Abstract
Aims and Hypothesis: This study aims to investigate the mechanism involved in intracellular regulation of EGFR degradation induced by EGF.
Methods: Phosphorylation of proteins related to EGFR signaling was examined by western blot analysis. Activation, connection between Rab35 and folliculin (FLCN) were assessed by pulldown, coimmunoprecipitation assays separately. The relationship between FLCN and cell growth was detected using gene overexpression and knock-down techniques.
Results: Here, we demonstrate that interfering with FLCN, a tumor suppressor, reduces the rate of EGF-induced EGFR degradation, resulting in prolonged activation of downstream signaling. Rab35 is also involved in these processes. Moreover, C-terminal of FLCN binds to and activates Rab35. Of special interest is the observation that erlotinib, a selective EGFR inhibitor, not only obstructs the EGFR-mediated cellular signaling, but also abolishes EGF-stimulated EGFR degradation. Further results reveal that EGF facilitates the activation of Rab35, and FLCN modulates EGF-dependent Rab35 activation and cell growth.
Conclusions: Taken together, our study proposes a negative-feedback regulation model in which FLCN mediates EGF-induced Rab35 activation, thereby increasing EGFR degradation and attenuating EGFR signaling.
Keywords: EGFR, degradation, FLCN, Rab35, feedback
Introduction
Epidermal growth factor receptor (EGFR), the first receptor tyrosine kinase (RTK) discovered more than three decades ago, has emerged as a central regulator of critical cellular processes including proliferation, differentiation and motility. EGFR achieves these functions mainly by activating RAF-MEK-ERK1/2 and PI3K-AKT signaling cascades (Ullrich et al., 1984; Hunter, 2000; Schlessinger, 2000). Excessive activation of EGFR and of the downstream signal transducers are found in many diverse cancers. Therefore, drugs that target the EGFR signaling pathway have been approved for the treatment of cancer (Tebbutt et al., 2013; Tomas et al., 2014). Unfortunately, an increasing number of studies revealed that a complex network of feedback regulation triggers the resistance to these target therapies and limits their overall clinical benefits (Avraham and Yarden, 2011; Lee et al., 2014; Li and Mansmann, 2014).
Receptor endocytosis is a major negative feedback loop that plays a key role in spatiotemporal regulation of signaling (Avraham and Yarden, 2011; Er et al., 2013; Jones and Rappoport, 2014). Binding of ligands to EGFR induces EGFR autophosphorylation at the plasma membrane and its internalization to the early endosome, where EGFR is sorted for recycling to the cell surface or degradation in the lysosome. The fate of the EGFR is influenced by cellular context, ligand type, concentration and duration of stimulation, and has significant consequences for cellular signaling outputs (Tomas et al., 2014). Although some reports showed that the inability of EGFR internalization and degradation is implicated in carcinogenesis, the mechanisms related to carcinogenesis remain incomplete (Deribe et al., 2009; Goueli et al., 2012).
Rab GTPases constitute the largest family of small GTPases. Together with their regulators, they control many aspects of intracellular trafficking. Rab GTPases cycle between active GTP-bound and inactive GDP-bound states by GEF, GAP, and other GTPase-activating proteins (Stenmark, 2009). Rab35, a highly conserved Rab during evolution, predominantly functions in endosomal trafficking by controlling the location of cargo such as transferrin, β1-integrin, cadherin (Patino-Lopez et al., 2008; Allaire et al., 2013; Charrasse et al., 2013; Argenzio et al., 2014; Tang et al., 2015). Aberrant Rab35 expression and gene mutation are associated with malignance, however, the oncogenic as well as tumor suppressive function of Rab35 have been reported previously (Sheach et al., 2009; Allaire et al., 2013; Zhu et al., 2013; Tang et al., 2015; Wheeler et al., 2015). In particular, Rab35 knockdown significantly enhances EGFR signaling and cell migratory capacity (Allaire et al., 2013; Wheeler et al., 2015). However, whether and how Rab35 contributes to EGFR trafficking remains largely unknown.
Folliculin (FLCN), a tumor suppressor, was originally identified from patients with Birt–Hogg–Dubé syndrome (Nickerson et al., 2002; Schmidt and Linehan, 2015). Subsequent studies demonstrated that it is mutated in various types of cancers including endometrial carcinoma, gastric cancer and colorectal cancer (Fujii et al., 2006; Jiang et al., 2007; Guda et al., 2015). Mechanically, FLCN deficiency in mice facilitates the activation of RAF, MEK, ERK1/2 and AKT (Baba et al., 2008; Hasumi et al., 2009), which are the key transducers of EGFR signaling. Interestingly, crystallographic and biochemical analysis revealed that DENN domain of FLCN has GEF activity toward Rab35 (Nookala et al., 2012). In this study, we hypothesize that FLCN may function as an activator of Rab35 and regulate EGFR intracellular trafficking and its downstream signaling transduction. Here, we show the C-terminal of FLCN binds to Rab35. We moreover establish a negative-feedback regulation model in which FLCN mediates EGF-induced Rab35 activation, thereby increasing EGFR degradation and attenuating EGFR signaling.
Materials and methods
Cell lines and cell culture
Human ovarian cancer cell line HeLa and human embryonic kidney cell line HEK293T were obtained from the Cell Biology Institute of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose; Gibco, Thermo Scientific, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco) and antibiotics (100 U/mL streptomycin and 100 μg/mL penicillin; Invitrogen, USA) in a humidified incubator at 37°C with 5% CO2.
Plasmids and siRNAs
The full-length FLCN plasmid was kindly provided by Dr. Laura S. Schmidt (Department of Urologic Oncology, NCI, USA). Full-length FLCN cDNA was amplified from this plasmid using the following primer set, sense: 5′-CCCAAGCTTATGAATGCCATCGTGGCTC-3′ and antisence: 5′-GCTCTAGATCAGTTCCGAGACTCCGAG-3′. The polymerase chain reaction (PCR) product was cloned into the pCMV-N-Flag (Beyotime, Nantong, China). FLCN cDNA fragments corresponding to the N-terminal (FLCN-NT), C-terminal (FLCN-CT) of FLCN were amplified using the following primer set, sense: 5′-CCCAAGCTTCGGAAGCTGCCAGTCTTC-3′ and antisence: 5′-GCTCTAGATCAGGGCTGCCAGCTCCCACA-3′ and sense: 5′-CCGGAATTCC GGAAGCTGCCAGTCTTC-3′ and antisence: 5′-CCGCTCGAGTCAGTTCCGAGACTCCGAG−3′ by PCR and ligated into pCMV-N-Flag. pEGFP-Rab35 (WT, S22N, and Q67L) plasmids were kindly provided by Dr. Matthew P. Scott (Department of Medicine, Stanford University, Stanford, California, USA). Full-length Rab35 cDNA was amplified from the Rab35 WT plasmid using the following primer set, sense: 5′-CGGAATTCATGGCCCGGGACTACGAC-3′ and antisence: 5′-CCGCTCGAGTTAGCAGCAGCGTTTCTT-3′. The PCR product was cloned into the pGEX-4T-1. The cells were seeded in six-well plate, cultured to 80% confluence, and then transfected with those plasmids by ExFect™ Transfection Reagent (Vazyme Biotech, Piscataway, NJ, China).
The small interfering RNA (siRNA) were chemically synthesized and purified by China GenePharma Co. FLCN: 5′-GAUAAAGAGACCUCCAUUAdTdT-3′; and Rab35: #1, 5′-GCAGCAACAACAGAACGAUdTdT-3′, #2, 5′-GCUCACGAAGAACAGUAAAdTdT-3′ and #3, 5′-GAUGAUGUGUGCCGAAUAUdTdT-3′. Cells were transfected with siRNA by Lipofectamine 2000 (Invitrogen).
Reagents and antibodies
EGF was purchased from R&D systems (Minneapolis, MN, USA). Cyclohexamide (CHX) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (USA). Erlotinib was purchased from ApexBio Technology (USA). Phospho-ERK1/2, Phospho-AKT (473), AKT, GFP, FLCN rabbit antibodies, and ERK1/2 mouse antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Normal mouse IgG, normal rabbit IgG, GAPDH and EGFR rabbit antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). GFP mouse antibody was purchased from EarthOx (USA). Rab35 rabbit antibody was purchased from Biogot technology (Nanjing, Jiangsu, China). Flag mouse antibody was purchased from Sigma. HRP-conjugated secondary antibody was purchased from Santa Cruz Biotechnology.
Western blot
Sample protein extraction and concentration determination of whole cells were performed as previously described (Deng et al., 2016). Briefly, equal amounts of protein were run on SDS polyacrylamide gels and transferred to nitrocellulose membrane. The resulting blots were blocked with 5% non-fat dry milk and incubated with primary antibodies overnight at 4°C. Protein bands were then detected by incubating with HRP-conjugated secondary antibodies for 2 h at room-temperature and visualized with ECL reagent (Millipore, Billerica, MA, USA) by Chemi Doc XRS and gel imaging system (Bio-Rad, USA). Densitometry analysis was performed using Quantity One software and band intensities were normalized to those of GAPDH.
In vitro pull down assays and immunoprecipitation
All cells were lysed with lysis buffer (20 mM Tris PH7.5, 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM EDTA, 1% Na3VO4, 0.5 μg/ml leupeptin, 1 mM PMSF). For in vitro binding assays, GST fusion proteins were first purified on MagneGST glutathione particles (Promega, Madison, WI). Five hundred micrograms of cell lysates transfected with target proteins were then incubated with GST fusion proteins. For immunoprecipitation assays, 500 μg of cellular proteins were precipitated and ralated protein were detected by western blot, as described previously (Duan et al., 2016). Antibodies against FLCN, Rab35 and GFP were used at dilutions of 1:100, 1:50, and 1:100 for immunoprecipitation, respectively.
Measurement of Rab35 activity
The Rab35-binding domain (RBD35) of mRUSC2 (aa 739–862) was amplified by PCR and ligated into pGEX-2T using EcoRI and SalI sites. It was generated as previously reported. GST-RBD35 was expressed in bacteria and purified by MagneGST glutathione particles at 4°C with constant rotation for 30 min. HeLa cells were serum-starved for 12 h and treated with 10 ng/mL EGF (5–60 min), then proteins were extracted and activation of Rab35 was measured as previously described (Deng et al., 2016).
MTT assay
Cells transfected with plasmids or siRNA were seeded at a density of 1 × 105 cells per well into 96-well plate and treated with EGF for the indicated times and doses. After culture, cells were washed, MTT was added and the plate was incubated in the dark for 4 h, followed by measurement of absorbance value at 490 nm using a microplate absorbance reader (Bio-Tek, Elx800, USA). The fold growth was calculated as the absorbance of drug treated sample/control sample absorbance × 100%.
EdU staining
Cell proliferation was measured using EdU staining kit according to the manufacturer's instruction (RiboBio, Guangzhou, China). In brief, cells were cultured in 96-well plate until reaching 70% confluence, then EdU was added to the culture media for 2 h. After fixing by paraformaldehyde, the cells were washed with PBS. Then, the cells were incubated with glycine and washed with PBS containing 0.5% Triton X-100. After the cells were counterstained with hochest33342, cells were mounted and imaged by fluorescence microscopy.
Statistical analysis
Data were presented as mean ± standard error of the mean (SD). Statistical analyses were performed using Prism 5.0 software (GraphPad Software, USA). Student's t-test was used for comparison between groups. Values of P < 0.05 were considered statistically significant. All experiments were repeated at least three times.
Results
Active Rab35 promotes EGFR degradation and attenuates EGFR signaling
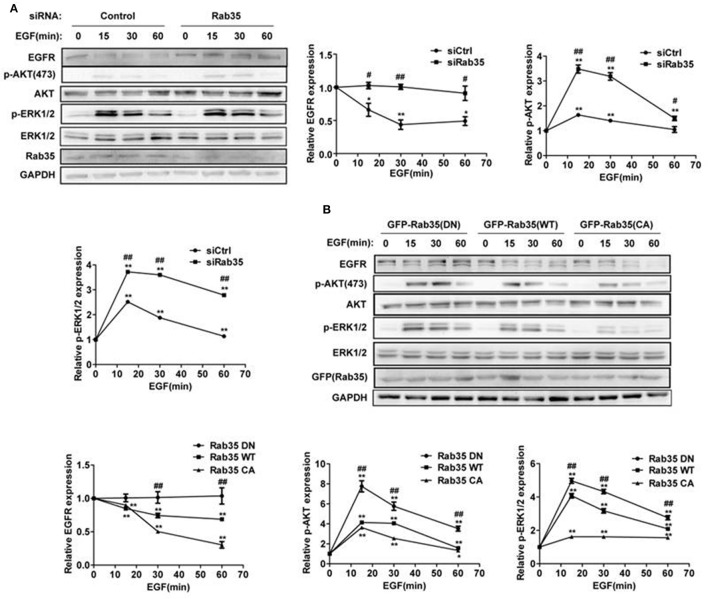
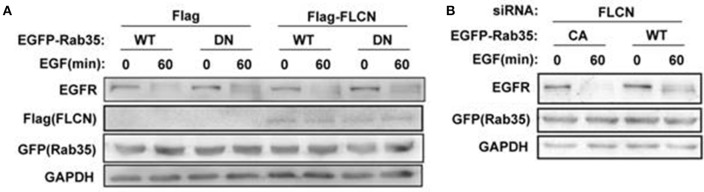
A previous study showed that knock down of Rab35 significantly enhanced the serum-induced EGFR recycling and signaling in both COS-7 and U251 cells (Allaire et al., 2013). In order to address whether Rab35 regulates EGFR abundance and signaling with EGF stimulation, we assessed EGFR degradation in HeLa cells, a model cell line with moderate EGFR abundance and complete endomembrane system (Sigismund et al., 2005; Spangler et al., 2010). Serum-starved HeLa cells were stimulated with EGF (10 ng/mL) for indicated times in the presence of the protein synthesis inhibitor cycloheximide (CHX). We observed EGF-induced degradation of EGFR, and this model was used in the following experiments. Compared with control cells, cells depleted of Rab35 by siRNA suppressed EGF-induced EGFR degradation and enhanced EGFR signaling (Figure 1A). To confirm these results, we repeated EGFR degradation assays by overexpression of three different constructs of Rab35 in HeLa cells. Dominant negative GDP-locked Rab35-S22N (Rab35 DN) consistently delayed EGFR degradation and enhanced the activation of ERK1/2 and AKT compared with wild-type Rab35 (Rab35 WT), while constitutively active GTP-locked Rab35-Q67L (Rab35 CA) facilitated EGFR degradation and attenuated EGFR signaling (Figure 1B). Together, these data suggest that active Rab35 is necessary to promote EGF-induced EGFR degradation and reduce EGFR signaling activation.
Figure 1.
Rab35 activation promotes EGFR degradation and attenuates EGFR signaling. (A) HeLa cells were starved overnight and then treated with cycloheximide for 30 min, then stimulated with 10 ng/mL EGF for the indicated time. EGFR, p-Akt and p-ERK1/2 levels were detected by western blot. Data were presented as mean ± SD of 3 independent determinations. (B) Overexpression of different plasmids of Rab35 (Rab35 DN, Rab35 WT, and Rab35 CA) to detect EGF-induced EGFR degradation and its downstream signaling. *P < 0.05 and **P < 0.01 show the comparison between groups at the different time points of EGF stimulation. #P < 0.05 and ##P < 0.01 show the comparison between different groups at a same time point.
FLCN regulates EGFR degradation and its downstream signaling
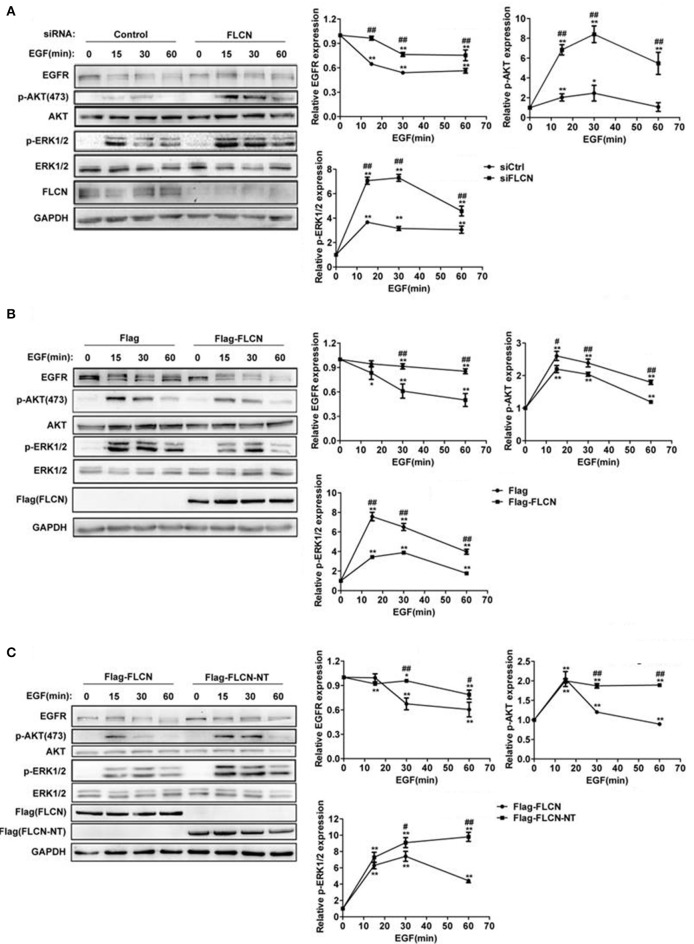
Considering FLCN has GEF activity toward Rab35 (Nookala et al., 2012), we hypothesized that FLCN might also affect the degradation of EGF-induced EGFR and its downstream signaling pathways. To test this, we used EGF to stimulate HeLa cells after transfected with FLCN siRNA pool. The results showed that EGF-stimulated EGFR degradation was significantly slowed down in FLCN-silencing HeLa cells, and knockdown of FLCN resulted in persisting activation of p-AKT and p-ERK (Figure 2A). Moreover, when FLCN is overexpressed, the EGF-induced EGFR degradation was accelerated and the expressions of p-AKT, p-ERK were significantly weakened (Figure 2B).
Figure 2.
FLCN promotes EGFR degradation and attenuates EGFR signaling. (A) HeLa cells were transfected with negative control siRNA or siRNA pool for FLCN, (B) pCMV-Flag empty vector or Flag-FLCN, (C) Flag-FLCN, or FLCN-NT. Cells were lysed and subjected to western blot analysis for EGFR expression and phosphorylation levels of its downstream signaling. *P < 0.05 and **P < 0.01 show the comparison between groups with EGF stimulation and without EGF stimulation. #P < 0.05 and ##P < 0.01 show the comparison between different groups at a same time point.
It has been reported that FLCN deficit was mostly due to BHD gene mutation in exon 11, resulting in a C-terminal deletion mutant of FLCN (Schmidt et al., 2005; Hasumi et al., 2009). To prove whether this mutant could affect EGFR degradation and its downstream signaling, we transiently transfected HeLa cells with FLCN full length (FLCN) and N-terminal (FLCN-NT), which is a truncated FLCN analogous to the BHD gene mutation to validate the function of FLCN (Nookala et al., 2012). The result showed that compared with the Flag-FLCN-NT, overexpression of full-length FLCN accelerates EGFR degradation and decreased p-AKT and p-ERK levels (Figure 2C). These results indicated that C-terminal deletion mutant of FLCN could not facilitate EGFR degradation and attenuate its downstream signaling.
FLCN binds to Rab35
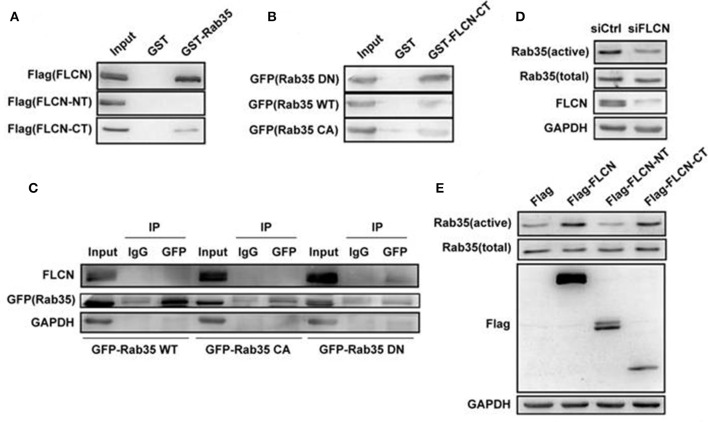
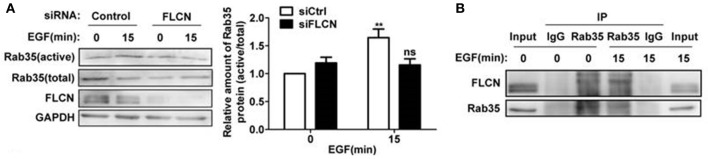
In order to further study the relationship between FLCN and Rab35, we overexpressed FLCN, FLCN-NT, FLCN-CT in HEK293T cells and performed GST-pulldown assays to detect the combination between Rab35 WT and different fragments of FLCN. Results showed that GST-Rab35 bound preferentially to full-length of FLCN, followed by FLCN-CT but not FLCN-NT (Figure 3A). To further validate the relationship of FLCN to Rab35, Rab35DN, and Rab35 WT plasmids were overexpressed in HEK293T cells, respectively. We found that FLCN-CT bound preferentially to Rab35 DN, rather than Rab35 WT or Rab35 CA (Figure 3B), which supports the idea that FLCN is a Rab35 GEF in cells. By immune coprecipitation, we demonstrated that endogenous FLCN binds to Rab35 DN, but not Rab35 WT or Rab35 CA (Figure 3C). Moreover, silencing of FLCN decreased Rab35 activity significantly (Figure 3D). We also found that overexpression of FLCN and FLCN-CT, but not FLCN-NT, significantly enhanced Rab35 activation (Figure 3E). Together, these results indicate that FLCN might act as GEF of Rab35 and its C-terminal domain might be required for its GEF activity.
Figure 3.
FLCN functions as an activator toward Rab35 in vitro. (A) HEK293T cells were transiently transfected with Flag-FLCN, Flag-FLCN-NT, or Flag-FLCN-CT plasmid. At 36 h after transfection, lysates of HEK-293T cells was incubated in vitro with GST-tagged Rab35 or GST alone, and coprecipitation of FLCN with GST fusion Rab35 proteins, bound to glutathione-beads, was analyzed by western blot using an anti-Flag antibody. GST, GST alone used as a control. (B) HEK293T cells were transiently transfected with Rab35 CA, Rab35 WT, or Rab35 DN plasmid. At 36 h after transfection, lysates of HEK-293T cells was incubated in vitro with GST-tagged FLCN-CT or GST alone, and coprecipitation of Rab35 with GST fusion FLCN-CT proteins, bound to glutathione-beads, was analyzed by western blot using an anti-GFP antibody. GST, GST alone used as a control. (C) Equal amounts of lysates from the indicated HeLa cells transiently transfected with Rab35 CA, Rab35 WT, or Rab35 DN plasmid were immunoprecipitated with an anti-GFP antibody or IgG, and then both unprocessed lysates (Input) and immunoprecipitates were resolved by western blot using the indicated antibodies. (D) HEK293T cells were transiently transfected with negative control siRNA or FLCN siRNA pool. At 36 h after transfection, cells were lysed and subjected to GST-pulldown analysis for the activity of Rab35. (E) HEK293T cells were transiently transfected with pCMV-Flag empty vector, Flag-FLCN, Flag-FLCN-NT, or Flag-FLCN-CT plasmid. At 36 h after transfection, cells were lysed and subjected to GST-pulldown analysis for the activity of Rab35.
EGF activates Rab35
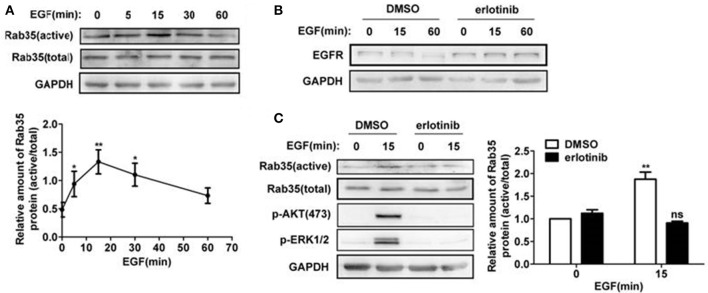
To study whether EGF activates Rab35, we stimulated HeLa cells with EGF for 0, 5, 15, 30, and 60 min and then detected Rab35 activity by GST-pulldown assays. As shown in Figure 4A, Rab35 activity reached a peak when EGF stimulated cells for 15 min. To further confirm the role of EGF-EGFR to Rab35, erlotinib was used to inhibit EGFR activity. The results showed that erlotinib inhibited EGFR degradation upon EGF stimulation (Figure 4B). This indicates that EGFR degradation depends on the activation of EGFR. Further experimental results show that erlotinib pretreatment also prevented EGF-induced Rab35 activity in HeLa cells (Figure 4C) as well as in the gastric cancer cell line SGC-7901 (data not shown).
Figure 4.
EGF activates Rab35 via EGFR. (A) HeLa cells were serum-starved for 12 h and stimulated with 10 ng/ml EGF for the indicated time. The GTP-bound form of endogenous Rab35 was precipitated by GST-RBD35 through GST-pulldown analysis. (B) HeLa cells were serum-starved and treated with DMSO or 1 μM erlotinib overnight, and then stimulated with 10 ng/ml EGF for the indicated time. Cells were lysed and subjected to western blot analysis for EGFR expression. *P < 0.05 and **P < 0.01 show the comparison between groups with EGF stimulation and without EGF stimulation. (C) HeLa cells were serum-starved and treated with DMSO or 1 μM erlotinib overnight, and then stimulated with 10 ng/ml EGF for 15 min or not. Cells were lysed and subjected to GST-pulldown analysis for the activity of Rab35. **P < 0.01 show the comparison between groups with EGF stimulation and without EGF stimulation, ns (not significant).
FLCN mediates EGF-induced Rab35 activation
In the above studies, we found that FLCN binds to Rab35, but whether FLCN can regulate Rab35 activity in the process of EGF-induced Rab35 activation is unknown. The results here showed that the increased Rab35 activity by EGF stimulation was attenuated when FLCN was silenced in HeLa cells (Figure 5A), suggesting that FLCN mediates EGF-stimulated activation of Rab35. To investigate the role of EGF in regulating the interaction between FLCN and Rab35, we detected the interaction status of FLCN and Rab35 by EGF stimulation. We found that the amount of endogenous Rab35 coprecipitated with FLCN were increased upon EGF stimulation for 15 min (Figure 5B).
Figure 5.
FLCN mediates EGF-induced Rab35 activation. (A) HeLa cells were transiently transfected with negative control siRNA or FLCN siRNA pool. At 36 h after transfection, cells were serum-starved overnight and stimulated with 10 ng/ml EGF for 15 min. Cells were lysed and subjected to GST-pulldown analysis for the activity of Rab35. **P < 0.01 show the comparison between groups with EGF stimulation and without EGF stimulation, ns (not significant). (B) HeLa cells were serum-free overnight and then treated with 10 ng/ml EGF for 15 min. Cells were lysed and anti-Rab35 or IgG immunoprecipitates were analyzed for coprecipitation of FLCN by western blot. Unprocessed lysates of HeLa cells was used as input.
Rab35 acts as a downstream effector to control EGFR degradation
To investigate whether FLCN regulates EGFR degradation through Rab35, Rab35 CA or Rab35 DN were transfected in FLCN-silencing or FLCN-overexpression cells to detect EGF-induced EGFR degradation separately. The results show that after transfection with Rab35 DN, the degradation of EGFR in HeLa cells was slowed down, whether Flag-FLCN existed in cells or not (Figure 6A). Interestingly, overexpression of Rab35 CA accelerates EGFR degradation when FLCN was interfered with siRNA (Figure 6B). Together, these results indicate that Rab35 may promote EGF-induced EGFR degradation via FLCN-independent manner.
Figure 6.
EGFR degradation requires Rab35 activation. (A) HeLa cells were transiently transfected with Rab35 WT or Rab35 DN plasmid and pCMV-Flag empty vector or Flag-FLCN at the same time. Cells were lysed and subjected to western blot analysis for EGFR expression. (B) FLCN-silenced HeLa cells were transiently transfected with Rab35 CA or Rab35 DN plasmid. Cells were lysed and subjected to western blot analysis for EGFR expression.
FLCN decreases EGF-induced cell growth
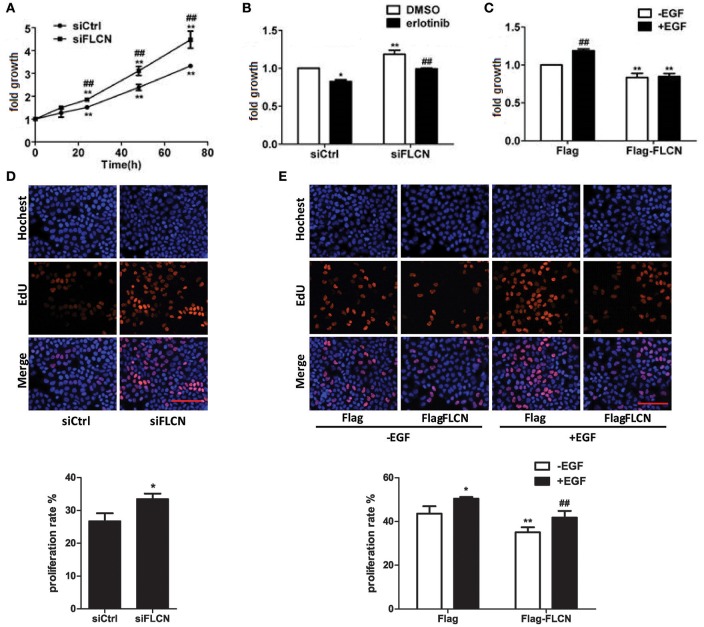
EGFR signaling is a central regulator of cellular processes such as viability and proliferation, According to the results of Peter S. McPherson's lab, knockdown of Rab35 significantly increases proliferation rates of COS-7 cells (Allaire et al., 2013). Then, we investigated the effect of FLCN on cell growth using MTT assay and EdU staining. The results showed that the cell growth rate increased after knockdown of FLCN (Figures 7A,D). However, when FLCN-silencing cells were pretreated with erlotinib, cell growth velocity was decreased (Figure 7B). On the contrary, overexpression of FLCN significantly repressed EGF-induced upregulation of cell growth (Figures 7C,E). Data from Gyorffy et al. also showed that expression of FLCN is positive correlation with patient survival in lung cancer and gastric cancer (Gyorffy et al., 2010). Overall, these results showed that FLCN may affect cell growth in an EGFR dependent manner.
Figure 7.
FLCN involves in EGF-induced cell growth. (A) HeLa cells were transiently transfected with negative control siRNA or FLCN siRNA pool. At the indicated time points after transfection, cells were analyzed by MTT assay. *P < 0.05 and **P < 0.01 show the comparison between the different time points and the 0 h point in the same group. #P < 0.05 and ##P < 0.01 show the comparison between different groups at a same time point. (B) HeLa cells were transiently transfected with negative control siRNA or FLCN siRNA pool and treated with DMSO or 1 μM erlotinib for 72 h, then cells were analyzed by MTT assay. *P < 0.05 and **P < 0.01 show the comparison with the group transfected with negative control siRNA and treated with DMSO. ##P < 0.01 show the comparison between groups treated with DMSO and 1 μM erlotinib. (C) HeLa cells were transiently transfected with pCMV-Flag empty vector or Flag-FLCN plasmid and stimulated with 10 ng/ml EGF for 72 h, then the cells were analyzed by MTT assay. **P < 0.01 show the comparison between groups transfected with pCMV-Flag empty vector and Flag-FLCN plasmid. ##P < 0.01 show the comparison between groups with EGF stimulation and without EGF stimulation. (D) HeLa cells were transiently transfected with negative control siRNA or FLCN siRNA pool for 72 h, then cells were analyzed by EdU staining. *P < 0.05 show the comparison with the group transfected with negative control siRNA and FLCN siRNA. Scale bar, 100 μm. (E) HeLa cells were transiently transfected with pCMV-Flag empty vector or Flag-FLCN plasmid and stimulated with 10 ng/ml EGF for 72 h, then the cells were analyzed by EdU staining. *P < 0.05, **P < 0.01 show the comparison with group transfected with pCMV-Flag empty vector with other groups. ##P < 0.01 show the comparison between EGF-incubation groups transfected with pCMV-Flag empty vector and Flag-FLCN plasmid. Scale bar, 100 μm.
Discussion
EGFR is shown mutated in a number of tumor types (Lynch et al., 2004; Walker et al., 2009), such mutation leads to its constant activation, which produces uncontrolled cell proliferation. This clinical observation led to the development of anticancer therapeutics directed against EGFR. However, until now, the precise mechanisms about intracellular regulation of EGFR content remain elusive. Similar to EGF (Levi-Montalcini and Cohen, 1960), mice with inactive FLCN in the epidermis have striking delays in eyelid opening (Medvetz et al., 2012), suggesting that FLCN may participate in EGF-EGFR signaling. FLCN has been used to modulate expression and localization of membrane proteins (Medvetz et al., 2012; Nahorski et al., 2012; Goncharova et al., 2014). Here, we propose a model in which negative feedback of FLCN regulates EGFR signaling. FLCN mediates EGF-induced Rab35 activation, leading to EGFR degradation and decreased activity of its downstream signaling. Our findings enrich the complicated network of EGFR signaling and uncover a molecular mechanism of FLCN in tumor suppression.
Increasing evidence has indicated that deletion of FLCN could lead to hyperactivation of various kinases, including ERK1/2 and AKT (Baba et al., 2008; Hasumi et al., 2009). Consistently, we demonstrate here that FLCN-deficiency reduces the rate of EGF-induced EGFR degradation, resulting in the continuous activation of ERK1/2 and AKT in an EGFR-dependent manner. Although biochemical analysis showed that the DENN domain, which is located in the C-terminal of FLCN, possesses GEF activity toward Rab35 (Nookala et al., 2012), activation of RAB35 by FLCN has never been validated in cells. Our results show that in HeLa cells, FLCN binds to Rab35 by its C-terminal, which plays a key role in mediating EGF-induced Rab35 activation and EGFR degradation.
Rab35 has been shown to regulate cargo recycling at endosomes, with an essential role in the regulation of actin cytoskeleton organization (Zhang et al., 2009; Chaineau et al., 2013). It is reported that Rab35 suppresses cell migration by controlling intracellular trafficking and cell surface levels of cadherins and β1-integrin in mammalian cells (Allaire et al., 2013; Charrasse et al., 2013; Argenzio et al., 2014; Tang et al., 2015). Consistent with these studies, our results show that Rab35 increases the rate of EGF-induced EGFR degradation and attenuates its downstream signaling. Furthermore, FLCN functions as an activator toward Rab35 in the process of EGF-stimulation. However, a recent report revealed that depletion of Rab35 inhibits AKT activation in response to growth factors through an underlying PI3K-dependent manner (Wheeler et al., 2015). The conflicting effect of Rab35 activation on AKT activity might depend on the cell context or experimental conditions. We previously demonstrated that Rab35 mediates Wnt5a-induced cell migration and EGF induces epithelial-mesenchymal transition via decreasing Wnt5a transcription (Zhu et al., 2013; Zhang et al., 2015). FLCN is also associated with Wnt signaling (Luijten et al., 2013). Our current observations, together with these previous studies, suggest that FLCN-Rab35 may play an essential role in the cross-talk between EGFR and Wnt signaling, which is necessary for further studies to elucidate the bilateral function of FLCN and Rab35 during oncogenic transformation.
When EGF-induced activation of EGFR signaling is completely blocked, EGFR trafficking is also obstructed in the cells pretreated with erlotinib. This suggests that the regulators of EGFR trafficking may sense EGFR signaling. Determining the upstream regulation mechanism of proteins associated with EGFR trafficking in detail will contribute to the comprehensive understanding of EGFR signal network. In summary, the finding that FLCN/Rab35 pathway contributes to the degradation of EGFR and attenuation of its downstream signaling advances our understanding of EGFR trafficking. However, it remains unclear whether FLCN and Rab35 act by binding to EGFR directly or via an indirect mechanism, further studies are required to investigate how the FLCN/Rab35 pathway promotes EGFR degradation and the association between EGFR and FLCN in clinical samples.
Author contributions
JZ designed the study. JZ, SS, JC, and YZ performed the experiments. JZ performed the statistical analysis. JD and BD drafted the manuscript, YZ supervised the experimental work. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81773107) to JD, the National Natural Science Foundation of China (No. 81602561) to YZ, the National Natural Science Foundation of China (No. 81372319), a Project Funded by Jiangsu Collaborative Innovation Center for Cancer Personalized Medicine to Luo Gu, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Allaire P. D., Seyed Sadr M., Chaineau M., Seyed Sadr E., Konefal S., Fotouhi M., et al. (2013). Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J. Cell Sci. 126(Pt 3), 722–731. 10.1242/jcs.112375 [DOI] [PubMed] [Google Scholar]
- Argenzio E., Margadant C., Leyton-Puig D., Janssen H., Jalink K., Sonnenberg A., et al. (2014). CLIC4 regulates cell adhesion and beta1 integrin trafficking. J. Cell Sci. 127, 5189–5203. 10.1242/jcs.150623 [DOI] [PubMed] [Google Scholar]
- Avraham R., Yarden Y. (2011). Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117. 10.1038/nrm3048 [DOI] [PubMed] [Google Scholar]
- Baba M., Furihata M., Hong S. B., Tessarollo L., Haines D. C., Southon E., et al. (2008). Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J. Natl. Cancer Inst. 100, 140–154. 10.1093/jnci/djm288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaineau M., Ioannou M. S., McPherson P. S. (2013). Rab35: GEFs, GAPs and effectors. Traffic 14, 1109–1117. 10.1111/tra.12096 [DOI] [PubMed] [Google Scholar]
- Charrasse S., Comunale F., De Rossi S., Echard A., Gauthier-Rouviere C. (2013). Rab35 regulates cadherin-mediated adherens junction formation and myoblast fusion. Mol. Biol. Cell 24, 234–245. 10.1091/mbc.E12-02-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Wang Y., Gu L., Duan B., Cui J., Zhang Y., et al. (2016). MICAL1 controls cell invasive phenotype via regulating oxidative stress in breast cancer cells. BMC Cancer 16:489. 10.1186/s12885-016-2553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deribe Y. L., Wild P., Chandrashaker A., Curak J., Schmidt M. H., Kalaidzidis Y., et al. (2009). Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci. Signal. 2:ra84. 10.1126/scisignal.2000576 [DOI] [PubMed] [Google Scholar]
- Duan B., Cui J., Sun S., Zheng J., Zhang Y., Ye B., et al. (2016). EGF-stimulated activation of Rab35 regulates RUSC2-GIT2 complex formation to stabilize GIT2 during directional lung cancer cell migration. Cancer Lett. 379, 70–83. 10.1016/j.canlet.2016.05.027 [DOI] [PubMed] [Google Scholar]
- Er E. E., Mendoza M. C., MacKey A. M., Rameh L. E., Blenis J. (2013). AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Sci. Signal. 6:ra45. 10.1126/scisignal.2004015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Jiang W., Matsumoto T., Miyai K., Sashara K., Ohtsuji N., et al. (2006). Birt-Hogg-Dube gene mutations in human endometrial carcinomas with microsatellite instability. J. Pathol. 209, 328–335. 10.1002/path.1992 [DOI] [PubMed] [Google Scholar]
- Goncharova E. A., Goncharov D. A., James M. L., Atochina-Vasserman E. N., Stepanova V., Hong S. B., et al. (2014). Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Rep. 7, 412–423. 10.1016/j.celrep.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goueli B. S., Powell M. B., Finger E. C., Pfeffer S. R. (2012). TBC1D16 is a Rab4A GTPase activating protein that regulates receptor recycling and EGF receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 109, 15787–15792. 10.1073/pnas.1204540109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guda K., Veigl M. L., Varadan V., Nosrati A., Ravi L., Lutterbaugh J., et al. (2015). Novel recurrently mutated genes in African American colon cancers. Proc. Natl. Acad. Sci. U.S.A. 112, 1149–1154. 10.1073/pnas.1417064112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B., Lanczky A., Eklund A. C., Denkert C., Budczies J., Li Q., et al. (2010). An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 123, 725–731. 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- Hasumi Y., Baba M., Ajima R., Hasumi H., Valera V. A., Klein M. E., et al. (2009). Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc. Natl. Acad. Sci. U.S.A. 106, 18722–18727. 10.1073/pnas.0908853106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (2000). Signaling–2000 and beyond. Cell 100, 113–127. 10.1016/S0092-8674(00)81688-8 [DOI] [PubMed] [Google Scholar]
- Jiang W., Fujii H., Matsumoto T., Ohtsuji N., Tsurumaru M., Hino O. (2007). Birt-Hogg-Dube (BHD) gene mutations in human gastric cancer with high frequency microsatellite instability. Cancer Lett. 248, 103–111. 10.1016/j.canlet.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Jones S., Rappoport J. Z. (2014). Interdependent epidermal growth factor receptor signalling and trafficking. Int. J. Biochem. Cell Biol. 51:23–28. 10.1016/j.biocel.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Zhuang G., Cao Y., Du P., Kim H. J., Settleman J. (2014). Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 26, 207–221. 10.1016/j.ccr.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Cohen S. (1960). Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann. N. Y. Acad. Sci. 85:324–341. [DOI] [PubMed] [Google Scholar]
- Li J., Mansmann U. R. (2014). A molecular signaling map and its application. Cell. Signal. 26, 2834–2842. 10.1016/j.cellsig.2014.08.022 [DOI] [PubMed] [Google Scholar]
- Luijten M. N., Basten S. G., Claessens T., Vernooij M., Scott C. L., Janssen R., et al. (2013). Birt-Hogg-Dube syndrome is a novel ciliopathy. Hum. Mol. Genet. 22, 4383–4397. 10.1093/hmg/ddt288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T. J., Bell D. W., Sordella R., Gurubhagavatula S., Okimoto R. A., Brannigan B. W., et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- Medvetz D. A., Khabibullin D., Hariharan V., Ongusaha P. P., Goncharova E. A., Schlechter T., et al. (2012). Folliculin, the Product of the Birt-Hogg-Dube Tumor Suppressor Gene, Interacts with the Adherens Junction Protein p0071 to Regulate Cell-Cell Adhesion. PLoS ONE 7:e47842. 10.1371/journal.pone.0047842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski M. S., Seabra L., Straatman-Iwanowska A., Wingenfeld A., Reiman A., Lu X., et al. (2012). Folliculin interacts with p0071 (plakophilin-4) and deficiency is associated with disordered RhoA signalling, epithelial polarization and cytokinesis. Hum. Mol. Genet. 21, 5268–5279. 10.1093/hmg/dds378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson M. L., Warren M. B., Toro J. R., Matrosova V., Glenn G., Turner M. L., et al. (2002). Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2, 157–164. 10.1016/S1535-6108(02)00104-6 [DOI] [PubMed] [Google Scholar]
- Nookala R. K., Langemeyer L., Pacitto A., Ochoa-Montano B., Donaldson J. C., Blaszczyk B. K., et al. (2012). Crystal structure of folliculin reveals a hidDENN function in genetically inherited renal cancer. Open Biol. 2, 120071. 10.1098/rsob.120071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Lopez G., Dong X., Ben-Aissa K., Bernot K. M., Itoh T., Fukuda M., et al. (2008). Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J. Biol. Chem. 283, 18323–18330. 10.1074/jbc.M800056200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. (2000). Cell signaling by receptor tyrosine kinases. Cell 103, 211–225. 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- Schmidt L. S., Linehan W. M. (2015). Molecular genetics and clinical features of Birt-Hogg-Dube syndrome. Nat. Rev. Urol. 12, 558–569. 10.1038/nrurol.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. S., Nickerson M. L., Warren M. B., Glenn G. M., Toro J. R., Merino M. J., et al. (2005). Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am. J. Hum. Genet. 76, 1023–1033. 10.1086/430842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheach L. A., Adeney E. M., Kucukmetin A., Wilkinson S. J., Fisher A. D., Elattar A., et al. (2009). Androgen-related expression of G-proteins in ovarian cancer. Br. J. Cancer 101, 498–503. 10.1038/sj.bjc.6605153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., et al. (2005). Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765. 10.1073/pnas.0409817102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler J. B., Neil J. R., Abramovitch S., Yarden Y., White F. M., Lauffenburger D. A., et al. (2010). Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc. Natl. Acad. Sci. U.S.A. 107, 13252–13257. 10.1073/pnas.0913476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525. 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- Tang Y., Lin Y., Li C., Hu X., Liu Y., He M., et al. (2015). MicroRNA-720 promotes in vitro cell migration by targeting Rab35 expression in cervical cancer cells. Cell Biosci. 5:56. 10.1186/s13578-015-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbutt N., Pedersen M. W., Johns T. G. (2013). Targeting the ERBB family in cancer: couples therapy. Nat. Rev. Cancer 13, 663–673. 10.1038/nrc3559 [DOI] [PubMed] [Google Scholar]
- Tomas A., Futter C. E., Eden E. R. (2014). EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 24, 26–34. 10.1016/j.tcb.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., et al. (1984). Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309, 418–425. 10.1038/309418a0 [DOI] [PubMed] [Google Scholar]
- Walker F., Abramowitz L., Benabderrahmane D., Duval X., Descatoire V., Henin D., et al. (2009). Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum. Pathol. 40, 1517–1527. 10.1016/j.humpath.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Wheeler D. B., Zoncu R., Root D. E., Sabatini D. M., Sawyers C. L. (2015). Identification of an oncogenic RAB protein. Science 350, 211–217. 10.1126/science.aaa4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Fonovic M., Suyama K., Bogyo M., Scott M. P. (2009). Rab35 controls actin bundling by recruiting fascin as an effector protein. Science 325, 1250–1254. 10.1126/science.1174921 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Du J., Zheng J., Liu J., Xu R., Shen T., et al. (2015). EGF-reduced Wnt5a transcription induces epithelial-mesenchymal transition via Arf6-ERK signaling in gastric cancer cells. Oncotarget 6, 7244–7261. 10.18632/oncotarget.3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Shen T., Liu J., Zheng J., Zhang Y., Xu R., et al. (2013). Rab35 is required for Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7 breast cancer cells. Cell. Signal. 25, 1075–1085. 10.1016/j.cellsig.2013.01.015 [DOI] [PubMed] [Google Scholar]