Hepatitis B virus (HBV) reactivation is a well known complication in patients with resolved HBV infection, i.e., HBsAg negative, hepatitis B core antibody (anti-HBc) positive ± antibodies against HBsAg (anti-HBs), undergoing chemotherapy (CT) and/or allogenic hematopoietic stem cell transplantation (HSCT) for onco-hematological diseases.1–3 In these patients, this risk can be prevented by either “pre-emptive anti-HBV therapy”, based on the monitoring of HBV DNA and/or HBsAg, followed by rescue therapy with anti-HBV regimens, or by “anti-HBV prophylaxis” based on the administration of nucleos(t)ides analogs (NUCs) during immunosuppression and for a consolidation time after the end of immunosuppressive drugs. As per standard procedure in our centre, all patients with hematological malignancies and a resolved HBV infection receive lamivudine (LMV) prophylaxis at the time of CT or HSCT to be maintained for at least 18 months after the discontinuation of immunosuppressive drugs.
Herein, we describe two patients with resolved HBV infection who, following allogenic HSCT and repeated cycles of CT for hematological malignancies, developed HBsAg seroreversion due to the late emergence of LMV-resistance (R) during long-term LMV prophylaxis.
Case A
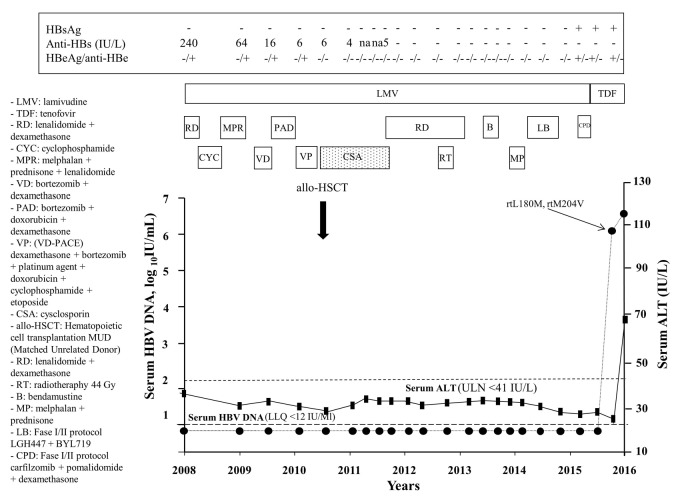
A 56-year-old Italian male was diagnosed with micromolecular IgG kappa multiple myeloma (MM) on June 2008. Since the virological profile was consistent with a resolved HBV infection with positive anti-HBe and anti-HBs (240 IU/L) and undetectable serum HBV DNA (<12 IU/mL), LMV prophylaxis was started concomitantly with CT initiation. Between January 2009 and December 2010, several cycles of CT were administered (Table 1 and Figure 1). In December 2010, a matched unrelated donor (MUD) HSCT was carried out (donor HBV profile: HBsAg negative, anti-HBs 715 IU/L, anti-HBc negative) and cysclosporin (CSA) as a prophylaxis and treatment of graft versus host disease (GvHD) was started to be progressively reduced and withdrawn in May 2012. However, in April 2012, MM relapsed requiring further cycles of CT without, nevertheless, achieving complete disease remission. In November 2015, the patient was enrolled in a phase I/II trial based on the co-adminstration of carfilzomib+pomalidomide+dexamethasone. Between 2008 and November 2015, the patient remained persistently negative for HBsAg, HBeAg and serum HBV DNA but with, however, a progressive decline of anti-HBs titers until undetectability in June 2012. For all this period, LMV was maintained owing to the fact that CT and/or CSA were not withdrawn for more than 18 months. After 3 months of carfilzomib+pomalidomide+dexamethasone combination, HBsAg seroreversion occurred (HBsAg 234 IU/mL) with detectable HBV DNA (772.850 IU/mL) and HBeAg, while alanine aminotransferase (ALT) levels remained within the normal range (19 IU/L). Molecular analysis by INNO-LIPA HBV Multi-DR confirmed the emergence of LMV-R (rtL180M, rtM204V). Despite the immediate switch from LMV to tenofovir disoproxil fumarate (TDF), serum HBsAg, HBV DNA and ALT increased (qHBsAg >52.000 IU/mL, HBV DNA 931.575 IU/mL, ALT 69 IU/L) with otherwise preserved hepatic function. Other possible causes of the ALT increase, i.e., hepatic GvHD, alcohol abuse, intake of herbal products, viral hepatitis other than HBV, were excluded. On April 2016, the patient died due to progression of the hematological malignancy (Figure 1).
Table 1.
Case A. Chemotherapy regimens and clinical outcomes.
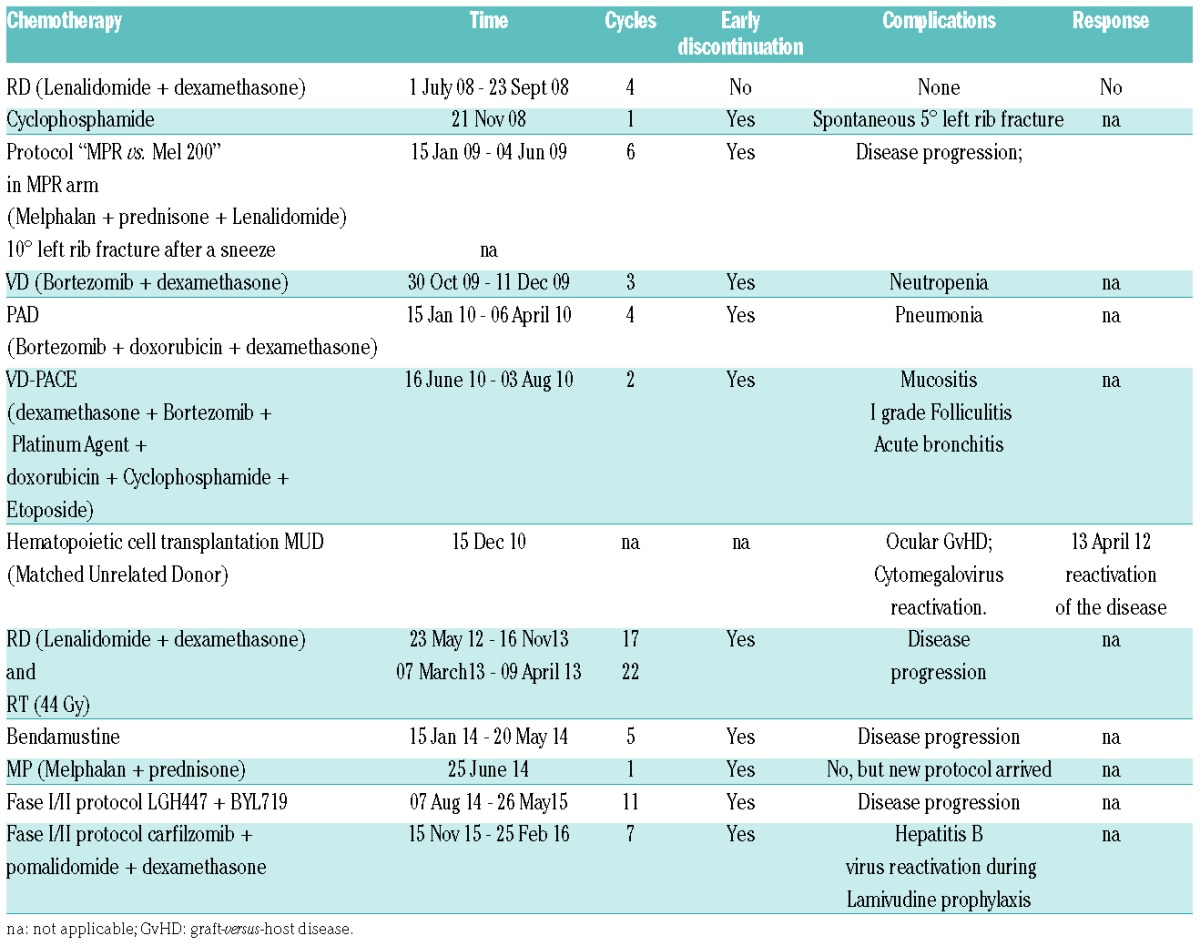
Figure 1.
Time course of serum ALT and HBV DNA in Case A. RD: lenalidomide+dexamethasone; CYC: cyclophosphamide; MPR: melphalan+prednisone+lenalidomide; VD: bortezomib+dexamethasone; PAD: bortezomib+doxorubicin+dexamethasone; VP: VD-PACE (dexamethasone+borte-zomib+platinum agent+doxorubicin+cyclophosphamide+etoposide); allo-HSCT: hematopoietic cell transplantation MUD (matched unrelated donor); CSA: cyclosporine; RT: radiotherapy; B: bendamustine; MP: melphalan+prednisone; LB: LGH447+BYL719; CPD: carfilzomib+pomalidomide+dexamethasone.
Case B
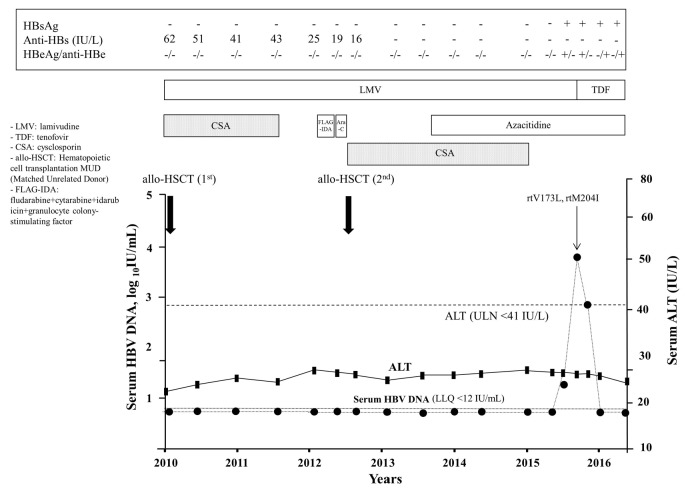
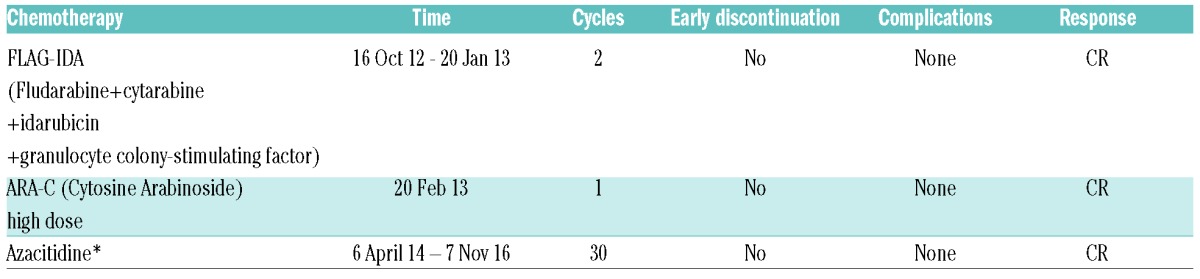
A 64-year-old Italian female with acute myeloid leukemia (AML) underwent MUD HSCT in August 2010 (donor HBV profile: HBsAg negative, anti-HBs 65 IU/L, anti-HBs positive). As the pre-stem cell transplantation virological profile was consistent with a resolved HBV infection (HBsAg negative, anti-HBs 62 IU/L, anti-HBc positive, HBV DNA <12 IU/mL), LMV prophylaxis was started concomitantly with CSA. Due to AML recurrence on October 2012, she received a salvage CT regimen which led to a complete remission of the hematological disease. In April 2013, the patient underwent a second allogenic HSCT from another matched unrelated donor (negative for HBsAg, anti-HBs and anti-HBc) and started CSA which was maintained until July 2015, and seven months (November 2013) after the second HSCT the patient developed a suspected liver GvHD. Owing to incomplete oncological response, a maintenance treatment with azacitidine was commenced in April 2014. Between 2010 and February 2016, the patient maintained a steady virological profile except for anti-HBs levels which progressively declined reaching undetectable levels in January 2014 (Figure 2). Despite LMV prophylaxis, in March 2016 the patient tested positive for HBsAg (1.2 IU/mL), HBeAg and HBV DNA (32 IU/mL). By April 2016, serum HBsAg increased to 6.5 IU/mL and HBV DNA levels further increased to 6.760 IU/mL (3.83 log10 IU/mL) (Figure 2), and occurrence of LMV-R (rtV173L, rtM204I) was confirmed by molecular analysis. Upon administration of TDF, virological markers progressively improved, HBV DNA rapidly became undetectable, and HBeAg serocoversion occurred. In December 2016, HBsAg was positive (70 IU/mL), HBeAg negative, anti-HBe positive, and serum HBV DNA undetectable (Figure 2).
Figure 2.
Time course of serum ALT and HBV DNA in Case B. CSA: cyclosporine; FLAG-IDA: fludarabine+cytarabine+idarubicin+granulocyte colony-stimulating factor; ARA-C: cytosine arabinoside high dose.
This report describes two patients with resolved HBV infection treated with repeated cycles of CT and allogenic HSCT for hematological malignancies that failed long-term LMV prophylaxis and, as a consequence, had HBsAg seroreversion. As far as we know, this is the first report of very late onset of LMV-R developing in patients with resolved HBV infection following heavy immunosuppressive therapy for hematological malignancies.
HBV reactivation may hit patients with resolved HBV infection undergoing CT and/or HSCT, at a rate of up to 50%.1–3 These patients, in fact, harbor low-level transcription and replication of HBV in the liver which may flare up on prolonged exposure to immunosuppressive therapies.4 While management to prevent HBV reactivation in such patients is not standardized, two available options include “pre-emptive anti-HBV therapy” and “universal anti-HBV prophylaxis”. However, patients with lymphoma and with resolved HBV infection, HBV reactivation and severe HBV-related hepatitis were not fully prevented by “pre-emptive anti-HBV therapy” with entecavir (ETV).5–7 Moreover, a randomized controlled study from Taiwan demonstrated a higher risk of HBV reactivation among the patients undergoing ETV as “pre-emptive therapy” compared to those who received this nucleoside analog as “prophylaxis” (18% vs. 2.4%, P=0.027).8 Similar findings were confirmed in a Spanish randomized prospective open label study in which TDF was used.9 Two Italian single center retrospective studies testing LMV as universal prophylaxis in patients with resolved HBV infection treated with Rituximab-based CT for NHL provided conflicting results. The former study demonstrated that LMV prophylaxis efficiently prevented HBV reactivation, both during and after withdrawal of CT, while the second showed a 10% risk of HBV reactivation.10,11
The risk of HBV reactivation in the onco-hematological settings requiring HSCT was investigated by a few studies. A recent Asian retrospective study of 173 patients with resolved HBV infection undergoing allogenic HSCT during a median follow up of 21 months showed no significant difference in the incidence of HBV reactivation between patients receiving “universal prophylaxis” with different NUCs and those managed with the “pre-emptive anti-HBV therapy” (5% vs. 5.3%).12 Two studies among Italian patients with resolved HBV infection undergoing HSCT reported that LMV prophylaxis efficiently prevented HBV reactivation in the vast majority of cases.13,14
Table 2.
Case B. Chemotherapy regimens and clinical outcomes.
As recommended by Italian guidelines,15 all patients referred to our Center due to a resolved HBV infection in need of CT for lymphoma, MM, AML, acute and chronic lymphocytic leukemia, and/or allogenic HSCT were considered for LMV prophylaxis to be maintained for at least 18 months after the end of the immunosuppression. Up to now, 318 patients have been managed with this approach and, herein, we report the only two cases (0.6%) that experienced HBV reactivation. To the best of our knowledge, these are the first ever reported cases of LMV-R developing after up to 8 years of prophylaxis in patients with resolved HBV infection and onco-hematological malignancies.
The interesting aspect of our report is that despite having protective anti-HBs titers and undetectable serum HBV DNA at baseline, both patients failed LMV after many years of effective prophylaxis, an event that was heralded by anti-HBs titer decline during CT and after HSCT. Boosting the risk of HBV reactivation in our patients could have been a number of factors, including the underlying malignancies, the pre-HSCT conditioning regimen, the intense and repeated cycles of CT including carfilzomib, bortezomib, pomalidomide, fludarabine, not to speak of occurrence and severity of GvHD which required long-term CSA. By contrast, it is unlikely that the virological profile of the donors played any role in HBV reactivation as seroconversion occurred many years after HSCT.
Whether universal prophylaxis with LMV remains the best option in a setting like this where new potent molecular target drugs are being developed, where the duration of immunosuppression cannot be easily anticipated, and where multiple and highly immunosuppressive regimens may be required, remains unclear. Universal prophylaxis with third generation NUCs like ETV or TDF in these patients could be taken into consideration although this strategy would be more expensive and it is not currently refunded by many National Health Systems. including ours. Only long-term prospective studies of large cohorts of such patients, aimed to define the risk and predictors of LMV failure, and the cost-effectiveness of LMV in comparison to ETV or TDF, will shed new light on this relevant issue.
In conclusion, universal prophylaxis with LMV monotherapy in patients with resolved HBV infection undergoing CT and/or HSCT for hematological malignancies is effective and safe but close, long-term virological and clinical monitoring is necessary to intercept and promptly rescue the few HBV reactivations due to virological breakthrough caused by LMV-resistance.
Selected onco-hematological patients with MM and leukemia and resolved HBV infection without oncologic response who need long-term chemotherapy or those who underwent HSCT requiring long-term immunosuppression due to chronic GvHD and, thereby, in need of long-term LMV prophylaxis, should be considered at very high risk of viral reactivation. These patients could be switched to third generation NUCs after 4 or 5 years of LMV prophylaxis, as no LMV-R has been reported before this time point in such a setting. Alternatively, third generation NUCs could be started as initial prophylaxis strategy.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Seto WK. Hepatitis B virus reactivation during immunosuppressive therapy: Appropriate risk stratification. World J Hepatol. 2015; 7(6):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raimondo G, Allain JP, Brunetto MR. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008; 49(4):652–657. [DOI] [PubMed] [Google Scholar]
- 3.Viganò M, Serra G, Casella G, Grossi G, Lampertico P. Reactivation of hepatitis B virus during targeted therapies for cancer and immune-mediated disorders. Expert Opin Biol Ther. 2016;16(7):917–926. [DOI] [PubMed] [Google Scholar]
- 4.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B infection. J Hepatol. 2007;46(1):160–170. [DOI] [PubMed] [Google Scholar]
- 5.Kusumoto S, Tanaka Y, Suzuki R, et al. Monitoring of hepatitis B virus (HBV) DNA and risk of HBV reactivation in B-cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61(5):719–729. [DOI] [PubMed] [Google Scholar]
- 6.Tamori A, Hino M, Kawamura E, et al. Prospective long-term study of hepatitis B virus reactivation in patients with hematologic malignancy. J Gastroenterol Hepatol. 2014;29(9):1715–1721. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C, Tsou HH, Lin SJ, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: A prospective study. Hepatology. 2014;59(6):2092–2100. [DOI] [PubMed] [Google Scholar]
- 8.Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31(22):2765–2772. [DOI] [PubMed] [Google Scholar]
- 9.Buti M, Manzano ML, Morillas RM, et al. Prevents HBV reactivation with tenofovir in anti-HBc positive patients withhematological malignancies treated with rituximab. Results final visit 18-months (PREBELIN STUDY). J Hepatol. 2016;64(suppl):S369. [Google Scholar]
- 10.Viganò M, Grossi G, Borsotti E, et al. Lamivudine prophylaxis prevents hepatitis B reactivation in HBsAg-negative/anti-HBc-positive patients undergoing Rituximab-based chemotherapy for non-Hodgkin’s B cell lymphoma. J Hepatol. 2015;6(suppl):S566. [Google Scholar]
- 11.Castelli R, Ferraris L, Pantaleo G, et al. High rate of hepatitis B viral breakthrough in elderly non-Hodgkin lymphomas patients treated with Rituximab based chemotherapy. Dig Liver Dis. 2016; 48(11):1394–1397. [DOI] [PubMed] [Google Scholar]
- 12.Yoo JJ, Cho EJ, Cho YY, et al. Efficacy of antiviral prophylaxis in HBsAg-negative, anti-HBc positive patients undergoing hematopoietic stem cell transplantation. Liver Int. 2015;35(12):2530–2536. [DOI] [PubMed] [Google Scholar]
- 13.Giaccone L, Festuccia M, Marengo A, et al. Hepatitis B virus reactivation and efficacy of prophylaxis with lamivudine in patients undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(6):809–817. [DOI] [PubMed] [Google Scholar]
- 14.Cerva C, Colagrossi L, Maffongelli G, et al. Persistent risk of HBV reactivation despite extensive lamivudine prophylaxis in haematopoietic stem cell transplant recipients who are anti-HBc-positive or HBV-negative recipients with an anti-HBc-positive donor. Clin Microbiol Infect. 2016;22(11):946.e1–946.e8 [DOI] [PubMed] [Google Scholar]
- 15.Marzano A, Angelucci E, Andreone P, et al. Prophylaxis and treatment of Hepatitis B in immunocompromised patients. Dig Liver Dis. 2007;39(5):397–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.