Abstract
Fumigant toxicity and sublethal effects of essential oils from Artemisia khorassanica Podl. and Artemisia sieberi Bess were investigated against adults of Sitotroga cerealella Olivier. To assess the sublethal effects, adult moths were exposed to the LC30 of each essential oil, and life table parameters of the surviving S. cerealella were studied. Higher fumigant toxicity of A. khorassanica (LC50: 7.38 µl/liter air) than A. sieberi (LC50: 9.26 µl/liter air) was observed against S. cerealella. Also, the insecticidal effects of A. khorassanica (LT50: 9.01 h) were faster than A. sieberi (LT50: 14.37 h). A significant extension was observed in the developmental time (egg to adult) of S. cerealella treated with the essential oils. In addition, fecundity of S. cerealella reduced by 25.29 and 35.78% following exposure to sublethal concentrations of A. sieberi and A. khorassanica, respectively. Both tested essential oils caused a significant reduction in the gross and net reproductive rates, intrinsic rate of increase (rm), and finite rate of increase of S. cerealella. The rm values following exposure to A. sieberi, A. khorassanica, and control were 0.098, 0.094, and 0.107 d−1, respectively. The results of this study suggest that tested essential oils have a good potential to apply in integrated pest management of S. cerealella.
Keywords: Sitotroga cerealella, botanical insecticide, population parameter, compositae
The Angoumois grain moth, Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae), is one of the most important and destructive stored product pests in the world (Shukle and Wu 2003). Feeding of S. cerealella larvae and producing fecal matter cause high crop damages on cereal grains (Throne and Weaver 2013).
The control of stored product insect pests is mainly based on the application of chemical insecticides, such as pyrethroids, phosphine, and dichlorvos (White and Leesch 1995, Nayak et al. 2003, Hori and Kasaishi 2005). However, the improper and indiscriminate application of these insecticides led to the long-term undesirable effects on human health, environment, and nontarget animals (Champ and Dyte 1977, Subramanyam and Hagstrum 1995, White and Leesch 1995). Because of the potential genotoxicity of phosphine and high toxicity to warm-blooded animals and potential ozone-depleting property of methyl bromide, the use of these insecticides became restricted (Dansi et al. 1984, Anonymous 1991, Bell and Wilson 1995, Meaklim 1998). Therefore, it seems wise to develop new types of safe and ecofriendly alternative management programs. Insecticide potential of many plant essential oils and their components has been investigated to be developed as new safe fumigants. These insecticides have many advantages over synthetic insecticides viz. low mammalian toxicity, rapid degradation, low pernicious effect on environment, and local availability (Isman 2006).
The toxicity of a large number of essential oils and their constituents has been evaluated against stored product insects (Hori and Kasaishi 2005, Rajendran and Srianjini 2008; Yang et al. 2014). The Artemisia genus is one of the largest and most widely distributed genera of the family Compositae (Kordali et al. 2006). Earlier studies showed that the essential oils of Artemisia have antifeedant activity, repellency, and insecticidal effects on the insects (Tripathi et al. 2000; Negahban et al. 2006, 2007; Borzoui et al. 2016). Moreover, essential oils and their constituents could have sublethal effects on the development, fecundity, survival, and life table parameters of stored product insects (Kordali et al. 2006, Stamopoulosa et al. 2007, Izakmehri et al. 2013, Borzoui et al. 2016, Nouri-Ganbalani and Borzoui 2017).
To date, no published information is available regarding the fumigant toxicity and sublethal effects of Artemisia species against S. cerealella. Therefore, the aim of this study was to assess the lethal effects of essential oils from Artemisia khorassanica Podl. and Artemisia sieberi Bess against S. cerealella under laboratory conditions. Also, the sublethal effects of these essential oils were studied on life history and life table parameters of this pest. The results of this study could be useful in the management programs of S. cerealella.
Materials and Methods
Insect Rearing
The initial population of S. cerealella was collected from stored wheat seeds from Ardabil, Iran. The insect’s population was reared on wheat seeds and maintained at 27 ± 2°C, relative humidity of 65 ± 5%, and a photoperiod of 14:10 (L:D) h. Before beginning of the experiments, the population of S. cerealella was reared for three generations on wheat (Gaskojen) cultivar.
Plant Materials and Extraction of Essential Oils
The essential oils of A. khorassanica and A. sieberi were extracted according to the method of Sahaf et al. (2008). The leaves of A. khorassanica were collected at full-flowering stage from Sabzevar region, Khorasane-Razavi (Iran), on June 2015. Also, the leaves of A. sieberi were collected during May 2015 from Ardabil region (Iran). The aerial parts of the plants were air-dried (at 26–28°C) in shadow for 1 wk. The dried leaves were then powdered and stored in sealed bags at 4°C until use. The ground powders were hydrodistilled using modified Clevenger-type apparatus and extracted with water for 4 h. Each hydrodistillation process was conducted using 50 g of ground powder and 600 ml of distilled water. Anhydrous sodium sulfate was used to remove water after extraction. The extracted oils were kept at 4°C and used in the experiments. The oil yield (1% w/w) was calculated on a dry weight basis.
Fumigant Toxicity Bioassay
The fumigant toxicity was studied to determine the median lethal concentrations of A. khorassanica and A. sieberi against adult of S. cerealella. After preliminary tests, the concentrations 12, 10.52, 9.16, 7.96, 6.96, and 6 µl/liter air for A. khorassanica and A. sieberi were calculated to apply as appropriate concentrations against adult of S. cerealella. Whatman filter papers (No. 1, diameter 2.0 cm) were impregnated with different concentrations, and then they were placed on the bottom of the screw cap of a glass vial (250 ml). Then, the caps were screwed onto the bottle, each of which included 15 adults (<24-h old). All treatments were then transferred to the incubator set at 27 ± 2°C, relative humidity of 65 ± 5%, and a photoperiod of 14:10 (L:D) h for 24 h. Control insects were kept under same conditions except for exposure to the essential oils. The number of dead insects was counted 24 h after exposure to the essential oils. Each concentration was replicated three times, and 15 adults were used in each concentration and control. The bioassay experiments were repeated three times.
To assay LT50 values, the highest concentration (12 µl/liter air) of A. khorassanica and A. sieberi essential oils was used against S. cerealella (Izakmehri et al. 2013). The LT50 assay was carried out in vials and conditions described above. The mortality of S. cerealella was checked at 6-h intervals. The control insects received all the conditions except for exposure to the essential oils. The experiments were performed in three replicates.
Effects of Essential Oils Low Concentration on Life Table Parameters
To assay the sublethal effects of A. khorassanica and A. sieberi essential oils on the life table parameters of S. cerealella, the LC30 were used because it is the mortality threshold (30%) suggested for insecticides use in integrated pest management (Desneux et al. 2006). The number of 110 newly mated adults (<24-h old) was exposed to the LC30 of A. khorassanica (6.34 µl/liter air) and A. sieberi (9.24 µl/liter air) using the exposure system described in the section ‘Fumigant toxicity bioassay’ for 24 h. After exposure, 25 pairs of adults (male and female) were singly transferred into the clean ovipostion containers (5 × 10 cm [diameter by depth]). Honey solution (10%) smeared on a cotton was provided for the adults’ feeding.
The oviposition containers were then inversely placed on the paper sheets (as an oviposition surface) and the paper sheets were replaced daily with new ones. The number of eggs laid was recorded every 24 h until the adult moths died, and recorded data were used for calculating life history and life table parameters. The developmental time, adult longevity, and fecundity (25 replicates) were recorded until the death of the last moth.
Data Analysis
The result of each trial was tested for curve fit using PROC GENMOD procedures (SAS Institute 2002, Robertson et al. 2007), and the data were analyzed using PROC PROBIT (SAS Institute 2002) to determine lethal concentrations (LC30, LC50, and LC90, values) on standard and log scales with associated 95 % fiducial limits. In addition, mortality of S. cerealella exposed to different concentrations of the tested essential oils was analyzed by one-way analysis of variance (ANOVA). If significant differences were detected, the means were separated at α = 0.05 by least significant difference (LSD) test. The data of LT50 were analyzed using SAS v. 9.2 program (PROC GLM, SAS Institute 2002) as mentioned for LC50. Resulting data were analyzed based on the age-stage, two-sex life table model developed by Chi and Liu (1985) and Chi (1988). The age-stage-specific survival rate (sxj) (where x = age in days and j = stage [stage 1 = egg, stage 2 = larva–pupa, stage 3 = adult female, stage 4 = adult male]) and the age-stage-specific fecundity (fxj) (daily number of eggs produced per female of age x) were calculated using the iterative bisection method described by Chi and Liu (1985). Bootstrap method was used to estimate the means, variances, and SEs of the population parameters (Efron and Tibshirani 1993). The obtained data were then analyzed by one-way ANOVA followed by comparison of the means with LSD and t-test at α = 0.05 using statistical software SAS version 9.2.
Results
Fumigant Toxicity Bioassay
The fumigant toxicity of essential oils from A. khorassanica and A. sieberi against adult of S. cerealella is shown in Table 1. The essential oil of A. khorassanica (LC50: 7.38 µl/liter air) caused higher mortality than A. sieberi (LC50: 9.26 µl/liter air) against adults of S. cerealella. The percentage mortality of S. cerealella exposed to different concentrations of tested essential oils is given in Table 2. As expected, the highest and lowest mortality of S. cerealella adults belong to the highest and lowest concentrations of A. khorassanica and A. sieberi essential oils. The percentage mortality of S. cerealella by different concentrations of A. khorassanica (F = 38.27; df = 5,17; P < 0.0001) and A. sieberi (F = 23.37; df = 5, 17; P < 0.0001) essential oils was significantly different. However, the percentage mortality of S. cerealella by A. khorassanica and A. sieberi essential oils was not significantly different at 12 (F = 6.12; df = 1, 5; P = 0.068) and 10.52 (F = 6.12; df = 1, 5; P = 0.068) µl/liter air concentrations (Table 2).
Table 1.
Fumigant toxicity of Artemisia khorassanica and Artemisia sieberi essential oils against the adult stage of Sitotroga cerealella
| Essential oil | n a | χ2 | Slope ± SE | Lethal concentrations (µL/liter air) | ||
|---|---|---|---|---|---|---|
| LC30 (95% FL) | LC50 (95% FL) | LC90 (95% FL) | ||||
| A. khorassanica | 315 | 31.49 | 4.86 ± 0.87 | 5.76 (4.61–6.48) | 7.38 (6.58–8.00) | 13.54 (11.74–17.85) |
| A. sieberi | 315 | 34.61 | 5.28 ± 0.91 | 7.37 (6.50–7.98) | 9.26 (8.60–10.11) | 16.20 (13.72–22.27) |
Lethal concentrations and 95% fiducial limits (FL) were estimated using logistic regression (SAS Institute 2002).
aThe total number of adult moths used for bioassay.
Table 2.
Mean (±SE) percentage mortality of S. cerealella exposed to different concentrations of A. khorassanica and A. sieberi essential oils
| Concentration (µL/liter air) | Treatment | |
|---|---|---|
| A. khorassanica | A. sieberi | |
| 12 | 91.11 ± 2.22 aA | 75.51 ± 5.87 aA |
| 10.52 | 75.56 ± 4.44 bA | 68.90 ± 5.87 aA |
| 9.16 | 64.44 ± 2.23 cA | 42.22 ± 2.18 bB |
| 7.96 | 55.56 ± 2.14 cdA | 35.56 ± 4.19 bcB |
| 6.96 | 48.89 ± 2.24 deA | 31.11 ± 2.20 bcB |
| 6 | 40.01 ± 3.84 eA | 22.22 ± 2.21cB |
Mean values in a column followed by different lowercase letters (LSD test, P < 0.05) and in a row followed by different uppercase letters (t-test, P < 0.05) are significantly different.
The LT50 values of A. khorassanica and A. sieberi essential oils on S. cerealella are listed in Table 3. The results indicated that the insecticidal effects of A. khorassanica oil (LT50: 9.01 h) were faster than A. sieberi (LT50: 14.37 h).
Table 3.
LT30, LT50 and LT90 values of A. khorassanica and A. sieberi essential oils against the adult stage of S. cerealella
| Essential oil | Concentration (µL/liter air) | χ2 | Slope ± SE | Lethal times (h) | ||
|---|---|---|---|---|---|---|
| LT30 (95% FL) | LT50 (95% FL) | LT90 (95% FL) | ||||
| A. khorassanica | 12 | 65.91 | 3.43 ± 0.42 | 6.34 (4.95–7.51) | 9.01 (7.63–10.28) | 21.29 (18.14–26.83) |
| A. sieberi | 12 | 45.60 | 3.20 ± 0.47 | 9.24 (7.06–10.94) | 14.37 (12.32–16.80) | 42.28 (31.57–72.05) |
Lethal times and 95% fiducial limits (FL) were estimated using logistic regression (SAS Institute 2002).
Effects of Essential Oils Low Concentration on Life Table Parameters
A significant increase was observed in egg incubation, larval–pupal period, and developmental time in treated insects when compared with the control. Moreover, adult life history parameters, such as female longevity, male longevity, fecundity, and oviposition period, were significantly reduced in treated insects when compared with the control (Table 4).
Table 4.
Sublethal effects (LC30) of A. khorassanica and A. sieberi essential oils on life history of S. cerealella
| Parameter (mean ± SE) | Treatment | Statistics of ANOVA | ||||
|---|---|---|---|---|---|---|
| A. khorassanica (5.76 µL/liter air) | A. Sieberi (7.37 µL/liter air) | Control | F | df | P | |
| Egg incubation (d) | 4.86 ± 0.12 a | 4.58 ± 0.09 b | 4 .24 ± 0.089 c | 10.22 | 2, 149 | <0.0001 |
| Larval and pupal period (d) | 24.24 ± 0.18 a | 23.96 ± 0.15 a | 23.16 ± 0.24 b | 8.75 | 2, 149 | <0.0001 |
| Developmental time (d) | 29.10 ± 0.22 a | 28.54 ± 0.17 a | 27.40 ± 0.25 b | 16.67 | 2, 149 | <0.0001 |
| Female longevity (d) | 6.64 ± 0.31b | 6.48 ± 0.28 b | 8.4 ± 0.31 a | 12.93 | 2, 74 | <0.0001 |
| Male longevity (d) | 5.24 ± 0.22 c | 6.01 ± 0.26 b | 7.76 ± 0.32 a | 24.04 | 2, 74 | <0.0001 |
| Fecundity (eggs laid) | 37.48 ± 2.49 b | 43.60 ± 1.95 b | 58.36 ± 2.96 a | 18.47 | 2, 74 | <0.0001 |
| Oviposition period (d) | 4.01 ± 0.26 c | 4.80 ± 0.24 b | 6.12 ± 0.21 a | 22.76 | 2, 74 | <0.0001 |
Mean values in a row followed by different lowercase letters are significantly different (LSD test).
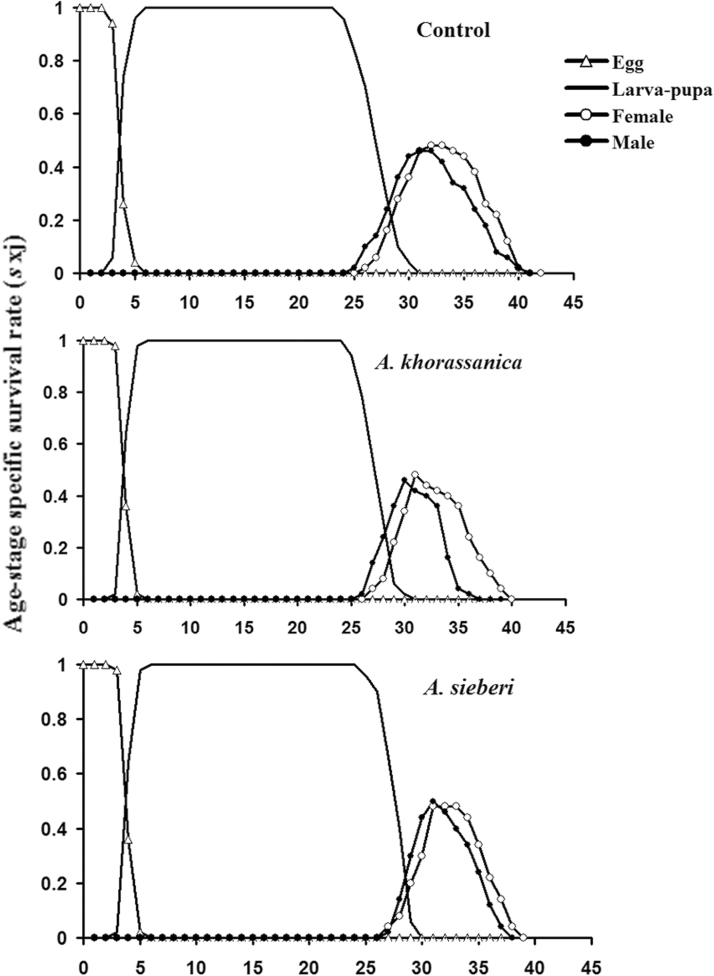
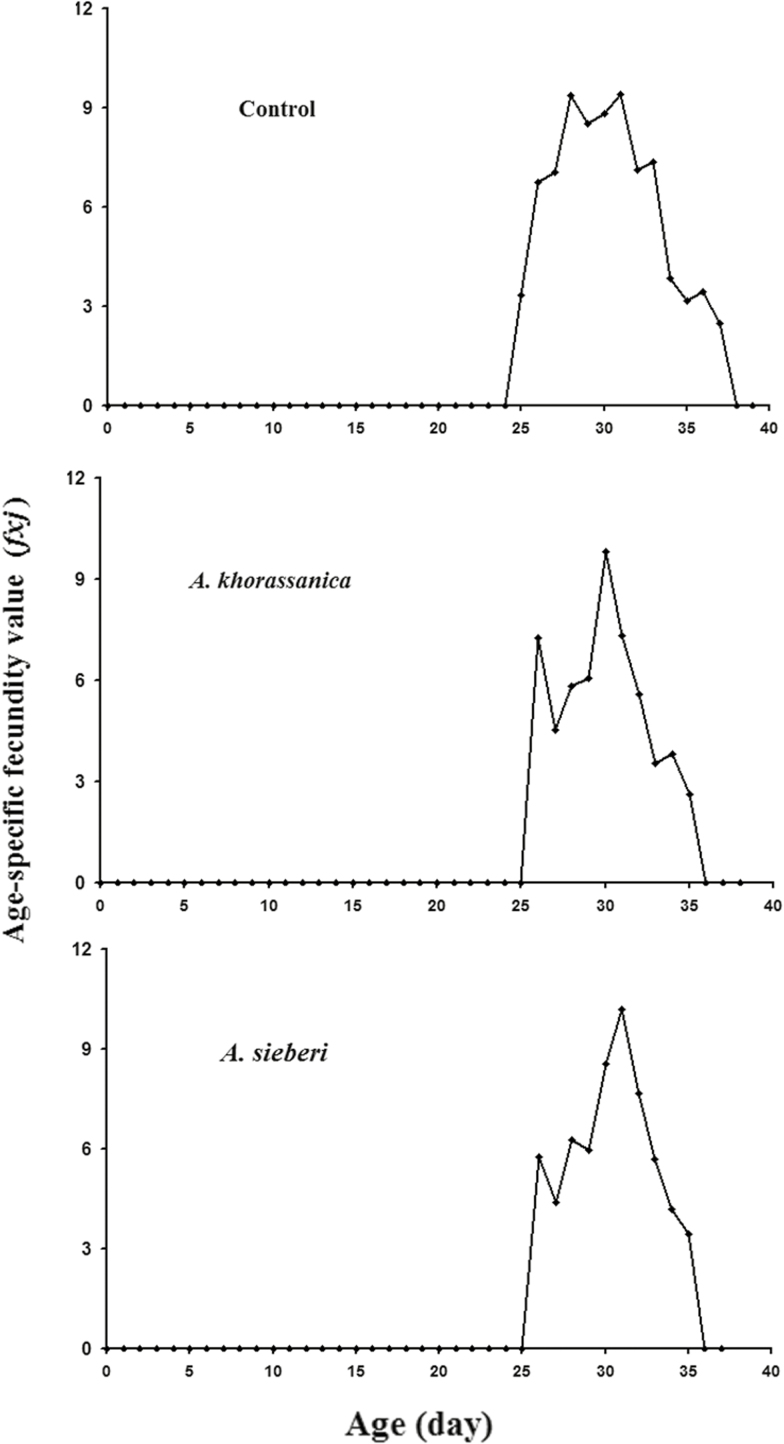
The age-stage-specific survival rate (sxj) of S. cerealella exposed to tested essential oils is shown in Fig. 1. Noticeable stage overlapping was observed because of variable development rates among the treatments. The highest sxj of larva–pupa, adult male, and female of S. cerealella was in the control insects (Fig. 1). The age-stage-specific fecundity (fx3) gives daily number of offspring produced by S. cerealella individual at age x and stage j. As only females produce offspring, there is only a single-curve fx3 (i.e., the adult female is the third life stage). The maximum fx3 in adults treated with A. khorassanica and A. sieberi oils, and control was 9.82, 10.21, and 9.42 eggs female−1 d−1, respectively, that occurred at the ages of 31, 30, and 31 d, respectively (Fig. 2).
Fig. 1.
Age-stage-specific survival rate (sxj) of Sitotroga cerealella after exposure to LC30 of Artemisia khorassanica and Artemisia sieberi essential oils.
Fig. 2.
Age-stage-specific fecundity (fxj) of Sitotroga cerealella after exposure to LC30 of Artemisia khorassanica and Artemisia sieberi essential oils.
The results of sublethal effects of A. khorassanica and A. sieberi essential oils on the two-sex life table attributes of S. cerealella are listed in Table 5. The sublethal concentration of both tested essential oils caused a significant reduction in gross reproductive rate, net reproductive rate (R0), intrinsic rate of increase (rm), and finite rate of increase (λ). However, sublethal concentrations of A. sieberi essential oil caused significant increase in generation time (T) of S. cerealella.
Table 5.
Sublethal effects (LC30) of A. khorassanica and A. sieberi essential oils on two-sex life table parameters of S. cerealella
| Parameter (mean ± SE) | Treatment | Statistics of ANOVA | ||||
|---|---|---|---|---|---|---|
| A. khorassanica (5.76 µL/liter air) | A. sieberi (7.37 µL/ liter air) | Control | F | df | P | |
| Gross reproductive rate (offspring) | 26.22 ± 0.172 b | 26.23 ± 0.187 b | 34.36 ± 0.241 a | 539.84 | 2, 1499 | <0.0001 |
| Net reproductive rate (R0) (offspring) | 18.81 ± 0.129 c | 21.84 ± 0.148 b | 28.91 ± 0.202 a | 1015.34 | 2, 1499 | <0.0001 |
| Intrinsic rate of increase (rm) (d−1) | 0.094 ± 0.0028 c | 0.098 ± 0.0022 b | 0.107 ± 0.0024 a | 914.65 | 2, 1499 | <0.0001 |
| Finite rate of increase (λ) (d−1) | 1.098 ± 0.0018 c | 1.103 ± 0.0025 b | 1.113 ± 0.0014 a | 918.32 | 2, 1499 | <0.0001 |
| Generation time (T) (d) | 31.089 ± 0.0149 c | 31.375 ± 0.0126 a | 31.203 ± 0.016 b | 97.49 | 2, 1499 | <0.0001 |
Mean values in a row followed by different lowercase letters are significantly different (LSD test).
Discussion
Plant essential oils have a high volatility and could be used as fumigants to control stored products insects. Because of low toxicity on mammals and nontarget species and no detrimental effects on environment, plant essential oils are a suitable alternative for chemical insecticides. The essential oils of A. khorassanica and A. sieberi were rich in monoterpenoids and esters. For example, camphor and 1,8-cineole were reported as major constituents of A. khorassanica and A. sieberi essential oils (Borzoui et al. 2016, Nouri-Ganbalani and Borzoui 2017), and could be used as new natural fumigants in controlling stored product insects (Obeng-Ofori et al. 1997, 1998; Lee et al. 2004; Rozman et al. 2007; Abdelgaleil et al. 2009; Wang et al. 2009). The mortality effects of plant essential oils are highly dependent to their constituents (Ahn et al. 1998; Lee et al. 2002a,b). In the earlier studies, the high mortality effects of Artemisia species were reported against different stored products insects (Kordali et al. 2006, Wang et al. 2006, Negahban et al. 2007, Borzoui et al. 2016).
The results of LC50 values showed higher toxicity of A. khorassanica essential oil than A. sieberi. Similar results were obtained by Borzoui et al. (2016), who found that A. khorassanica (LC50: 9.6 µl/liter air) essential oil had more insecticidal effect than Vitex pseudo-negundo (Hausskn) (LC50: 23.05 µl/liter air) against Plodia interpunctella Hübner. By comparing the LC50 values, toxicity of A. khorassanica essential oil (LC50: 7.38 µl/liter air) on S. cerealella, in this study, was higher than that reported for P. interpunctella (LC50: 9.60 µl/liter air) (Borzoui et al. 2016) and Tribolium confusum Jacquelin du Val (LC50: 22.45 µl/liter air) (Saeidi and Moharramipour 2013). Differences in the tested insect species and in the experimental conditions could explain such inconsistency. Moreover, fumigant toxicity of A. sieberi essential oil (LC50: 9.26 µl/liter air), in this study, was higher than that reported for Tribolium castaneum Herbst (LC50: 16.76 µl/liter) (Negahban et al. 2007) and Trogoderma granarium Everts (LC50: 33.50 µl/liter) (Nouri-Ganbalani and Borzoui 2017), suggesting that the beetles such as T. castaneum and T. granarium were more tolerant than Lepidoptera species to A. sieberi essential oil.
The higher concentration of the essential oils, in this study, had much more mortality effects than lower concentration against S. cerealella. This finding was confirmed by Kordali et al. (2006), who found a high toxicity (80–90%) of Artemisia species against Sitophilus granarius L. (Coleoptera: Curculionidae) at a dose of 9 µl/liter air after 48 h of exposure. Consistent with the fumigant toxicity test, the LT50 evaluations revealed that the insecticidal effect of A. khorassanica oil was much faster than A. sieberi. These results are in agreement with the findings of Saeidi and Moharramipour (2013), who reported the LT50 of 9.63 h for A. khorassanica against T. confusum.
Studies of sublethal effects of pesticides could provide more useful knowledge than lethal effects on bioactivity of pesticides on the insects. Demographic toxicology is an important tool for accurate assessment of the total effects of an insecticidal compound (Abedi et al. 2014). In this study, sublethal effects of A. khorassanica and A. sieberi essential oils were detected on life history and life table parameters of S. cerealella. In agreement with the findings of Izakmehri et al. (2013) and Borzoui et al. (2016), significant extensions observed in the developmental time of treated S. cerealella could lengthen the exposing time to the essential oils before mating and producing next generation. In consistent with Borzoui et al. (2016), a significant reduction in the adult’s longevity and ovipostion period of S. cerealella treated with tested oils could decrease number of eggs laid, and eventually could negatively affect the population of the next generation. In addition, the fecundity of S. cerealella treated with A. khorassanica and A. sieberi essential oils reduced by 35.78 and 25.29%, respectively. As fecundity plays an important role in population of the next generation, its reduction could suppress the population increase of the insects. These results are in agreement with those reported by Izakmehri et al. (2013), who expressed that exposure to Eucalyptus camaldulensis Dehnh. and Heracleum persicum Desf. essential oils reduced fecundity of Callosobruchus maculates (Fabricius).
The life table parameters, especially the intrinsic rate of increase (rm), are the most useful parameters to evaluate the population growth potential of insect species (Southwood 1966, Ricklefs and Miller 2000). The lower rm value in S. cerealella treated with tested essential oils than the control is mainly attributed to the lower survivorship, longer developmental time of immature stages, and lower fecundity of the pest. Reduction in this parameter can decrease the speed of population growth of S. cerealella. Similar results were reported by Borzoui et al. (2016) for P. interpunctella exposed to A. khorassanica and V. pseudo-negundo essential oils.
In conclusion, both examined essential oils showed high lethal and sublethal effects on S. cerealella. However, more studies are needed to improve our knowledge about human, environmental, and nontarget animals’ safety of these essential oils. For the future, it is necessary to evaluate the impacts of these essential oils on S. cerealella under storage systems.
Acknowledgments
The work received financial support by the University of Mohaghegh Ardabili, which is greatly appreciated.
References Cited
- Abdelgaleil S. A. M., Mohamed M. I. E., Badawy M. E. I., and El-Arami S. A. A.. 2009. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 35: 518–525. [DOI] [PubMed] [Google Scholar]
- Abedi Z., Saber M., Gharekhani G. H., Mehrvar A., and Kamita G.. 2014. Lethal and sublethal effects of azadirachtin and cypermethrin on Habrobracon hebetor (Hymenoptera: Braconidae). J. Econ. Entomol. 107: 638–645. [DOI] [PubMed] [Google Scholar]
- Ahn Y. I., Lee S. B., Lee H. S., and Kim G. H.. 1998. Insecticidal and acaricidal activity of caravacrol and thujaplicine derived from Thujopsis dolabrata var. hondai sawdust. J. Chem. Ecol. 24: 1–90. [Google Scholar]
- Anonymous. 1991. Scientific assessment of ozone depletion. World Meteological Organization Report. World Meteological Organizations of the United Nations, Geneva, Switzerland. [Google Scholar]
- Bell C. H., and Wilson S. M.. 1995. Phosphine tolerance and resistance in Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Stored Prod. Res. 31: 199–205. [Google Scholar]
- Borzoui E., Naseri B., Abedi Z., and Karimi-Pormehr M. S.. 2016. Lethal and sublethal effects of essential oils from Artemisia khorassanica and Vitex pseudo-negundo against Plodia interpunctella (Lepidoptera: Pyralidae). Environ. Entomol. 45: 1220–1226. [DOI] [PubMed] [Google Scholar]
- Champ B. R., and Dyte C. E.. 1977. FAO global survey of pesticide susceptibility of stored grain pests. FAO Plant Prot. Bull. 25: 49–67. [Google Scholar]
- Chi H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17: 26–34. [Google Scholar]
- Chi H., and Liu H.. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24: 225–240. [Google Scholar]
- Dansi L., Van Velson F. L., Vander Geuden C. A.. 1984. Methyl bromide: carcinogenetic effects in the rat forestomach. Toxicol Appl. Pharmacol. 72: 262–271. [DOI] [PubMed] [Google Scholar]
- Desneux N., O’neil R. J., and Yoo H. J. S.. 2006. Suppression of population growth of the soybean aphid, Aphis glycines Matsumura, by predators: the identification of a key predator and the effects of prey dispersion, predator abundance, and temperature. Environ. Entomol. 35: 1342–1349. [Google Scholar]
- Efron B., and Tibshirani R. J.. 1993. An introduction to the Bootstrap. Chapman and Hall, New York, NY, 436 pp. [Google Scholar]
- Hori M., and Kasaishi Y.. 2005. Estimation of the phosphine resistance level of the cigarette beetle, Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae), by the knockdown time of adult. Appl. Entomol. Zool. 40: 557–561. [Google Scholar]
- Isman M. B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51: 45–66. [DOI] [PubMed] [Google Scholar]
- Izakmehri K., Saber M., Mehrvar A., Hassanpouraghdam M. B., and Vojoudi S.. 2013. Lethal and sublethal effects of essential oils from Eucalyptus camaldulensis and Heracleum persicum against the adults of Callosobruchus maculatus. J. Insect Sci. 13: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordali S., Aslan I., Calmasur O., and Cakir A.. 2006. Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Ind. Crops Prod. 23: 162–170. [Google Scholar]
- Lee B. H., Lee S. E., Annis P. C., Pratt S. J., Park B. S., and Tumaalii F.. 2002a. Fumigant toxicity of essential oils and monoterpenes against the red flour beetle, Tribolium castaneum Herbst. J. Asia-Pac. Entomol. 5: 237–240. [Google Scholar]
- Lee S., Peterson C. J., and Coats J. R.. 2002b. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res. 39: 77–85. [Google Scholar]
- Lee B. H., Annis P. C., Tumaalii F., and Choi W. S.. 2004. Fumigant toxicity of essential oils from the Myrtaceae family and 1,8-cineole against 3 major stored-grain insects. J. Stored Prod. Res. 40: 553–564. [Google Scholar]
- Meaklim J. 1998. Phosphine toxicity: are phosphine users, or the general community, at risk of adverse health effects, pp. 119–125. InBanks H. J., Wright E. J., Damcevski K. A., (eds.), Stored Grain in Australia. In Proceedings of the Australia Postharvest Technical Conference, 26–29 May 1998, Canberra, Australia. [Google Scholar]
- Nayak M. J., Collins P. J., Pavic H., and Kopittke R. A.. 2003. Inhibition of egg development by phosphine in the cosmopolitan pest of stored products Liposcelis bostrychophila (Psocoptera: Liposcelididae). Pest Manag. Sci. 59: 1191–1196. [DOI] [PubMed] [Google Scholar]
- Negahban M., Moharramipour S., and Sefidkon F.. 2006. Chemical composition and insecticidal activity of Artemisia scoparia essential oil against three coleopteran stored-product insects. J. Asia-Pac. Entomol. 9: 1–8. [Google Scholar]
- Negahban M., Moharramipour S., and Sefidkon F.. 2007. Insecticidal activity of essential oil from Artemisia sieberi Beser against three stored-product insects. J. Stored Prod. Res. 43: 123–128. [Google Scholar]
- Nouri-Ganbalani G., and Borzoui E.. 2017. Acute toxicity and sublethal effects of Artemisia sieberi Besser on digestive physiology, cold tolerance and reproduction of Trogoderma granarium Everts (Col.: Dermestidae). J. Asia-Pac. Entomol. 20: 285–292. [Google Scholar]
- Obeng-Ofori D., Reichmuth C., Bekele J., and Hassanali A.. 1997. Biological activity of 1,8-cineole, a major component of essential oil of Ocimum kenyense (Ayobangira) against stored product beetles. J. Appl. Entomol. 121: 237–243. [Google Scholar]
- Obeng-Ofori D., Reichmuth C. H., Bekele A. J., Hassanali A.. 1998. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest Manag. 44: 203–209. [Google Scholar]
- Rajendran S., and Srianjini V.. 2008. Plant products as fumigants for stored-product insects control. J. Stored Prod. Res. 44: 126–135. [Google Scholar]
- Ricklefs R. E., and Miller G. L.. 2000. Ecology. 3rd ed Freeman and Company, New York, NY. [Google Scholar]
- Robertson J. L., Russell R. M., Preisler H. K., and Savin N. E.. 2007. Bioassay with arthropods. CRC, London, United Kingdom. [Google Scholar]
- Rozman V., Kalinovic I., and Korunic Z.. 2007. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 43: 349–355. [Google Scholar]
- Saeidi M., and Moharramipour S.. 2013. Insecticidal and repellent activities of Artemisia khorassanica, Rosmarinus officinalis and Mentha longifolia essential oils on Tribolium confusum. J. Crop Prot. 2: 23–31. [Google Scholar]
- Sahaf B. Z., Moharramipour S., and Meshkatalsadat M. H.. 2008. Fumigant toxicity of essential oil from Vitex pseudo-negundo against Tribolium castaneum (Herbst) and Sitophilus oryzae (L.). J. Asia-Pac. Entomol. 1: 175–179. [Google Scholar]
- SAS Institute 2002. The SAS system for Windows. SAS Institute, Cary, NC. [Google Scholar]
- Shukle R. H., and Wu L.. 2003. The role of protease inhibitors and parasitoids on the population dynamics of Sitotroga cerealella (Lepidoptera: Gelechiidae). Environ. Entomol. 32: 488–498. [Google Scholar]
- Southwood T. R. E. 1966. Ecological methods with particular reference to the study of insect populations. Methuen, London, United Kingdom. [Google Scholar]
- Stamopoulosa D. C., Damosb P., and Karagianidoub G.. 2007. Bioactivity of five monoterpenoid vapours to Tribolium confusum (du Val) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 43: 571–577. [Google Scholar]
- Subramanyam B., and Hagstrum D. W.. 1995. Resistance measurement and management, in integrated management of insects in stored products. Marcel Dekker, New York, NY. [Google Scholar]
- Throne J. E., and Weaver D. K.. 2013. Impact of temperature and relative humidity on life history parameters of adult Sitotroga cerealella (Lepidoptera: Gelechiidae). J. Stored Prod. Res. 55: 128–133. [Google Scholar]
- Tripathi A. K., Prajapati V., and Aggarwal K. K.. 2000. Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. J. Econ. Entomol. 93: 43–47. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhua F., Zhoua X. M., Niua C. Y., and Lei C. L.. 2006. Repellent and fumigant activity of essential oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 42: 339–347. [Google Scholar]
- Wang J. L., Li Y., and Lei C. L.. 2009. Evaluation of monoterpenes for the control of Tribolium castaneum (Herbst) and Sitophilus zeamais Motschulsky. Nat. Prod. Res. 23: 1080–1088. [DOI] [PubMed] [Google Scholar]
- White N. D. G., and Leesch J. G.. 1995. Chemical control, in integrated management of insects in stored products. Marcel Dekker, New York, NY. [Google Scholar]
- Yang K., Sun R. Q., Guo S. S., Du S. S., Liu Z. L., and Deng Z. W.. 2014. Bioactivity of essential oil of Litsea cubeba from China and its main compounds against two stored product insects. J. Asia-Pac. Entomol. 17: 459–466. [Google Scholar]