Abstract
Individuals with schizophrenia often demonstrate theory of mind (ToM) impairment relative to healthy adults. However, the exact nature of this impairment (first- vs. second-order ToM and cognitive vs. affective ToM) and the extent to which ToM abilities deteriorate with illness chronicity is unclear. Furthermore, little is known about the relationships between clinical symptoms and ToM error types (overmentalising, reduced mentalising and no ToM) in early and chronic schizophrenia. This study examined the nature and types of ToM impairment in individuals with early (n = 26) and chronic schizophrenia (n = 32) using a novel virtual reality task. Clinical participants and demographically-matched controls were administered the Virtual Assessment of Mentalising Ability, which provides indices of first- and second-order cognitive and affective ToM, and quantifies three different types of mentalising errors (viz., overmentalising, reduced mentalising, and no ToM). Individuals with early schizophrenia performed significantly poorer than healthy controls on first-order affective and second-order cognitive and affective ToM, but significantly higher than individuals with chronic schizophrenia on all ToM subscales. Whereas a lack of mental state concept was associated with negative symptoms, overmentalising was associated with positive symptoms. These findings suggest that ToM abilities selectively deteriorate with illness chronicity and error types are related to these individuals' presenting symptomology. An implication of the findings is that social-cognitive interventions for schizophrenia need to consider the nature, time course and symptomatology of the presenting patient.
Keywords: Theory of mind, Mentalising error types, Schizophrenia, Virtual reality
1. Introduction
Theory of mind (ToM) is the ability to understand and predict other's mental states and behaviour (Mancuso et al., 2011). It consists of two neurobiologically dissociable subcomponents (Shamay-Tsoory et al., 2006, Shamay-Tsoory et al., 2004). Whereas cognitive ToM refers to the ability to make inferences about the beliefs and intentions of others, affective ToM requires an additional appreciation of another person's subjective emotional context to infer how they feel. As such, the latter may be more engaging than the former and requires additional neural processes. These two components can be divided into first- (i.e., inferring thoughts and feelings of another person) and second-order (i.e., reasoning what another person thinks a third party is thinking or feeling) processes (Shamay-Tsoory et al., 2007). Compared to its first-order counterpart, the second-order component is more cognitively complex as it requires higher-level reasoning.
Researchers have distinguished different kinds of ToM errors (Fretland et al., 2015, Montag et al., 2011). ‘Overmentalising’ refers to the tendency to excessively attribute intentions or self-referential meaning to others (Frith, 2004). ‘Undermentalising’ refers to the ways individuals can demonstrate diminished mentalising ability. Individuals can either lack the capacity to represent mental states, ‘no ToM’, or have difficulty applying social knowledge despite an intact capacity to represent mental states, ‘reduced mentalising’ (Montag et al., 2011).
Two systematic reviews (Brüne, 2005, Harrington et al., 2005), and a number of meta-analyses (Bora and Pantelis, 2013, Bora et al., 2009, Fett et al., 2011, Sprong et al., 2007) have shown that nearly all published studies report ToM impairment in early and chronic schizophrenia. Although there is evidence that individuals with schizophrenia perform poorly on ToM tasks, little is known about the trajectory of ToM impairments over the course of illness. Similar to other cognitive impairments, ToM impairments in individuals with schizophrenia are expected to deteriorate with increasing duration of illness, however, evidence is needed to confirm this finding. Green et al. (2011) explored the stability of first-order ToM ability across prodromal, early (viz., FEP), and chronic phases of schizophrenia. Although results indicated impairment at each stage of illness, there was no evidence of a deterioration or improvement over time. Bora and Pantelis (2013) found that first-order cognitive and affective ToM impairments in individuals with FEP were comparable to those of individuals with chronic schizophrenia.
Contrary to these results, recent findings suggest that impairments in first- and second-order ToM are differentially affected early in the course of illness. Ho et al. (2015) examined the extent to which first- and second-order cognitive and affective ToM are impaired in individuals with FEP and their unaffected siblings. Results indicated that individuals with FEP did not significantly differ from unaffected siblings and healthy controls in first-order cognitive ToM, but performed significantly poorer in first-order affective ToM, and second-order cognitive and affective ToM. These results suggest that first-order cognitive ToM may be preserved early in the course of schizophrenia. Ho et al. (2015) results highlight the importance of comprehensively assessing first- and second-order cognitive and affective ToM in both early and chronic stages of illness.
Given the modern conceptualisation of error types, recent research has also started to examine the nature of ToM impairments in schizophrenia. Montag et al. (2011) found that individuals with chronic schizophrenia selected more undermentalising (i.e., both reduced mentalising and no ToM) responses, but not more overmentalising responses, than healthy controls. In addition, they found that positive symptoms were associated with overmentalising, and negative symptoms were associated with no ToM. Fretland et al. (2015) found that within the undermentalising domain, individuals with chronic schizophrenia performed more reduced mentalising than no ToM errors, suggesting that schizophrenia is characterized by accuracy problems rather than a lack of mental state concept. Similar to Montag et al. (2011), Fretland et al. (2015) also found that overmentalising was associated with positive symptoms. Although undermentalising error types were not associated with symptoms, the frequency of reduced mentalising errors showed a non-significant trend in associating with disorganisation. Few studies have investigated the full range of ToM error types in schizophrenia, which is likely due to a lack of psychometrically sound and ecologically valid measures that allow for a detailed assessment and analysis of aberrant mentalising styles.
The use of simulated social interaction paradigms, such as the Virtual Assessment of Mentalising Ability VAMA, (Canty et al., 2015), enhances the ecological validity of social cognitive assessment. The VAMA contextualises social cognitive content in an interactive, dynamic protocol, and adopts the first-person perspective of the test-taker within social scenarios. This format assesses online ToM processes which are representative of real-world abilities. Other advantages of the VAMA include the ability to provide parallel assessments of first- and second-order cognitive and affective ToM using a range of mental state modalities (e.g., false belief, faux pas, and sarcasm), provision for quantifying different types of mentalising errors, and evidence of reliability and validity.
1.1. Aims
The first aim of this study was to compare ToM impairments in individuals with early and chronic schizophrenia using the VAMA. It was hypothesised that (a) first-order ToM cognitive ToM would be intact whereas first-order affective ToM and second-order ToM processes would be impaired in individuals with early schizophrenia, and (b) both first- and second-order ToM processes would be significantly impaired in individuals with chronic schizophrenia. The second aim was to examine the relationships between clinical symptoms and specific ToM error types in early and chronic schizophrenia. It was hypothesised that (a) overmentalising would be most strongly associated with positive symptoms and undermentalising errors would be most strongly associated with disorganised/negative symptoms.
2. Method
2.1. Participants
All clinical participants had a DSM-5 (American Psychiatric Association, 2013) diagnosis of schizophrenia and were recruited from an early psychosis intervention or mental health rehabilitation program in Queensland, Australia. Clinical participants were stabilised on atypical antipsychotic medications for at least 1 month prior to participation.
The early schizophrenia group included 15 inpatients and 11 outpatients (13 males). All had experienced their first psychotic episode within two years of participating in this study. The chronic schizophrenia group included 15 inpatients and 18 outpatients (22 males). All had experienced multiple psychotic episodes and were within 5 to 15 years of illness.
All clinical participants understood English and exhibited no physical or language impairment. Individuals with schizoaffective, schizophreniform, and bipolar disorder, IQ < 70, or histories of neurological disorder or brain injury were excluded. Individuals were also excluded if there was evidence of alcohol and/or substance dependence in the past 6 months and if psychotic symptoms were drug induced.
Two groups of healthy adult controls, matched to clinical participants on age, gender, and education level were included in the study. Exclusion criteria included a previously identified DSM-5 diagnosis of a psychotic, mood, anxiety, or personality disorder, and current or past alcohol or substance dependence. Those who reported using psychotropic medication ≤ 6 months prior to participation, had histories of neurological disorder or brain injury, developmental disability, limited fluency in English, or a first-degree relative with a psychotic disorder were also excluded. All participants provided informed written consent before taking part in the study.
The demographic and clinical features of the clinical participants and controls are summarised in Table 1. The Early Schizophrenia Control Group (ES-Control, n = 26) was comparable to the early schizophrenia group with respect to age (t(50) = 0.74, p = 0.46), education level (t(50) = − 1.21, p = 0.23) and estimated IQ (t(50) = 0.25, p = 0.81). The Chronic Schizophrenia Control Group (CS-Control, n = 33) was comparable to the chronic schizophrenia group with respect to age (t(64) = 1.79, p = 0.08) and education level (t(64) = 1.76, p = 0.08), but had a significantly higher estimated IQ (t(64) = 3.69, p < 0.001). The gender ratio of each clinical group was identical to that of their control group. Individuals with early schizophrenia were significantly younger than individuals with chronic schizophrenia (t(39.85) = 5.09, p < 0.001). Individuals with early schizophrenia did not differ from individuals with chronic schizophrenia in terms of gender distribution (χ2(1) = 1.67, p = 0.29), age of onset (t(57) = 1.10, p = 0.28), education level (t(57) = 1.80, p = 0.07), or estimated IQ (t(57) = 1.98, p = 0.053).
Table 1.
Descriptive statistics for demographic and clinical characteristics of individuals with early and chronic schizophrenia.
| Variable | Early schizophrenia M (SD) |
Chronic schizophrenia M (SD) |
ES-Control M (SD) |
CS-Control M (SD) |
|---|---|---|---|---|
| Male:female | 13:13 | 22:11 | 13:13 | 22:11 |
| Age, years | 23.19 (2.84) | 31.64 (8.97) | 23.85 (3.46) | 35.09 (6.51) |
| Education, years | 12.19 (2.02) | 11.30 (1.70) | 11.62 (1.36) | 12.15 (2.15) |
| Estimated IQ (WASI) | 99.04 (12.30) | 92.79 (11.88) | 99.73 (7.08) | 102.30 (8.83) |
| Duration of illness, years | 0.62 (1.21) | 10.98 (6.16) | ||
| Age of onset, years | 22.27 (3.11) | 20.91 (5.63) | ||
| Number of psychotic episodes | 1.62 (1.33) | 9.13 (8.56) | ||
| PANSS | ||||
| Positive symptoms factor | 8.00 (3.48) | 11.70 (4.48) | ||
| Negative symptoms factor | 11.76 (4.54) | 13.63 (5.29) | ||
| Disorganisation symptoms factor | 6.16 (2.82) | 8.77 (2.88) | ||
| Chlorpromazine equivalent (daily) | 466.58 (373.49) | 627.14 (399.61) |
Note. PANSS = Positive and Negative Syndrome Scale. WASI = Wechsler Abbreviated Scale of Intelligence.
2.2. Measures
2.2.1. Symptom assessment
Symptom severity was assessed with the PANSS (Kay et al., 1987). The positive (items P1, P3, P5, G9), negative (items N1, N2, N3, N4, N6, G7), and disorganised (items P2, N5, G11) factors were used (Fretland et al., 2015).
2.2.2. Theory of mind assessment
The VAMA assesses first- and second-order cognitive and affective ToM (10 items per scale) via a virtual interface that simulates the demands of real-life social interactions (Canty et al., 2015). It involves ongoing and ToM components. The former requires participants to navigate a virtual shopping centre and complete a list of errands. The latter involves responding to four multiple-choice questions each time a social interaction occurs between the test taker and his/her virtual ‘friends’ (number of interactions = 10). Multiple choice response options include content that reflects an accurate interpretation of the interaction, a conceptual deficit (no ToM), difficulty applying mental state knowledge (reduced mentalising), and exaggerated mental state attribution (overmentalising). Scales were scored dichotomously, whereby each accurate ToM response was allocated a score of 1 and all other responses were scored as 0. Scores can range for 0 to 10 for each subscale, with higher scores indicate better performance. The total number of no ToM, reduced mentalising, and overmentalising responses was also recorded (0 to 40).
Two neutral interactions are presented after the third and eighth ToM interactions and followed by questions that require non-mental state reasoning. These serve as control questions to check for accurate encoding of task content and the ability to undertake non-ToM reasoning tasks. The VAMA has been found to have adequate construct validity (showing relationships with other similar tests such as the Faux Pas Recognition Test and the Hinting Task), internal consistency (Cronbach's alphas ranged from 0.69 to 0.84), and four-week test-retest reliability (ICCs ranged from 0.93 to 0.99) in a sample of healthy adults (Canty et al., 2015).
2.2.3. Neurocognitive assessment
Measures of attention (Digit Span from the WAIS-IV; Wechsler, 2003), mental flexibility (Trail Making Test [TMT]: Part B; Reitan and Wolfson, 1985), verbal fluency (Controlled Oral Word Association Test [COWAT]; Benton et al., 1978), verbal response inhibition (Hayling Sentence Completion Test; Burgess and Shallice, 1997), working memory (Letter Number Sequencing [LNS] subtest of the WMS-III Wechsler, 2003), and estimated IQ (measured by the Vocabulary and Matrix Reasoning subtests from the WASI-II; Wechsler and Hsiao-pin, 2011) were obtained.
2.3. Procedure
Participants were individually administered all measures during a 2-h assessment session. The VAMA was administered first, followed by the neurocognitive assessment battery. Participants were reimbursed AUD$40 or, in the case of student participants, awarded course credit.
2.4. Data analyses
A composite measure of neurocognition was used as a statistical control in all analyses. The main and interactive effects between group (early schizophrenia, chronic schizophrenia, and the two matched control groups), order (first and second), and ToM type (cognitive and affective) as measured by the VAMA were tested using a 4 × 2 × 2 ANCOVA. Partial correlations between the VAMA error scores and clinical symptoms were evaluated with Pearson's r.
3. Results
There was no significant difference between groups in accuracy on the VAMA control questions (F(3, 114) = 1.19, p = 0.32), indicating that clinical and healthy participants were similar in their comprehension of task content and non-social reasoning.
The ANCOVA indicated that accuracy did not differ between cognitive and affective ToM, as reflected by the absence of a significant main effect of Type (F(1, 113) = 1.39, p = 0.24), Group × Type (F(3, 113) = 1.87, p = 0.14), or Type × Order × Group interaction (F(3, 113) = 1.19, p = 0.32). Accuracy was poorer for second-order than for first-order items, as reflected in a significant main effect of Order (F(1, 113) = 107.10, p < 0.001, η2p = 0.49). Further, accuracy differed between groups as reflected in a main effect of Group (F(3, 113) = 88.54, p < 0.001, η2p = 0.70) and Group × Order interaction (F(3, 113) = 6.48, p < 0.001, η2p = 0.15). Additionally, accuracy on first- and second-order items differed with regards to whether cognitive or affective ToM was being assessed, as indicated by a significant Type × Order interaction (F(1, 113) = 24.90, p < 0.001, η2p = 0.18). Results indicated that first-order cognitive ToM was significantly more difficult than first-order affective ToM (F(1, 116) = 9.39, p = 0.003), whereas second-order cognitive ToM was significantly less difficult than second-order affective ToM (F(1, 116) = 17.28, p < 0.001).
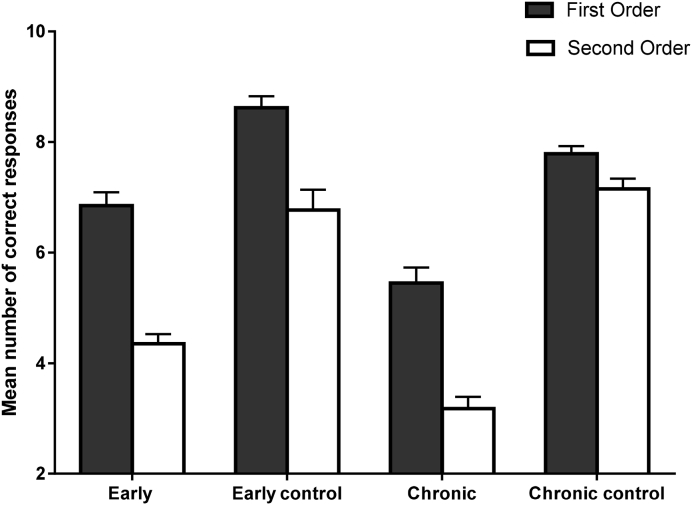
Further analyses were conducted to compare Groups across Order by conducting univariate ANCOVAs. As seen in Fig. 1, Fig. 2, individuals with early schizophrenia performed similarly to the ES-Control Group on first-order cognitive (F(1, 49) = 3.64, p = 0.06), but significantly poorer on first-order affective (F(1, 49) = 28.70, p < 0.001, η2p = 0.37), second-order cognitive (F(1, 49) = 55.53, p < 0.001, η2p = 0.53), and second-order affective ToM (F(1, 49) = 31.97, p < 0.001, η2p = 0.40).
Fig. 1.
Performance of clinical and healthy participants on first-order and second-order cognitive ToM as measured by the Virtual Assessment of Mentalising Ability. Error bars represent ± 1 SE.
Fig. 2.
Performance of clinical and healthy participants on first- and second-order affective ToM as measured by the Virtual Assessment of Mentalising Ability. Error bars represent ± 1 SE.
Individuals with chronic schizophrenia performed significantly poorer than individuals with early schizophrenia on all VAMA subscales (first-order cognitive, F(1, 56) = 12.26, p < 0.001, η2p = 0.18; first-order affective, F(1, 56) = 14.53, p < 0.001, η2p = 0.21; second-order cognitive, F(1, 56) = 14.28, p = 0.001, η2p = 0.20; second-order affective, F(1, 56) = 16.50, p < 0.001, η2p = 0.23). Similarly, individuals with chronic schizophrenia performed poorer than the CS-Control Group on all subscales (first-order cognitive, F(1, 63) = 26.08, p < 0.001, η2p = 0.29; first-order affective, F(1, 63) = 48.77, p < 0.001, η2p = 0.44; second-order cognitive, F(1, 63) = 103.46, p < 0.001, η2p = 0.62; second-order affective, F(1, 63) = 137.01, p < 0.001, η2p = 0.69).
Significant differences were observed between the clinical and control groups in how frequently they selected the different error types. Although individuals with early and chronic schizophrenia did not significantly differ from one another, these groups selected significantly more overmentalising (chronic schizophrenia, t(64) = 2.31, p < 0.05, d = 0.57; early schizophrenia, t(38.66) = 5.60, p < 0.001, d = 1.55), reduced mentalising (chronic schizophrenia, t(41.26) = 18.83, p < 0.001, d = 4.62; early schizophrenia, t(35.11) = 10.36, p < 0.001, d = 2.87), and no ToM (chronic schizophrenia, t(44.35) = 9.65, p < 0.001, d = 2.97; early schizophrenia, t(30.93) = 8.71, p < 0.001, d = 2.42) errors than their respective control groups (see Table 2).
Table 2.
Mean number of error types selected by clinical and control groups.
| Group | Overmentalising M (SD) |
Reduced mentalising M (SD) |
No mentalising M (SD) |
|---|---|---|---|
| Early schizophrenia | 7.50 (3.17) | 8.19 (2.79) | 6.80 (2.96) |
| ES-Control | 3.54 (1.73) | 1.96 (1.28) | 0.77 (1.11) |
| Chronic schizophrenia | 6.09 (2.73) | 9.79 (2.36) | 7.42 (2.54) |
| CS-Control | 4.73 (2.02) | 1.52 (0.91) | 1.36 (0.37) |
Table 3 summarises the partial correlations between clinical symptoms and mentalising error types (with neurocognition controlled). The frequency of overmentalising errors significantly correlated with positive symptoms for both clinical groups, and with disorganised symptoms (negatively) for individuals with chronic schizophrenia. Frequency of reduced mentalising errors also significantly correlated with disorganised symptoms for individuals with chronic schizophrenia. Frequency of no ToM errors significantly correlated with negative symptoms and positive symptoms (negatively) in both individuals with early and chronic schizophrenia.
Table 3.
Partial correlations between clinical symptoms and mentalising error types for individuals with early and chronic schizophrenia.
| Positive symptoms | Negative symptoms | Disorganised symptoms | ||||
|---|---|---|---|---|---|---|
| Early | Chronic | Early | Chronic | Early | Chronic | |
| Error types | ||||||
| Overmentalising | 0.51⁎⁎ | 0.61⁎⁎ | − 0.38 | − 0.47⁎⁎ | − 0.40 | − 0.59⁎⁎ |
| Reduced mentalising | − 0.13 | − 0.43 | − 0.18 | 0.05 | 0.35 | 0.50⁎⁎ |
| No ToM | − 0.58⁎⁎ | − 0.52⁎⁎ | 0.63⁎⁎ | 0.60⁎⁎ | 0.24 | 0.27 |
Note. ToM = theory of mind. Control variable = neurocognition.
p < 0.01.
4. Discussion
This study offers new and exciting insights into the nature and breadth of ToM in early and chronic schizophrenia. Individuals with early schizophrenia had intermediate performance between matched controls and individuals with chronic schizophrenia on first-order affective, and second-order cognitive and affective ToM, after controlling for neurocognition and IQ. The frequency of overmentalising responses significantly correlated with positive symptoms, whereas the frequency of undermentalising responses significantly correlated with negative and disorganised symptoms.
The impairment in first-order affective ToM is consistent with some previous research (e.g., Ho et al., 2015, Shamay-Tsoory et al., 2007), and may indicate that unlike cognitive ToM, affective ToM deteriorates earlier in schizophrenia than cognitive ToM. As mentioned in the Introduction, this could be because compared to cognitive ToM, affective ToM may require additional neural processes relating to the additional emotional contents. This finding is consistent with evidence of emotion recognition and perception deficits in early schizophrenia and may suggest that aberrant processing of emotional information could be linked to the evolution of psychopathology (Kohler et al., 2010).
As hypothesised, individuals with early schizophrenia were impaired on both types of second-order ToM. Moreover, individuals with chronic schizophrenia performed significantly poorer than individuals with early schizophrenia and matched controls on first- and second-order cognitive and affective ToM. As mentioned in the Introduction, second-order ToM is more cognitive complex and demanding because it involves a higher-level of reasoning. Therefore, second-order ToM impairments are likely to manifest in difficulties understanding and managing group dynamics in occupational and social settings. Such results corroborate existing evidence for ToM impairments in chronic schizophrenia (Fretland et al., 2015, Montag et al., 2011), and provide new evidence that ToM abilities may deteriorate with illness chronicity. Our findings, combined with recent evidence of social cognitive impairments in individuals at high risk for developing schizophrenia (Bora and Pantelis, 2013), indicate that early targeted social cognitive interventions may prevent deterioration in social abilities or significantly reduce the severity of social impairment that parallels illness chronicity.
The associations between positive symptoms and high frequency of overmentalising and low frequency of no ToM errors are complementary and provide support to etiological concepts of delusion formation and maintenance (Frith, 2004). Further, our results support evidence that individuals with high negative symptoms may lack a functional concept of ToM similar to that observed in autism (Fretland et al., 2015). Such findings lend evidence to a more complex ToM impairment in schizophrenia, whereby individuals with dominantly positive, negative, and disorganised symptoms may produce unique types of errors (Fretland et al., 2015, Montag et al., 2011). It is likely that these individuals make both over- and undermentalising errors and have a mixed symptom profile (e.g., severe positive and disorganised symptoms). Inspecting profiles of error types has the potential to enhance treatments tailored to improving ToM in schizophrenia.
This study is the first to use ecologically valid virtual reality technology to examine differences in first- and second-order cognitive and affective ToM in different stages of schizophrenia. Results indicated that individuals with early schizophrenia are impaired on most ToM subprocesses, but maintain higher ToM functioning relative to individuals in more chronic stages of the illness. This pattern of findings suggests a gradient effect, whereby ToM abilities differentially deteriorate with illness chronicity. Furthermore, although ToM impairments are likely to be a trait marker of schizophrenia, the specific types of ToM errors an individual makes may vary according to their symptomology and stage of illness. These associations provide new insights into potential causes of the clinical presentation of schizophrenia and may explain the heterogeneity of ToM impairment in this population (Fretland et al., 2015). Longitudinal research including prodromal samples will advance clinical understanding of the nature and course of ToM impairment in schizophrenia.
This study has three limitations. First, the samples are small so this could have limited the power of our study in detecting some higher order interactions. Second, individuals in the early schizophrenia group were younger, more likely to be female, had less severe clinical symptoms, and were receiving less antipsychotic treatment than individuals in the chronic schizophrenia group. These differences are potential confounders of the findings of our study and should be controlled or taken into consideration when attributing ToM performance differences between the two groups of individuals with schizophrenia. Third, the use of a cross-sectional design precludes conclusions concerning causality and trajectory of ToM impairments.
Conflict of interest statement
None of the authors reported any conflicts of interest in producing this research.
Contributor Information
Allana L. Canty, Email: a.canty@griffithuni.edu.au.
David L. Neumann, Email: d.neumann@griffith.edu.au.
David H.K. Shum, Email: d.shum@griffith.edu.au.
References
- American Psychiatric Association . American Psychiatric Association; Washington, D.C: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Benton A.L., Varney N.R., de Hamsher K. Lateral differences in tactile directional perception. Neuropsychol. Res. J. 1978;16(1):109–114. doi: 10.1016/0028-3932(78)90049-0. [DOI] [PubMed] [Google Scholar]
- Bora E., Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr. Res. 2013;144(1):31–36. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J. Affect. Disord. 2009;113(1):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Brüne M. Theory of mind in schizophrenia: a review of the literature. Schizophr. Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Burgess P.W., Shallice T. Thames Valley Test Company; Bury St Edmunds, UK: 1997. The Hayling and Brixton Tests. [Google Scholar]
- Canty A., Neumann D., Fleming J., Shum D. Evaluation of a newly developed measure of theory of mind: the virtual assessment of mentalising ability. Neuropsychol. Rehabil. 2015 doi: 10.1080/09602011.2015.1052820. https://doi.org/10.1080/09602011.2015.1052820 (Advance online publication) [DOI] [PubMed] [Google Scholar]
- Fett A.J., Viechtbauer W., Dominguez M., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fretland R.A., Andersson S., Sundet K., Andreassen O.A., Melle I., Vaskinn A. Theory of mind in schizophrenia: error types and associations with symptoms. Schizophr. Res. 2015;162(1):42–46. doi: 10.1016/j.schres.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Frith C.D. Schizophrenia and theory of mind. Psychol. Med. 2004;34(03):385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- Green M.F., Bearden C.E., Cannon T.D. Social cognition in schizophrenia, part 1: performance across phase of illness. Schizophr. Bull. 2011;38(4):854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L., Siegert R., McClure J. Theory of mind in schizophrenia: a critical review. Cogn. Neuropsychiatry. 2005;10(4):249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- Ho K., Lui S., Hung K., Wang Y., Li Z., Cheung E., Chan R. Theory of mind impairments in patients with first-episode schizophrenia and their unaffected siblings. Schizophr. Res. 2015;166(1–3):1–8. doi: 10.1016/j.schres.2015.05.033. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opfer L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kohler C., Walker J., Martin E., Healey K., Moberg P. Chapter 2: facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso F., Horan W.P., Kern R.S., Green M.F. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr. Res. 2011;125(2):143–151. doi: 10.1016/j.schres.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Dziobek I., Richter I.S. Different aspects of theory of mind in paranoid schizophrenia: evidence from a video-based assessment. Psychiatry Res. 2011;186(2):203–209. doi: 10.1016/j.psychres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Reitan R., Wolfson D. Neuropsychology Press; Tuscon: 1985. The Halstead-Reitan Neuropsycholoigcal Test Battery: Theory and Clinical Interpretation. [Google Scholar]
- Shamay-Tsoory S.G., Tomer R., Goldsher D., Berger B.D., Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. J. Clin. Exp. Neuropsychol. 2004;26(8):1113–1127. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Tibi-Elhanany Y., Aharon-Peretz J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Soc. Neurosci. 2006;1(3–4):149–166. doi: 10.1080/17470910600985589. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Shur S., Barcai-Goodman L., Medlovich S., Harari H., Levkovitz Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007;149(1):11–23. doi: 10.1016/j.psychres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Sprong M., Schothorst P., Vos E., Hox J., Van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br. J. Psychiatry. 2007;191(1):5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2003. Wechsler Memory Scale–Third Edition (WMS-III) [Google Scholar]
- Wechsler D., Hsiao-pin C. NCS Pearson; San Antonio, TX: 2011. WASI-II: Wechsler Abbreviated Scale of Intelligence. [Google Scholar]