of ibrutinib and acalabrutinib on apoptosis, natural killer-cell degranulation and antibody-dependent cellular cytotoxicity. (A) Peripheral blood mononuclear cells (PBMC) from CLL patients were plated in the presence or absence of 0.1–10 μM acalabrutinib (ACP) or ibrutinib (IBT). After 48 h, cells were stained with CD19-FITC, permeabilized and incubated with cleaved PARP-Alexafluor 647 antibody. The data show the percentages of cleaved PARP in CD19+ cells detected by flow cytometry and are the means and standard deviations of four independent experiments. (B) PBMC from healthy donors were treated for 1 h with the indicated concentrations of ACP and IBT and then stimulated by the addition of MEC1 CLL B cells (1:1 ratio of PBMC:MEC1 cells), opsonized with 1 μg/mL obinutuzumab (OBZ) or cetuximab (CET), an irrelevant, control antibody. Natural killer (NK)-cell degranulation was measured by flow cytometry after 4 h at 37°C, as the percentage of CD56+ /CD107a+ double-positive cells with respect to total CD56+ NK cells. (C) Whole blood from healthy donors was drawn in 50 μg/mL desirudin9 and plated with 0.1–10 μM ACP or IBT. One hour later CLL B cells opsonized with 1 μg/mL OBZ or CET were added. After a further 4 h of incubation at 37°C, samples were analyzed for CD107a expression on CD56+ NK cells. The results are shown as percentage of NK-cell degranulation in the presence BTK inhibitors with respect to control treated with CLL+ OBZ alone without inhibitors. (D) For the antibody-dependent cellular cytotoxicity (ADCC) assays, PBMC from healthy donors were pre-incubated with increasing concentrations of ACP or IBT for 1 h. Then MEC1 cells loaded with calcein-AM were added together with 1 μg/mL OBZ or CET control antibody. Calcein release was measured after 4 h of incubation at 37°C. All results (A–D) are the means and standard deviations of three to five independent experiments, as indicated in each panel. Statistical significance was determined using a paired Student t-test, comparing single doses of ACP with IBT, or comparing drug-treated versus untreated samples. The statistical significance versus untreated controls is shown by colored asterisks placed near the relevant experimental condition, whereas significance between ACP and IBT at specific doses is shown using brackets that indicate the specific comparisons. *P<0.05; ** P<0.01; *** P<0.001.
We and others have previously demonstrated that ibrutinib strongly inhibits natural killer (NK)-cell degranulation as well as ADCC induced by anti-CD20 antibodies.4,5 We therefore analyzed the effect of acalabrutinib on NK-cell degranulation in comparison with that of ibrutinib in our standard assays. We first used purified peripheral blood mononuclear cells from healthy donors as effectors, anti-CD20 obinutuzumab and the MEC1 CLL B-cell line as the target. As previously observed, ibrutinib strongly inhibited NK-cell degranulation in these conditions, with an IC50 of about 0.1 μM and about 90% inhibition at 1 μM (P<0.001). In contrast, acalabrutinib was only mildly inhibitory, with 20% and 35% inhibition at the higher 1 and 10 μM drug doses (Figure 1B) (P<0.05). The difference between the effects of the two drugs at the 1 and 10 μM doses was also statistically significant (P<0.05). NK-cell degranulation was also analyzed in whole blood assays using obinutuzumab-opsonized CLL B-cell targets. Ibrutinib inhibited NK-cell degranulation in whole blood, with 90–95% inhibition at 5 and 10 μM with respect to controls and an IC50 of about 2–3 μM (Figure 1C). In contrast inhibition by acalabrutinib at the same doses was marginal (about 2–25%) and not statistically significant. The difference between the effects of acalabrutinib and ibrutinib was statistically significant at the 5 and 10 μM doses (P<0.05) (Figure 1C).
Finally, the effect of BTK inhibitors on ADCC was measured using the MEC1 CLL B-cell line as the target and obinutuzumab. Ibrutinib was strongly inhibitory on ADCC, with an IC50 of about 1 μM, as previously reported4 (P<0.05) (Figure 1D). In contrast, inhibition by acalabrutinib was marginal even at 10 μM. The difference between the effects of ibrutinib and acalabrutinib at 10 μM was statistically significant (Figure 1D) (P<0.05). Similar results were obtained in the presence of rituximab (data not shown).
The data on NK-cell degranulation and ADCC suggest that ibrutinib at standard doses, but not acalabrutinib, may significantly inhibit NK-cell activation and cytotoxicity in patients.3,4 The different behaviors of ibrutinib and acalabrutinib in ADCC are probably due to the different off-target effects of these drugs on kinases such as ITK or TEC, known to be differentially inhibited by ibrutinib and acalabrutinib,1 and implicated in T- and NK-cell activation.7 This hypothesis needs to be investigated further.
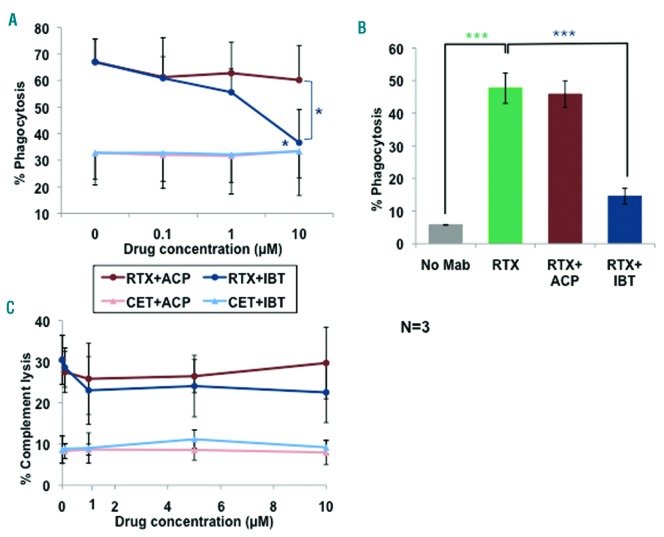
We next analyzed the effects of acalabrutinib and ibrutinib on phagocytosis, by human macrophages, of CLL B cells opsonized with rituximab. In flow cytometry assays, rituximab induced 67% phagocytosis of CLL B-cell targets (Figure 2A). Ibrutinib inhibited phagocytosis with respect to the control by about 90% at 10 μM (P<0.05). In contrast, acalabrutinib did not inhibit phagocytosis significantly at any dose. We also performed phagocytosis assays in chamber slides, counting phagocytosing macrophages directly under the microscope. As shown in Figure 2B, 10 μM ibrutinib, but not acalabrutinib, significantly inhibited phagocytosis of rituximabopsonized CLL B cells by macrophages. These data confirm previous reports that ibrutinib inhibits antibody-dependent phagocytosis with an IC50 of 1–3 μM.4,6 Although monocytes/macrophages express BTK, the lower IC50 for ibrutinib and the fact that acalabrutinib had little effect on phagocytosis indicate that kinases situated downstream of Fcg receptors other than BTK, such as Hck, FgR or Lyn, and reported to be differentially inhibited by acalabrutinib and BTK,1 may be involved in this phenomenon. This hypothesis does, however, remain to be investigated in detail.
Figure 2.
Effects of ibrutinib and acalabrutinib on phagocytosis by macrophages and complement-dependent cytotoxicity. (A) Human macrophages, differentiated in vitro by macrophage colony-stimulating factor, were pretreated with increasing concentrations of ibrutinib (IBT) or acalabrutinib (ACP) for 1 h at 37°C, and then incubated with CFSE-labeled CLL B cells opsonized with 1 μg/mL cetuximab (CET) or rituximab (RTX) antibodies.4 After a further 2 h at 37°C, cells were stained with CD11b-PE and CD19-APC and phagocytosis was measured by flow cytometry. Percentage phagocytosis, defined as CD11b+CFSE+CD19− cells with respect to total CD11b+ cells, is shown. (B) In some experiments phagocytosis assays were performed in chamber slides, incubating macrophages and unlabeled CLL B cells with 1 μg/mL RTX in the presence or absence of 10 μM BTK inhibitors, for 2 h at 37°C; cells were then fixed and stained with hematoxylin-eosin. Macrophages having engulfed at least one CLL B cell were counted under the microscope as percentage of total macrophages. (C) To measure complement-dependent cytotoxicity (CDC), MEC1 cells were incubated with 0.1, 1, 5 or 10 μM ACP or IBT for 1 h at 37°C, after which 10 μg/mL RTX or control CET and 20% human serum as a source of complement were added and incubation continued for a further 6 h. 7-AAD was then added and CDC was measured by flow cytometry. All results (A–C) are the means and standard deviation of three independent experiments. Statistical analyses were performed with the Student t-test. Symbols are as described in the legend to Figure 1 and colors refer to the specific conditions tested.
We also analyzed whether the BTK inhibitors had any effect on human complement-dependent cytotoxicity (CDC) of the MEC1 CLL B-cell line, induced by rituximab. CDC reached a mean of 30% with rituximab and human serum (Figure 2C). Neither acalabrutinib nor ibrutinib inhibited CDC, even at the highest doses of 10 μM. These data are in agreement with our previous data.4 We cannot, however, exclude that modifications of CD20 expression at later time points may affect CDC, as suggested for ibrutinib by some groups.8
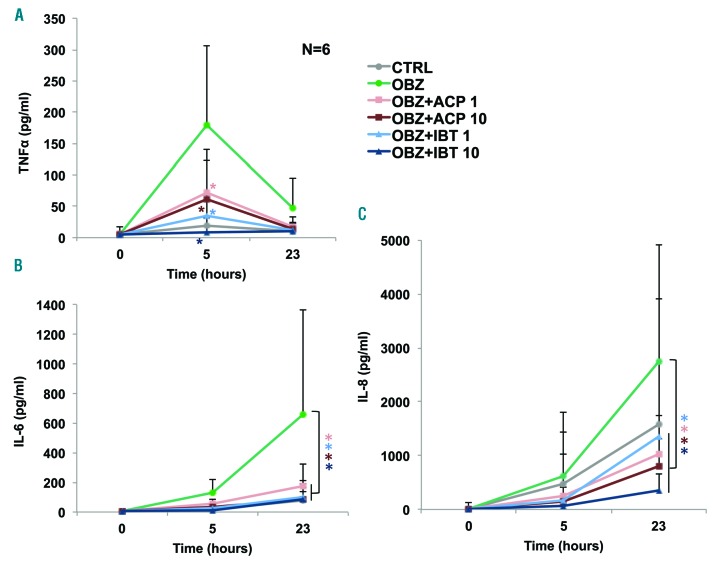
Finally, to evaluate the effect of BTK inhibitors on cytokine release in CLL patients, we treated freshly isolated whole blood samples from six untreated CLL patients (Online Supplementary Table S2) with 1 and 10 μM of the drugs and 1 h later with obinutuzumab. Supernatants were collected before (T=0) as well as 5 and 23 h after the addition of obinutuzumab and interleukin (IL)-6, IL-8, IL-1β, tumor necrosis factor (TNF)-α, IL-10 and IL12p70 concentrations in plasma were measured by flow cytometry. As previously reported,9 IL-10, IL-12p70 and IL-1b were not detected reproducibly and at significant levels in the presence or absence of obinutuzumab (data not shown). TNF-α was induced by obinutuzumab with a peak at 5 h. Both ibrutinib and acalabrutinib at the concentrations of 1 and 10 μM inhibited TNF-α induction consistently in all patients at this time point (Figure 3A) (P<0.05 for both drugs at both 1 and 10 μM). There was no significant difference between acalabrutinib and ibrutinib. IL-6 and IL-8 were induced above control levels between 5 and 23 h. IL-6 was strongly inhibited by both drugs to a similar extent, at both time points, and this was statistically significant at all doses tested and for both drugs (Figure 3B) (P<0.05). The induction of IL-8 by obinutuzumab was also significantly inhibited by acalabrutinib and ibrutinib to a similar extent (Figure 3C) (P<0.05 for both drugs at all doses). IL-6, IL-8 and TNF-α are induced by rituximab and obinutuzumab in patients, suggesting that our whole blood assay may adequately reflect the in vivo situation. Of note, some CLL cases (3/14) showed very high spontaneous cytokine release which was not further enhanced by obinutuzumab (data not shown). These data were not analyzed here and the reason for this diverse behavior is unclear at present.
Figure 3.
Ibrutinib and acalabrutinib inhibit the induction of tumor necrosis factor-α, interleukin-6 and interleukin-8 by obinutuzumab in whole blood from patients with chronic lymphocytic leukemia. Whole blood samples from CLL patients, drawn into desirudin, were treated with 1 or 10 μM acalabrutinib (ACP) or ibrutinib (IBT) for 1 h followed by 10 μg/mL obinutuzumab (OBZ) (T=0). Aliquots were collected at 5 and 23 h after the OBZ stimulus and centrifuged. TNF-α (A), IL-6 (B) and IL-8 (C) were measured in the supernatants by flow cytometry using the CBA bead array kit (BD Biosciences).9 The results are the means and standard deviations of six independent experiments using samples from six different CLL patients. Statistical analyses were performed using repeated-measures analysis-of-variance (ANOVA), with subjects nested into groups and significance is shown with respect to samples treated with OBZ only; *P<0.05, the asterisks indicate the significance of drug-treated samples versus those samples incubated with OBZ only, the colors corresponding to each condition.
The inhibition of cytokine release, as observed here, may have several functional consequences in vivo. IL-8 is produced by CLL B cells and has been implicated as an autocrine survival factor in CLL correlating with prognosis.10 Thus inhibition of IL-8 by acalabrutinib and ibrutinib may contribute to control disease. The role of IL-6 in CLL B-cell proliferation and survival, and its prognostic value, are still controversial issues. However, IL-6 is a major mediator of life-threatening cytokine release syndrome.11 TNF-α is also a strong pro-inflammatory mediator. Based on our data, we propose that inhibition of IL-6 and TNF-α release by the BTK inhibitors acalabrutinib and ibrutinib may help to mitigate the immediate toxicities associated with anti-CD20 treatment, as shown for ibrutinib during therapy with chimeric antigen receptor T cells.12
Acalabrutinib monotherapy has shown promising activity in animal models of CLL and lymphoma13,14 and in CLL patients.1,15 Like ibrutinib, acalabrutinib is already being tested in combination with anti-CD20 monoclonal antibodies, in particular in B-cell non-Hodgkin lymphoma (www.clinicaltrials.com). The data presented here suggest that acalabrutinib may be usefully combined with these antibodies. Acalabrutinib may show advantages over ibrutinib, since it does not interfere with any of the mechanisms of action of anti-CD20 monoclonal antibodies, at least in the short term. Furthermore, like ibrutinib, acalabrutinib may mitigate the cytokine-mediated early toxicity induced by anti-CD20 antibodies. Finally acalabrutinib has little toxicity on platelets which is an off-target effect of ibrutinib potentially leading to platelet dysfunction and bleeding.1
It should be noted that we did not investigate in detail the direct effects of acalabrutinib on CLL cells, including apoptosis induction, alone and in combination with anti-CD20 monoclonal antibodes. This should be done in future work, using samples from a large panel of CLL patients sensitive or resistant to ibrutinib. Similarly the efficacy of acalabrutinib and ibrutinib needs to be investigated and compared in vivo in animal models. Finally acalabrutinib will need to be tested in comparison with ibrutinib in clinical trials, alone or in combination with different anti-CD20 antibodies.
Supplementary Material
Acknowledgments
We thank Mr. Todd Covey, Acerta Pharma, for his advice on cleaved PARP measurements and Dr. A. Carrobbio for helping in statistical analyses. We also thank the numerous patients and healthy volunteers who kindly donated peripheral blood for these experiments and the nurses for their precious help.
Footnotes
Funding: this work was funded in part by Acerta Pharma, San Carlos, CA, USA, the “Associazione Italiana Ricerca contro il Cancro” (AIRC Investigator grant to JG) and the “Associazione Italiana contro le Leucemie-linfomi e myeloma (AIL)-sezione Paolo Belli”, Bergamo, Italy
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016; 374(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel V, Balakrishnan K, Bibikova E, et al. Comparison of acalabrutinib, a selective Bruton tyrosine kinase inhibitor, with ibrutinib in chronic lymphocytic leukemia cells. Clin Cancer Res. 2017;23(14):3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da Roit F, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015; 100(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014; 123(12):1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borge M, Belen Almejun M, Podaza E, et al. Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages. Haematologica. 2015;100(4):e140–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khurana D, Arneson LN, Schoon RA, Dick CJ, Leibson PJ. Differential regulation of human NK cell-mediated cytotoxicity by the tyrosine kinase Itk. J Immunol. 2007;178(6):3575–3582. [DOI] [PubMed] [Google Scholar]
- 8.Skarzynski M, Niemann CU, Lee YS, et al. Interactions between ibrutinib and anti-CD20 antibodies: competing effects on the outcome of combination therapy. Clin Cancer Res. 2016;22(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golay J, Da Roit F, Bologna L, et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013; 122(20):3482–3491. [DOI] [PubMed] [Google Scholar]
- 10.Francia di Celle P, Mariani S, Riera L, Stacchini A, Reato G, Foa R. Interleukin-8 induces the accumulation of B-cell chronic lymphocytic leukemia cells by prolonging survival in an autocrine fashion. Blood. 1996;87(10):4382–4389. [PubMed] [Google Scholar]
- 11.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruella M, Kenderian SS, Shestova O, et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia. 2017;31(1):246–248. [DOI] [PubMed] [Google Scholar]
- 13.Herman SE, Montraveta A, Niemann CU, et al. The Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia. Clin Cancer Res. 2017;23(11):2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington BK, Gardner HL, Izumi R, et al. Preclinical evaluation of the novel BTK inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma. PLoS One. 2016;11(7):e0159607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014; 370(24):2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.