Abstract
As an important endogenous gaseous signaling molecule, hydrogen sulfide (H2S) exerts various effects in the body. A variety of pathological changes, such as cancer, glycometabolic disorders, and diabetes, are associated with altered endogenous levels of H2S, especially decreased. Therefore, the supplement of H2S is of great significance for the treatment of diseases containing the above pathological changes. At present, many efforts have been made to increase the in vivo levels of H2S by administration of gaseous H2S, simple inorganic sulfide salts, sophisticated synthetic slow-releasing controllable H2S donors or materials, and using H2S stimulating agents. In this article, we reviewed the recent development of H2S releasing/stimulating reagents and their potential applications in two common pathological processes including cancer and glycometabolic disorders.
Keywords: hydrogen sulfide, donor, cancer, glucose metabolism
Introduction
For a long time, hydrogen sulfide (H2S) was considered to be a colorless, flammable, water-soluble, and highly toxic environmental hazard with the characteristic smell of rotten eggs. However, research conducted over the last decade suggests that this gaseous molecule can be endogenously generated and exerts very important roles in organisms (Szabo, 2007, 2012; Wang, 2012; Lv et al., 2017). In mammals, endogenous H2S is mainly produced through the metabolism of L-cysteine and homocysteine by the catalysis of two pyridoxal-5′-phosphate (PLP)-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (Chiku et al., 2009; Singh et al., 2009). It can also be generated from the PLP-independent 3-mercaptopyruvate sulfurtransferase (3-MST), or cysteine aminotransferase (CAT) in the presence of α-ketoglutarate. The optimum pH value of 3-MST and CAT for the catalytic H2S production is about 9.7, while the physiological pH value of human blood ranges from 7.35 to 7.45. Additionally, 3-mercaptopyruvate, a substrate of 3-MST, is quite unstable. Therefore, the biological significance of this enzymatic reaction remains controversial, especially under physiological conditions (Mikami et al., 2011; Yadav et al., 2013).
These H2S-producing enzymes are distributed throughout the body in a tissue-specific manner. In murine liver and kidney tissues, CBS and CSE are both expressed, but the protein level of CSE is significantly higher than that of CBS. At high substrate concentrations (up to 20 mM cysteine or homocysteine) the capacity for liver H2S production is approximately equal for CBS and CSE, whereas in the kidney CBS constitutes the major source of H2S (Kabil et al., 2011). A number of studies have revealed that H2S can exert a variety of biological effects. For example, H2S is able to reduce vascular tone and stimulate angiogenesis (Lefer, 2007; Polhemus and Lefer, 2014). The genetic deletion of CSE in mice decreased H2S generation and led to pronounced hypertension and impaired endothelium-dependent vasorelaxation (Yang et al., 2008). In inflammatory response, H2S seems to be a double-edged sword. It can reduce inflammation and promote tissue repair by targeting many elements of the inflammatory cascade (Wallace et al., 2015) whereas in endotoxic shock, H2S can trigger or facilitate inflammation response in the lungs (Li et al., 2005). All of these findings suggest that the regulation of H2S content in the body may have great therapeutic value.
To understand the functions of H2S and develop H2S-related therapy, reagents that can be used to produce H2S in vitro and in vivo are often needed. Those compounds are known as H2S donors or stimulating reagents. The development of such chemicals has received much attention from medicinal chemists and chemical biologists. A number of different H2S donors have been reported and the topic of H2S donors have been reviewed several times (Li et al., 2008; Zhao et al., 2014; Steiger et al., 2016; Zhao and Pluth, 2016; Zheng et al., 2016). Herein, we provide an overview on current understanding of commonly used H2S donors and stimulating reagents. We focus our discussion on recent development of H2S donors, donor materials, and stimulating reagents. It is worthwhile to note that cancer and glycometabolic disorders have become an increasing public health concern throughout the world. Recent studies have revealed some unique functions of H2S in these diseases. Therefore, in this article we also reviewed the studies and results of applying H2S in these pathophysiological processes.
Donors of Hydrogen Sulfide
Gaseous H2S
H2S gas can be inhaled by testing animals. Therefore, experiment animals can be put into an H2S-riched environment to observe H2S’s physiological effects or toxicity. For example, it was found that when mice were exposed to 80 ppm of H2S for 6 h, their oxygen consumption dropped by ∼50%, and the metabolic rate and core body temperature were also significantly decreased into a suspended animation state (Blackstone et al., 2005). This effect is associated with the inhibition of cytochrome C oxidase of the electron transport chain during oxidative phosphorylation (Beauchamp et al., 1984). Notably, lowering metabolic demand could be useful for the reduction of physiological damage caused by trauma and improve outcomes after surgery (Blackstone et al., 2005). However, a later study of various larger species, such as sheep, swine, and human, indicated that H2S only exerted thermoregulatory effects (Wagner et al., 2011). H2S has good solubility in water (110 mM/atm at room temperature; 210 mM/atm at 0°C). Therefore, solutions of H2S gas are often used in studies. For example, in type 2 diabetes H2S gas solutions were used and it was found that they could promote glucose uptake through amelioration of insulin resistance and reduce renal injury (Xue et al., 2013). It should be noted that solutions with precise H2S concentrations are difficult to obtain, as H2S gas can easily escape from the solutions leading to a decreased concentration. In addition, H2S is a highly toxic gas, especially at high concentrations. These problems limit the use of H2S gas as a suitable reagent for many researchers.
Inorganic Sulfide Salts
Under physiological pH, H2S is in fast equilibrium with HS- in aqueous solutions. The proportions of HS- and H2S are 81 and 19%, respectively. Therefore, inorganic sulfide salts, such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), are often used as H2S equivalents in many studies. These salts are easy to obtain and widely used in the preparation of H2S solutions. However, these salts are considered to be fast H2S donors, as they produce H2S immediately when dissolved in aqueous solutions. Moreover, H2S molecule can rapidly escape from the buffers under a variety of experimental conditions, such as in the studies of tissue culture plates, muscle myograph baths, and Langendorff perfused heart apparatus (DeLeon et al., 2012). This loss of H2S is mainly due to the rapid volatilization of H2S. This problem may explain the discrepancy between low H2S concentrations in blood and tissues versus high concentrations of exogenous H2S (when sulfide salts are used) required to produce physiological responses (DeLeon et al., 2012). When exposed to high concentrations of H2S for a short period of time, tissues and cells may be damaged or show different responses, therefore, it is hard to investigate the effects of physiological concentrations of H2S (at low μM or nM levels). Another problem is that H2S tends to oxidize, particularly in the presence of metal contaminants in solutions. It is therefore recommended to prepare solutions in anaerobic water/buffers. The addition of heavy metal chelators, like DTPA (diethylenetriaminepentaacetic acid), can chelate metal ions and stabilize H2S in solutions. The use of DTPA is highly recommended when working with H2S solutions.
Nevertheless, NaHS has been used as a standard H2S donor in many studies. For example, it was demonstrated that NaHS could alleviate amyloid beta-peptide (Aβ) 25-35-induced neural lesion in an Alzheimer’s disease cellular model (Tang et al., 2008). In doxorubicin-induced cardiotoxicity, NaHS was found to reduce cardiomyocyte injury through inhibition of endoplasmic reticulum (ER) stress (Wang et al., 2012). In hypoxic skin damage, NaHS could exert anti-inflammatory effects through inhibition of reactive oxygen species (ROS)-activated nuclear factor kappa B (NF-κB)/cyclooxygenase (COX)-2 (Yang et al., 2011). Meanwhile, some researchers prefer using Na2S as the H2S donor. For example, Lefer and colleagues suggested that Na2S could attenuate mouse myocardial ischemia-reperfusion injury by preservation of mitochondrial function (Elrod et al., 2007). In another study on inflammatory modulation, the administration of Na2S (28 μmol/kg) was proved to suppress carrageenan-induced rat paw edema by producing H2S (Zanardo et al., 2006). Notably, the intravenous administration of Na2S (0.005–0.20 mg/kg, infused over 1 min) to healthy human volunteers increased blood sulfide and thiosulfate concentrations, as well as exhaled H2S gas (Toombs et al., 2010). Clinically, the elevated amount of exhaled H2S gas by patients was used to predict a non-eosinophilic phenotype of chronic obstructive pulmonary disease (Zhang et al., 2015) and the airway inflammation of asthma (Zhang et al., 2014).
Garlic-Derived Sulfur-Containing Compounds
Garlic is rich in sulfur-containing compounds and can be considered as an active “H2S” pool. Allicin (diallyl thiosulfinate) is the most well-characterized compound in garlic. In aqueous solutions allicin decomposes to a number of reactive sulfur-containing compounds, including diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) (Amagase, 2006), which can release H2S in different manners. DATS reacts rapidly with GSH to release H2S through thiol-disulfide exchange followed by allyl perthiol reduction by GSH. However, DADS only releases a minute amount of H2S via a sluggish reaction with GSH through an alpha-carbon nucleophilic substitution pathway (Liang et al., 2015). In human erythrocytes, garlic-derived organic polysulfides could be converted into H2S through formation of a key intermediate, hydropolysulfide (RSnH) (Benavides et al., 2007). In recent years, DADS and DATS have often been used as H2S donors to investigate their biologic actions. For example, it was shown that DADS can inhibit tumor cell proliferation and improve tissue repair (Ciocci et al., 2016). Since this class of H2S donors have been used clinically, the donation of H2S from them may have vital clinical significance.
Synthetic Slow-Releasing H2S Donors
Given the problems encountered with using H2S gas or sulfide salts in studies, researchers have explored a number of synthetic small molecules as H2S-releasing agents, e.g., H2S donors. These compounds are usually stable in physiological buffers. When applied to biological systems, they can release H2S under different mechanisms, such as hydrolysis, photolysis, or via the interaction with biomolecules like biothiols or enzymes. Some representative synthetic H2S donors are shown in Figure 1. GYY4137, a Lawesson’s reagent derivative, is perhaps the most well-known H2S donor. It has been used by many researchers as the standard slow H2S production source. The release of H2S from GYY4137 is very slow in aqueous solutions as well as after intravenous or intraperitoneal administration in animals (Li et al., 2008; Chen et al., 2016). GYY4137’s H2S release mechanism is suggested to be due to direct hydrolysis or interacting with un-identified biomolecules but the details still need to be elucidated. A sulfur-containing heterocycle dithiolethione is another often-used H2S-releasing motif. It has been used particularly in the construction of hybrid drugs with non-steroidal anti-inflammatory drugs (NSAIDs). H2S-releasing NSAIDs are designed with the primary goal of using H2S to overcome gastric side-effects of traditional NSAIDs through improvement of blood flow, attenuation of oxidation and inflammation (Liu et al., 2012). A series of H2S-releasing NSAIDs have been developed, such as HS-aspirin (ACS14) (Figure 1), HS-sulindac, HS-ibuprofen, HS-naproxen, S-sulindac, S-ibuprofen, and S-naproxen, and their inhibitory effects on cancer cells have also been described in detail (Chattopadhyay et al., 2012; Kodela et al., 2015b; Vannini et al., 2015; De Cicco et al., 2016). Besides the gastric protection and the effects against cancer, these hybrids have shown more potentials for relieving inflammatory pain than the traditional NSAIDs (Fonseca et al., 2015). However, to date, it is still unclear how H2S is generated from dithiolethiones nor is the by-product known. Therefore, this lack of information may be a problem in developing these compounds for clinic uses.
FIGURE 1.
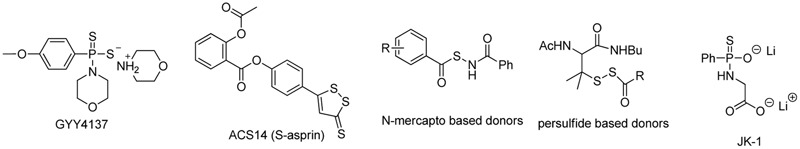
The structures of representative synthetic H2S donors.
H2S release from the aforementioned donors is considered non-controllable, as the release is either due to the donor’s spontaneous hydrolysis or the mechanism is still unknown. Recently, some controllable H2S donors have been developed. The representative examples are persulfide-based donors (Zhao et al., 2013), N-mercapto-based donors (Zhao et al., 2015), and JK type donors (Kang et al., 2016; Yang et al., 2017a), which are also shown in Figure 1. These are considered “controllable” donors because their H2S release mechanisms are clearly exploited and the structural modifications could lead to H2S release profile changes (faster or slower). As such, these compounds can potentially be used for precisely delivering H2S in biological systems. Nevertheless these synthetic donors have been reviewed multiple times. Interested readers can refer to those review articles (Zhao et al., 2014; Zheng et al., 2017). Herein we would like to focus on some recent developments in this field.
Ammonium Tetrathiomolybdate
Thiomolybdate salts have been used as thiol transfer reagents in organic synthesis (Prabhu et al., 2002). The four sulfur atoms in the structures also make them excellent copper chelators. Ammonium tetrathiomolybdate (TTM) (Figure 2) has been used clinically as a treatment for copper toxicity, especially for Wilson’s disease (Brewer, 2003), a genetically recessive disease of copper accumulation. TTM’s pharmacology and toxicology have been thoroughly studied (Brewer, 2009). Also, it is known that TTM can produce H2S under strong acidic conditions such as 5% H2SO4 (Wang et al., 1997). As such, it is possible to repurpose TTM as a unique inorganic complex-based H2S donor, analogous to the discovery of sodium nitroprusside as a widely used nitric oxide (NO) donor (Hottinger et al., 2014). To this end, we studied TTM’s H2S-generation and relevant activities under physiological conditions (Xu et al., 2016). Using a modified methylene blue method, commercially purchased TTM (99.97% purity from Sigma-Aldrich) was used to test H2S release in phosphate-buffered saline under pH 5, 6, 7.4, and 8. The results showed that TTM released H2S upon solvation, and H2S concentrations in all pH levels were maintained at a steady level for up to 15 h. These results suggest that TTM is a slow H2S releaser, similar to GYY4137. It was also found that acidic pH could accelerate TTM’s H2S release. Further studies in cellular models showed TTM could protect cells under oxidative stress (treated by H2O2). These preliminary results indicate TTM is a promising H2S donor (Xu et al., 2016). A recent study has shown that TTM could attenuate myocardial and cerebral injury induced by ischemia/reperfusion (Dyson et al., 2017). More studies on it are expected to come in the next years.
FIGURE 2.
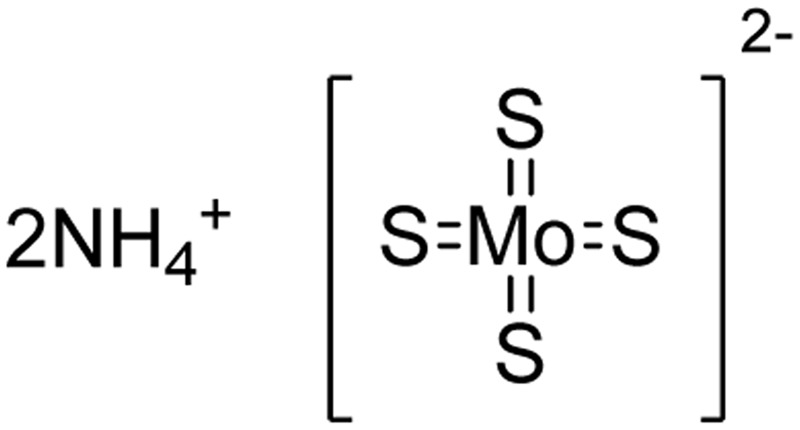
The chemical structure of TTM.
Reactive Oxygen Species Activated H2S Donors
In 2016, Pluth and colleague designed several interesting ROS activated H2S donors (Zhao and Pluth, 2016). These caged thiocarbamate compounds bear a pinacol boron functional group, which can be oxidized and cleaved to hydroxyl (-OH) by ROS, like hydrogen peroxide (H2O2). A tandem reaction will then occur and release carbonyl sulfide (COS), as well as quinone and amine byproducts (Figure 3). It is worth noting that COS can easily undergo hydrolysis to produce H2S if carbonic anhydrase (CA) is presented (Steiger et al., 2016). This work represents the first ROS triggered H2S donors. It was found that the reaction rates could be tuned by electronic modulation of thiocarbamates. Interestingly, further studies showed CA is not required for the donors’ H2S release as H2O2 can also trigger the rapid transformation of COS to H2S. Cellular imaging experiments demonstrated the donors, such as Peroxy TCM, can release H2S with activation of both exogenous H2O2 and endogenous H2O2 in cells. The donors can increase cell viability under H2O2-induced oxidative stress, while the control compounds without aryl boron moiety, which produces H2O instead of H2S, show little or no protective effects against H2O2 induced damage. These results indicate that these donors are effective under ROS stimulation.
FIGURE 3.
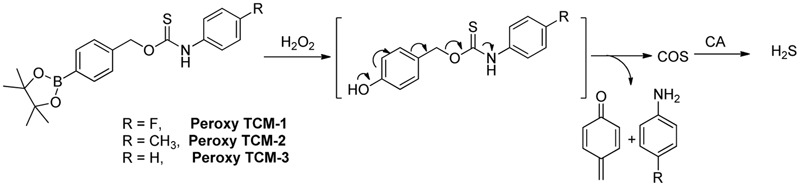
H2O2-mediated COS/H2S release from Peroxy TCM donors.
Enzyme Activated H2S Donors
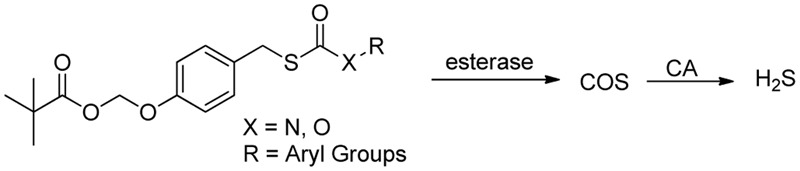
Also in 2016, Wang and colleagues reported a series of esterase activated H2S donors (Figure 4) (Zheng et al., 2016). These donors contain a thioacid moiety and an ester-masked nucleophilic phenol in a “trimethyl lock” template. After the ester group is removed by an esterase, a spontaneous intramolecular lactonization occurs, resulting in the release of H2S. Experimental data showed the donors require porcine liver esterase or cell culture media containing FBS to release H2S, confirming the donors’ selectivity and chemical stability. Additionally, their H2S-releasing rates can be tuned by adjusting ester substituents and the trimethyl lock structure. In RAW 264.7 cells, these donors inhibited lipopolysaccharide-induced tumor necrosis factor (TNF)-α secretion, suggesting the H2S-related anti-inflammatory effects, while control compounds without the H2S-releasing moiety did not show such effects. These esterase activated donors can be conjugated to NSAIDs to form H2S–NSAID hybrids. Such hybrids, like HP-105, could utilize H2S’s anti-inflammatory and anti-oxidative effects to ameliorate NSAIDs-induced gastric damage. However, detailed biological evaluations of these new H2S–NSAID hybrids have yet to be seen.
FIGURE 4.
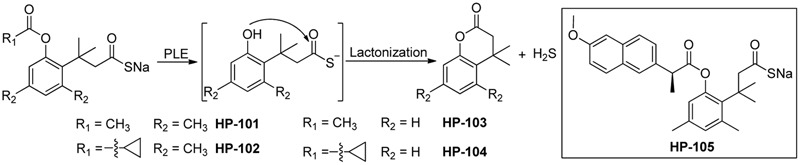
H2S release from esterase-activated donors.
Besides Wang’s work (Zheng et al., 2016), Chakrapani and colleagues also designed esterase-activated H2S donors (Figure 5), utilizing carbamothioates and carbonothioates to generate COS as the H2S precursor (Chauhan et al., 2017). COS is then hydrolyzed by CA to produce H2S. These donors’ ability of generating H2S when esterase and CA are both presented has been proven by various studies (Steiger et al., 2017a,b). Kinetics studies showed the decomposition of the compounds may go through a short-lived intermediate.
FIGURE 5.
Esterase-activated COS/H2S release.
Nitroreductase (NTR)-activated H2S donors were recently reported by Chakrapani and colleagues (Shukla et al., 2017). The donors contain 4-nitroaryl masking groups, which are good substrates for NTR. Upon nitro reduction, a gem-dithiol will be produced, and further converted to H2S (Figure 6). Fluorescent and monobromobimane assays proved the donors’ H2S generation in cell-free conditions in the presence of bacterial NTR. The donors also generate H2S in bacterial cells that express NTR, but fail to generate H2S in bacterial cells or mammalian cells deficient in NTR. These donors were used as chemical tools to study H2S’s role in antimicrobial resistance.
FIGURE 6.
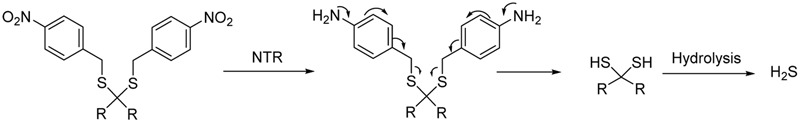
Nitroreductase-activated H2S donors.
These ROS- and enzyme-activated donors utilize interesting chemistry, and make their H2S release more controllable. They can be useful chemical tools. However, there are very few studies about their biological applications or therapeutic potentials. We look forward to these studies in the near future.
H2S-Releasing Materials
Compared to small molecule drugs, material-based drugs have some unique advantages, such as improved water solubility and stability, slower clearance rates, and reduced toxicity. Material-based drug delivery has been extensively studied. However, H2S-releasing biomaterials are relatively new in this field. In the past several years, a few such materials have been developed, which are summarized below.
In 2014, Matson and colleague prepared S-aroylthiooxime (SATO)-based polymers A (Figure 7) by functionalizing aldehyde-containing polymers with S-aroylthiohydroxylamines (Foster and Matson, 2014). In the presence of biological thiols, such as cysteine or GSH, the SATO moiety on the polymer can degrade to release H2S. The kinetics can be controlled by tuning electronics on SATO substituents. Recently, the same strategy was also employed to prepare SATO-based micelles by the same group (Foster et al., 2017). In this case, SATO was formed on polymer amphiphiles to give B, which undergoes self-assembly in THF-water solutions and forms micelles. Similarly, these micelles release H2S upon activation by biothiols. The micelles showed slower but more sustained H2S release than A and small molecule SATOs. This could be attributed to the more difficult diffusion of biothiols to the hydrophobic cores of micelles. In the presence of cysteine, the H2S-micelles showed cytotoxicity toward cancer cells, such as HCT 116 cells, but much reduced toxicity for “normal” cells such as NIH/3T3 cells, suggesting that H2S-micelles can selectively target cancer cells.
FIGURE 7.
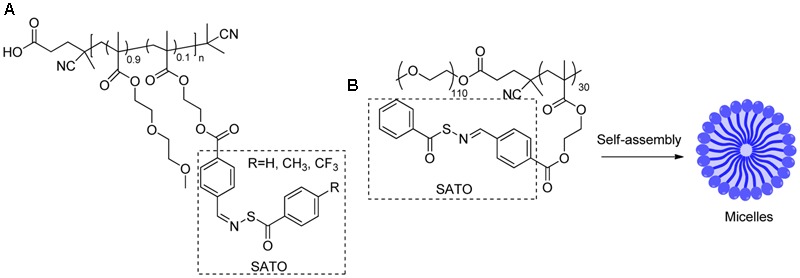
(A) SATO based polymers. (B) SATO based micelles.
In 2016, Matson’s group developed polyNTA (Figure 8), a random polymer that can release H2S via the COS intermediate (Powell et al., 2016). This polymer contains N-thiocarboxyanhydrides (NTAs), which undergoes a ring-opening reaction with biological nucleophiles, such as amines to release COS. Then COS can be hydrolyzed to produce H2S in the presence of CA. PolyNTA was shown to generate H2S in buffers when glycine and CA were present. However, it failed to promote endothelial cell proliferation like small molecule H2S donors.
FIGURE 8.
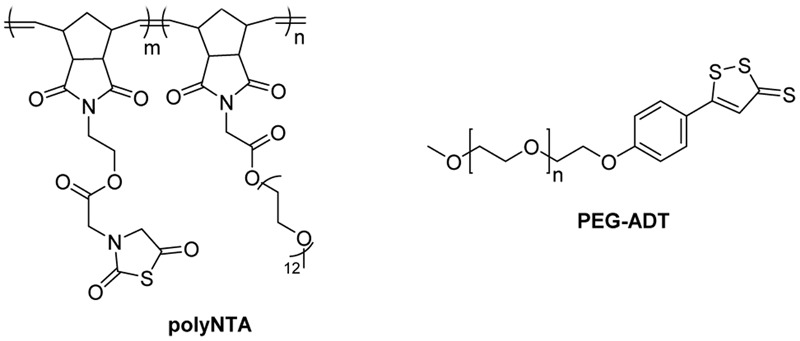
The structures of polyNTA and PEG-ADT.
Another example of an H2S-relasing polymer is PEG-ADT (Figure 8) developed by van der Vlies and colleague (Hasegawa and van der Vlies, 2014). In this work, H2S donor 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH) was conjugated to polyethylene glycol (PEG). PEG-ADT showed lower H2S generating capacity than its parent compound ADT-OH and reduced cytotoxicity. Cell imaging studies suggested PEG-ADT could enter cells via endocytosis and remain in the endolysosome, whereas ADT-OH enters the cytoplasm via membrane diffusion. This difference may lead to reduced toxicity of PEG-ADT.
Apart from chemical conjugations, Wang et al. (Feng et al., 2015; Wu et al., 2016b) reported H2S-releasing nanofibers by physically adding H2S donor molecules to polycaprolactone (PCL) solutions and fabricated the materials with electrospinning. Two H2S-releasing nanofibers, thiol-activated H2S-fibers (Feng et al., 2015) and pH-activated PCL-JK1 (Wu et al., 2016b) were prepared. These fibers showed prolonged H2S release compared to their parent small molecules. Notably, they also showed significant cytoprotection and improved wound healing capacity.
SG-1002
SG-1002 (Figure 9) is an inorganic mixture, containing S8, Na2SO4, Na2S2O3, Na2S3O6, Na2S4O6, and Na2S5O6. It was developed by SulfaGENIX as an oral H2S donor for the treatment of heart diseases, like myocardial I/R injury and heart failure. In a pre-clinic study, SG-1002 effectively restored H2S and sulfane sulfur levels in mice with heart failure triggered by transverse aortic constriction (TAC) (Kondo et al., 2013). SG-1002 treated mice suffered less cardiac enlargement and left ventricular (LV) dilation, and showed reduced fibrosis compared with mice fed by a control diet after TAC. Additionally, the treatment with SG-1002 upregulated the vascular endothelial growth factor (VEGF), protein kinase B (Akt), endothelial NO synthase, NO and cGMP pathway, and attenuated mitochondrial respiratory dysfunction and oxidative stress induced by TAC.
FIGURE 9.
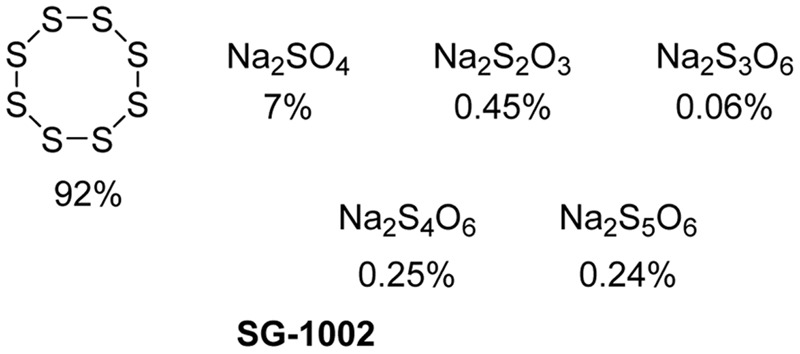
SG-1002 Components.
In another model study, SG-1002 attenuated cardiac dysfunction induced by a high fat diet (HFD) in mice (Barr et al., 2015). HFD fed mice showed decreased sulfide levels, metabolic perturbation such as increased serum glucose level and glucose intolerance, as well as cardiac dysfunction such as decreased LV function fraction and increased circumferential stress. Morphological histological analysis showed cardiac enlargement and fibrosis in HFD fed mice. However, the treatment with SG-1002 markedly ameliorated some of the metabolic perturbations and attenuated cardiac dysfunction. Further study showed that the treatment could restore adiponectin levels and suppress cardiac ER stress.
In a Phase I clinical trial (Polhemus et al., 2015), the effects of SG-1002 were tested on both healthy subjects and subjects with heart failure with dosages up to 800 mg twice daily for 7 days. Both groups showed increased H2S levels with only minor adverse effects observed. In addition, a double blind, placebo controlled Phase I trial (ClinicalTrials.gov Identifier: NCT01989208) is in process.
Despite the promise, no present study has described the H2S releasing mechanism of SG-1002 in vivo. We look forward to studies that elucidate the mechanism, which should provide new insight in H2S-related therapies.
H2S-Stimulating Agents
In addition to donors that directly degrade to produce H2S via different chemical mechanisms, some compounds are known to stimulate H2S production in vivo. For example, L-cysteine is an important natural substrate of H2S-producing enzymes. When the amino group of L-cysteine is acetylated, the resulted product N-acetyl-L-cysteine (NAC) is one of the essential medicines listed by the World Health Organization (WHO). Although NAC has been used clinically as an expectorant (Sheffner, 1963) or an antioxidant in hepatic failure (Saito et al., 2010), no clinical trial has used it as a H2S donor. However, NAC was found to suppress leukocyte infiltration in an air pouch model through generation of endogenous H2S (Zanardo et al., 2006).
S-allyl-L-cysteine (SAC) (Kim et al., 2006) and S-propargyl-L-cysteine (SPRC) (Gong et al., 2011) (Figure 10) are two other cysteine derivatives that can be used as CSE substrates to produce H2S. The classical effects of H2S including antioxidation and cardioprotection were demonstrated in SAC treated rats suffering from isoproterenol toxicity (Padmanabhan and Prince, 2006). Zhu and colleagues further found that SAC’s cardioprotective effects were associated with induction of CSE expression and enhancement of H2S generation (Chuah et al., 2007). A recent study showed that SAC could suppress hepatocyte growth factor-induced migration and invasion in nasopharyngeal cancer cells (Cho et al., 2015). SAC could also exert anti-diabetic effects by means of improving beta-cell function and reducing glycemia (Kim et al., 2017).
FIGURE 10.
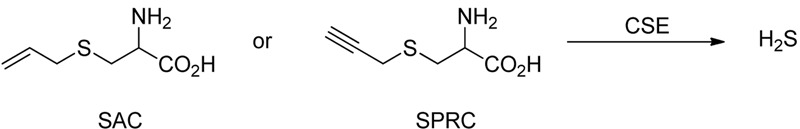
The Structures of SAC and SPRC and their H2S release reaction.
SPRC is a structural analog of SAC and has expected similar effects as SAC, such as enhanced CSE expression and plasma H2S concentrations, as well as cardioprotection (Wang et al., 2009) and anti-cancer effects (Ma et al., 2011). Recently, it was documented that SPRC could prevent doxorubicin-induced cardiotoxicity (Wu et al., 2016a), which is similar to the effects of NaHS used in previous studies (Wang et al., 2012). In addition, SPRC exhibited anti-inflammation (Wang et al., 2016), anti-apoptosis and anti-oxidation in high glucose treated cardiomyocytes (Yang et al., 2015).
Vitamin D (VD), a key endogenous hormone, has been thought to regulate calcium and phosphate metabolism contributing to bone growth and remodeling for a long time. In recent years, the extraskeletal effects of VD have been documented, such as cancer progression, cardiovascular action, and immune regulation (Christakos et al., 2016). In fact, H2S can exert some coincident effects in the body with VD. Notably, Wilinski et al. (2012) found that cholecalciferol, also known as VD3, could increase tissue H2S concentration in mouse heart, brain and kidney. Meanwhile, another report suggested that VD3 had ability to upregulate glucose transporter type 4 (GLUT4) and decreased glycemia in diabetes through induction of CSE expression and H2S generation (Manna and Jain, 2012). Moreover, in high glucose and methylglyoxal (MGO)-induced diabetic peripheral neuropathy, VD could exert significant protective roles through restoration of CBS expression and endogenous H2S formation (Zhang et al., 2016).
Table 1 provides a brief summary of H2S donors and stimulating reagents covered in this review.
Table 1.
Summary of H2S releasing/stimulating reagents.
| Reagents | Potential applications | Advantages | Disadvantages |
|---|---|---|---|
| Gaseous H2S | Reduce body temperature and metabolic rate; improve glucose uptake in type 2 diabetes | Clean H2S source; no byproducts; easy administration; good absorbability | High volatility; difficult to control concentrations; uncontrolled H2S release |
| Inorganic sulfide salts | |||
| NaHS, Na2S | Widely used in cellular and animal disease models; intravenous administration to healthy volunteers | Good solubility; easy to handle; clean H2S source, no byproducts | High volatility; difficult to control concentrations; Uncontrolled H2S release |
| Garlic-derived sulfur containing compounds | |||
| DAS, DADS, DATS | Proliferative inhibition in tumor cells; improvement of tissue repair and myocardial function | Extensive data on a variety of models | Stability of polysulfides is a concern; interfere with GSH |
| Synthetic slow-releasing H2S donors | |||
| GYY4137, ACS14 | Cytoprotection; anti-cancer and anti-inflammation activities | Good stability; slow H2S release; ACS14 overcomes side effects of NSAIDs | Uncontrolled release; unclear release mechanism; produce byproducts |
| Novel controllable H2S donors | |||
| N-mercapto/persulfide-based donors, JK donors | Myocardial/gastric preservation | Good stability; controlled H2S release | Produce byproducts |
| Tetrathiomolybdate | A common Cu2+ chelator; dermal, myocardial and cerebral preservation | Good stability and safety; a clinically used drug | Unclear byproducts could cause side effects |
| ROS-/enzyme-activated H2S donors | Cytoprotection and anti-inflammation | Good stability; controlled H2S release | Produce byproducts; lacking in vivo data |
| H2S-releasing materials | |||
| SATO-based polymers/micelles, polyNTA, PEG-ADT, PCL-JK1 | Anti-cancer activity; improvement of wound healing; cardioprotection | Good solubility, stability, membrane permeability; slow clearance rates | More in vivo data are needed |
| H2S-stimulating agents | |||
| L-cysteine derivatives, Vitamin D | Anti-cancer, anti-diabetes, anti-oxidation, anti-inflammation, and cardioprotection | Transformed into endogenous substances | |
From the above sections, we can see that numerous H2S releasing/stimulating reagents have been developed so far and many of them have been used in cell- or animal-based disease models. However, very few of them have been further advanced into pre-clinic studies, indicating the development of H2S as therapeutics is still at the early stage. Nevertheless these studies have revealed interesting sulfur-related biological mechanisms, which can potentially impact future research in this area. In the following section, we intend to review the applications of H2S releasing/stimulating reagents in the studies of cancer and glycometabolic disorders, two very common health concerns in the world.
Hydrogen Sulfide and Cancer
Effects of H2S on Cell Cycle and Proliferation
The cell cycle represents a series of tightly integrated events, which regulate the transition from cellular quiescence to proliferation, and thus ensure high fidelity of the genetic transcript (Schwartz and Shah, 2005). In eukaryotic cells, the cell cycle includes four conventional phases, i.e., Gap phase 1 (G1); DNA synthesis phase (S); Gap phase 2 (G2), during which the cell prepares itself for division, and mitosis phase (M), during which the chromosomes separate and the cell divides. Dysregulation of the cell cycle is an important cause of cellular overproliferation and cancer (Schwartz and Shah, 2005; Malumbres and Barbacid, 2009).
The treatment of oral squamous cell carcinoma cells, Cal27, GNM, and WSU-HN6, with a H2S donor, NaHS, significantly downregulated cell cycle regulatory genes, RPA70 and RB1, and upregulated proliferating cell nuclear antigen and cyclin-dependent kinase 4 (CDK4), resulting in cell proliferation (Ma et al., 2015). In human colon cancer HCT 116 cells and hepatocellular carcinoma cells, H2S-mediated proliferation was investigated through addition of NaHS and induction of CSE expression, respectively. It was found that the effects of H2S on tumorigenesis were associated with the decreased proportion of cells in G0–G1 phase, downregulation of cyclin-dependent kinase inhibitor p21, and with the increase in proportion of S phase cells (Cai et al., 2010; Yin et al., 2012). A recent study showed that by inhibiting endogenous CBS/H2S pathway quinolone-indolone conjugate (QIC)2 could significantly attenuate hepatoma cell proliferation (Jia et al., 2017). These findings suggest that H2S participates in the development of cancer through shortening cell cycle and inducing proliferation.
On the other hand, some researchers used SPRC as a H2S stimulating reagent, and found that the treatment with SPRC could induce cell cycle arrest at G1/S phase and consequently inhibit proliferation of SGC-7901 gastric cancer cells (Ma et al., 2011). The synthetic slow-releasing H2S donor, GYY4137, also showed similar anti-proliferation in hepatocellular carcinoma model (Lu et al., 2014). In addition, the exposure to GYY4137 for 8 days can induce cell cycle arrest at the G2/M phase in human breast adenocarcinoma MCF-7 cells (Lee et al., 2011). Some studies introduced H2S-releasing moieties to NSAIDs and found that these hybrids have enhanced anti-tumor activity and gastrointestinal (GI) safety compared with the prodrugs (Kodela et al., 2015a,b). Of note, a study indicated that H2S-releasing NSAIDs inhibited cellular proliferation in a tissue type-independent manner (Chattopadhyay et al., 2012). TTM also exerts anti-cancer effects through chelating and reducing copper, which can promote angiogenesis and feed an expanding tumor (Beauchamp et al., 1984; Garber, 2015). However, it is still unclear if H2S is involved in TTM’s anti-cancer effects.
From these studies, it has been indicated that H2S has both pro-proliferation and anti-proliferation, even in the same tumor cells, which seems to be conflicting. Notably, in the reports of H2S’s pro-proliferation, the inorganic sulfide salts, such as NaHS and Na2S, were usually used. However, in the reports of H2S’s anti-proliferation, the slow-releasing H2S donors such as GYY4137 and dithiolethione were used. Besides the different H2S release profiles, the former donors (e.g., NaHS and Na2S) are simple donors, whose byproduct can almost be neglected. However, the latter synthetic donors are structurally complicated and may produce biologically active byproducts after H2S release, or the donor molecules themselves can have H2S independent activities. Further studies are needed to clarify these effects.
Effects of H2S on Cellular Apoptosis
It is necessary for multicellular organisms to keep a balance between proliferation and apoptotic cell death. If this well controlled balance is disrupted, the development of cancer may be triggered (Cotter, 2009). Induction of apoptosis has become an effective strategy for cancer prevention and treatment (Li et al., 2011; Fernald and Kurokawa, 2013; Fulda, 2015). However, if the apoptosis is suppressed in tumor cells, these cells will survive and even resist chemotherapy. Studies have shown that the treatment with various forms of H2S donors could induce cell apoptosis in cancer. For example, the exposure of oral cancer cells to H2S gas for 72 h markedly led to apoptotic cell death, but did not affect healthy oral keratinocytes (Murata et al., 2014). It was found that the expression of CSE in human melanoma cells was elevated and the overexpressed CSE provoked spontaneous apoptosis. Importantly, exogenously applied H2S donor, DATS, or CSE substrate, L-cysteine, can both inhibit tumor growth in mice (Panza et al., 2015). In addition, the slow-releasing donor GYY4137 could trigger apoptosis of many types of cancer cells, including HeLa, HCT-116, Hep G2, HL-60, MCF-7, MV4-11, and U2OS, causing significant inhibition of tumor growth (Lee et al., 2011).
On the other hand, several reports showed that H2S of GI tract could inhibit the phytochemical agent β-phenethyl isothiocyanate-induced cellular apoptosis in human colon cancer HCT116 cells (Rose et al., 2005). Moreover, the application of exogenous H2S, in the form of NaHS at concentration of 500 μM, dramatically increased cell viability but decreased the number of apoptotic cells through activation of NF-κB pathway in PLC/PRF/5 hepatoma cells (Zhen et al., 2015).
From these studies, it can be noticed that the effects of H2S on cellular apoptosis are also contradictory, similar to its effects on proliferation. Therefore, more related and designed research has become imperative.
Effects of H2S on Angiogenesis
In cancer progression, including tumor growth, invasion, and metastasis, angiogenesis plays a vital role through supplement of oxygen and nutrients (Zhang G. et al., 2013). The proliferation and migration of endothelial cells and fibroblasts are the base for angiogenesis, and this progress is regulated by a number of growth factors, such as VEGF, fibroblast growth factor, and epidermal growth factor (Carmeliet and Jain, 2011; Weis and Cheresh, 2011). H2S, in the form of NaHS, could induce VEGF expression and promote angiogenesis in soluble fms-like tyrosine kinase 1 (sFlt1)-mediated renal injury (Holwerda et al., 2014) or in hind limb ischemic rats (Wang et al., 2010). Since VEGF-mediated angiogenesis contributes to cancer progression, H2S’s pro-angiogenic activity may cause cancer, which is supported by some recent studies. In colon cancer, H2S produced by CBS in tumor can stimulate angiogenesis, increase tumor blood flow and ATP generation, resulting in tumor growth. The pharmacological inhibition of H2S generation with a selective CSE inhibitor, aminooxyacetic acid, could impede endothelial cell migration and tumor cell proliferation, migration, and invasion (Szabo and Hellmich, 2013; Szabo et al., 2013). In ovarian cancer, similar H2S pro-angiogenic effects on tumor growth were also demonstrated (Bhattacharyya et al., 2013; Hellmich et al., 2015). Besides VEGF induction, H2S can activate its receptor VEGFR2 via breaking Cys1045–Cys1024 disulfide bond (Tao et al., 2013). Despite the aforementioned anti-proliferation and pro-apoptosis, H2S may be used in cancer therapy. However, its proangiogenesis will impede the use in this field. In combination of VEGF neutralizing antibody or VEGFR2 antagonist may overcome this effect of H2S on angiogenesis and contribute to its anti-cancer effects.
Hydrogen Sulfide and Glycometabolism Disorder
Diabetes mellitus (DM) is another common issue affecting public health. As reported by WHO, the number of patients suffering DM has exceeded 420 million throughout the world. It is noteworthy that diabetic patients can carry carcinoma more easily than healthy people (Ben et al., 2011; Jiang et al., 2011; Xu et al., 2013; Tsilidis et al., 2015). Disorders of glucose metabolism are one of the most important features of DM. A growing body of evidence has shown H2S plays an important role in the disordered glucose metabolism (Wallace and Wang, 2015; Untereiner and Wu, 2017). In this section, the effects of H2S on glucose metabolism are discussed.
Effects of H2S on Insulin Secretion
In the human body, there are many hormones which can increase blood glucose, such as growth hormone, glucocorticoid, and glucagon. However, insulin is the only hormone that decreases blood glucose. Consequently, insulin’s abnormal secretion or/and dysfunction is the significant reason for glycometabolism disorder. In general, H2S can affect insulin-secreting beta cells in two ways, i.e., inhibiting secretion of insulin from the cells (Yang et al., 2005; Tang et al., 2013), and preventing them against various stimuli-induced cellular apoptosis (Lipson et al., 2006; Okamoto et al., 2013).
It is documented that in streptozotocin (STZ)-induced diabetic rats, the expression of H2S synthetases, including CSE and CBS, in the liver and pancreas is significantly upregulated comparing with the control rats. The induction of the two enzymes is restored by the exogenous supplement of insulin (Yusuf et al., 2005). In an in vitro study, the administration of H2S to INS-1E cells, a beta cell line, obviously attenuates insulin secretion triggered by a high concentration of glucose (Yang et al., 2005). In another beta cell line, HIT-T15 cells, similar findings have been demonstrated. In addition, it is reported that the inhibitory effects of H2S on insulin secretion depend on opening KATP channels (Ali et al., 2007). These studies indicate that under diabetic conditions, the elevated levels of endogenous H2S can open KATP channels in islet beta cell membrane, resulting in hyperpolarization of membrane potential and reduction of insulin secretion (Yang et al., 2005). Besides working as a KATP channel opener, H2S provided by NaHS can directly inhibit L-type voltage-dependent Ca2+ channels, thereby reducing insulin secretion in a KATP channel-independent manner (Tang et al., 2013). In MIN6 cells, a pancreatic beta cell line, through decreasing the intracellular ATP content, the enhancement of H2S generation by L-cysteine can also indirectly open KATP channels and then inhibit insulin secretion (Kaneko et al., 2006). All of these studies manifest that H2S inhibits insulin secretion by targeting various links, including inhibition of ATP synthesis, activation of KATP channels and inactivation of L-type voltage-dependent Ca2+ channels, respectively (Wallace and Wang, 2015; Figure 11).
FIGURE 11.
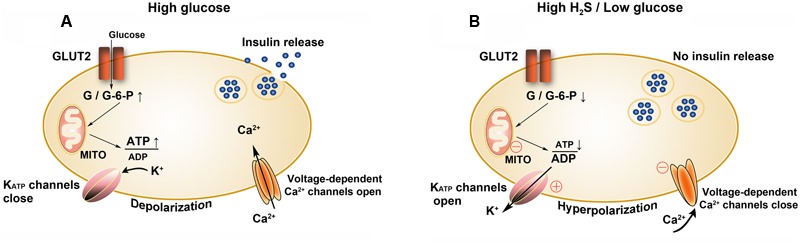
Effects of H2S on insulin secretion by beta cells. (A) Insulin secretion is stimulated at high glucose levels and (B) inhibited at high H2S or low glucose levels. GLUT2, glucose transporter type 2; G, glucose; G-6-P, glucose 6-phosphate; MITO, mitochondria; ○, targets of H2S; ⊕, to activate; Θ, to inhibit.
Similar to STZ-induced diabetic model, Zucker DM rats have higher endogenous H2S content than obese rats. Notably, the inhibition of endogenous H2S synthesis in Zucker DM rats improves insulin release and relieves hyperglycemia (Wu et al., 2009). These studies demonstrate that H2S can inhibit insulin secretion, and the downregulation of H2S system seemingly benefits diabetic prevention and treatment.
Actually, endogenous H2S does not always work as a foe. For example, some researchers have shown that H2S can prevent islet beta cells from various stimuli-induced injury (Kaneko et al., 2009; Taniguchi and Niki, 2011). CBS is widely expressed in the whole pancreas, while CSE is mainly expressed in the islets of pancreas. In the isolated pancreatic beta cells, basal CSE expression is very low, but can be elevated by the treatment with high concentrations of glucose. Additionally, both exogenous administration of H2S (NaHS) and stimulating endogenous H2S generation with L-cysteine can impede oxidation-induced cellular apoptosis (Kaneko et al., 2009). Besides excessive apoptotic death of beta cells, the persistently elevated inflammatory response can damage islet cells and cause many diabetic complications. Therefore, DM has been accepted as an inflammatory response disease and thus anti-inflammation is a very important way to treat DM (Donath, 2014, 2016; Esser et al., 2015). Interestingly, the application of NaHS to release H2S can significantly inhibit proinflammatory factors-induced injury in primary cultured pancreatic beta cells and MIN6 cells (Taniguchi et al., 2011). In HFD-induced type 2 DM mice, the knockout of CSE (to reduce H2S) aggravates oxidation-related insults without promotion of insulin secretion or reduction of blood glucose levels (Okamoto et al., 2013). Our studies indicate that in diabetic skin complications, H2S provided by NAC or NSHD-1, a synthetic slow-releasing H2S donor, can exert protective effects against diabetes-like injury (Yang et al., 2014, 2017b). However, some other studies have shown that H2S participates in ER stress-mediated cellular apoptosis through activation of p38 MAPK pathway (Yang et al., 2007), which is not completely consistent with the report by Taniguchi et al. (2011).
Based on the above studies, it can be summarized that the effects of H2S on insulin secretion vary and may be dependent on different stages of diabetic development. At the early stage, hyperglycemia-induced CSE upregulation may be a protective mechanism in the body. The increased H2S level can exert anti-oxidation, anti-inflammation, and inhibition of autoimmune response, consequently protecting pancreatic beta cells. With the diabetic development, the content of H2S further increases, and the elevated H2S will inhibit insulin secretion and then reduce the overload of diabetic beta cells via reduction of ATP content, activation of KATP channels, or inhibition of L-type voltage-dependent calcium channels (Okamoto et al., 2015). However, if the hyperglycemia state persists, the endogenous content of H2S will exceed its threshold, which triggers ER stress response and resultant apoptosis (Yang et al., 2007). Therefore, the regulation of endogenous H2S generation is very important, i.e., at the early stage of DM, the administration of H2S may be beneficial, while at its late stage inhibiting H2S generation may be a reasonable treatment strategy.
Effects of H2S on Hepatic Glucose Metabolism
The liver is an important organ to control blood glucose homeostasis, by balancing glycogen synthesis, to reduce blood glucose levels, and gluconeogenesis, to raise blood glucose levels. Hepatic malfunction can also lead to abnormal glucose metabolism, even diabetic development. Importantly, H2S plays a potential role in the glucose metabolism in the liver. In HepG2 hepatocytes, H2S (NaHS) can inhibit glucose uptake and glycogen storage through reduction of glucokinase activity (Zhang L. et al., 2013). Additionally, NaHS can induce gluconeogenesis and glycogen breakdown via phosphoenolpyruvate carboxykinase in liver (Untereiner et al., 2016). By these means, H2S can increase blood glucose levels. However, it was also found that under physiological conditions, the effects on CSE/H2S signal was inhibited by insulin, and perhaps H2S does not significantly regulate hepatic glucose output (Zhang L. et al., 2013). When hyperglycemia persists or insulin resistance develops, the CSE/H2S signal is obviously activated. A further study showed that the elevated H2S content can cause pyruvate carboxylase S-sulfhydration and activation, increasing gluconeogenesis and blood glucose levels. Interestingly, the expression of CSE can be increased by glucose deprivation, but decreased by a HFD, indicating that hepatic H2S generation is significant to maintain glycemic homeostasis (Ju et al., 2015).
Effects of H2S on Glucose Metabolism of Adipose Tissues
When blood glucose increases, the adipose tissues can store the excessive glucose as body fat. Conversely, when blood glucose decreases, some fat stored in adipose tissues can be mobilized and used for gluconeogenesis and energy. Therefore, the adipose tissues usually serve as another buffer for blood glucose in the body alongside the liver. It was found that CSE is expressed in adipose cells and preadipocytes. Stimulating endogenous H2S production with L-cysteine can inhibit glucose uptake by adipose tissues via reducing insulin sensitivity (Feng et al., 2009). A 2-deoxy-[(3)H] glucose uptake experiment in vivo showed that the basal and insulin-stimulated glucose uptake by adipose tissues of Kir 6.2(-/-) mice, deficient in the function of KATP channel, is enhanced compared with that of wild-type mice (Miki et al., 2002), which is supported by an earlier study (Tayo, 1976). As a KATP agonist, it is reasonable that H2S has an opposite effect comparing with the knockout of Kir 6.2. However, Feng et al.’s (2009) study showed that the effects of H2S do not depend on KATP channels, but are rather associated with PI3K signaling pathway. Besides inhibition of glucose uptake, H2S is involved in TNFα-induced insulin resistance in 3T3-L1 adipocytes (Huang et al., 2013). Therefore, for the regulation of glucose uptake by adipose tissues H2S does not seem to play a beneficial role.
It is worthwhile to note that some recent studies showed the opposite action of H2S on glucose regulation by adipose tissues. Manna and Jain (2011, 2012) found that the administration of H2S (Na2S) or its stimulating agent (L-cysteine), even VD, can enhance the ability of adipose tissues to take up glucose. This effect is associated with increases in insulin sensitivity and GLUT4 activity. In another in vivo study, H2S (NaHS) was found to promote adipocytes to uptake glucose and thus reduce fasting blood glucose levels and increase glucose tolerance (Xue et al., 2013). Besides the improvement of adipocyte function, H2S can protect the adipocytes against high concentration of glucose-induced adipocyte dysfunction, evidenced by the restored monocyte chemotactic protein (MCP)-1 and adiponectin secretion (Pan et al., 2014). In addition, H2S can modulate adipogenesis and adipocyte maturation (Tsai et al., 2015). Hence, the roles of H2S in glucose homeostasis regulated by adipose tissues are very complicated and even contradictory. The reason for this may be related to the amount of H2S, processing time, experimental model, and diabetic development stage.
Future Directions
To Develop More Selective CSE/CBS Modulator
In recent years, H2S has received much increased attentions in biomedical fields because of its functions in physiological and pathophysiological processes. As discussed above, endogenous H2S not only plays many beneficial roles, but also exerts a great number of adverse effects in the body. Thereby, its fine modulation may have great therapeutic significance for some diseases. Genetic approaches are very useful for basal studies in animals or cells, by modulating the deficiency or overexpression of CSE/CBS. However, in clinical trials, pharmacological approaches should be more promising by modulating CSE/CBS expression or activity with a selective agonist/antagonist (Wallace et al., 2009), which is still quite scarce although there are a handful of non-selective ones. Searching for more selective inhibitors or stimulators of CSE/CBS should be attractive for researchers.
To Develop H2S Donor Materials with Targeted Delivery
Another pharmacological approach is what we have mainly focused on in the present article, i.e., H2S releasing agents. Studies in this field have made fantastic progress, especially in recent years. Through this article, we can see that present donors have gradually been improved in water-solubility, stability, releasing controllability, and byproduct toxicity. Nevertheless, one should bear in mind that targeted delivery is critically affecting clinical usage. For example, some compounds have rather unique effects in cellular experiments or local administration in animals, while no effects were found when used systemically. Additionally, it is local rather than systemic H2S changes that lead to the pathology or disease. Consequently, a rise in local H2S content will be more valuable in ex vivo application of H2S. In this regard, ROS-activated (Zhao and Pluth, 2016) and pH-controlled H2S donors (Kang et al., 2016) may partially possess this feature. Targeted delivery of H2S donors or materials should be another exciting research topic.
Conclusion
H2S has been considered as one of the endogenous gaseous signaling molecules with various effects in the body. In some pathological process, e.g., cancer and glycometabolic malfunction, different studies show that H2S may exert different or even opposite actions. The reasons may be complex or even perplexing; thereby more related studies are still required to reveal the mystery. Nevertheless, reduced endogenous H2S has been linked to the cause of many diseases or their stage-dependent injury. Therefore, to enhance endogenous H2S content is of great pharmacological significance with the use of H2S releasing/stimulating reagents. It is gratifying to see the development of H2S releasing reagents has made great progress in recent years. It is anticipated that this will continue to be an active research topic. We expect to see more interesting work arising in this field.
Author Contributions
All authors listed have made substantial contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by National Institutes of Health of United States (R01HL116571) to MX, Natural Science Foundation of Guangdong Province in China (2017A030313892) to C-tY, Science and Technology Planning Project of Guangzhou city in China (201510010201) to C-tY, Projects of Colleges and Universities directly under the Guangzhou Education Bureau (1201620148), National Funds for developing local colleges and universities (B16056001), and Teaching Reform Projects of Higher Education in Guangdong Colleges and Universities (2016430).
References
- Ali M. Y., Whiteman M., Low C. M., Moore P. K. (2007). Hydrogen sulphide reduces insulin secretion from HIT-T15 cells by a KATP channel-dependent pathway. J. Endocrinol. 195 105–112. 10.1677/JOE-07-0184 [DOI] [PubMed] [Google Scholar]
- Amagase H. (2006). Clarifying the real bioactive constituents of garlic. J. Nutr. 136(Suppl. 3) 716S–725S. [DOI] [PubMed] [Google Scholar]
- Barr L. A., Shimizu Y., Lambert J. P., Nicholson C. K., Calvert J. W. (2015). Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide 46 145–156. 10.1016/j.niox.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. O., Jr., Bus J. S., Popp J. A., Boreiko C. J., Andjelkovich D. A. (1984). A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 13 25–97. 10.3109/10408448409029321 [DOI] [PubMed] [Google Scholar]
- Ben Q., Xu M., Ning X., Liu J., Hong S., Huang W., et al. (2011). Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur. J. Cancer 47 1928–1937. 10.1016/j.ejca.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Benavides G. A., Squadrito G. L., Mills R. W., Patel H. D., Isbell T. S., Patel R. P., et al. (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 104 17977–17982. 10.1073/pnas.0705710104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Saha S., Giri K., Lanza I. R., Nair K. S., Jennings N. B., et al. (2013). Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLOS ONE 8:e79167 10.1371/journal.pone.0079167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone E., Morrison M., Roth M. B. (2005). H2S induces a suspended animation-like state in mice. Science 308 518 10.1126/science.1108581 [DOI] [PubMed] [Google Scholar]
- Brewer G. J. (2003). Tetrathiomolybdate anticopper therapy for Wilson’s disease inhibits angiogenesis, fibrosis and inflammation. J. Cell. Mol. Med. 7 11–20. 10.1111/j.1582-4934.2003.tb00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J. (2009). Zinc and tetrathiomolybdate for the treatment of Wilson’s disease and the potential efficacy of anticopper therapy in a wide variety of diseases. Metallomics 1 199–206. 10.1039/b901614g [DOI] [PubMed] [Google Scholar]
- Cai W. J., Wang M. J., Ju L. H., Wang C., Zhu Y. C. (2010). Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol. Int. 34 565–572. 10.1042/cbi20090368 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Jain R. K. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473 298–307. 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M., Kodela R., Nath N., Dastagirzada Y. M., Velazquez-Martinez C. A., Boring D., et al. (2012). Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem. Pharmacol. 83 715–722. 10.1016/j.bcp.2011.12.018 [DOI] [PubMed] [Google Scholar]
- Chauhan P., Bora P., Ravikumar G., Jos S., Chakrapani H. (2017). Esterase activated carbonyl sulfide/hydrogen sulfide (H2S) donors. Org. Lett. 19 62–65. 10.1021/acs.orglett.6b03336 [DOI] [PubMed] [Google Scholar]
- Chen S., Bu D., Ma Y., Zhu J., Sun L., Zuo S., et al. (2016). GYY4137 ameliorates intestinal barrier injury in a mouse model of endotoxemia. Biochem. Pharmacol. 118 59–67. 10.1016/j.bcp.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. (2009). H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284 11601–11612. 10.1074/jbc.M808026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho O., Hwang H. S., Lee B. S., Oh Y. T., Kim C. H., Chun M. (2015). Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor. Radiat. Oncol. J. 33 328–336. 10.3857/roj.2015.33.4.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. (2016). Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 96 365–408. 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah S. C., Moore P. K., Zhu Y. Z. (2007). S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am. J. Physiol. Heart Circ. Physiol. 293H2693–H2701. 10.1152/ajpheart.00853.2007 [DOI] [PubMed] [Google Scholar]
- Ciocci M., Iorio E., Carotenuto F., Khashoggi H. A., Nanni F., Melino S. (2016). H2S-releasing nanoemulsions: a new formulation to inhibit tumor cells proliferation and improve tissue repair. Oncotarget 7 84338–84358. 10.18632/oncotarget.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter T. G. (2009). Apoptosis and cancer: the genesis of a research field. Nat. Rev. Cancer 9 501–507. 10.1038/nrc2663 [DOI] [PubMed] [Google Scholar]
- De Cicco P., Panza E., Ercolano G., Armogida C., Sessa G., Pirozzi G., et al. (2016). ATB-346, a novel hydrogen sulfide-releasing anti-inflammatory drug, induces apoptosis of human melanoma cells and inhibits melanoma development in vivo. Pharmacol. Res. 114 67–73. 10.1016/j.phrs.2016.10.019 [DOI] [PubMed] [Google Scholar]
- DeLeon E. R., Stoy G. F., Olson K. R. (2012). Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem. 421 203–207. 10.1016/j.ab.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Donath M. Y. (2014). Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13 465–476. 10.1038/nrd4275 [DOI] [PubMed] [Google Scholar]
- Donath M. Y. (2016). Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia 59 679–682. 10.1007/s00125-016-3873-z [DOI] [PubMed] [Google Scholar]
- Dyson A., Dal-Pizzol F., Sabbatini G., Lach A. B., Galfo F., dos Santos Cardoso J., et al. (2017). Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLOS Med. 14:e1002310 10.1371/journal.pmed.1002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod J. W., Calvert J. W., Morrison J., Doeller J. E., Kraus D. W., Tao L., et al. (2007). Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 104 15560–15565. 10.1073/pnas.0705891104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N., Paquot N., Scheen A. J. (2015). Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 24 283–307. 10.1517/13543784.2015.974804 [DOI] [PubMed] [Google Scholar]
- Feng S., Zhao Y., Xian M., Wang Q. (2015). Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 27 205–213. 10.1016/j.actbio.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Chen Y., Zhao J., Tang C., Jiang Z., Geng B. (2009). Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem. Biophys. Res. Commun. 380 153–159. 10.1016/j.bbrc.2009.01.059 [DOI] [PubMed] [Google Scholar]
- Fernald K., Kurokawa M. (2013). Evading apoptosis in cancer. Trends Cell Biol. 23 620–633. 10.1016/j.tcb.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M. D., Cunha F. Q., Kashfi K., Cunha T. M. (2015). NOSH-aspirin (NBS-1120), a dual nitric oxide and hydrogen sulfide-releasing hybrid, reduces inflammatory pain. Pharmacol. Res. Perspect. 3:e00133 10.1002/prp2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. C., Matson J. B. (2014). Functionalization of methacrylate polymers with thiooximes: a robust postpolymerization modification reaction and a method for the preparation of H2S-releasing polymers. Macromolecules 47 5089–5095. 10.1021/ma501044b [DOI] [Google Scholar]
- Foster J. C., Radzinski S. C., Zou X., Finkielstein C. V., Matson J. B. (2017). H2S-releasing polymer micelles for studying selective cell toxicity. Mol. Pharm. 14 1300–1306. 10.1021/acs.molpharmaceut.6b01117 [DOI] [PubMed] [Google Scholar]
- Fulda S. (2015). Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 31 84–88. 10.1016/j.semcancer.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Garber K. (2015). BIOMEDICINE. Targeting copper to treat breast cancer. Science 349 128–129. 10.1126/science.349.6244.128 [DOI] [PubMed] [Google Scholar]
- Gong Q. H., Wang Q., Pan L. L., Liu X. H., Xin H., Zhu Y. Z. (2011). S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav. Immun. 25 110–119. 10.1016/j.bbi.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Hasegawa U., van der Vlies A. J. (2014). Design and synthesis of polymeric hydrogen sulfide donors. Bioconjug. Chem. 25 1290–1300. 10.1021/bc500150s [DOI] [PubMed] [Google Scholar]
- Hellmich M. R., Coletta C., Chao C., Szabo C. (2015). The therapeutic potential of cystathionine beta-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 22 424–448. 10.1089/ars.2014.5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda K. M., Burke S. D., Faas M. M., Zsengeller Z., Stillman I. E., Kang P. M., et al. (2014). Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J. Am. Soc. Nephrol. 25 717–725. 10.1681/ASN.2013030291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottinger D. G., Beebe D. S., Kozhimannil T., Prielipp R. C., Belani K. G. (2014). Sodium nitroprusside in 2014: a clinical concepts review. J. Anaesthesiol. Clin. Pharmacol. 30 462–471. 10.4103/0970-9185.142799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Y., Yao W. F., Wu W. G., Lu Y. L., Wan H., Wang W. (2013). Endogenous CSE/H2S system mediates TNF-alpha-induced insulin resistance in 3T3-L1 adipocytes. Cell Biochem. Funct. 31 468–475. 10.1002/cbf.2920 [DOI] [PubMed] [Google Scholar]
- Jia H., Ye J., You J., Shi X., Kang W., Wang T. (2017). Role of the cystathionine beta-synthase/H2S system in liver cancer cells and the inhibitory effect of quinolone-indolone conjugate QIC2 on the system. Oncol. Rep. 37 3001–3009. 10.3892/or.2017.5513 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Ben Q., Shen H., Lu W., Zhang Y., Zhu J. (2011). Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 26 863–876. 10.1007/s10654-011-9617-y [DOI] [PubMed] [Google Scholar]
- Ju Y., Untereiner A., Wu L., Yang G. (2015). H2S-induced S-sulfhydration of pyruvate carboxylase contributes to gluconeogenesis in liver cells. Biochim. Biophys. Acta 1850 2293–2303. 10.1016/j.bbagen.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Kabil O., Vitvitsky V., Xie P., Banerjee R. (2011). The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 15 363–372. 10.1089/ars.2010.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Kimura T., Taniguchi S., Souma M., Kojima Y., Kimura Y., et al. (2009). Glucose-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett. 583 377–382. 10.1016/j.febslet.2008.12.026 [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Kimura Y., Kimura H., Niki I. (2006). L-cysteine inhibits insulin release from the pancreatic beta-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes Metab. Res. Rev. 55 1391–1397. 10.2337/db05-1082 [DOI] [PubMed] [Google Scholar]
- Kang J., Li Z., Organ C. L., Park C. M., Yang C. T., Pacheco A., et al. (2016). pH-Controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 138 6336–6339. 10.1021/jacs.6b01373 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Yu S. H., Cho Y. J., Pan J. H., Cho H. T., Kim J. H., et al. (2017). Preparation of S-allylcysteine-enriched black garlic juice and its antidiabetic effects in streptozotocin-induced insulin-deficient mice. J. Agric. Food Chem. 65 358–363. 10.1021/acs.jafc.6b04948 [DOI] [PubMed] [Google Scholar]
- Kim J. M., Lee J. C., Chang N., Chun H. S., Kim W. K. (2006). S-Allyl-L-cysteine attenuates cerebral ischemic injury by scavenging peroxynitrite and inhibiting the activity of extracellular signal-regulated kinase. Free Radic. Res. 40 827–835. 10.1080/10715760600719540 [DOI] [PubMed] [Google Scholar]
- Kodela R., Chattopadhyay M., Velazquez-Martinez C. A., Kashfi K. (2015a). NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 98 564–572. 10.1016/j.bcp.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodela R., Nath N., Chattopadhyay M., Nesbitt D. E., Velazquez-Martinez C. A., Kashfi K. (2015b). Hydrogen sulfide-releasing naproxen suppresses colon cancer cell growth and inhibits NF-kappaB signaling. Drug Des. Devel. Ther. 9 4873–4882. 10.2147/DDDT.S91116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Bhushan S., King A. L., Prabhu S. D., Hamid T., Koenig S., et al. (2013). H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 127 1116–1127. 10.1161/CIRCULATIONAHA.112.000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Z. W., Zhou J., Chen C. S., Zhao Y., Tan C. H., Li L., et al. (2011). The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLOS ONE 6:e21077 10.1371/journal.pone.0021077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer D. J. (2007). A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. U.S.A. 104 17907–17908. 10.1073/pnas.0709010104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Zhao J., Wang C. Z., Searle J., He T. C., Yuan C. S., et al. (2011). Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 301 185–192. 10.1016/j.canlet.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Bhatia M., Zhu Y. Z., Zhu Y. C., Ramnath R. D., Wang Z. J., et al. (2005). Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 19 1196–1198. 10.1096/fj.04-3583fje [DOI] [PubMed] [Google Scholar]
- Li L., Whiteman M., Guan Y. Y., Neo K. L., Cheng Y., Lee S. W., et al. (2008). Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117 2351–2360. 10.1161/CIRCULATIONAHA.107.753467 [DOI] [PubMed] [Google Scholar]
- Liang D., Wu H., Wong M. W., Huang D. (2015). Diallyl trisulfide is a fast H2S donor, but diallyl disulfide is a slow one: the reaction pathways and intermediates of glutathione with polysulfides. Org. Lett. 17 4196–4199. 10.1021/acs.orglett.5b01962 [DOI] [PubMed] [Google Scholar]
- Lipson K. L., Fonseca S. G., Urano F. (2006). Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr. Mol. Med. 6 71–77. [DOI] [PubMed] [Google Scholar]
- Liu L., Cui J., Song C. J., Bian J. S., Sparatore A., Soldato P. D., et al. (2012). H2S-releasing aspirin protects against aspirin-induced gastric injury via reducing oxidative stress. PLOS ONE 7:e46301 10.1371/journal.pone.0046301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Gao Y., Huang X., Wang X. (2014). GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int. J. Oncol. 44 1259–1267. 10.3892/ijo.2014.2305 [DOI] [PubMed] [Google Scholar]
- Lv W., Yang L., Xu C., Shi Z., Shao J., Xian M., et al. (2017). Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Front. Plant Sci. 8:232 10.3389/fpls.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Liu Y., Zhu Q., Liu C. H., Duan J. L., Tan B. K., et al. (2011). H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLOS ONE 6:e20525 10.1371/journal.pone.0020525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Bi Q., Wang Y. (2015). Hydrogen sulfide accelerates cell cycle progression in oral squamous cell carcinoma cell lines. Oral Dis. 21 156–162. 10.1111/odi.12223 [DOI] [PubMed] [Google Scholar]
- Malumbres M., Barbacid M. (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9 153–166. 10.1038/nrc2602 [DOI] [PubMed] [Google Scholar]
- Manna P., Jain S. K. (2011). Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Czeta/lambda (PKCzeta/lambda) in 3T3l1 adipocytes. J. Biol. Chem. 286 39848–39859. 10.1074/jbc.M111.270884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P., Jain S. K. (2012). Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J. Biol. Chem. 287 42324–42332. 10.1074/jbc.M112.407833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami Y., Shibuya N., Kimura Y., Nagahara N., Ogasawara Y., Kimura H. (2011). Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 439 479–485. 10.1042/BJ20110841 [DOI] [PubMed] [Google Scholar]
- Miki T., Minami K., Zhang L., Morita M., Gonoi T., Shiuchi T., et al. (2002). ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 283 E1178–E1184. 10.1152/ajpendo.00313.2002 [DOI] [PubMed] [Google Scholar]
- Murata T., Sato T., Kamoda T., Moriyama H., Kumazawa Y., Hanada N. (2014). Differential susceptibility to hydrogen sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer cell lines and oral keratinocytes: role of PHLDA1 as an apoptosis suppressor. Exp. Cell Res. 320 247–257. 10.1016/j.yexcr.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Okamoto M., Ishizaki T., Kimura T. (2015). Protective effect of hydrogen sulfide on pancreatic beta-cells. Nitric Oxide 46 32–36. 10.1016/j.niox.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Okamoto M., Yamaoka M., Takei M., Ando T., Taniguchi S., Ishii I., et al. (2013). Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem. Biophys. Res. Commun. 442 227–233. 10.1016/j.bbrc.2013.11.023 [DOI] [PubMed] [Google Scholar]
- Padmanabhan M., Prince P. S. (2006). Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: a histopathological study. Toxicology 224 128–137. 10.1016/j.tox.2006.04.039 [DOI] [PubMed] [Google Scholar]
- Pan Z., Wang H., Liu Y., Yu C., Zhang Y., Chen J., et al. (2014). Involvement of CSE/ H2S in high glucose induced aberrant secretion of adipokines in 3T3-L1 adipocytes. Lipids Health Dis. 13:155 10.1186/1476-511X-13-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza E., De Cicco P., Armogida C., Scognamiglio G., Gigantino V., Botti G., et al. (2015). Role of the cystathionine gamma lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 28 61–72. 10.1111/pcmr.12312 [DOI] [PubMed] [Google Scholar]
- Polhemus D. J., Lefer D. J. (2014). Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 114 730–737. 10.1161/CIRCRESAHA.114.300505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus D. J., Li Z., Pattillo C. B., Gojon G. Sr., Gojon G., Jr., Giordano T., et al. (2015). A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 33 216–226. 10.1111/1755-5922.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. R., Foster J. C., Okyere B., Theus M. H., Matson J. B. (2016). Therapeutic delivery of H2S via COS: small molecule and polymeric donors with benign byproducts. J. Am. Chem. Soc. 138 13477–13480. 10.1021/jacs.6b07204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu K. R., Devan N., Chandrasekaran S. (2002). Chemistry of tetrathiomolybdate: applications in organic synthesis Synlett 2002 1762–1778. 10.1055/s-2002-34863 [DOI] [Google Scholar]
- Rose P., Moore P. K., Ming S. H., Nam O. C., Armstrong J. S., Whiteman M. (2005). Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 11 3990–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C., Zwingmann C., Jaeschke H. (2010). Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51 246–254. 10.1002/hep.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. K., Shah M. A. (2005). Targeting the cell cycle: a new approach to cancer therapy. J. Clin. Oncol. 23 9408–9421. 10.1200/jco.2005.01.5594 [DOI] [PubMed] [Google Scholar]
- Sheffner A. L. (1963). The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-L-cysteine. Ann. N. Y. Acad. Sci. 106 298–310. [DOI] [PubMed] [Google Scholar]
- Shukla P., Khodade V. S., SharathChandra M., Chauhan P., Mishra S., Siddaramappa S., et al. (2017). “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci. 8 4967–4972. 10.1039/C7SC00873B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Padovani D., Leslie R. A., Chiku T., Banerjee R. (2009). Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284 22457–22466. 10.1074/jbc.M109.010868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A. K., Marcatti M., Szabo C., Szczesny B., Pluth M. D. (2017a). Inhibition of mitochondrial bioenergetics by esterase-triggered COS/H2S donors. ACS Chem. Biol. 12 2117–2123. 10.1021/acschembio.7b00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A. K., Pardue S., Kevil C. G., Pluth M. D. (2016). Self-immolative thiocarbamates provide access to triggered H2S donors and analyte replacement fluorescent probes. J. Am. Chem. Soc. 138 7256–7259. 10.1021/jacs.6b03780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A. K., Zhao Y., Pluth M. D. (2017b). Emerging roles of carbonyl sulfide in chemical biology: sulfide transporter or gasotransmitter? Antioxid. Redox Signal. 10.1089/ars.2017.7119 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C. (2007). Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6 917–935. 10.1038/nrd2425 [DOI] [PubMed] [Google Scholar]
- Szabo C. (2012). Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 17 68–80. 10.1089/ars.2011.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Coletta C., Chao C., Modis K., Szczesny B., Papapetropoulos A., et al. (2013). Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 110 12474–12479. 10.1073/pnas.1306241110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Hellmich M. R. (2013). Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle 12 2915–2916. 10.4161/cc.26064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Zhang L., Yang G., Wu L., Wang R. (2013). Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 56 533–541. 10.1007/s00125-012-2806-8 [DOI] [PubMed] [Google Scholar]
- Tang X. Q., Yang C. T., Chen J., Yin W. L., Tian S. W., Hu B., et al. (2008). Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin. Exp. Pharmacol. Physiol. 35 180–186. 10.1111/j.1440-1681.2007.04799.x [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Kang L., Kimura T., Niki I. (2011). Hydrogen sulphide protects mouse pancreatic beta-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br. J. Pharmacol. 162 1171–1178. 10.1111/j.1476-5381.2010.01119.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S., Niki I. (2011). Significance of hydrogen sulfide production in the pancreatic beta-cell. J. Pharmacol. Sci. 116 1–5. 10.1254/jphs.11R01CP [DOI] [PubMed] [Google Scholar]
- Tao B. B., Liu S. Y., Zhang C. C., Fu W., Cai W. J., Wang Y., et al. (2013). VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid. Redox Signal. 19 448–464. 10.1089/ars.2012.4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayo F. M. (1976). Failure of glibenclamide to stimulate glucose uptake by adipose tissue in vitro West. Afr. J. Pharmacol. Drug Res. 3 79–80. [PubMed] [Google Scholar]
- Toombs C. F., Insko M. A., Wintner E. A., Deckwerth T. L., Usansky H., Jamil K., et al. (2010). Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br. J. Clin. Pharmacol. 69 626–636. 10.1111/j.1365-2125.2010.03636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. Y., Peh M. T., Feng W., Dymock B. W., Moore P. K. (2015). Hydrogen sulfide promotes adipogenesis in 3T3L1 cells. PLOS ONE 10:e0119511 10.1371/journal.pone.0119511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilidis K. K., Kasimis J. C., Lopez D. S., Ntzani E. E., Ioannidis J. P. (2015). Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607 10.1136/bmj.g7607 [DOI] [PubMed] [Google Scholar]
- Untereiner A., Wu L. (2017). Hydrogen sulfide and glucose homeostasis: a tale of sweet and the stink. Antioxid. Redox Signal. 10.1089/ars.2017.7046 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Untereiner A. A., Wang R., Ju Y., Wu L. (2016). Decreased gluconeogenesis in the absence of cystathionine gamma-lyase and the underlying mechanisms. Antioxid. Redox Signal. 24 129–140. 10.1089/ars.2015.6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini F., MacKessack-Leitch A. C., Eschbach E. K., Chattopadhyay M., Kodela R., Kashfi K. (2015). Synthesis and anti-cancer potential of the positional isomers of NOSH-aspirin (NBS-1120) a dual nitric oxide and hydrogen sulfide releasing hybrid. Bioorg. Med. Chem. Lett. 25 4677–4682. 10.1016/j.bmcl.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K., Georgieff M., Asfar P., Calzia E., Knoferl M. W., Radermacher P. (2011). Of mice and men (and sheep, swine etc.): the intriguing hemodynamic and metabolic effects of hydrogen sulfide (H2S). Crit. Care 15 146 10.1186/cc10079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Blackler R. W., Chan M. V., Da Silva G. J., Elsheikh W., Flannigan K. L., et al. (2015). Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antioxid. Redox Signal. 22 398–410. 10.1089/ars.2014.5901 [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Vong L., McKnight W., Dicay M., Martin G. R. (2009). Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137 569–578 578.e1 10.1053/j.gastro.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Wang R. (2015). Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 14 329–345. 10.1038/nrd4433 [DOI] [PubMed] [Google Scholar]
- Wang H. W., Skeldon P., Thompson G. E., Wood G. C. (1997). Synthesis and characterization of molybdenum disulphide formed from ammonium tetrathiomolybdate. J. Mater. Sci. 32 497–502. 10.1023/A:1018538424373 [DOI] [Google Scholar]
- Wang M., Tang W., Xin H., Zhu Y. Z. (2016). S-propargyl-cysteine, a novel hydrogen sulfide donor, inhibits inflammatory hepcidin and relieves anemia of inflammation by inhibiting IL-6/STAT3 pathway. PLOS ONE 11:e0163289 10.1371/journal.pone.0163289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. J., Cai W. J., Li N., Ding Y. J., Chen Y., Zhu Y. C. (2010). The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid. Redox Signal. 12 1065–1077. 10.1089/ars.2009.2945 [DOI] [PubMed] [Google Scholar]
- Wang Q., Liu H. R., Mu Q., Rose P., Zhu Y. Z. (2009). S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: the contribution of the hydrogen sulfide-mediated pathway. J. Cardiovasc. Pharmacol. 54 139–146. 10.1097/FJC.0b013e3181ac8e12 [DOI] [PubMed] [Google Scholar]
- Wang R. (2012). Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92 791–896. 10.1152/physrev.00017.2011 [DOI] [PubMed] [Google Scholar]
- Wang X. Y., Yang C. T., Zheng D. D., Mo L. Q., Lan A. P., Yang Z. L., et al. (2012). Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol. Cell. Biochem. 363 419–426. 10.1007/s11010-011-1194-6 [DOI] [PubMed] [Google Scholar]
- Weis S. M., Cheresh D. A. (2011). Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17 1359–1370. 10.1038/nm.2537 [DOI] [PubMed] [Google Scholar]
- Wilinski B., Wilinski J., Somogyi E., Piotrowska J., Opoka W. (2012). Vitamin D3 (cholecalciferol) boosts hydrogen sulfide tissue concentrations in heart and other mouse organs. Folia Biol. 60 243–247. [DOI] [PubMed] [Google Scholar]
- Wu J., Guo W., Lin S. Z., Wang Z. J., Kan J. T., Chen S. Y., et al. (2016a). Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell Death Dis. 7 e2339. 10.1038/cddis.2016.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Li Y., He C., Kang J., Ye J., Xiao Z., et al. (2016b). Novel H2S releasing nanofibrous coating for in vivo dermal wound regeneration. ACS Appl. Mater. Interfaces 10.1021/acsami.6b06466 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wu L., Yang W., Jia X., Yang G., Duridanova D., Cao K., et al. (2009). Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Invest. 89 59–67. 10.1038/labinvest.2008.109 [DOI] [PubMed] [Google Scholar]
- Xu S., Yang C. T., Meng F. H., Pacheco A., Chen L., Xian M. (2016). Ammonium tetrathiomolybdate as a water-soluble and slow-release hydrogen sulfide donor. Bioorg. Med. Chem. Lett. 26 1585–1588. 10.1016/j.bmcl.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wu J., Mao Y., Zhu Y., Hu Z., Xu X., et al. (2013). Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLOS ONE 8:e58079 10.1371/journal.pone.0058079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Hao D. D., Sun J. P., Li W. W., Zhao M. M., Li X. H., et al. (2013). Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid. Redox Signal. 19 5–23. 10.1089/ars.2012.5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P. K., Yamada K., Chiku T., Koutmos M., Banerjee R. (2013). Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 288 20002–20013. 10.1074/jbc.M113.466177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Yang Z., Zhang M., Dong Q., Wang X., Lan A., et al. (2011). Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-kappaB/COX-2 pathway. PLOS ONE 6:e21971 10.1371/journal.pone.0021971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. T., Lai Z. Z., Zheng Z. H., Kang J. M., Xian M., Wang R. Y., et al. (2017a). A novel pH-controlled hydrogen sulfide donor protects gastric mucosa from aspirin-induced injury. J. Cell. Mol. Med. 10.1111/jcmm.13166 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. T., Meng F. H., Chen L., Li X., Cen L. J., Wen Y. H., et al. (2017b). Inhibition of methylglyoxal-induced AGEs/RAGE expression contributes to dermal protection by N-acetyl-L-cysteine. Cell. Physiol. Biochem. 41 742–754. 10.1159/000458734 [DOI] [PubMed] [Google Scholar]
- Yang C. T., Zhao Y., Xian M., Li J. H., Dong Q., Bai H. B., et al. (2014). A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction. Cell. Physiol. Biochem. 34 1304–1317. 10.1159/000366339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., et al. (2008). H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322 587–590. 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Yang W., Wu L., Wang R. (2007). H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 282 16567–16576. 10.1074/jbc.M700605200 [DOI] [PubMed] [Google Scholar]
- Yang H., Mao Y., Tan B., Luo S., Zhu Y. (2015). The protective effects of endogenous hydrogen sulfide modulator, S-propargyl-cysteine, on high glucose-induced apoptosis in cardiomyocytes: a novel mechanism mediated by the activation of Nrf2. Eur. J. Pharmacol. 761 135–143. 10.1016/j.ejphar.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Yang W., Yang G., Jia X., Wu L., Wang R. (2005). Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 569(Pt 2) 519–531. 10.1113/jphysiol.2005.097642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P., Zhao C., Li Z., Mei C., Yao W., Liu Y., et al. (2012). Sp1 is involved in regulation of cystathionine gamma-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell. Signal. 24 1229–1240. 10.1016/j.cellsig.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Yusuf M., Kwong Huat B. T., Hsu A., Whiteman M., Bhatia M., Moore P. K. (2005). Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem. Biophys. Res. Commun. 333 1146–1152. 10.1016/j.bbrc.2005.06.021 [DOI] [PubMed] [Google Scholar]
- Zanardo R. C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J. L. (2006). Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 20 2118–2120. 10.1096/fj.06-6270fje [DOI] [PubMed] [Google Scholar]
- Zhang G., Panigrahy D., Mahakian L. M., Yang J., Liu J. Y., Stephen Lee K. S., et al. (2013). Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. U.S.A. 110 6530–6535. 10.1073/pnas.1304321110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhuang X. D., Meng F. H., Chen L., Dong X. B., Liu G. H., et al. (2016). Calcitriol prevents peripheral RSC96 Schwann neural cells from high glucose & methylglyoxal-induced injury through restoration of CBS/H2S expression. Neurochem. Int. 92 49–57. 10.1016/j.neuint.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang X. M., Chen Y. H., Yao W. Z. (2014). Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology 19 1165–1169. 10.1111/resp.12372 [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang X. M., Chen Y. H., Yao W. Z. (2015). Exhaled hydrogen sulfide predicts airway inflammation phenotype in COPD. Respir. Care 60 251–258. 10.4187/respcare.03519 [DOI] [PubMed] [Google Scholar]
- Zhang L., Yang G., Untereiner A., Ju Y., Wu L., Wang R. (2013). Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology 154 114–126. 10.1210/en.2012-1658 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bhushan S., Yang C., Otsuka H., Stein J. D., Pacheco A., et al. (2013). Controllable hydrogen sulfide donors and the activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 8 1283–1290. 10.1021/cb400090d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Biggs T. D., Xian M. (2014). Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem. Commun. 50 11788–11805. 10.1039/c4cc00968a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Pluth M. D. (2016). Hydrogen sulfide donors activated by reactive oxygen species. Angew. Chem. Int. Ed. Engl. 55 14638–14642. 10.1002/anie.201608052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yang C., Organ C., Li Z., Bhushan S., Otsuka H., et al. (2015). Design, synthesis, and cardioprotective effects of N-mercapto-based hydrogen sulfide donors. J. Med. Chem. 58 7501–7511. 10.1021/acs.jmedchem.5b01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y., Pan W., Hu F., Wu H., Feng J., Zhang Y., et al. (2015). Exogenous hydrogen sulfide exerts proliferation/anti-apoptosis/angiogenesis/migration effects via amplifying the activation of NF-kappaB pathway in PLC/PRF/5 hepatoma cells. Int. J. Oncol. 46 2194–2204. 10.3892/ijo.2015.2914 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Yu B., De La Cruz L. K., Choudhury M. R., Anifowose A., Wang B. (2017). Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med. Res. Rev. 10.1002/med.21433 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Yu B., Ji K., Pan Z., Chittavong V., Wang B. (2016). Esterase-sensitive prodrugs with tunable release rates and direct generation of hydrogen sulfide. Angew. Chem. Int. Ed. Engl. 55 4514–4518. 10.1002/anie.201511244 [DOI] [PMC free article] [PubMed] [Google Scholar]


