Abstract
Cervical cancer is the third most common cancer in women worldwide. However, the underlying mechanism of occurrence and development of cervical cancer is obscure. In this study, we observed that miR-30e was downregulated in clinical cervical cancer tissues and cervical cancer cells. Next, overexpression of miR-30e reduced the cervical cancer cell growth through MTT, colony formation, EdU, and Transwell assay in SiHa and Caski cells. Subsequently, UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase 7 (GALNT7) was identified as a potential miR-30e target by bioinformatics analysis. Moreover, we showed that miR-30e was able to bind to the 3′UTR of GALNT7 by luciferase reporter assay. In addition, the mRNA and protein levels of GALNT7 in cervical cancer cells were downregulated by miR-30e. And we validated that downregulation of GALNT7 repressed the proliferation of SiHa and Caski cells by MTT, colony formation, and Transwell assay. We identified that the restoration of GALNT7 expression was able to counteract the effect of miR-30e on cell proliferation of cervical cancer cells. Furthermore, we found that the expression levels of GALNT7 were frequently upregulated and negatively correlative to those of miR-30e in cervical cancer tissues. In addition, we validated that restoration of GALNT7 rescued the miR-30e–suppressed growth of cervical cancer xenografts in vivo. In conclusion, the current results suggest that miR-30e may function as tumor suppressors in cervical cancer through downregulation of GALNT7. Both miR-30e and its novel target, GALNT7, may play an important role in the process of cervical cancer.
Introduction
Cervical carcinoma is a malignancy with the second highest morbidity and mortality in women worldwide [1], [2]. The incidence of cervical cancer in developing countries is high [3]. It is estimated that approximately 500,000 new cases of cervical cancer are reported annually and about 230,000 women die of cervical cancer annually [4]. Cervical cancer is a complex disease involving the abnormal expression of many oncogenes and tumor suppressor genes [5], [6], [7], [8]. Previous studies have revealed several genes associated with human cervical cancer [9], [10], [11], [12], [13]. However, the detailed molecular pathogenesis of cervical carcinoma is obscure. Further studies to explore cervical cancer pathogenesis are needed.
MicroRNAs (miRNAs) are evolutionarily conserved, endogenous small noncoding and double-stranded RNA molecules that function as posttranscriptional gene regulators [14]. MiRNA dysregulation occurs in numerous human malignancies and is associated with altered malignant potential, affecting survival, proliferation, apoptosis, and invasion [15], [16], [17]. MiRNAs can bind to the 3-untranslated region (3′UTR) of target messenger RNAs (mRNAs) and suppress their expressions. Recently, global profiling studies have enabled microRNA-based stratifications of cancer types and patient outcomes [18], [19], [20]. Aberrant miRNA expressions can be pathogenic and occur in various human malignancies including cervical cancer [21], [22], [23]. MiR-30e is abnormally expressed in several cancers, including lung cancer, breast cancer, and hepatocellular carcinoma [24], [25], [26]. However, the function and target genes of miR-30e in cervical cancer remain to be elucidated.
GALNT7, a member of the acetylgalactosaminyltransferase family, acts as a glycosyltransferase in protein O-GlcNAcylation. Aberrant glycosylation is a well-described hallmark of many human cancers and is associated with cell growth, differentiation, transformation, adhesion, metastasis, and tumor immune surveillance [27]. The O-glycosylation of these glycoproteins is considered to play a key role in determining tumor properties in many cases of cancer, such as cervical cancer, hepatocellular carcinoma, and esophageal squamous cell cancer [17], [28], [29]. It has been reported that GALNT7 was targeted by some of miRNAs, such as miR-214, miR-494, and miR-17, in different cancers [17], [28], [30]. However, the expression and the regulation of GALNT7 in cervical cancer remain obscure.
In the current study, we investigated the expression profile and correlation of miR-30e and GALNT7 in cervical cancer patient tissues. Furthermore, the cellular and molecular functions of miR-30e and one of its key targets, GALNT7, were elucidated. Our findings indicate that miR-30e may be an important biomarker and target for diagnosis and treatment of cervical cancer patients.
Materials and Methods
Patient Sample
Thirty cervical cancer tissue samples and their corresponding adjacent nontumorous tissues were obtained from Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) after surgical resection. Written consent approving the use of tissue samples for research purposes was obtained from patients. The information of patients with cervical cancer is presented in supporting information. The study protocol was approved by the Institute Research Ethics Committee at Tianjin Medical University Cancer Institute and Hospital.
Cell Culture and Transfection
The human normal epithelial cell line Hacat was cultured in Dulbecco's modified Eagle medium (DMEM) (HyClone, Logan, UT) with 10% fetal bovine serum and 100 IU/ml penicillin G and 100 μg/ml streptomycin (Sigma-Aldrich). The human cervical cancer cell lines Hela, SiHa, and Caski were cultured separately in RPMI 1640 medium (Gibco) with 10% fetal bovine serum and 100 IU/ml penicillin G and 100 μg/ml streptomycin. All of the cell lines were incubated at 37°C in a humidified atmosphere with 5% CO2. All of the cell lines were obtained from the American Type Culture Collection. Transfection was performed using the Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer's protocol.
Analysis of Cell Proliferation
SiHa and Caski cells were seeded into 96-well plates (1000 cells/well) for 24 hours before transfection, and 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell proliferation every day from the first day until the third day after transfection. 5-Ethynyl-2′-deoxyuridine (EdU) incorporation assay was carried out using the Cell-Light TM EdU imaging detecting kit according to the manufacturer's instructions (RiboBio, Guangzhou, China).
Plasmid Construction
The CDS sequence of GALNT7 was amplified by PCR from the cDNA of SiHa using specific primers and was cloned into the pcDNA3.1 (+) vector. To construct luciferase reporter plasmids of GALNT7, the wild type or mutant of GALNT7 mRNA 3′UTR which was targeted by miR-30e was amplified by PCR and was inserted into the pGL3-Control vector to generate pGL3-GALNT7-wt and pGL3-GALNT7-mut, respectively. Primers used were as follows: 5′-GCTCTAGAGAAACCTGCTGCAACTAT-3′, 5′-ACATTATCATCTGCAGTTACTAAAAG GAGACTGC-3′, 5′-GCAGTCTCCTTTTAGTAACTGCAGATGATAATGT-3′, and 5′-GGGGGC CGGCCGTAATCCCAAAGAAGACAA-3′.
Luciferase Reporter Gene Assay
Luciferase reporter gene assay was performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. Cells were transferred into 24-well plates at 3 × 104 cells per well. After 48 hours, the cells were transiently cotransfected with 50 ng/well of pRL-TK plasmid (Promega, Madison, WI) containing the Renilla luciferase gene used for internal normalization, pGL3-GALNT7-wt or pGL3-GALNT7-mut (100 ng/well). All experiments were performed at least three times.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with PrimeScript RT Reagent Kit (Perfect Real Time) (TaKaRa Biotechnology, Dalian, China). All steps were according to the manufacturer's protocol. Primers were as follows: GALNT7 forward: 5′-TGCTGGAGGAGATTCCCAGAA-3′, GALNT7 reverse: 5′-GCACAGGATCATGGTAGGTG AA-3′; MiR-30e forward: 5′-TGTAAACATCCTTGACTGGAAG-3′, MiR-30e reverse: 5′-GCGAGCACAGAATTAAT ACGAC-3′; U6 forward: 5′-CTCGCTTCGGCAGCACA-3′, U6 reverse: 5′-AACGCTTCA CGAATTTGCGT-3′; GAPDH forward: 5′-TGTGGGCATCAATGG ATTTGG-3′, GAPDH reverse: 5′-ACACCATGTAT TCCGGGTCAAT-3′.
Western Blotting
Cancer cells were lysed in RIPA buffer at 4°C for 30 minutes and centrifuged at 12,000×g at 4°C for 15 minutes to obtain total protein lysates for immunoblot analysis. Cell lysates were separated by using 10% SDS-PAGE and transferred onto PVDF membranes. Proteins were then subjected to immunoblotting. All primary and secondary antibodies were diluted in PBS. Primary antibody incubation was performed at 4°C overnight, and secondary antibody incubation was performed at room temperature for 2 hours. The immunoreactive blots were visualized using an enhanced chemiluminescence reagent. The anti-GALNT7 antibody mouse monoclonal antibody was obtained from Abcam, and anti-actin was purchased from Proteintech (Wuhan, China).
Cell Invasion Assay
Matrigel (1:5, 50 μl/well, BD Bioscience, San Jose, CA) was added to Transwell chambers in 24-well plate. A total of 2 × 104 posttransfection SiHa and Caski cells in100 μl of serum-free DMEM medium were plated onto the upper chambers (24-well insert, pore size 8 μm, Corning), and the lower chambers were filled with 500 μl of DMEM medium with 10% FBS. After 24 hours of incubation, the membranes were fixed with methanol, stained by 0.1% crystal violet, and washed three times. Finally, the stained cells were visualized under a microscope and counted.
Tumor Xenograft Experiment
We obtained 18 BALB/c-nude mice (6-7 weeks old, female) from Beijing Huafukang Bioscience Co. Inc. (Beijing, China). The mice were housed in an SFP room that was maintained at a constant temperature (23°C-25°C) and humidity (40%-50%). The mice were randomly divided into three groups (each group, n = 6). SiHa Cells were harvested and resuspended at 2× 107 cells/ml. Then, 0.2-ml aliquots were injected subcutaneously into the flank of each mouse. Tumor growth was measured using a Vernier caliper after 6 days from injection and then every 6 days. At the end of experiment, mice were killed and tumors were weighted. The volume was calculated with the following formula: V = (length × width2)/2.
Statistical Analysis
All replicates are true biological replicates. The Student's t test was used to analyze the statistical significance of the results. *P < .05 and ** P < .01 were considered statistically significant. Spearman correlation coefficients were used for relation statistics.
Results
Downregulation of miR-30e in Cervical Cancer Tissues and Cervical Cancer–Derived Cell Lines
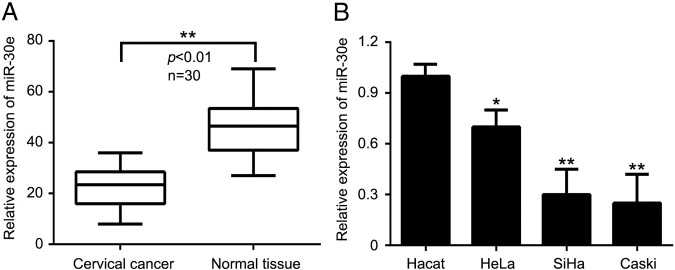
We measured the expression of miR-30e in 30 human cervical cancer tissues and their counterparts using quantitative RT-PCR. MiRNA-30e exhibited apparently low level expression in cancer tissues relative to those of normal tissues (Figure 1A). Moreover, we tested the miR-30e expression in one normal epithelial cell line, Hacat, and three cervical cancer–derived cells, including HeLa, SiHa, and Caski. Our data showed that miR-30e was downregulated in cervical cancer cells relative to normal epithelial cell (Figure 1B). The results imply that miR-30e is reduced in cervical cancer tissues and may be serve as antitumor gene involved in cervical carcinogenesis.
Figure 1.
Expression of miR-30e mRNA in cervical tissues and cell lines. (A) qRT-PCR of miR-30e relative to U6 expression in cervical cancer and normal tissues. (B) qRT-PCR of miR-30e relative to U6 expression in immortalized cervical epithelial cell line and cervical cancer cell lines. *P < .05, **P < .01, Student's t test. The experiment was repeated at least three times.
MiR-30e Inhibits Proliferation and Invasion Ability of SiHa and Caski Cells
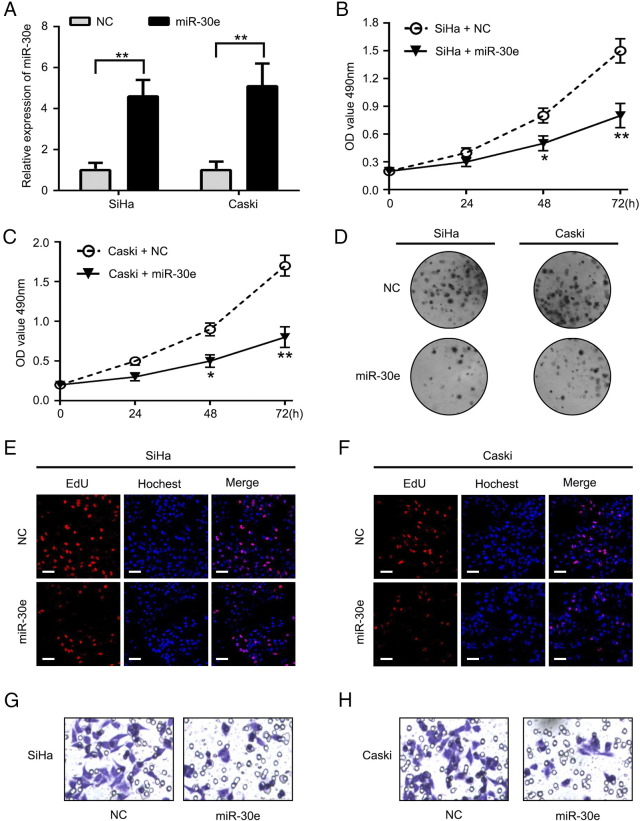
In order to test the effect of miR30e in cervical cancer, firstly, we transfected miR-30e mimics and control to SiHa and Caski cells and detected miR-30e level by real-time PCR. Our data showed that the miR-30e expression level was obviously increased in miR-30e mimics–transfected group compared to NC group (Figure 2A). Next, we used MTT assay to determine the effect of miR-30e on cell viability. Cells transfected with miR-30e had decreased cell viability (Figure 2, B and C). Consistent with this result, we confirmed the effect of miR-30e on the long-term proliferative capacity of SiHa and Caski cells with colony formation. The colony numbers of above-mentioned cells with miR-30e mimics were both decreased relative to control group (Figure 2D). Furthermore, EdU assay was used to analyze effect of miR-30e on the cell proliferation of SiHa and Caski cells. Overexpression of miR-30e resulted in a reduced number of SiHa and Caski cells compared to the NC group (Figure 2, E and F). In addition, we observed that invasion ability of SiHa and Caski cells was suppressed after overexpressed miR-30e (Figure 2, G and H). These results demonstrate that miR-30e can repress the growth of cervical cancer cells.
Figure 2.
MiR-30e represses proliferation and colony formation of SiHa and Caski cells. (A) miR-30e mimics or NC was transfected into the cells, and qRT-PCR was performed to determine miR miR-30e expression. (B) The effects of overexpression or knockdown of miR-30e on SiHa cell growth viability. (C) The effects of overexpression or knockdown of miR-30e on Caski cell growth viability. (D) Effect of miR-30e on cell proliferation was determined by colony formation assays in SiHa and Caski cells. (E, F) Effect of miR-30e on cell proliferation was determined by EdU assays in SiHa and Caski cells, respectively (scale bar = 100 μm). (G, H) Cell invasion. *P < .05, **P < .01, Student's t test. The experiment was repeated at least three times.
MiR-30e Negatively Regulates GALNT7 Through Directly Targeting its 3′UTR
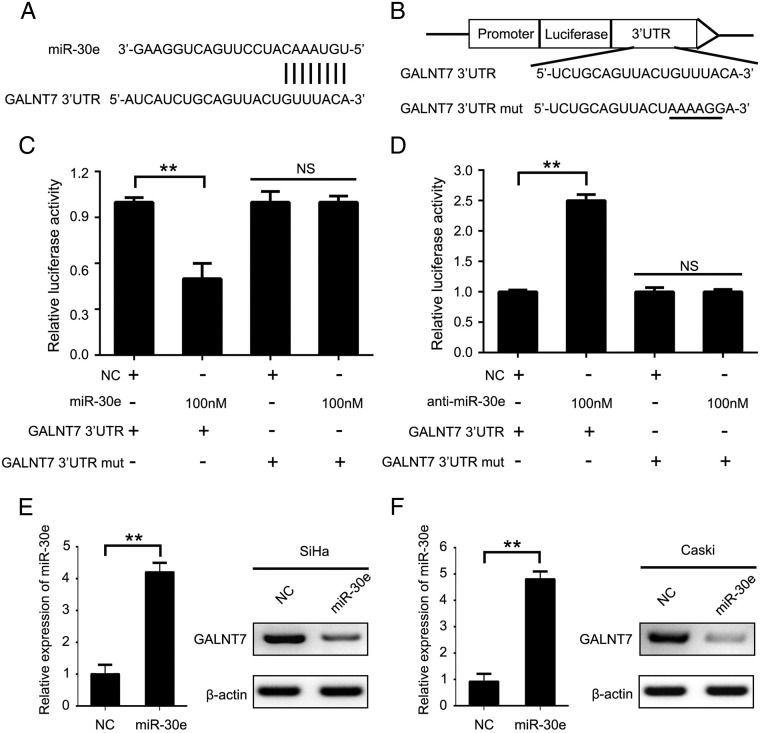
Next, we screened the target genes of miR-30e using Targetscan, Miranda, and Pictar software. Interestingly, we observed that GALNT7 might be a putative target gene of miR-30e (Figure 3A). Then, we cloned the 3′UTR of GALNT7 mRNA (or its mutants) into downstream of pGL3-control luciferase reporter gene vector named GALNT7 3′UTR and GALNT7 3′UTR- mut, respectively (Figure 3B). The luciferase reporter gene assays revealed that miR-30e significantly suppressed the firefly luciferase activities of GALNT7 3′UTR in SiHa cells, whereas it failed to work when the seed sequence was mutated (Figure 3C). The inhibition of endogenous miR-30e by anti–miR-30e resulted in the increases of firefly luciferase activities of GALNT7 3′UTR in SiHa cells but not the mutant (Figure 3D). Then, we performed the transient transfection of miR-30e in SiHa and Caski cells to evaluate the effect of miR-30e on GALNT7. Our data showed that the overexpression of miR-30e was able to suppress the expression of GALNT7 at the levels of mRNA and protein in the above cells (Figure 3, E and F). Taken together, we conclude that miR-30e negatively regulates GALNT7 through directly targeting its 3′UTR.
Figure 3.
MiR-30e directly targets GALNT7 and inhibits its expression. (A and B) A schematic of the bioinformatics predicted seed region in the 3′UTR of GALNT7, as well as mutated 3′UTR used in this study. (C and D) Effect of miR-30e mimics or anti–miR-30e on the luciferase activity of the plasmid GALNT7–3′UTR and GALNT7–3′UTR-mut in SiHa cells. (E and F) qRT-PCR analysis the efficiency of overexpression miR-30e and Western blot shows suppression of the GALNT7 protein by miR-30e mimics. **P < .01, Student's t test.
Knockdown of GALNT7 Inhibits Proliferation and Invasion Ability of SiHa and Caski Cells
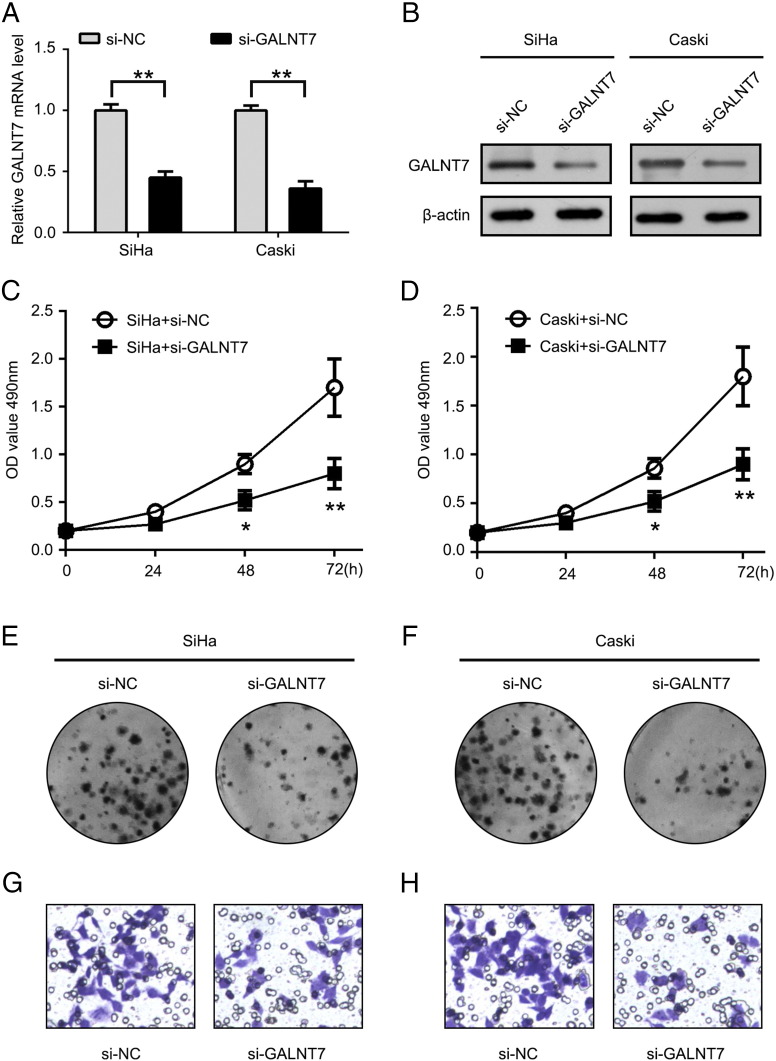
It has been reported that GALNT7 was abnormally increased in human cervical cancer. Therefore, we detected the effect of GALNT7 knockdown on the growth and colony formation ability of SiHa and Caski cells. Both mRNA and protein levels of GALNT7 were decreased after treatment with si-GALNT7 in SiHa and Caski cells (Figure 4, A and B). MTT assay showed that the cell proliferation was significantly decreased, compared with negative control transfection, in SiHa and Caski cells, respectively, when GALNT7 was silenced by siRNA (Figure 4, C and D). Transfection of si-GALNT7 also significantly decreased the colony numbers of SiHa and Caski cells (Figure 4, E and F). Moreover, we validated that the cell invasion ability was significantly decreased, compared with negative control transfection, in SiHa and Caski cells, respectively, when GALNT7 was silenced by siRNA (Figure 4, G and H). We conclude that knockdown of GALNT7 inhibits proliferation and invasion ability of SiHa and Caski cells.
Figure 4.
Knockdown of GALNT7 restrains the cervical cancer cells proliferation and colony formation. (A) qRT-PCR analysis the GALNT7 mRNA expression level in SiHa and Caski cells after treatment with si-GALNT7. (B) Western blot shows the GALNT7 protein expression level in SiHa and Caski cells after treatment with si-GALNT7. (C and D) Cell growth was measured by MTT assay at different time intervals in SiHa and Caski cells, respectively. (E and F) Effect of si-GALNT7 on cell proliferation was determined by colony formation assays in SiHa and Caski cells, respectively. (G and H) Cell invasion. *P < .05, **P < .01, Student's t test. The experiment was repeated at least three times.
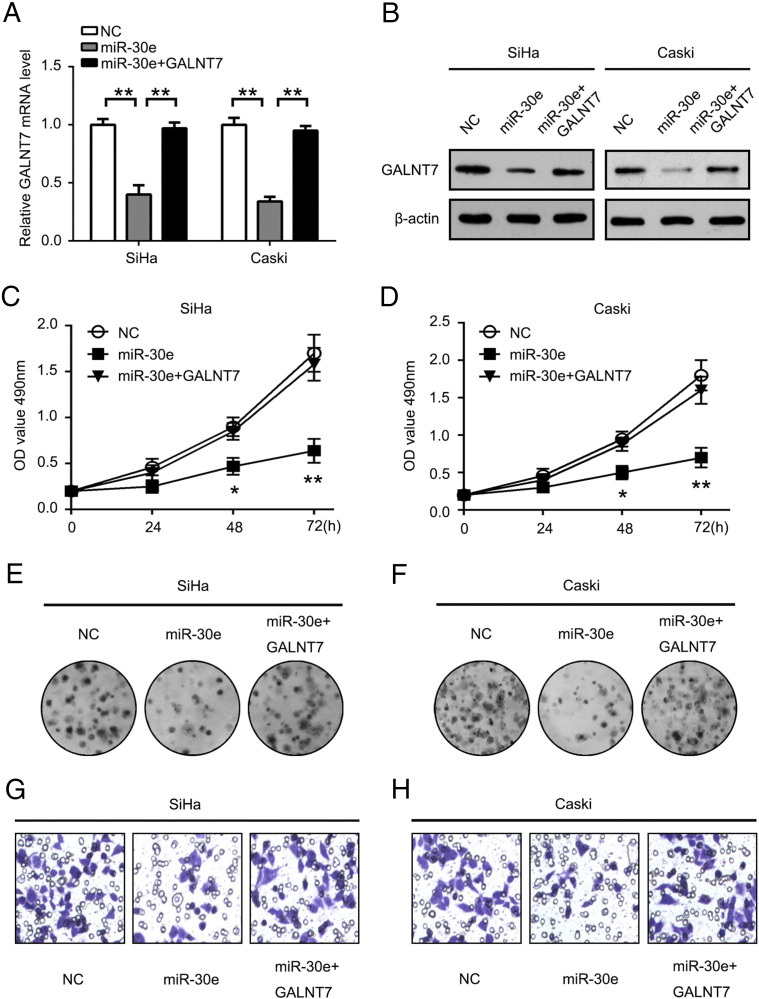
Restoration of GALNT7 Counteracts Effects of miR-30e Expression
To confirm that the effects of miR-30e on the proliferation and colony formation of SiHa and Caski cells are mediated through GALNT7, we constructed a eukaryotic expression vector containing the GALNT7 CDS without the 3′UTR to avoid the influence of miRNAs. Transfection of SiHa and Caski cells with this eukaryotic expression vector construct reversed the negative effects of miR-30e on GALNT7 mRNA and protein levels (Figure 5, A and B). The MTT assay showed that overexpression miR-30e could inhibit the growth of SiHa and Caski cells. Conversely, the event caused by miR-30e was abrogated when co-transfected with the GALNT7 vector in SiHa and Caski cells (Figure 5, C and D). Moreover, colony formation assay showed a similar effect of the above mentioned in SiHa and Caski cells (Figure 5, E and F). In addition, we observed that overexpression of both miR-30e and GALNT7 could rescue the inhibition effect by miR-30e in SiHa and Caski cells (Figure 5, G and H). Thus, we conclude that restoration of GALNT7 counteracts effects of miR-30e expression in SiHa and Caski cells.
Figure 5.
GALNT7 rescues miR-30e–induced cellular phenotypes in cervical cancer cells. (A) Cells were co-transfected with the GALNT7 vector with or without miR-30e mimics. (B) The GALNT7 protein level was measured by Western blot at 48 hours after transfection. (C and D) MTT assay shows the effect of above-mentioned treatment in SiHa and Caski cells, respectively. (E and F) Colony formation assay was used to evaluate the cell viability in SiHa and Caski cells, respectively. (G and H) Cell invasion. *P < .05, **P < .01, Student's t test. The experiment was repeated at least three times.
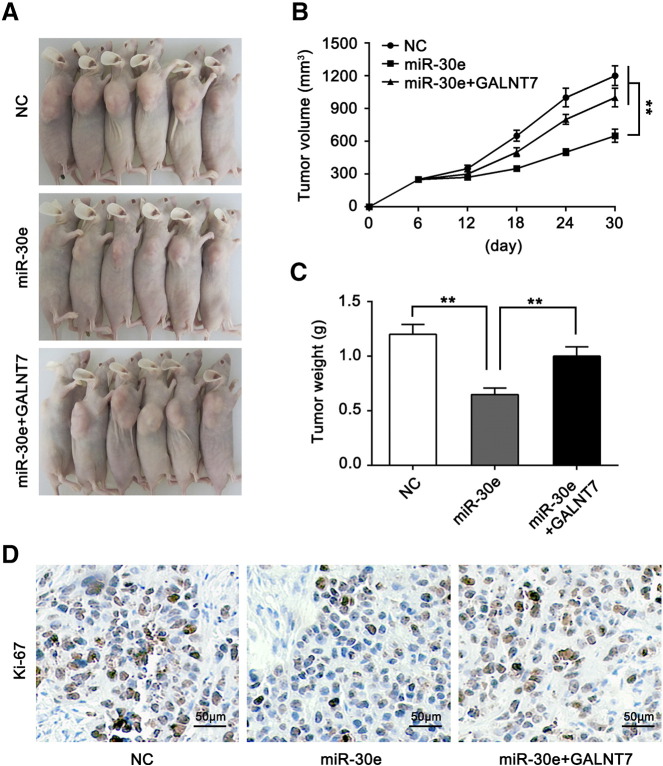
Restoration of GALNT7 Can Rescue the miR-30e–Suppressed Growth of Cervical Cancer Xenografts In Vivo
To further assess the effects of miR-30e on the proliferation of cervical cells through GALNT7. Nude mice were injected subcutaneously with pretreated SiHa cells. After 30 days, the mice were mercifully killed (Figure 6A). As shown in Figure 6B, the tumors formed by the miR-30e–transfected SiHa cells grew much slower than those formed by the negative control SiHa cells (Figure 6B). However, the event caused by miR-30e was rescued when co-transfected with the GALNT7 vector in SiHa cells (Figure 6B). At the termination of the experiment, the mice were sacrificed and the tumors were excised. Then, the tumors weights were recorded. The weights of the tumor obtained from miR-30e–transfected group were lower than those of negative control group (Figure 6C). The weights of the tumor due to the GALNT7-overexpressing cells were much heavier than those due to the miR-30e cells (Figure 6C). Moreover, the expression of Ki67, a well-known cell proliferation marker, was examined in the tumor xenografts tissues by immunohistochemical staining. As shown in Figure 6D, the expression of Ki67 in the tumor tissues formed by the miR-30e–transfected SiHa cells was decreased compared with the negative control cells. In contrast, many more Ki67-positive cells were found in the tumor tissues formed by the GALNT7 and miR-30e–co-transfected SiHa cells than in those formed by the miR-30e–transfected SiHa cells (Figure 6D). All these data suggest that GALNT7 can rescue the miR-30e–suppressed growth of cervical cancer xenografts in vivo.
Figure 6.
Restoration of GALNT7 can rescue the miR-30e–suppressed growth of cervical cancer xenografts in vivo. (A) The image of tumors in nude mice (n = 6). (B) The tumor growth curve of cervical cells injected into female nude mice is shown. (C) The average tumor weight of each group is shown. (D) Immunohistochemical staining for Ki67 in tumor tissues; scale bar, 50 μm. ** P < .01, Student's t test.
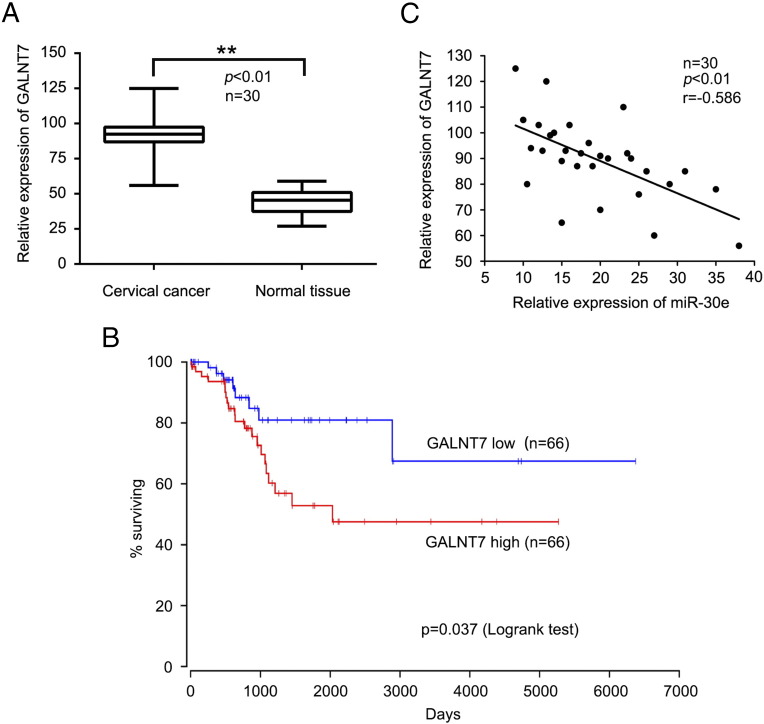
MiR-30e is Negatively Correlated to GALNT7 in Human Cervical Cancer Tissues
To further detect the level of GALNG7 in human cervical cancer tissues, we utilized qRT-PCR to detect the GALNT7 expression level in human cervical cancer tissues and adjacent normal tissues. We observed that expression levels of GALNT7 mRNA were significantly increased in all the 30 cervical cancer samples relative to their peritumor counterparts (Figure 7A). Then, we analyzed the data of GALNT7 expression level in human cervical cancer from OncoLnc (http://www.oncolnc.org) (Figure 7B), suggesting that high levels of GALNT7 are associated with poor survival of cervical cancer. Furthermore, we validated the relationship between miR-30e and GALNT7 mRNA in 30 clinical cervical cancer tissues using qRT-PCR analysis. As expected, we found that the levels of miR-30e exhibited a significant negative correlation with those of GALNT7 mRNA (Figure 7C). This result supports that GALNT7 is one of the target genes of miR-30e in cervical cancer. Taken together, we conclude that miR-30e is negatively correlated to GALNT7 in human cervical cancer tissues.
Figure 7.
MiR-30e is negatively correlated to GALNT7 in cervical cancer tissues. (A) GALNT7 mRNA expression levels were analyzed by qRT-PCR in cervical cancer tissues and adjacent normal tissues. (B) Kaplan-Meier analysis of overall survival in cervical cancer patients. (C) Correlation of miR-30e levels with GALNT7 mRNA levels was examined by qRT-PCR analysis in 30 cases of clinical cervical cancer tissues (Pearson's correlation coefficient, r = −0.586). **P < .01, Student's t test.
Discussion
Cervical cancer is the second women common malignancy worldwide, especially in developing countries, rank only second to breast cancer [3]. MiRNAs have been demonstrated to play important roles in multiple biological processes [31], [32], [33]. Various miRNAs have been reported to regulate cervical cancer through different mechanisms [34], [35]. Some miRNAs modulate HCC by directly regulating the mRNA expression levels. Recent studies showed us that miR-30e was connected with proliferation, invasion, and migration of tumor cells because its dysregulation was found in various types of tumors [24]. However, the effect of miR-30e in cervical carcinoma has not been elucidated.
In this study, we found that the expression of miR-30e was significantly downregulated in tumor specimens compared to nontumor specimens. Moreover, qRT-PCR analysis was used to test the expression level of miR-30e in cervical cancer–derived cells. We demonstrated that miR-30e might act as a cancer suppressor in cervical cancer.
Glycosyltransferases are a group of enzymes that catalyze the addition of monosaccharides to core proteins. In a previous report, GALNT7 is targeted by miR-214 in esophageal squamous cell cancer [17]. MiR-214 also suppresses growth and invasiveness of cervical cancer cells by targeting GALNT7 [36]. Our data showed that GALNT7 was a new target of miR-30e in cervical cancer. The expression level of GALNT7 was dramatically decreased in SiHa and Caski cells after overexpression of miR-30e. It has been reported that GALNT7 plays a role in carcinogenesis and metastasis in many common cancers [30], [37]. Meanwhile, GALNT7 is found to be upregulated in cancers such as laryngeal carcinoma and cervical cancer [36], [38]. Recent studies manifested that suppressing the expression of GALNT7 can inhibit cancer cell invasion and metastasis [17]. In addition, we tested whether the effects of miR-30e on the proliferation of cervical cells are mediated through GALNT7 using a xenografts mice model in vivo. Our results demonstrated that miR-30e could suppress the growth of cervical cancer. In contrast, GALNT7 can rescue the miR-30e–suppressed growth of cervical cancer xenografts in vivo. In our study, we also found that GALNT7 was upregulated in cervical cancer patient tissues and was positively correlated with poor survival time. Cervical cancer cells exhibited decreased growth and colony formation ability compared to control group when transfected with GALNT7 siRNA. Otherwise, GALNT7 could rescue the effect of miR-30e overexpression in SiHa and Caski cells.
Altogether, our study demonstrates that miR-30e expression is significantly decreased in cervical cancer tissues and cervical cancer cells. The miR-30e inhibits the proliferation and metastasis of cervical cancer cells. Furthermore, GALNT7 is a direct target of miR-30e in cervical cancer. In sum, miR-30e exerts its inhibitory effects on cervical cancer proliferation and metastasis, at least in part, by targeting GALNT7.
Conclusions
To conclude, we recognize miR-30e downregulation as a biomarker for cervical cancer. Moreover, miR-30e is able to suppress the proliferation and invasion ability of cervical cancer cells through targeting 3′UTR of GALNT7 mRNA. Our findings may provide insight into a novel mechanism of cervical carcinogenesis. MiR-30e and its new target GALNT7 could be biomarker and therapeutic target for cervical cancer in the future time.
Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
This work was supported by the National Key Clinical Specialist Construction Programs of China.
References
- 1.Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac J Cancer Prev. 2015;16:7401–7407. doi: 10.7314/apjcp.2015.16.17.7401. [DOI] [PubMed] [Google Scholar]
- 2.Pandey D, Shetty J, Sambhaji C, Saxena PU, Mishra D, Chawla A. Cervical cancer as a silent killer: a rare case report with review of literature. J Cancer Res Ther. 2015;11:653–656. doi: 10.4103/0973-1482.137997. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Dmoch-Gajzlerska E, Kozakiewicz B, Chadzynska M. Women's knowledge regarding the effects of cigarette smoking and human papillomavirus infection on the development of cervical cancer. Clin Oncol. 2014;26:356–358. doi: 10.1016/j.clon.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, Xie W, Wang F, Li RH, Cui L, Wang H, Fu X, Song J. Epidemiological investigation and risk factors for cervical lesions: cervical cancer screening among women in rural areas of Henan Province China. Med Sci Monit. 2016;22:1858–1865. doi: 10.12659/MSM.894663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wuerthner BA, Avila-Wallace M. Cervical cancer: screening, management, and prevention. Nurse Pract. 2016;41:18–23. doi: 10.1097/01.NPR.0000490390.43604.5f. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Wang S, Fu Y, Tang W, Jin H, Meng Q, Zhang C, Cui M, Cao X, Li X. A novel mechanism of rs763110 polymorphism contributing to cervical cancer risk by affecting the binding affinity of CEBP/beta and OCT1 complex to chromatin. Int J Cancer. 2016;140:756–763. doi: 10.1002/ijc.30490. [DOI] [PubMed] [Google Scholar]
- 8.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol. 2016;76:49–55. doi: 10.1016/j.jcv.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao JL, Zhang L, Guo X, Wang JH, Zhou W, Liu M, Li X, Tang H. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life. 2015;67:380–394. doi: 10.1002/iub.1381. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Liu M, Sun GC, Yang X, Qian Q, Feng S, Mackey LV, Coy DH. Notch signaling activation in cervical cancer cells induces cell growth arrest with the involvement of the nuclear receptor NR4A2. J Cancer. 2016;7:1388–1395. doi: 10.7150/jca.15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Guo X, Que S, Yang X, Fan H, Liu M, Li X, Tang H. LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget. 2016;8:43768–43781. doi: 10.18632/oncotarget.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi Y, Li H, Lv Q, Wu K, Zhang W, Zhang J, Zhu D, Liu Q, Zhang W. miR-202 inhibits the progression of human cervical cancer through inhibition of cyclin D1. Oncotarget. 2016;7:72067–72075. doi: 10.18632/oncotarget.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying TH, Lee CH, Chiou HL, Yang SF, Lin CL, Hung CH, Tsai JP, Hsieh YH. Knockdown of Pentraxin 3 suppresses tumorigenicity and metastasis of human cervical cancer cells. Sci Rep. 2016;6:29385–29397. doi: 10.1038/srep29385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non–protein-coding RNA. Biochim Biophys Acta. 2010;1799:597–615. doi: 10.1016/j.bbagrm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Lin CW, Chang YL, Chang YC, Lin JC, Chen CC, Pan SH, Wu CT, Chen HY, Yang SC, Hong TM. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4:1877–1891. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 16.Chu Y, Ouyang Y, Wang F, Zheng A, Bai L, Han L, Chen Y, Wang H. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J Cell Biochem. 2014;115:847–853. doi: 10.1002/jcb.24726. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q, Xu L, Li C, Yuan Y, Huang S, Chen H. miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer. Tumour Biol. 2016;37:14605–14614. doi: 10.1007/s13277-016-5320-7. [DOI] [PubMed] [Google Scholar]
- 18.Gu XY, Wang J, Luo YZ, Du Q, Li RR, Shi H, Yu TP. Down-regulation of miR-150 induces cell proliferation inhibition and apoptosis in non–small-cell lung cancer by targeting BAK1 in vitro. Tumor Biol. 2014;35:5287–5293. doi: 10.1007/s13277-014-1688-4. [DOI] [PubMed] [Google Scholar]
- 19.Shen G, Jia H, Tai Q, Li Y, Chen D. miR-106b downregulates adenomatous polyposis coli and promotes cell proliferation in human hepatocellular carcinoma. Carcinogenesis. 2013;34:211–219. doi: 10.1093/carcin/bgs320. [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Qi RQ, Liu M, Xu YP, Li G, Weiland M, Kaplan DH, Mi QS. microRNA miR-17-92 cluster is highly expressed in epidermal Langerhans cells but not required for its development. Genes Immun. 2014;15:57–61. doi: 10.1038/gene.2013.61. [DOI] [PubMed] [Google Scholar]
- 21.Lai XJ, Cheng XY, Hu LD. microRNA 421 induces apoptosis of c-33a cervical cancer cells via down-regulation of Bcl-xL. Genet Mol Res. 2016;15:1–10. doi: 10.4238/gmr15048853. [DOI] [PubMed] [Google Scholar]
- 22.Yan S, Li X, Jin Q, Yuan J. MicroRNA-145 sensitizes cervical cancer cells to low-dose irradiation by downregulating OCT4 expression. Exp Ther Med. 2016;12:3130–3136. doi: 10.3892/etm.2016.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan JY, Fan YJ, Wang XL, Xie H, Gao HJ, Zhang Y, Liu M, Tang H. miR-429 is involved in regulation of NF-kappaB activity by targeting IKKbeta and suppresses oncogenic activity in cervical cancer cells. FEBS Lett. 2016;591:118–128. doi: 10.1002/1873-3468.12502. [DOI] [PubMed] [Google Scholar]
- 24.Feng G, Shi H, Li J, Yang Z, Fang R, Ye L, Zhang W, Zhang X. MiR-30e suppresses proliferation of hepatoma cells via targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) mRNA. Biochem Biophys Res Commun. 2016;472:516–522. doi: 10.1016/j.bbrc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Sohn D, Peters D, Piekorz RP, Budach W, Janicke RU. miR-30e controls DNA damage-induced stress responses by modulating expression of the CDK inhibitor p21WAF1/CIP1 and caspase-3. Oncotarget. 2016;7:15915–15929. doi: 10.18632/oncotarget.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Aiuto F, Callari M, Dugo M, Merlino G, Musella V, Miodini P, Paolini B, Cappelletti V, Daidone MG. miR-30e is an independent subtype-specific prognostic marker in breast cancer. Br J Cancer. 2015;113:290–298. doi: 10.1038/bjc.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munkley J, Vodak D, Livermore KE, James K, Wilson BT, Knight B, McCullagh P, McGrath J, Crundwell M, Harries LW. Glycosylation is an androgen-regulated process essential for prostate cancer cell viability. EBioMedicine. 2016;8:103–116. doi: 10.1016/j.ebiom.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan SW, Fang L, Shatseva T, Rutnam ZJ, Yang X, Du W, Lu WY, Xuan JW, Deng Z, Yang BB. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J Cell Sci. 2013;126:1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 29.Peng RQ, Wan HY, Li HF, Liu M, Li X, Tang H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem. 2012;287:14301–14309. doi: 10.1074/jbc.M111.337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie GH, Luo L, Duan HF, Li XQ, Yin MJ, Li Z, Zhang W. GALNT7, a target of miR-494, participates in the oncogenesis of nasopharyngeal carcinoma. Tumor Biol. 2016;37:4559–4567. doi: 10.1007/s13277-015-4281-6. [DOI] [PubMed] [Google Scholar]
- 31.Du X, Lin LI, Zhang L, Jiang J. microRNA-195 inhibits the proliferation, migration and invasion of cervical cancer cells via the inhibition of CCND2 and MYB expression. Oncol Lett. 2015;10:2639–2643. doi: 10.3892/ol.2015.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aalaeiandabili SH, Fabbri M, Rezaei N. Reciprocal effects of Toll-like receptors and miRNAs on biological processes in human health and disease: a systematic review. Immunotherapy. 2015;5:1127–1142. doi: 10.2217/imt.13.112. [DOI] [PubMed] [Google Scholar]
- 33.Sun K, Jee D, de Navas LF, Duan H, Lai EC. Multiple in vivo biological processes are mediated by functionally redundant activities of Drosophila mir-279 and mir-996. PLoS Genet. 2015;11:e1005245. doi: 10.1371/journal.pgen.1005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YK, Xi WY, Xi RX, Li JY, Li Q, Gao YE. MicroRNA-494 promotes cervical cancer proliferation through the regulation of PTEN. Oncol Rep. 2015;33:2393–2401. doi: 10.3892/or.2015.3821. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Q, Zhai YX, Liu HQ, Shi YA, Li XB. MicroRNA-491-5p suppresses cervical cancer cell growth by targeting hTERT. Oncol Rep. 2015;34:979–986. doi: 10.3892/or.2015.4013. [DOI] [PubMed] [Google Scholar]
- 36.Peng R, Wan H, Li H, Liu M, Li X, Tang H. MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem. 2012;287:14301–14309. doi: 10.1074/jbc.M111.337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Ma H, Sun J. MicroRNA-34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol Med Rep. 2014;9:1293–1298. doi: 10.3892/mmr.2014.1929. [DOI] [PubMed] [Google Scholar]
- 38.Luo M, Shen D, Zhou X, Chen X, Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153:836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]