Abstract
Background
To ascertain the validity of kidney paired donations (KPDs) as an alternative strategy for increasing living donor kidney transplantations (LDKTs) in an LDKT-dominated transplant programme since directed kidney transplantation, ABO-incompatible or crossmatch-positive pairs are not feasible due to costs and infectious complications.
Methods
This was a prospective single-centre study of 77 KPD transplantations (25 two-way, 7 three-way and 1 six-way exchange) from 1 January 2015 to 1 January 2016 of 158 registered donor recipient pairs. During this period, a total of 380 kidney transplantations [71 deceased donor kidney transplantations (DDKTs), 309 LDKTs] were performed. The reasons for opting for KPD were ABO incompatibility (n = 45), sensitization (n = 26) and better matching (n = 6).
Results
KPD matching was facilitated in 62% (n = 98) of transplants. In all, 48.7% (n = 77) of the transplants were completed in 2015, whereas 13.3% (n = 21) of the matched patients were to undergo transplant surgery in early 2016 after getting legal permission. The waiting time for KPD was shorter compared with DDKT. The death-censored graft survival and patient survival were 98.7% (n = 76) and 93.5% (n = 72), respectively. In all, 14.2% (n = 11) of patients had acute rejection. Match rates among sensitized (n = 60) and O group patients (n = 62) were 58.3% (n = 35) and 41.9% (n = 26), respectively. Of these, 43.3% (n = 26) and 29% (n = 18) of transplants were completed and 15% (n = 9) and 12.9% (n = 8), respectively, are waiting for legal permission.
Conclusions
LDKT increased by 25% in 1 year in our single-centre KPD programme. Our key to success was the formation of a KPD registry, awareness and active counselling programs and developing a dedicated team.
Keywords: developing country, kidney paired donation, living donor kidney transplantation, renal replacement therapy
Introduction
For end-stage renal disease (ESRD) patients in developing countries such as India, ABO-compatible living donor kidney transplantation (LDKT) is the only cost-effective renal replacement therapy (RRT) with the best long-term outcomes, whereas worldwide, kidney paired donation (KPD) has resulted in increased access to LDKT in national [1–9] and single-centre programmes [9–12].
In an LDKT-dominated transplant programme, KPD may be a cost-effective and valid alternative strategy for increasing LDKT in countries such as India with limited resources. This is because directed kidney transplantation (KT), ABO-incompatible (ABOi) or crossmatch-positive pairs are not feasible due to greater costs and infectious complications here. Another reason could also be that deceased donor kidney transplantation (DDKT) is in its initial stages of development in India.
The challenges faced in expanding KPD throughout India include the lack of a national database for incompatible pairs, lack of harmony and coordination between transplant centres, lack of a dedicated KPD team to facilitate the process between transplant units, human leucocyte antigen (HLA) laboratories and authorization committees; differences in policies across different transplant centres about donor selection and fitness of recipients; manual allocation and lack of sophisticated computer algorithms to increase match rates. Additional challenges faced in India are that bigger surgical teams are required to carry out simultaneous KT and administrative challenges such as legal permissions are often encountered [10–13]. Hence, the single-centre KPD programme commonly practiced in India has inherent limitations for expanding the donor pool. In this study we report our experience of performing 77 KPD transplantations in 1 year and how that led to a 25% increase in LDKT at one transplant centre in India.
Materials and methods
This was a prospective single-centre observational study of 77 KPD transplantations (25 two-ways, 7 three-ways and 1 six-way exchange) performed at our centre in Ahmedabad, India. This study was approved by government and institutional ethical review boards and all participants gave written informed consent prior to inclusion in the study.
Counselling of LDKT, registration and manual allocation of KPD pairs
Generally, approximately one-third of potential healthy, willing living donors are rejected for directed LDKT due to ABO incompatibility or sensitization. The goal of our KPD programme was to increase LDKT for incompatible pairs. We maintained a database that included blood group, age, gender, state of domicile, status of medical fitness and contact information, HLA and antibody profile by Luminex single-antigen assay for all sensitized donor recipient pairs (DRPs) in our KPD registry. Each incompatible pair could register in the KPD registry prior to a medical fitness exam done by the transplant team. Early registration helps the incompatible pair by giving them more time to prepare all the documents required for obtaining the legal permissions and arranging the monetary funds required for LDKT from the government. Patients were given an explanation about the various transplant options (DDKT, ABOi KT, desensitization protocol) and their cost-effectiveness and the long-term benefits resulting from living donor KPD transplants.
Allocation rules for our KPD programme were reported previously [11–13]. Manual allocation was the responsibility of a sole nephrologist who was supervised by the ethical review board to ensure equitable allocation. Medical fitness of the patients and donors was mandatory for donor allocation, immunological compatibility testing and permission from the authorization committee. This avoided potential breakup of the KPD pairs for medical reasons such as undiagnosed comorbid conditions being detected in the pairs. If a match was found, then nephrologist would inform the pairs. Different DRPs were allowed to meet each other and share the medical reports of the donors (e.g. creatinine, nuclear scan for glomerular filtration rate) with each other. Each recipient could meet their intended donor in the presence of the transplant team to discuss any anticipated issues and to encourage development of basic trust between the various participants. There was no room for unexpected behavioural factors such as the DRP not agreeing due to non-medical reasons (caste, religion, etc.). Only after this discussion, and if the DRP agreed to the exchange, were the immunological compatibility with donor-specific antibody (DSA), flow crossmatch (FCM) and lymphocyte crossmatch (LCM) performed. If the patients did not undergo LDKT in the KPD registry within 6 months, they could continue to wait in the KPD registry or consider an alternative course of action after discussing the options with their nephrologist and transplant team. KPD transplants were only done when directed and living donations from other healthy, willing first-degree family members were not feasible. Compatible pairs (n = 6) benefitted by better HLA-matched or age-matched donors. Consented compatible pairs were matched only if a better age- and HLA-matched donor was available immediately. Marginal living donors or donors with comorbid conditions such as hypertension and altruistic donors were not included in the KPD programme.
We previously reported challenges and solutions for the KPD programme in India [11–13]. The many factors responsible for the success of our single-centre KPD programme were developing a KPD registry, awareness and prospective active counselling about the advantages of the living donor KPD programme, early registration, a dedicated KPD team for evaluating the donors and the recipients, lending support and helping the patients overcome a variety of logistical barriers, an expert transplant coordinator, a dedicated HLA/immunology laboratory, complete workup of KPD pairs before the final allocation, robust immunological compatibility testing, non-anonymous allocation, only exchanging kidneys of similar quality and performing simultaneous surgeries. The patient mentorship programme increased awareness about KPD and this resulted in 10 patients, including 2 international patients, coming to our centre for KPD transplantation through the information available on social networking sites. The patient mentorship programme, which was initiated to increase awareness about KPD, apparently resulted in 15 additional KPD transplants.
Induction immunosuppression consisted of methyl pred-nisolone and rabbit thymoglobulin; prednisolone + tacrolimus + mycophenolate was commonly used for the maintenance immunosuppression regimen.
Results
Patient and donor characteristics (Table 1)
Table 1.
Demographics and outcome
| Patients, n | 77 |
| Age, mean ± SD (range), years | 38.5 ± 10.8 (15–65) |
| Gender | 64 males:14 females |
| Dialysis duration | 6 months |
| Weight, mean ± SD (range), kg | 52.8 ± 11.1 (32–80) |
| Time from registration in KPD to find donor | 30 days |
| Time from KPD donor to KT | 45 days |
| HLA matching | |
| DR (0/2) | 38 patients |
| DR (1/2) | 36 patients |
| DR (2/2) | 3 patients |
| A, B, DR, mean ± SD (range) | 1.3 ± 1 (0–4) |
| A, B, DRB1, DRB3, DRB4, DRB5, mean ± SD (range) | 2.9 ± 1.7 (0–8) |
| A, B, Bw, Cw, DRB1, DRB3, DRB4, DRB5, DQ B1, mean ± SD (range) | 4.4 ± 2.1 (1–10) |
| Outcome | |
| Graft survival, % (n) | 98.7 (76) |
| Patient survival, % (n) | 93.5 (72) |
| Biopsy-proven acute rejection, % (n) | 14.2 (11) |
| Serum creatinine (mg/dL) | 1.1 |
| Donors, n | 77 |
| Age, mean ± SD (range) years | 45 ± 9.1 (25–65) |
| Gender | 14 male:63 female |
| Weight, mean ± SD, kg | 50 ± 10.1 |
| GFR. mean ± SD (right/left) | 51.8 ± 4.2/50.7 ± 3.5 |
| Creatinine, mean ± SD (range), mg/dL | 0.7 ± 0.1 (0.5–1.1) |
| Laparoscopic donor nephrectomy, % (n) | 100 (77) |
The domicile states of the patients were Gujarat (n = 49), Chhattisgarh (n = 1), Uttar Pradesh (n = 1), Madhya Pradesh (n = 2), Bihar (n = 3), Rajasthan (n = 19) and others (n = 2). The mean ± SD age of the patients (64 males and 13 females) enrolled in this study was 38.5 ± 10.8 (range 15–65) years. In all, seven of the transplants were in paediatric patients. The basic kidney diseases leading to ESRD were hypertension (n = 25), diabetes (n = 11), unknown aetiology (n = 20), obstructive uropathy (n = 8), lupus nephritis (n = 3) and others (n = 10). A total of eight patients were pre-transplant hepatitis C virus (HCV)enzyme-linked immunosorbent assay positive and negative HCV viral load after antiviral therapy prior to their KT. A total of 10 patients were on anti-tuberculosis therapy before their KT.
The mean ± SD age of the donors (14 males and 63 females) enrolled in this study was 45 ± 9.1 (range 25–65) years. The relationships of living donors to the recipients were spouse (n = 48), parents (n = 24) and others (n = 5).
KPD registry
Figure 1 shows the blood group distribution of the registered and matched recipients (Figure 1A) and donors (Figure 1B) and their reasons for joining the KPD registry. The patient ABO blood group types were AB (n = 4), O (n = 18), A (n = 24) and B (n = 31). The donor ABO blood group types were AB (n = 0), O (n = 21), A (n = 21) and B (n = 35). The number of transplants for each blood group were A to A (n = 21), B to B (n = 30), O to O (n = 18), O to A (n = 3), O to B (n = 1), A to AB (n = 1), B to AB (n = 3). In all, 10 O group patients with their non-O group donors (8 B group, 2 A group donors) and 8 O group patients who were sensitized with their O group donor benefitted from an O group donor in KPD. The number of pairs with an O group donor allocated to a non-O group recipient was four (three were sensitized). A total of 10 pairs with O group patients and non-O group donors benefitted from KPD transplantation. These included three sensitized patients.
Fig. 1.
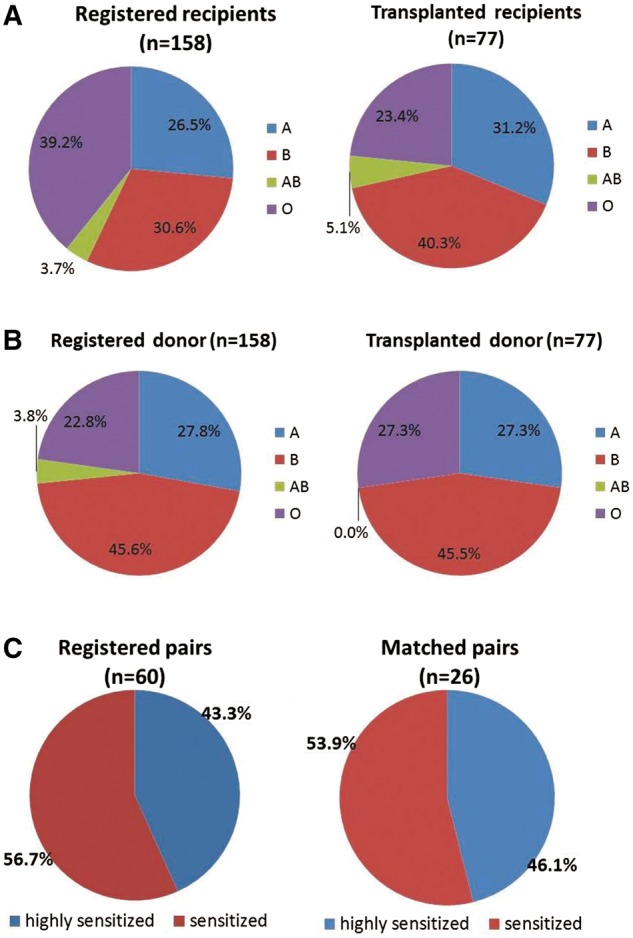
(A) Blood type distribution of recipients in KPD registry. (B) Blood type distribution of donors in KPD registry. (C) Level of sensitization of registered and matched pairs.
The reasons for joining the KPD programme given by registered pairs (n = 158) were ABO incompatibility (n = 92) or HLA incompatibility (sensitization) (n = 60) and better matching (n = 6) and those given by matched pairs were ABO incompatibility (n = 45), HLA incompatibility (sensitization) (n = 26) and better (HLA/age) matching (n = 6). Of the 26 sensitized patients, 10 underwent the desensitization protocol but did not respond, whereas 16 patients joined the KPD programme without undergoing desensitization. In cases of unsuccessful desensitization attempts (n = 10), rescue KPD was used for salvaging failed desensitization treatment and increasing the transplantation rate (n = 10). A total of 12 patients [one six-way, exchange and two three-way exchanges (2 × 3 = 6)] underwent non-simultaneous LDKT and the remaining underwent simultaneous KT (n = 65). In all, three chains (one six-way, one three-way and one four-way kidney exchange) collapsed due to the death of a patient in each chain after allocation of the KPD donor and before KT due to the long waiting time for securing permission from the authorization committee. All 13 patients were repaired, 11 KTs were completed and 2 patients were waiting for permission from the authorization committee.
We performed a total of 380 KTs (71 deceased donor, 309 living donor) from 1 January 2015 to 1 January 2016. KPD constituted 24.9% of LDKTs in 2015. As of 1 January 2016, 158 pairs were registered and the match efficiency of our KPD programme remained high, with 62% (n = 98) of registered pairs finding a match. In all, 77 (48.7%) patients were successfully transplanted. A total of 21 (13.3%) matched patients were scheduled to undergo transplant surgery in early 2016. They were medically fit and had their intended KPD donors ready but did not progress to LDKT as they awaited permission from the authorization committee. The transplant match rate among the O group patients (n = 62) was 41.9% (n = 26), of which 29% (n = 18) of KTs were completed and 12.9% (n = 8) were still waiting for their documents and permissions from the authorization committee. The transplant match rate among the sensitized patients (n = 60) was 58.3% (n = 35), of which 43.3% (n = 26) of KTs were completed and 15% (n = 9) were still waiting for their documents and permissions from the authorization committee.
Level of sensitization of registered and matched pairs (Figure 1C)
We do not routinely perform calculated panel reactive antibody (cPRA) testing in our centre. The sensitized patients were divided according to LCM, FCM and DSA. Broadly sensitized patients had an LCM of 90% + positive FCM >300 median channel shifts (MCSs) + 3 DSA >5000 mean fluorescence intensity (MFI), while narrowly sensitized patients had 2 or 3 DSA >5000 MFI ± FCM positive with normal LCM. From 2012 to 2015, an increasing number of sensitized (3, 4, 12 and 26) and O group patients (5, 8, 10 and 26) have been transplanted in our KPD programme.
Outcome of unmatched registered pairs still waiting (Figure 2)
Fig. 2.
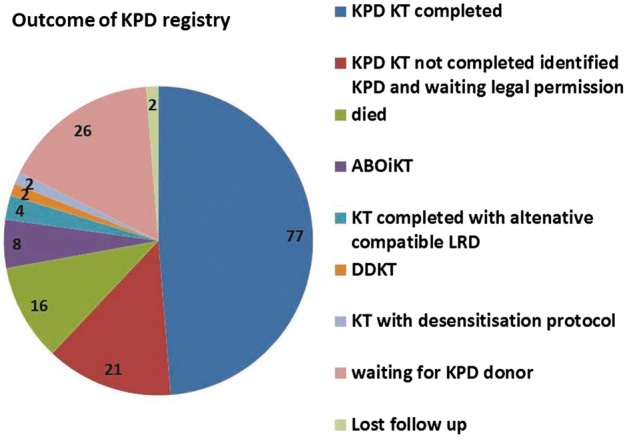
Outcomes of patients who were registered in our single-centre KPD registry.
LRD, living related donation.
The mortality at 1 year in the patients without KT was very high (n = 16) mainly due to infections and poverty. The available data show that the median ABO titres of patients registered in KPD were 1:256. Eight patients were not willing to undergo KPD and underwent ABOi KT due to low ABO titre ≤1:128. The majority of patients remaining unmatched in the KPD registry were O group (n = 36) and sensitized patients (n = 25).
Outcome of KPD transplant (Table 1, Figure 2)
The waiting time for KPD was short (median 2 months in non-O group patients and 6 months in O group patients) as compared with DDKT (median 24 months in non-O group patients and 36 months in O group patients). The death-censored graft survival and patient survival were 98.7% (n = 76) and 93.5% (n = 72), respectively. One highly sensitized patient lost a graft from immune injury. Despite normal functioning grafts, five patients died due to ischaemic heart disease (n = 1), intracranial bleeding (n = 1) and infections (n = 3). In all, 11 (14.2%) patients had biopsy-proven acute rejection and 1 patient had chronic rejection. The mean creatinine was 1.1 mg/dL on the last follow-up in our hospital. Infections [cytomegalovirus (n = 1), herpes (n = 2), tuberculosis (n = 3), pneumonia (n = 3) and urinary tract infection (n = 5)] were more common in patients who underwent pre-transplant desensitization. Donor survival was 100%. Of note, there are currently no pairs with A patient and B donor and vice versa without sensitization in the KPD registry since all of these were transplanted by KPD.
Discussion
Our data validate the short-term outcomes of KPD transplants as being comparable to those of directed LDKT and similar to other KPD programmes in India [12]. In a high-volume LDKT programme, all A and B blood group DRPs without sensitization can be transplanted with KPD within a reasonable waiting time with manual allocation [15]. The absolute numbers are not necessarily the best metric to evaluate the performance of a KPD programme, rather it is the ability to match and transplant the highest proportion of highly sensitized patients and O group patients and the transplant rate. In 2015, the transplant match rate of our KPD programmes was 62%, which is significantly higher than the national KPD programmes of Australia, Canada, The Netherlands and the UK (49, 44, 37 and 27%, respectively) [2, 3].
The team of transplant authorization and ethical committee members and social workers and the transplant co-coordinator ensured that no commercial transactions were involved in KPD transplantation between the international patients and compatible pairs. They confirmed donor autonomy of voluntarily donation and only near relatives (spouse, mother, father, sister, brother, daughter, son and grandparents) were allowed to donate, as per the Indian Transplant of Human Organs Act (THOA). The national registry was searched first before allowing for international swapping for Indian patients. The permission for international swapping was obtained from the government of Gujarat, India and the foreign nations involved [14]. Of the 77 patients, 5 were <18 years of age and their outcomes, immunosuppression results and follow-ups were similar to those of the adult patients. In patients with pre-transplant tuberculosis, ABO-incompatible KT or desensitization therapy was not performed due to economic constraints and the risk of reactivation after KT. DDKT was not performed due to higher morbidity and mortality on long-term dialysis. There was no reactivation of tuberculosis after KT with optimum medical care before and after transplantation and proper selection of patients. With the use of social media, help from local nephrologists/physicians and regular counselling and checking for adherence at each visit, we were able to arrange for successful follow-up and adherence despite long distances, lack of education and poor transport facilities in many communities. It is difficult to expand the DDKT and LDKT programmes without focusing on KPD in India. KPD is useful in centres with a shortage of DDKTs. Every year we perform >300 KTs. In 2015, the total number of LDKTs decreased in our centre, mainly due to patients who were ABO or HLA incompatible. The KPD increase observed is unlikely to be due to secular trends secondary to other factors such as social awareness or media attention in our state through government initiatives since the number of LDKTs and DDKTs performed generally in the whole state remained stable over the study period. This suggests that the observed increase in KPD at our centre could be due to the impact of the dedicated KPD team and KPD registry. Living donor KPD transplant also reduced the waiting list in DDKT for those who have no living donor available.
What we learned from our KPD registry
There is a higher frequency of group B blood in the Indian and central Asian population (B≥ O > A > AB), in contrast to blood group O (O > A > B> AB) in the developed world (USA/Europe or Australia) [15]. There is a greater impact of similar donor age in our KPD allocation and patients are less willing to accept older donors despite better HLA matching. Patients are also less willing to participate and wait in longer chains that result in increased pre-transplant time. In India, the majority of the transplant centres have limited transplant teams (operating rooms/surgical staff) and infrastructure and it is difficult to do simultaneous KPD in long chains (>three-way exchange). Simultaneous KPD should be the standard practice. Multicentre simultaneous KPD should be encouraged over single-centre, non-simultaneous KPD. Non-simultaneous KPD should be performed in exceptional cases when the transplant unit has the capacity to provide DDKT on a priority basis. Since patients develop ESRD at younger ages in India, HLA-based KPD transplantation in the national KPD programme will improve long-term outcomes. Compatible pairs from spousal donors will increase the number of transplants of O group and sensitized recipients. All the compatible pairs can also be offered the advantage of better HLA matching and younger donor ages.
Potential and sustainability of a single-centre KPD programme: comparison with other successful single-centre programmes like San Antonio [9–12]
The Methodist San Antonio KPD Program reported outcomes of 134 KPD transplants (17 compatible and 117 incompatible pairs) performed over a 3-year period (November 2007–February 2011) [12, 13]. From 2013 to 2015 we performed 189 paired donor transplants in our centres (Figure 1). The volume and percentage of KPD transplants consistently increased over the 3 years and substantially contributed to the growth of our LDKT programme (15.8, 18.1 and 24.9%) as well as in the Methodist San Antonio KPD Program (11, 27 and 35%). These data also validate the impact of our single-centre KPD programme.
There has been an accumulation of hard-to-match pairs. Compatible pairs [16, 17], a combination of KPD and desensitization [18], international KPD [14], non-simultaneous KPD [19] and an acceptable mismatch programme were the strategies implemented by us, which were similar to other successful single-centre programmes such as San Antonio [9]. However, we did not have other key features of the Methodist San Antonio KPD Program, such as computer allocation, storage of blood specimens for future crossmatch testing, use of an A2 donor for O patients and more compatible pairs. A higher percentage of sensitized patients were registered (63% vs. 34.8%) in the Methodist San Antonio KPD Program, whereas a higher percentage of ABO incompatible (65.2% vs. 37%) pairs were registered in our centre. In the Methodist San Antonio KPD Program, they even had increased access to KPD transplantation for traditionally disadvantaged cohorts of patients [female recipient (61%) and previous transplant (32%)].
Limitations
In our study, manual allocation without computerized software and being a single-centre KPD programme may have led to a lower match rate for O group and sensitized patients. Manual allocation can lead to man-made errors in cases of a large donor pool and the most appropriate pairs may not be identified. These impressive results may be part of the honeymoon phase of KPD previously described [20]. Transplant and match rates in the early stages of a KPD registry can be reversed as registries become older.
Future of KPD
The Indian Society of Nephrology and the Indian Society of Organ Transplantation, in collaboration with the International Transplantation Society and the International Society of Nephrology mentorship, should take the lead in expansion of KPD, as it will increase LDKT by >25%. The optimal use of list exchange will also increase the transplantation rate [21].
Conclusion
KPD increased the LDKT rate by 25% in 1 year. To the best of our knowledge, this is largest number of KPD transplantations in 1 year in any single centre in the world. KPD should be promoted to overcome the organ crisis and shortage of DDKT programmes.
Authors’ contributions
V.B.K. and P.R.S. maintained the KPD registry and wrote the manuscript and all authors contributed to the clinical management of patients, acquisition of data and approval of this manuscript.
Conflict of interest statement
None declared.
References
- 1. Flechner SM, Leeser D, Pelletier R. et al. The incorporation of an advanced donation program into kidney paired exchange: initial experience of the National Kidney Registry. Am J Transplant 2015; 15: 2712–2717 [DOI] [PubMed] [Google Scholar]
- 2. Cantwell L, Woodroffe C, Holdsworth R. et al. Four years of experience with the Australian kidney paired donation programme. Nephrology 2015; 20: 124–131 [DOI] [PubMed] [Google Scholar]
- 3. Cole EH, Nickerson P, Campbell P. et al. The Canadian kidney paired donation program: a national program to increase living donor transplantation. Transplantation 2015; 99: 985–990 [DOI] [PubMed] [Google Scholar]
- 4. Ferrari P, Weimar W, Johnson RJ. et al. Kidney paired donation: principles, protocols and programs. Nephrol Dial Transplant 2015; 30: 1276–1285 [DOI] [PubMed] [Google Scholar]
- 5. Gentry SE, Montgomery RA, Segev DL.. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis 2011; 57: 144–151 [DOI] [PubMed] [Google Scholar]
- 6. de Klerk M, Witvliet MD, Haase-Kromwijk BJ. et al. A flexible national living donor kidney exchange program taking advantage of a central histocompatibility laboratory: the Dutch model. Clin Transpl 2008: 69–73 [PubMed] [Google Scholar]
- 7. Johnson RJ, Allen JE, Fuggle SV. et al. Early experience of paired living kidney donation in the United kingdom. Transplantation 2008; 86: 1672–1677 [DOI] [PubMed] [Google Scholar]
- 8. Segev DL, Kucirka LM, Gentry SE. et al. Utilization and outcomes of kidney paired donation in the United States. Transplantation 2008; 86: 502–510 [DOI] [PubMed] [Google Scholar]
- 9. Wallis CB, Samy KP, Roth AE. et al. Kidney paired donation. Nephrol Dial Transplant 2011; 26: 2091–2009 [DOI] [PubMed] [Google Scholar]
- 10. Bingaman AW, Wright FH Jr, Kapturczak M. et al. Single-center kidney paired donation: the Methodist San Antonio experience. Am J Transplant 2012; 12: 2125–2132 [DOI] [PubMed] [Google Scholar]
- 11. Kute VB, Patel HV, Shah PR . et al. National kidney paired donation programme in India: challenges, solution, future direction. Nephrology 2015; 20: 442. [DOI] [PubMed] [Google Scholar]
- 12. Kute VB, Shah PS, Vanikar AV. et al. Increasing access to renal transplantation in India through our single-center kidney paired donation program: a model for the developing world to prevent commercial transplantation. Transpl Int 2014; 27: 1015–1021 [DOI] [PubMed] [Google Scholar]
- 13. Kute VB, Vanikar AV, Shah PR. et al. Facilitators to national kidney paired donation program. Transpl Int 2013; 26: e38–e39 [DOI] [PubMed] [Google Scholar]
- 14. Kute VB, Patel HV, Shah PR. et al. International kidney paired donation transplantations to increase kidney transplant of O group and highly sensitized patient: first report from India. World J Transplant 2017; 7: 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roodnat JI, van de Wetering J, Claas FH. et al. Persistently low transplantation rate of ABO blood type O and highly sensitised patients despite alternative transplantation programs. Transpl Int 2012; 25: 987–993 [DOI] [PubMed] [Google Scholar]
- 16. Varyani UT, Kute VB, Patel HV. et al. Participation of compatible donor to improve HLA matching can increase kidney transplant rate of o blood group patients. Clin Queries Nephrol 2015; 4: 38 [Google Scholar]
- 17. Kute VB, Vanikar AV, Gumber MR. et al. Successful three-way kidney paired donation with compatible pairs to increase donor pool. Ren Fail 2014; 36: 447-450 [DOI] [PubMed] [Google Scholar]
- 18. Kute VB, Patel HV, Shah PR. et al. Increasing access to kidney transplantation for sensitized recipient through three-way kidney paired donation with desensitization: the first Indian report. World J Clin Cases 2016; 4: 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kute VB, Patel HV, Varyani UT. et al. Six end-stage renal disease patients benefited from first non-simultaneous single center six-way kidney exchange transplantation in India. World J Nephrol 2016; 5: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gentry SE, Segev DL.. The honeymoon phase and studies of non-simultaneous chains in kidney-paired donation. Am J Transplant 2011; 11: 2778–2779; author reply 2780-1 [DOI] [PubMed] [Google Scholar]
- 21. Kute VB, Patel HV, Shah PR. et al. A potential solution to make the best use of a living donor-deceased donor list exchange. Am J Transplant 2016; 16: 3580. [DOI] [PubMed] [Google Scholar]


