Recent advances have been made in the identification of molecular determinants of the rare hemolytic disease, dehydrated hereditary stomatocytosis (DHSt), also called hereditary xerocytosis (HX). This well-known red blood cell (RBC) pathology is characterized by an abnormal cation leak resulting in KCl loss and red blood cell dehydration.1 It leads to cell fragility and hemolytic anemia. Two different genes have been linked to this phenotype: PIEZO1 and KCNN4 coding, respectively, for Piezo1, a non-selective cation channel activated by mechanical forces,2 and a calcium activated K+ channel (KCNN4) also known the Gardos channel in RBCs.3 So far, three different point mutations in KCNN4 have been linked to HX.4–6 The mutated KCNN4 is over-activated in patient RBCs leading to an increased K+ loss and, hence, water loss and cell dehydration.7 About a dozen mutations in Piezo1 are linked to HX,8–10 and some of them have been shown to change the kinetics of channel gating.10,11 In normal RBCs, Piezo1 appears to be a major factor in cell response to mechanical stress (by controlling calcium influx),12,13 and there is a functional connection between Piezo1 and KCNN4 through the modification of intracellular calcium concentration. Our present study was designed to evaluate in HX the functional link between mutated Piezo1 and KCNN4 and to assess the efficiency of a KCNN4 blocker, Senicapoc,14 to treat HX whatever the molecular cause.
Our study focused on three independent index cases with a typical HX clinical and biological phenotype (Online Supplementary Table S1 and Figure 1); two were unreported cases (patients 1 and 2), and one was previously described (patient 3).9 Patient 1 was a 35-year-old man presenting with undiagnosed compensated hemolytic anemia and iron overload. He was investigated due to an unexplained fatal hydrops history during his wife’s second pregnancy; his first son was well and unaffected. Patient 2 was a 38-year-old women investigated for undiagnosed compensated hemolytic anemia. PIEZO1 sequencing for patient 1 and 2 revealed two new missense mutations : a c.1792G>A mutation in exon 14 in patient 1, leading to pVal598Met (predicted as tolerated by SIFT, score 0.1, and disease causing by Mutation taster, P value 0.998) and a c.2042T>C mutation in exon 16 in patient 2, leading to pPhe681Ser substitution (predicted as deleterious by SIFT, score 0) (Online Supplementary Figure S2). No mutation was identified in KCNN4. The third patient carried two known Piezo1 substitutions, G782S and R808Q.9 In contrast to those previously studied, these mutations are in the N-terminal part of the channel, i.e., the extracellular domain lying on the plasma membrane, the “blade” domain, which is proposed to sense shear forces on the membrane.
Figure 1.
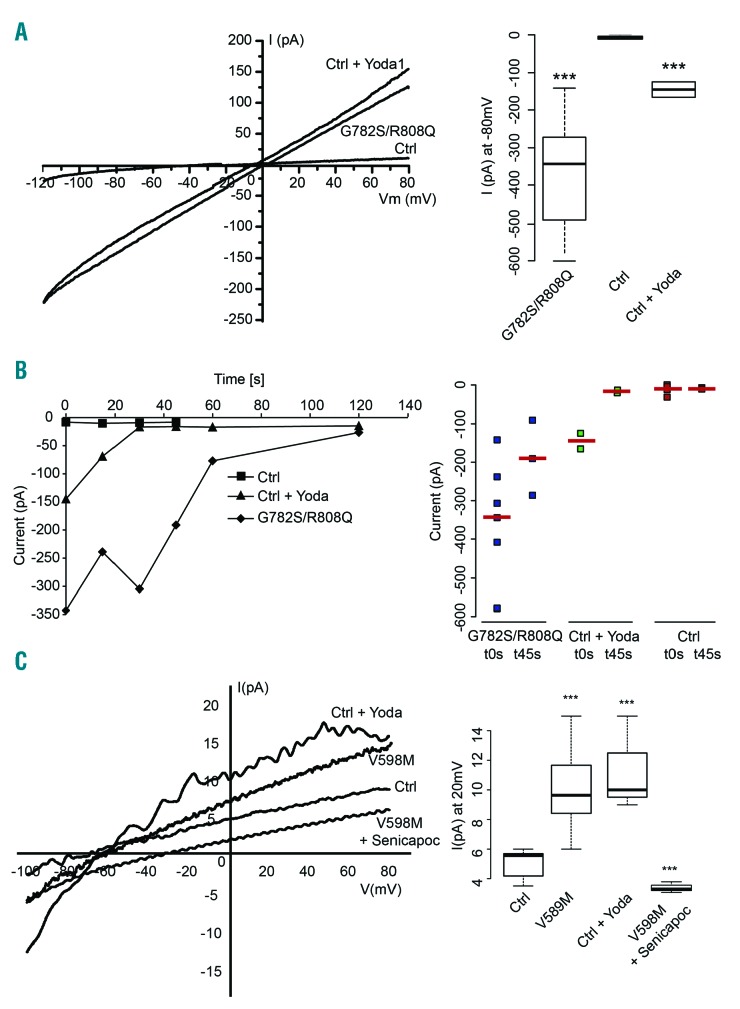
Electrical features of patient red blood cells with Piezo1 mutations. All patch-clamp experiments were performed with a PC-controlled EPC 9 patch-clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). Currents were acquired and analyzed with Pulse and Pulsefit software (HEKA). Whole-cell configuration was used and hematocrit was around 0.1% (10μl of a 30% suspension in 2ml dish). Glass pipettes (Brand, Wertheim, Germany) were made on a horizontal pipette puller (PIP5 HEKA, Germany) to give a final resistance ranging from 18 to 20 MΩ. The bath solution was in mM: NaCl 150, KCl 5, MgCl2 1, CaCl2 1, Hepes 10, buffered with NaOH pH7.4 (320 mOsm). The intracellular solution was in mM: KCl 140, NaCl 5, MgCl2 1, Hepes 10 pH 7.2 adjusted with NaOH, 0.5 μM CaCl2. Currents were measured at room temperature using a ramp protocol from −120 to +80 mV from a holding potential of −60 mV for 250 ms every 5 s (sampling frequency 10 kHz; filtered 1 kHz). (A) Representative I/V curves of patient or control red blood cells. Currents were recorded just after cell break-in. Bath solution containing 15 μM Yoda1, a specific Piezo1 activator (Syeda R. et al., Elife 2015;4) was prepared just before adding the cell suspension. Quantification: currents recorded at −80 mV, n=5 (ctrl) 7 (patient) 2 (Ctrl+Yoda1). B) left panel: time course of currents measured at −80 mV for control, control+15 μM Yoda1 or patient with G782S/R808Q Piezo1. Data are median currents n=5 (ctrl) 7 (patient) 2 (Ctrl+Yoda1). For clarity purpose the dispersion around median was not shown but each individual currents with corresponding median values are reported for t=0 and t=45 s in the graph on the right. C) Representative I/V curves of patient (V598M mutation) or control red blood cells recorded after extinction of the large conductance between 90 and 120 s depending on cells. Inset median currents recorded at +20 mV, n=5 (ctrl) 7 (patient) 3 (patient + Senicapoc) 3 (ctrl+ Yoda1).
The electrical features of the patients’ red blood cells were studied by patch clamp experiments in whole-cell configuration. The intracellular calcium was maintained at a concentration around the threshold for KCNN4 activation (0.5 μM),4 whilst extracellular calcium was present at 1 mM. Figure 1 and Online Supplementary Figure S3 illustrate the I/V curves for patient and control RBCs. Just after whole-cell configuration was reached, patient erythrocytes showed a large current with reverse potential close to zero mV, whilst control RBCs exhibited a smaller current with a −29±14 mV (n=5) reverse potential (Figure 1A). However, the activation of Piezo1 by Yoda1 in control RBCs induced a large linear current similar to the current in RBCs with mutated Piezo1. This large conductance was transient, as shown in figure 1B, but the current decrease was much faster in control RBCs activated by Yoda1 compared to patient RBCs. Following this large conductance decrease, a rectified current with reverse potential around −60 mV was observed in patient as in control RBCs stimulated by Yoda1. This current exhibited KCNN4 current features and was sensitive to 0.4 μM Senicapoc. Thus, the electrical signature of patient RBCs was mimicked by activating Piezo1 in control RBCs.
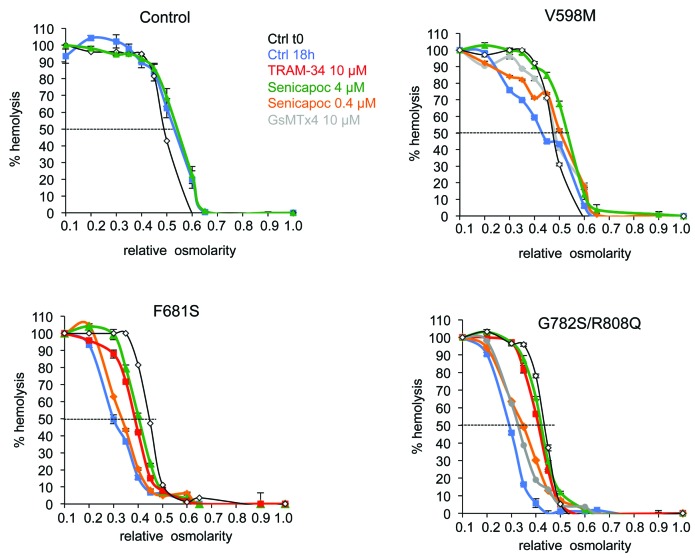
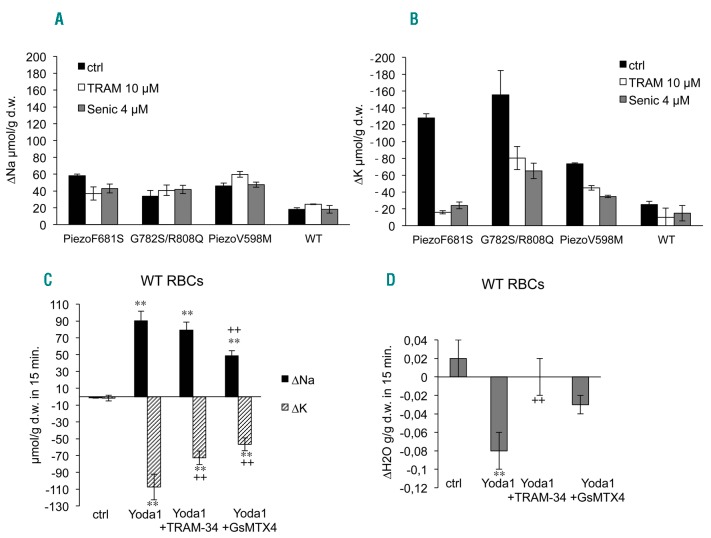
RBC osmotic resistance was assessed in Ca2+ containing medium after 18 hours’ incubation at 37°C. Different drugs blocking KCNN4, TRAM-34 or Senicapoc, were added to the incubating medium. The spider toxin GsMTx4, inhibitor of Piezo1 channel, was also assessed in some patients’ RBCs. Control RBCs showed a rightward shift in osmotic resistance insensitive to 4 μM Senicapoc after 18 hours’ incubation at 37°C (Figure 2). In contrast, RBCs with the different Piezo1 mutations showed a leftward shift of the osmotic resistance curve after incubation (50% hemolysis for a relative osmolarity between 0.3 and 0.4 for Piezo1 mutated RBCs compared to 0.50 for control). This leftward shift was inhibited by Senicapoc in a dose- dependent manner, and by TRAM-34. The GsMTx4 was able to slightly prevent dehydration in RBCs from patients with G782S/R808Q as well as V598M mutations. It was not assessed on F681S mutant. Of note, the blunt slope of the osmotic resistance curve for V598M mutant differed from the other two mutants, suggesting heterogeneity in this patient’s RBCs. In parallel, RBC Na+ and K+ contents were measured at time zero (Online Supplementary Table S2) before running the 18h incubation, and also after. Following incubation, whatever the mutations in Piezo1, the Na+ uptake was significantly increased compared to control (Figure 3A). This Na+ uptake was neither sensitive to Senicapoc nor TRAM-34 in patient as in control RBCs. The K+ loss was greatly enhanced in all RBCs with Piezo1 mutations (Figure 3B). This increased K+ loss was similarly sensitive to 10 μM TRAM-34 or 4 μM Senicapoc. Except in control, there was no correlation between the Na+ uptake and the K+ loss; this latter exceeded Na+ uptake in all patients’ RBCs. However, in presence of KCNN4 inhibitors, the K+ loss tended to equilibrate the Na+ uptake for all the studied Piezo1 mutations. During the incubation time in absence of energy supply, the progressive slowing down of pump activity is expected to increase Na+ content and K+ loss. However, RBCs with Piezo1 mutations were much more leaky to these cations than WT RBCs. In addition, the part of K+ loss exceeding the Na+ uptake was mediated by KCNN4 (as shown by its sensitivity to these channel blockers), and blocking KCNN4 prevented patients’ RBC dehydration.
Figure 2.
Osmotic resistance tests. Fresh venous blood was obtained by venipuncture from informed patients and healthy volunteers in EDTA collecting tubes kept at 4°C until analysis. Experiments were performed 24h after withdrawn. Fresh blood was washed 4 times at room temperature in medium containing in mM: NaCl 147, KCl 5, MgCl2 1, CaCl2 1, Hepes 10, buffered with NaOH pH7.4 (320 mOsm). RBC suspension (40% hematocrit) was then incubated at 37°C for 18 hours. Blue squares: control at t=18h, red squares: 10 μM TRAM-34 18 h incubation, orange diamonds: 0.4 μM Senicapoc (MedChem Express) 18h incubation, Green triangles: 4 μM Senicapoc 18h incubation, grey circles: 10 μM GsMTx4 (Grammostola spatulata toxin, specific blocker of Piezo1: Bae C. et al. Biochemistry 2011; 50(29)) 18h incubation. The black line with white diamonds represent the osmotic resistance curve done at blood reception, on total blood, before washing in saline buffer and starting the incubation at 37°C. Data are means±sem n=3.
Figure 3.
Variation in intracellular Na+ and K+ contents in control or patient red blood cells following 18 hours’ incubation at 37°C (A and B) or after stimulation of Piezo1 by Yoda1 in control RBCs (C and D). Variation in intracellular Na+ (A) and K+ (B) contents in blood samples used for osmotic resistance tests, i.e., RBC suspension at 40% hematocrit. Intracellular ion contents were measured as previously described4 at t=0 and at the end of the incubation time and the variations in 18h are plotted in the bar graph. Data are means±sem, n=3. Black bars: control condition, white bars: 10 μM TRAM-34, grey bars: 4 μM Senicapoc. Statistical analyses were done with Mann and Whitney tests comparing control condition between WT and mutated Piezo1 on one hand and inhibitors versus control condition on the other hand. Na+ and K+ contents were significantly changed in mutant versus WT red blood cells, P<0.05 for Na+ and P<0.001 for K+. Inhibitors did not significantly change Na+ contents whereas they significantly modified K+ contents in mutant red blood cells (P<0.001) but not in control red blood cells. Cation contents at t=0 are given in Online supplementary Table S3. Cation (C) and water (D) movements in control RBCs stimulated by Yoda1. Washed RBC suspension was set to 30% hematocrit and 0.5 μM ouabain (Sigma-Aldrich) was added. At time zero, 15 μM of Yoda1 (Sigma-Aldrich) was added to cell suspension either containing 10 μM TRAM-34 (MedChemExpress), 10 μM GsMTx4 (Smartox, Grenoble, France) or DMSO. Samples were collected 15, 30 and 60 minutes after Yoda1 addition and cell water, Na+ and K+ contents were measured. Data showed the variation in 15 minutes, means ± sem of 4 experiments. **P<0.001 comparison of control condition with the 3 conditions with Yoda1. ++P<0.001 comparison between Yoda1 and Yoda1+inhibitors. Mann and Whitney test. The kinetic is shown on Online Supplementary Figure S4.
In order to assess the involvement of Piezo1 channel in RBC cation permeability, control RBCs were treated by Yoda1 in the presence of ouabain to prevent cation recirculation through the Na+/K+ ATPase. Na+ and K+ contents were measured and the effects of TRAM-34 and GsMTx4 were assessed. In 15 minutes, Yoda1 was able to reverse the cation gradient leading to a large K+ loss that slightly exceeded Na+ uptake, which is corroborated by an 8% water loss (Figure 3C–D). In presence of TRAM-34, the Na+ uptake induced by Yoda1 was not significantly affected, whilst K+ loss was reduced by a third. K+ loss in presence of TRAM-34 compensated the Na+ uptake; there was no net cation movement and, consequently, no water loss. In contrast, GsMTx4 inhibited the Na+ uptake and the K+ loss similarly. Of note, the inhibition was not complete; only 45% inhibition was observed at 10 μM (the effect of a lower concentration, 4 μM, was not statistically significant). Thus, Piezo1 activation by Yoda1 induced a rapid change in Na+ and K+ contents independently of a mechanic stimulus. A steady state was reached within about 15 minutes for cell volume and cation content, and the Na+ uptake was counter-balanced by the K+ leak in presence of KCNN4 inhibitors. This shows that Piezo1 activation leads to a Ca2+ uptake which is able to subsequently activate KCNN4, as observed in mouse RBCs by S. Cahalan and colleagues.12 According to whole-cell recordings in control RBCs, the Yoda1 effect on Piezo1 activation was transient. Moreover, the K+ loss was no longer sensitive to KCNN4 inhibitor after 15 minutes incubation with Yoda1 (Online Supplementary Figure S4), suggesting that Yoda1 does not change intracellular calcium concentration after a certain period of time. Remarkably, the cell volume was only slightly affected by Piezo1 activation, showing that the calcium rise results in a moderate response of KCNN4. The efficiency of the Ca2+ pump to maintain a low level of intracellular Ca2+ together with a transient activity of Piezo1, might explain this observation.
In contrast to the previously described Piezo1 mutants, the mutations in the N-terminal part are constitutively leaky to cations, with no stimulus needed to activate Piezo1 and to observe stimulation of KCNN4 in patient RBCs. Piezo1 activation, due to either mutations or to Yoda1, induces an important change in Na+ and K+ contents which is expected to challenge the Na+ K+ pump and, hence, RBC metabolism. In addition, the calcium leak is subsequently able to stimulate KCNN4, which appears as the only effector of RBC dehydration following Piezo1 activation. All the Piezo1 mutations described in HX generate inappropriate activity of the channel responsible for the higher KCNN4 activity, and RBC dehydration is only due to this higher KCNN4 activity. The ability of RBCs to cope with hyper-active Piezo1 and KCNN4 will depend on many issues such as metabolism and pump efficiency, which could explain phenotype variability between patients. Senicapoc has already been shown to prevent RBC dehydration due to gain of function mutations in KCNN4,7,15 the other molecular cause of HX. Herein, we have shown that this drug could also be used to treat HX due to gain of function mutations in Piezo1.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Miller DR, Rickles FR, Lichtman MA, La Celle PL, Bates J, Weed RI. A new variant of hereditary hemolytic anemia with stomatocytosis and erythrocyte cation abnormality. Blood. 1971;38(2):184–204. [PubMed] [Google Scholar]
- 2.Coste B, Xiao B, Santos JS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;4 83(7388):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher AD, Kuchel PW. The Gárdos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35(8):1182–1197. [DOI] [PubMed] [Google Scholar]
- 4.Rapetti-Mauss R, Lacoste C, Picard V, et al. A mutation in the Gardos channel is associated with hereditary xerocytosis. Blood. 2015; 126(11):1273–1280. [DOI] [PubMed] [Google Scholar]
- 5.Andolfo I, Russo R, Manna F, et al. Novel Gardos channel mutations linked to dehydrated hereditary stomatocytosis (xerocytosis). Am J Hematol. 2015;90(10):921–926. [DOI] [PubMed] [Google Scholar]
- 6.Glogowska E, Lezon-Geyda K, Maksimova Y, Schulz VP, Gallagher PG. Mutations in the Gardos channel (KCNN4) are associated with hereditary xerocytosis. Blood. 2015;126(11):1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapetti-Mauss R, Soriani O, Vinti H, Badens C, Guizouarn H. Senicapoc: a potent candidate for the treatment of a subset of hereditary xerocytosis caused by mutations in the Gardos channel. Haematologica. 2016;101(11):e431–e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarychanski R, Schulz VP, Houston BL, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andolfo I, Alper SL, De Franceschi L, et al. Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood. 2013;121(19):3925–3935. [DOI] [PubMed] [Google Scholar]
- 10.Albuisson J, Murthy SE, Bandell M, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demolombe S, Duprat F, Honoré E, Patel A. Slower Piezo1 inactivation in dehydrated hereditary stomatocytosis (xerocytosis). Biophys J. 2013;105(4):833–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahalan SM, Lukacs V, Ranade SS, Chien S, Bandell M, Patapoutian A. Piezo1 links mechanical forces to red blood cell volume. Elife. 2015;4:e07370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci USA. 2015;112(38):11783–11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101(6):2412–2418. [DOI] [PubMed] [Google Scholar]
- 15.Rivera A, Vandorpe DH, Shmukler BE, et al. Erythrocytes from Hereditary Xerocytosis patients heterozygous for KCNN4 V282M exhibit increased spontaneous Gardos channel-like activity inhibited by Senicapoc. Am J Hematol. 2017;92(6):E108–E110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.