Abstract
Regulatory T cells (Tregs) are critical modulators of immune homeostasis. Tregs maintain peripheral tolerance to self-antigens, thereby preventing autoimmune disease. Furthermore, Tregs suppress excessive immune responses deleterious to the host. Recent research has deepened our understanding of how Tregs function at the ocular surface. This manuscript describes the classification, the immunosuppressive mechanisms, and the phenotypic plasticity of Tregs. We review the contribution of Tregs to ocular surface autoimmune disease, as well as the function of Tregs in allergy and infection at the ocular surface. Finally, we review the role of Tregs in promoting allotolerance in corneal transplantation.
Keywords: Allergic eye disease, Corneal transplantation, Regulatory T cells
1. INTRODUCTION
Regulatory T cells (Tregs) have emerged as key modulators of immune homeostasis, playing an essential role in maintaining peripheral tolerance and controlling the immune response [1]. The critical role of Tregs in preventing autoimmunity has been demonstrated in both murine models [2] and by the fatal human disorder of immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) resulting from FOXP3 mutations [3]. In addition to promoting tolerance to self antigens and thereby preventing autoimmune disease, Tregs can limit constructive immune responses to neoplastic disease and vaccinations [4,5]. Due to the potential beneficial and deleterious effects of Tregs based on the context in which they function, their immunological mechanisms have been the focus of considerable attention over the past two decades.
This article reviews the current knowledge of the function of regulatory T cells at the ocular surface. First we consider the classification, immunosuppressive mechanisms, and the phenotypic plasticity of Tregs. We then address the function of Tregs in autoimmune disease of the ocular surface. Next, the role of Tregs in allergy and infection of the ocular surface are examined. Finally, we focus on the function of Tregs in corneal transplantation.
1.1. Classification
The proposal that thymic-derived lymphocytes promoted tolerance was first presented almost five decades ago [6], but research on suppressor T cells lay dormant until Sakaguchi’s identification of CD25 as a phenotypic marker for CD4+ suppressor T cells [7]. Forkhead box protein 3 (Foxp3) was subsequently recognized as a transcription factor that defined this important regulatory T cell lineage in both mice [8,9] and humans [10,11].
Foxp3+ Tregs are further classified as naturally occurring, thymus-derived (nTreg) or produced extrathymically at peripheral sites (pTregs) [12]. Foxp3+ Tregs constitute approximately 5–10% of peripheral CD4+ T cells, the vast majority of which are nTregs, with pTregs representing a smaller population [13]. In vitro-induced Tregs (iTregs) are generated by stimulation of conventional T cells (CD4+Foxp3−) in vitro in the presence of TGF-β [12].
1.1. Mechanism of action
Tregs orchestrate their regulatory function by a number of mechanisms: (i) suppression by cytolysis, (ii) suppression by releasing soluble factors, (iii) suppression by modulation of dendritic cell (DC) function, and (iv) by metabolic competition (Fig. 1) [1]. Tregs express granzyme B and kill T cells and antigen-presenting cells (APCs) by a perforin-dependent pathway [14]. In addition to Treg-induced cytolysis by granzyme-B and perforin-mediated mechanisms, Tregs may induce apoptosis of effector T cell through a TRAIL-DR pathway [15] and by galectin-induced cell death [16]. Key inhibitory cytokines expressed by Tregs include interleukin-10 (IL-10), IL-35 and TGF-β. IL-10 potently inhibits macrophage and T effector cells, and plays an important role in suppressing mucosal immune responses to environmental antigens [17]. In addition to its immunosuppressive function, IL-35 has the capacity to propagate infectious tolerance by expanding a subpopulation of IL-35-expressing Tregs [18]. TGF-β is vital for the differentiation of Tregs both in vivo and in vitro, maintains Foxp3 expression of nTreg cells, and suppresses inflammatory cells [19,20]. Modulation of DC function is achieved via constitutively expressed cell surface proteins, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) [1]. This inhibitory cell surface receptor has greater affinity for the DC ligands CD80 and CD86 than the costimulatory cell surface protein CD28 [21]. CTLA-4 thereby inhibits DC function either by concealing CD80 and CD86, or by trans-endocytosis of these costimulatory ligands (removal from DC membrane) [21]. Furthermore, DC expression of indoleamine 2,3-dioxygenase is induced by reverse signalling from interactions of CTLA-4 with CD80 and CD86 [21]. This immunosuppressive enzyme catabolises tryptophan into proapoptotic metabolites [22]. Finally, Tregs can suppress effector T cells function by metabolic competition [1]. Of particular importance in this heterogeneous group of mechanisms is the high expression of CD25 by Tregs, which allows them to compete for IL-2, resulting in cytokine deprivation-mediated apoptosis [23].
Figure 1.
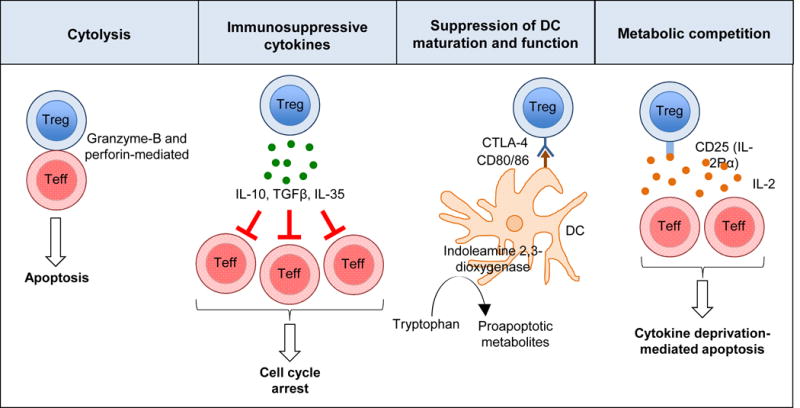
Tregs use four key mechanisms to exert their immunoregulatory function: (i) suppression by cytolysis, (ii) suppression by releasing soluble factors, (iii) suppression of dendritic cell (DC) function and (iv) suppression by metabolic competition. The expression of indoleamine 2,3-dioxygenase by DCs is induced by reverse signalling from interactions of CTLA-4 with CD80 and CD86. Indoleamine 2,3-dioxygenase is an immunosuppressive enzyme that catabolises tryptophan into proapoptotic metabolites. The high affinity of CTLA-4 for the DC ligands CD80 and CD86 results in impaired co-stimulation of effector T cells via the costimulatory cell surface protein CD28.
It is widely accepted that Treg suppression of effector T cells is principally contact-dependent, and the extent to which inhibitory cytokines such as IL-10 and TGF-β contribute to Treg function remains open to debate [1,24]. Cell-cell contact is fostered by the migration of Tregs to the secondary lymphoid compartment, which in turn is dependent on Treg expression of C-C chemokine receptor type 7 (CCR7) [25]. CCR7 is a protein receptor that plays an essential role in the homing of Tregs to draining lymph nodes (DLNs) via high endothelial venules [26]. Deficiency of CCR7 has been demonstrated to compromise the ability of Tregs to moderate the priming phase of the immune response, and results in the accumulation of Tregs in peripheral, inflamed sites [27]. Failure of Treg migration to the DLNs results in impaired suppression of antigen-induced effector T cell responses [25].
1.1. Plasticity
The paradigm of clonal populations of CD4+ T cells performing discrete and fixed functions, as determined by specific cytokine profiles, is not entirely accurate [28]. It has been established that CD4+ T cells can adopt particular phenotypes, while maintaining the ability to repolarize towards mixed or alternative fates [28]. This phenotypic plasticity permits CD4+ T cells to undergo functional adaptations according to cues from the microenvironment [29].
Th17 cells and Tregs demonstrate a particularly high magnitude of plasticity, and share a reciprocal developmental relationship [30]. TGF-β promotes Treg differentiation, while IL-6 and IL-21 inhibit Treg differentiation and induce the development of Th17 cells [31]. The molecular basis for the reciprocal relationship involves Foxp3-mediated inhibition of retinoic acid receptor-related orphan receptor γt (RORγt), the lineage-specific transcription factor for Th17 cells [32]. The conversion of immunosuppressive Foxp3+ Tregs to pro-inflammatory Th17 cells has been identified as a critical factor in the pathogenesis of autoimmune diseases [30]. Moreover, in transplantation immunology, the balance between T effectors and Tregs is recognized as a key determinant of allograft fate [33].
The functional stability of Foxp3 expression by Tregs has been called into question [34]. Using murine models that permit Foxp3 lineage tracing, studies have demonstrated a population of Tregs that lose Foxp3 expression either partially or completely [29,35]. These ‘exTreg’ cells exhibit strong T cell receptor engagement with autoantigens and express pro-inflammatory cytokines [35]. Experiments involving the adoptive transfer of exTregs in murine models have demonstrated the pathogenicity of these cells – exTregs have been shown to induce type 1 diabetes [36], arthritis [37], experimental autoimmune encephalitis [38], and colitis [39].
2. TREGS IN AUTOIMMUNITY
Aberrant activation of the immune system to self-antigens situated at the ocular surface and associated tissues can result in autoimmune disease. The resulting pathology may be either ocular-specific (e.g., dry eye disease [DED]) or systemic (e.g., Sjögren syndrome, rheumatoid arthritis, systemic lupus erythematosus).
2.1. Ocular-specific autoimmune disease
DED is a chronic multifactorial disorder of the ocular surface [40]. The pathogenesis of DED has not been fully defined; nevertheless, it is evident that immune-mediated inflammation plays an important role in disease induction and amplification [41–45]. APC migration from the cornea to the DLNs is believed to be critical for the induction of DED [46]. APCs activate naïve T cells in the lymphoid compartment; these effector T cells subsequently acquire chemokine receptors (e.g., CCR5 and CXCR3) that drive their migration and homing to the ocular surface [43]. Tregs modulate this pro-inflammatory system in the secondary lymphoid compartment. The expression of homing receptors CD62L and CCR7 is upregulated in tTregs exiting the thymus, and guides their migration toward secondary lymphoid tissue [47]. In the presence of self-antigens at secondary lymphoid tissue, Tregs generate stable interactions with antigen-bearing DCs. These stable contacts prevent the interactions between naïve T cells and DCs that are essential for T cell priming [21]. Tregs impair the capacity of DCs to activate effector T cells (by down-modulating the expression of the co-stimulatory molecules CD80 and CD86) and induce DCs to produce pro-apoptotic molecules (via the expression of the immunosuppressive enzyme indoleamine 2,3-dioxygenase) [21,22].
The importance of immune-mediated mechanisms in DED has been confirmed by data from the Pflugfelder-Stern-Niederkorn collaboration, which demonstrated that DED could be induced in healthy T cell-deficient nude mice by the adoptive transfer of CD4+ T cells derived from mice exposed to desiccating stress (following depletion of CD25+ T cells) [48]. Further investigations have illustrated the importance of Tregs in curbing the autoimmune inflammatory milieu of DED. In mice exposed to desiccating stress, depletion of CD4+CD25hiFoxp3+ Tregs results in exacerbated disease [48]. Adoptive transfer of CD4+ T-effector cells isolated from donor mice exposed to desiccating stress increases the tear concentration of proinflammatory cytokines IL-12, IFN-γ, and TNF-α — an effect which is abrogated by the co-transfer of CD4+CD25hiFoxp3+ Tregs [49]. Other studies have evaluated the functional competence of Tregs in DED. It has been shown that, despite the frequencies of Tregs in DED and naïve mice being similar, CD4+CD25hiFoxp3+ Tregs in DED mice are less effective at suppressing the activation of pathogenic T cells relative to Tregs in naïve mice [45]. This data implies that the impaired immunoregulatory capacity of Tregs in DED is due to a qualitative rather than quantitative deficit.
IL-17-secreting Th17 cells have emerged as the principal pathogenic effector cells in DED [45,50]. The ocular surface of patients with DED expresses higher concentrations of cytokines that induce Th17 cells (IL-6, TGF-β, IL-23 and IL-17A). Moreover, an increase in the concentration of IL-17-producing cells has been demonstrated at the ocular surface in a murine model of DED [50]. Importantly, Tregs from DED mice have been shown to be particularly inefficient at suppressing the proliferation of IL-17-producing CD4+ T cells [45]. Consistent with other reports, this finding suggests that Tregs may be less successful at suppressing Th17 cells relative to other T cell subsets [51,52]. The interaction between Th17 effector cells and Tregs at the ocular surface may provide therapeutic opportunities to interrupt the autoimmune pathogenesis of DED.
The prevalence of DED, as with many other autoimmune conditions, increases with age [53,54]. Recent data suggest that dysfunctional Tregs may contribute to increased autoimmunity in aging. There is evidence from murine studies that, although Tregs from aged mice are capable of suppressing IFN-γ–producing CD4+ T cells, they demonstrate impaired suppression of IL-17– producing CD4+ T cells [55]. This important finding implies that Tregs may demonstrate function-specific impairment with age – their ability to control immune activation against infection and tumors might be intact, yet they fail to suppress IL-17-driven autoimmunity [56]. Using NOD.B10.H2b mice, Coursey and colleagues showed that dacryoadenitis in aging is associated with a significant increase in Treg frequencies, despite worsened lacrimal gland pathology [57]. Furthermore, the investigators identified a population of Tregs that, despite continued expression of Foxp3, had impaired suppressive function and expressed the pro-inflammatory cytokines IL-17 and IFN-γ. The pathogenicity of these inflammatory cytokine-producing Tregs was confirmed in adoptive transfer experiments, in which recipients of either aged Tregs or aged effector T cells developed DED, whereas recipients of young Tregs or young effector T cells did not [57]. The plasticity of Tregs towards an effector cell phenotype may be an important factor contributing to the increased prevalence of DED with age.
2.2. Systemic autoimmune disease with ocular manifestations
Ocular surface disease can occur as a manifestation of systemic autoimmune conditions, such as Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus. Sjögren syndrome is a common systemic autoimmune disease that can lead to sight-threatening DED. It can occur either as an independent disease entity, primary Sjögren syndrome, or in combination with another autoimmune condition, secondary Sjögren syndrome. Secondary Sjögren syndrome occurs in 17–29% of patients with rheumatoid arthritis and in 6.5–19% of patients with systemic lupus erythematosus [58].
In addition to DED, rheumatoid arthritis has a tendency to cause episcleritis, scleritis, and corneal ulceration [59]. Other systemic autoimmune conditions frequently associated with ocular surface disease include scleroderma, vasculitis, inflammatory bowel disease, and relapsing polychondritis, among others [60]. Although these conditions represent a heterogeneous grouping, they share an autoimmune pathogenesis, which results from failure of the mechanisms governing peripheral tolerance [61]. There is mounting data from murine models concerning the role of Tregs in these systemic autoimmune diseases.
Scurfy mice develop lethal multi-organ inflammation due to a mutation in Foxp3, resulting in the total deficiency of CD4+CD25+Foxp3+ regulatory T cells [8]. These mice develop excessive Th1, Th2 and Th17 immunity and have a life expectancy of 3–4 weeks [62]. The generalized autoimmune disorder manifest in scurfy mice affects almost every organ system, including the ocular surface. Interestingly, inflammation of the eyelids is the first physical manifestation of disease following adoptive transfer of lymph node cells from scurfy donors into Rag1−/− recipients [63].
CD25 knockout mice have defective CD4+CD25+ Tregs [64]. These mice spontaneously develop lymphocytic infiltration of their lacrimal and salivary glands, and have been proposed as an animal model of Sjögren syndrome. In a study investigating ocular surface pathology in these mice, they were demonstrated to spontaneously develop T-cell infiltration of the lacrimal gland, conjunctiva and cornea with a concomitant increase in corneal irregularity [65]. The investigators found these changes to be greater than or equal to those detected in C57BL/6 mice exposed to desiccating stress [50,66]. One hypothesis to explain these findings is that defective Tregs have an impaired capacity to modulate the self-reactive T cell response.
Autoimmune keratitis is an ocular manifestation of systemic autoimmune diseases, most commonly due to collagen vascular disorders such as rheumatoid arthritis. Spontaneous autoimmune keratitis frequently develops in female C57BL/10 mice that lack γδ T cells (B10.TCRδ−/− mice) [67]. It has been shown that the frequency of Tregs (both proportionately and by absolute number) is reduced in B10.TCRδ−/− mice compared to matched wildtype controls [68]. Furthermore, the investigators found evidence of functional impairment of Tregs from B10.TCRδ−/− mice by demonstrating reduced expression of IL-2Rα and IL-2Rβ relative to controls. IL-2Rα and IL-2Rβ are vital to Treg differentiation and maintenance [69]. Interestingly, the expression of IL-2Rα and IL-2Rβ was also reduced when Tregs from keratitic B10.TCRδ−/− mice were compared to Tregs from non-keratitic B10.TCRδ−/− mice [68]. These findings implicate Treg deficit and functional incompetence in the increased prevalence of autoimmune keratitis in B10.TCRδ−/− mice.
3. TREGS IN ALLERGY
Allergic eye disease comprises a spectrum of disorders, including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, vernal keratoconjunctivitis, and atopic keratoconjunctivitis. Allergic conjunctivitis (AC) represents one component of systemic hypersensitivity to environmental antigens [70]. Allergies are typically chronic conditions, with over one-fifth of the US population having been diagnosed with allergic rhinitis [71].
In the allergic response, IgE antibodies bind to high affinity Fcε receptors on the surface of mast cells, triggering the release of vasoactive mediators, cytokines, and chemotactic factors. Allergen-reactive type 2 helper T cells (Th2) play an important role in initiating this process [72]. CD4+CD25+Foxp3+ Tregs have been shown to have an anti-inflammatory function in murine models of allergy [73,74]. In a murine model of experimental allergic conjunctivitis, increased expression of Tregs has been associated with disease suppression [75]. IL-10 is an immunosuppressive cytokine released by regulatory T cells, which has been reported to regulate mast cell development and function [76]. In a model of allergic conjunctivitis using IL-10 knockout mice, investigators have examined the susceptibility of mast cells to degranulate in response to a secretagogue Compound 48/80 [77]. The data demonstrate increased conjunctival mast cell degranulation in IL-10 knockout mice relative to wild type mice. The stabilizing effects of IL-10 on conjunctival mast cells were further demonstrated when reconstitution of IL-10 knockout mice with recombinant IL-10 abrogated the secretagogue’s degranulatory effects [77]. In addition to studying the role of IL-10 in allergic eye disease, investigators have considered whether allergy alters the susceptibility of other T cell subsets to modulation by Tregs. In a study exploring how allergic conjunctivitis might exacerbate corneal allograft rejection, Reyes and colleagues showed that exogenous IL-4 reduced Treg suppression of CD4+ effector T cells both in vitro and in vivo [78]. Furthermore, the authors demonstrate that IL-5 and IL-13 have no effect on Treg suppressive function. The authors note that allergic eye disease is a risk factor for corneal allograft rejection and propose that by blocking IL-4 with antibody, the immune privilege of the anterior chamber might be restored, with a concomitant improvement in corneal transplant survival [78].
4. TREGS IN INFECTIOUS DISEASE
Infectious challenges are met with a wide array of antimicrobial immune responses. These humoral and cellular mechanisms are potentially vigorous and can result in collateral tissue damage. By constraining the magnitude of effector responses, Tregs function to maintain immune homeostasis. In doing so, however, Tregs can compromise the immune system’s ability to adequately control infection. Although modulation of the immune response by Tregs has been found in viral, bacterial, protozoan, helminthic, and fungal infections [79]; studies conducted at the ocular surface have largely focused on viral infections, particularly herpes simplex virus (HSV).
HSV is a double-stranded, linear DNA virus that causes keratoconjunctivitis. The clinical manifestation involves dendritic corneal ulcers, with corneal opacity secondary to stromal edema and neovascularization [80]. Studies conducted in mouse models have shown that HSV-1-induced corneal inflammation is predominantly mediated by T lymphocytes, specifically IFN-γ+ Th1 cells [81,82]. Indeed, intracorneal infection with HSV-1 does not cause keratitis in T-cell-deficient mice unless the mice have received adoptive transfer of HSV-1 immune T lymphocytes [83,84]. There is some evidence that CD8+ T cells play an important role in eliminating HSV-1 from corneas at late time-points following infection [85]. Although the immunopathological mechanisms of HSV infection have not been fully determined, it has been clearly demonstrated that expression of the Treg-associated cytokines IL-10 and TGF-β can modulate disease severity [86,87].
The role of Tregs in HSV infection has been investigated by comparing the result of HSV infection in naïve and nTreg-depleted mice [88]. Suvas and colleagues infected mice with an immunodominant peptide of HSV following depletion of nTregs with anti-CD25 antibody, and evaluated the CD8+ T cell response [89]. The CD8+ T cell response was enhanced between three-and fourfold in nTreg-depleted mice relative to control. This effect was noted in both the acute and memory phases of the immune response. Furthermore, CD8+ T cells were shown to retain an activation phenotype for longer periods in CD25+-depleted animals. By investigating the CD8+ T cell response to HSV immunization, it has been shown that CD25+ Tregs have both a quantitative and qualitative effect in modulating vaccine-generated immunity [90].
Low-dose interleukin-2 (IL-2) and anti-IL-2 antibody immune complex has been used to expand the population of Foxp3+ Tregs [91,92]. Gaddipati and colleagues investigated the effect of Treg expansion of the development and progression of HSV stromal keratitis by systemically administering IL-2/anti-IL-2 antibody immune complex to C57BL/6 mice prior to corneal HSV-1 infection [93]. Their results demonstrated a reduced viral load in corneas from the immune complex-treated group, as well as a decreased influx of CD4+ T cells to the inflamed corneas. Finally, a significant reduction in the number of HSV-1 specific IFN-γ-producing CD4+ T cells was found in the draining lymph nodes and spleen of the immune complex-treated mice relative to controls. The authors propose that expansion of Tregs by systemic treatment with IL-2/anti-IL-2 antibody immune complex is an efficacious prophylactic method to control HSV-1 stromal keratitis.
The translational potential for Treg immunomodulatory therapy for infectious disease has been considered previously. By adoptive transference of in vitro-generated antigen-specific Tregs, Sehrawat and colleagues have demonstrated how Tregs are effective in suppressing lesion severity in HSV stromal keratitis [94]. Inhibition of DNA methyltransferase activity with 5-azacytidine has been shown to reduce numbers of pro-inflammatory T cells and the expression of associated cytokines, as well as decreasing nonlymphoid inflammatory cells [95]. In this study, Varanasi and colleagues demonstrated an increase in the ratio of Tregs to effector Th1 cells in 5-azacytidine-treated mice, as well as increased Treg suppressor activity in vitro. This corresponded with greater epigenetic variation in the Treg-specific demethylated region (TSDR) of Foxp3 in the 5-azacytidine-treated mice, which was associated with heightened phenotypic stability when the cells were exposed to inflammatory cytokines [95]. The observation that the therapeutic effects of 5-azacytidine were abrogated by Treg depletion confirmed the investigators’ hypothesis that Tregs played an essential role in mediating the anti-inflammatory effects of DNA methyltransferase inhibition with 5-azacytidine.
5. TREGS IN CORNEAL TRANSPLANTATION
Corneal transplantation is the most common form of tissue grafting, with over 150,000 performed annually worldwide [96]. In patients receiving their first graft, the 5-year graft survival rate exceeds 90% in non-vascularized and uninflamed host beds (low-risk transplantation) [97]. In contrast, rejection rates exceed 50% in patients with a history of graft rejection, or in grafts performed in vascularized and inflamed host beds (high-risk) [98,99]. A number of factors have been recognized that differentiate high-risk from low-risk transplants, including: increased trafficking of APCs from graft site to host lymphatics [100], phenotypic maturity and sensitivity of APCs which promote host T cell sensitization [101], an amplified direct pathway of allosensitization [102,103], and an abundant network of lymphatics and blood vessels that facilitate the trafficking of lymphocytes [104]. More recently, the function of Tregs in promoting allotolerance has attracted attention [105–107].
There is ample evidence that Tregs play a critical role in suppressing immune responses directed towards alloantigens [108,109]. Tregs function to downregulate the efferent phase of the delayed-type hypersensitivity response, contributing to the phenomenon of anterior chamber-associated immune deviation (ACAID) [106]. ACAID describes the aberrant systemic immune response induced when antigens enter the anterior chamber [110]. Experiments involving Treg adoptive transfer have demonstrated that tolerance can be induced in naïve hosts who subsequently receive a corneal allograft [107]. Chauhan and colleagues have shown that corneal allograft survival is closely correlated with Treg expression of Foxp3 [107]. In this study, the investigators demonstrated that Foxp3hi Tregs from accepted grafts exhibit greater suppression of naïve T cell proliferation and higher expression of IL-10 and TGF-β [107]. Using an orthotopic murine model of corneal transplantation, Cunnusamy and colleagues have determined that IL-17A (a proinflammatory cytokine known for its role in the pathogenesis of autoimmunity) is required for the generation of Tregs [111]. The investigators established that Tregs require IL-17A to mediate contact-dependent suppression. Furthermore, treatment with monoclonal anti-IL-17A was shown to result in rejection of 90% of corneal allografts. These findings are consistent with other transplantation immunology reports of IL-17 playing an important role in the tolerance of cardiac and renal allografts [112,113].
A myriad of studies have confirmed the importance of soluble suppressive molecules IL-10 and TGF-β in promoting graft survival [114–117]. Indeed, in an investigation of the function of tTregs and pTregs in a high-risk model of corneal transplantation, it has been demonstrated that the frequency and function of pTregs were suppressed in high-risk transplants and that this corresponded to reduced expression of IL-10 and TGF-β [118]. Data from this study suggest that antigen-specific pTregs (but not tTregs) are liable to dysfunction in an inflammatory microenvironment, such as occurs in a high-risk host bed. This distinction between tTregs and pTregs is of critical importance, as it supports the proposition that pTregs maintain peripheral tolerance to allografts in the low-risk setting, but their function is subverted by graft site inflammation, in which case they promote rejection [118].
Tregs from allograft acceptors have been shown to express higher levels of CCR7, and to preferentially localize to the paracortical region of draining lymph nodes in close association with APCs, relative to Tregs from graft rejectors [119]. Moreover, in vitro Treg suppression assay has demonstrated superior suppressive potential of CCR7hi Tregs [119]. In this study, the investigators established that CCR7 expression could be upregulated by in vitro stimulation of Tregs with the CCR7 ligand CCL21, and demonstrate that these conditioned Tregs have improved homing to DLNs with attendant enhanced corneal allograft survival [119].
Treg immunotherapy offers a potential therapeutic tool in promoting allotolerance. Adoptive transfer of in vitro expanded Tregs has been shown to suppress corneal allograft rejection [120]. Treatment with low-dose IL-2 has been established as a viable means of in vivo expansion of Tregs, and has been demonstrated to increase allograft survival [94]. IL-2 is known to maintain Treg suppressive function with the promotion of expression of Foxp3 and immunoregulatory cytokines [121,122]. Low-dose IL-2 immunotherapy has been considered as a means of abrogating the accelerated T cell sensitization (and concomitant graft failure) that occurs in the high-risk corneal transplantation setting [94]. Hildebrand and colleagues have proposed the local application of Tregs as a therapeutic strategy for preventing corneal graft rejection, following their observation that subconjunctivally administered Tregs increased graft survival in a rat model of penetrating keratoplasty [123]. These data cumulatively suggest that immunosuppressive therapies involving Tregs offer promising approaches to promote corneal graft survival; yet it is vital to consider the implications of Treg plasticity in this setting, and the risk of conversion to pro-inflammatory phenotypes.
The possibility that Tregs can repolarize towards the Th17 phenotype in inflammatory environments is potentially problematic for immunomodulatory strategies employing Tregs to promote graft tolerance [28]. Indeed, in cases there the local microenvironment remains inflamed, Tregs may be prone to change phenotype. Th17 cells have been proposed as mediating an alternative pathway of allograft rejection [124], with IL-17 implicated in transplant rejection [112,113]. Indeed, IL-17 antagonism has been demonstrated to delay graft rejection in murine models of transplantation [125,126]. Data from our laboratory (unpublished investigations) indicate that inflammation in the ocular tissue (as occurs in high-risk corneal transplantation) leads to the loss of Foxp3 expression by Tregs, with conversion to exTregs that express pro-inflammatory cytokines such as IFN-γ. These exTregs are phenotypically identical to the effector Th1 cells that mediate graft rejection. Although the pathogenicity of exTregs in autoimmunity has been established [36–39], these data are novel in implicating exTregs in the loss of corneal immune privilege and promotion of allograft rejection in the high-risk setting.
5. CONCLUSIONS
Foxp3+ Tregs are critical to immune homeostasis and the prevention of autoimmunity and chronic inflammation. Treg immunotherapy is an enticing prospect at the ocular surface, particularly in the setting of autoimmune disease and high-risk corneal transplantation. Despite enormous progress in our understanding of Treg lineage differentiation and function, there remain key unanswered questions. Further investigation of the immunosuppressive mechanisms, phenotypic plasticity, and functional adaptability of this potent subset of CD4+ T cells is essential if their therapeutic potential is going to be exploited.
Acknowledgments
The National Institutes of Health/National Eye Institute Grant EY012963 supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no financial or proprietary interests in any concept or product discussed in this article.
References
- 1.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182(1):18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 3.Ochs HD, Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 4.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212(1):163–9. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7(11):875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 6.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18(5):723–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science (80−) 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 11.Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling K-L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35(6):1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 12.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259(1):88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DAA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66(16):2603–22. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4+CD25+ regulatory T cells. Cell Death Differ. 2007;14(12):2076–84. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 16.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 17.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: A key mediator of suppression and the propagation of infectious tolerance. Front Immunol. 2013;4:315. doi: 10.3389/fimmu.2013.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang W, Beckett O, Ma Q, Li MO. Transforming Growth Factor-β Signaling Curbs Thymic negative selection promoting regulatory T cell development. Immunity. 2010;32(5):642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. “Yin-Yang” functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell DJ. Control of regulatory T cell migration, function, and homeostasis. J Immunol. 2015;195(6) doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 23.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation–mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4 + CD25 + regulatory T cells. J Exp Med. 2007;204(4):735–45. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 27.Menning A, Höpken UE, Siegmund K, Lipp M, Hamann A, Huehn J. Distinctive role of CCR7 in migration and functional activity of naive- and effector/memory-like Treg subsets. Eur J Immunol. 2007;37(6):1575–83. doi: 10.1002/eji.200737201. [DOI] [PubMed] [Google Scholar]
- 28.DuPage M, Bluestone JA. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16(3):149–63. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 29.Sawant DV, Vignali DAA. Once a Treg, always a Treg? Immunol Rev. 2014;259(1):173–91. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25(4):305–12. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27(1):485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453(7192):236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17–regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14(4):326–31. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 34.da Silva Martins M, Piccirillo CA. Functional stability of Foxp3+ regulatory T cells. Trends Mol Med. 2012;18(8):454–62. doi: 10.1016/j.molmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Guo J, Zhou X. Regulatory T cells turn pathogenic. Cell Mol Immunol. 2015;12(5):525–32. doi: 10.1038/cmi.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2013;20(1):62–8. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 38.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39(5):949–62. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi R, Nishimoto S, Muto G, Sekiya T, Tamiya T, Kimura A, et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-γ and IL-17A production. J Exp Med. 2011;208(10):2055–67. doi: 10.1084/jem.20110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.No authors listed; The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson W, Chauhan SK, Dana R. Dry Eye Disease. Arch Ophthalmol. 2012;130(1):90. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31(3):271–85. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50(8):3802–7. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Chauhan SK, Soo Lee H, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7(1):38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182(3):1247–52. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaumburg CS, Siemasko KF, De Paiva CS, Wheeler LA, Niederkorn JY, Pflugfelder SC, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187(7):3653–62. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 47.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169(5):2461–5. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 48.Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, et al. Desiccating Stress Induces T Cell-Mediated Sjögren’s Syndrome-Like Lacrimal Keratoconjunctivitis. J Immunol. 2006;176(7) doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 49.Siemasko KF, Gao J, Calder VL, Hanna R, Calonge M, Pflugfelder SC, et al. In vitro expanded CD4+CD25+Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammation. Invest Ophthalmol Vis Sci. 2008;49(12):5434–40. doi: 10.1167/iovs.08-2075. [DOI] [PubMed] [Google Scholar]
- 50.De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243–53. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 54.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US Men. Arch Ophthalmol. 2009;127(6):763. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Hurez VJ, Thibodeaux SR, Kious MJ, Liu A, Lin P, et al. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012;11(3):509–19. doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 56.Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology. 2014;60(2):130–7. doi: 10.1159/000355303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, de Paiva CS. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 2017;10(3):743–756. doi: 10.1038/mi.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247–55. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol. 2005;140(5):808–13. doi: 10.1016/j.ajo.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 60.Hamideh F, Prete PE. Ophthalmologic manifestations of rheumatic diseases. Semin Arthritis Rheum. 2001;30(4):217–41. doi: 10.1053/sarh.2001.16639. [DOI] [PubMed] [Google Scholar]
- 61.Bach JF. Autoimmune diseases as the loss of active “self-control”. Ann N Y Acad Sci. 2003 Sep;998:161–77. doi: 10.1196/annals.1254.017. [DOI] [PubMed] [Google Scholar]
- 62.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003 Apr;4(4):3. 337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 63.Sharma R, Sung S, Fu SM, Ju S-T. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J Biomed Sci. 2009;16(1):20. doi: 10.1186/1423-0127-16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furtado GC, de Lafaille MAC, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196(6) doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Paiva CS, Hwang CS, Pitcher JD, Pangelinan SB, Rahimy E, Chen W, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49(2):246–58. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Paiva CS, Corrales RM, Villarreal AL, Farley W, Li D-Q, Stern ME, et al. Apical Corneal Barrier Disruption in Experimental Murine Dry Eye Is Abrogated by Methylprednisolone and Doxycycline. Investig Opthalmology Vis Sci. 2006;47(7):2847. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 67.O’Brien RL, Taylor MA, Hartley J, Nuhsbaum T, Dugan S, Lahmers K, et al. Protective role of gammadelta T cells in spontaneous ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50(7):3266–74. doi: 10.1167/iovs.08-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Yang Z, Huang C, McGowan J, Casper T, Sun D, et al. γδ T Cell-Dependent Regulatory T Cells Prevent the Development of Autoimmune Keratitis. J Immunol. 2015;195(12):5572–81. doi: 10.4049/jimmunol.1501604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(10):1105–11. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 70.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. 2008 Allergy. [DOI] [PubMed] [Google Scholar]
- 71.Nathan RA, Meltzer EO, Derebery J, Campbell UB, Stang PE, Corrao MA, et al. The prevalence of nasal symptoms attributed to allergies in the United States: Findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600–8. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 72.Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. 1998;3(4):233–44. doi: 10.1016/s1380-2933(97)10005-7. [DOI] [PubMed] [Google Scholar]
- 73.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202(11):1549–61. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leech MD, Benson RA, De Vries A, Fitch PM, Howie SEM. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J Immunol. 2007;179(10):7050–8. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 75.Fukushima A, Sumi T, Fukuda K, Yamaguchi T, Kumagai N, Nishida T, et al. Modulation of murine experimental allergic conjunctivitis by treatment with α-galactosylceramide. Immunol Lett. 2006;107(1):32–40. doi: 10.1016/j.imlet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173(2):507–10. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bundoc VG, Keane-Myers A. IL-10 confers protection from mast cell degranulation in a mouse model of allergic conjunctivitis. Exp Eye Res. 2007;85(4):575–9. doi: 10.1016/j.exer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Reyes NJ, Chen PW, Niederkorn JY. Allergic conjunctivitis renders CD4 + T cells resistant to T regulatory cells and exacerbates corneal allograft rejection. Am J Transplant. 2013;13(5):1181–92. doi: 10.1111/ajt.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maizels RM, Smith KA. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hendricks RL, Epstein RJ, Tumpey T. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Invest Ophthalmol Vis Sci. 1989;30(1):105–15. [PubMed] [Google Scholar]
- 81.Chang E, Galle L, Maggs D, Estes DM, Mitchell WJ. Pathogenesis of herpes simplex virus type 1-induced corneal inflammation in perforin-deficient mice. J Virol. 2000;74(24):11832–40. doi: 10.1128/jvi.74.24.11832-11840.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149(9):3035–9. [PubMed] [Google Scholar]
- 83.Doymaz MZ, Rouse BT. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthalmol Vis Sci. 1992;33(7):2165–73. [PubMed] [Google Scholar]
- 84.Mercadal CM, Bouley DM, DeStephano D, Rouse BT. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67(6):3404–8. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conrady CD, Zheng M, Stone DU, Carr DJJ. CD8+ T Cells Suppress Viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J Immunol. 2012;189(1):425–32. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuklin NA, Daheshia M, Chun S, Rouse BT. Immunomodulation by mucosal gene transfer using TGF-beta DNA. J Clin Invest. 1998 Jul;102(2):15. 438–44. doi: 10.1172/JCI2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tumpey TM, Elner VM, Chen SH, Oakes JE, Lausch RN. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. J Immunol. 1994;153(5):2258–65. [PubMed] [Google Scholar]
- 88.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212(1):272–86. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 89.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4 + CD25 + T Cells regulate virus-specific primary and memory CD8 + T cell responses. J Exp Med. 2003;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78(23):13082–9. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2–mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tahvildari M, Omoto M, Chen Y, Emami-Naeini P, Inomata T, Dohlman TH, et al. In vivo expansion of regulatory t cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. transplantation. 2016;100(3):525–32. doi: 10.1097/TP.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaddipati S, Estrada K, Rao P, Jerome AD, Suvas S. IL-2/anti-IL-2 antibody complex treatment inhibits the development but not the progression of herpetic stromal keratitis. J Immunol. 2015;194(1):273–82. doi: 10.4049/jimmunol.1401285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82(14):6838–51. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varanasi SK, Reddy PBJ, Bhela S, Jaggi U, Gimenez F, Rouse BT. Azacytidine treatment inhibits the progression of herpes stromal keratitis by enhancing regulatory T cell function. Longnecker RM, editor. J Virol. 2017;91(7):e02367–16. doi: 10.1128/JVI.02367-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 97.Price FW, Whitson WE, Collins KS, Marks RG. Five-year corneal graft survival. A large, single-center patient cohort. Arch Ophthalmol (Chicago, Ill 1960) 1993;111(6):799–805. doi: 10.1001/archopht.1993.01090060087029. [DOI] [PubMed] [Google Scholar]
- 98.No authors listed; The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992 Oct;110(10):1. 1392–403. [PubMed] [Google Scholar]
- 99.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000;19(5):625–43. doi: 10.1097/00003226-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II–positive dendritic cells derived from MHC Class II–negative grafts. J Exp Med. 2002;195(2):259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173(7):4464–9. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 102.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74(2):172–8. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 103.Hamrah P, Liu Y, Zhang Q, Dana MR, DNJ H, RM S, et al. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121(8):1132. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 104.Dana MR. Angiogenesis and lymphangiogenesis—implications for corneal immunity. Semin Ophthalmol. 2006;21(1):19–22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 105.Jin Y, Chauhan SK, Saban DR, Dana R, AMD LA. Role of CCR7 in facilitating direct allosensitization and regulatory T-cell function in high-risk corneal transplantation. Invest Opthalmol Vis Sci. 2010 Feb;51(2):1. 816. doi: 10.1167/iovs.09-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196(10):3983–91. doi: 10.4049/jimmunol.1600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–53. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114(10):1398–403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wood KJ, Sakaguchi S. Regulatory Lymphocytes: Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 110.Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest Ophthalmol Vis Sci. 1996;37(13):2700–7. [PubMed] [Google Scholar]
- 111.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-Dependent CD4+CD25+ Regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186(12):6737–45. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162(1):577–84. [PubMed] [Google Scholar]
- 113.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14(5):287–98. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 114.Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol. 2009;21(4):379–91. doi: 10.1093/intimm/dxp005. [DOI] [PubMed] [Google Scholar]
- 115.Cunnusamy K, Chen PW, Niederkorn JY. Paradigm shifts in the role of CD4+ T cells in keratoplasty. Discov Med. 2010;10(54):452–61. [PMC free article] [PubMed] [Google Scholar]
- 116.Li B, Tian L, Diao Y, Li X, Zhao L, Wang X. Exogenous IL-10 induces corneal transplantation immune tolerance by a mechanism associated with the altered Th1/Th2 cytokine ratio and the increased expression of TGF-β. Mol Med Rep. 2014;9:2245–2250. doi: 10.3892/mmr.2014.2073. [DOI] [PubMed] [Google Scholar]
- 117.Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001 Apr;166(7):1. 4327–33. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 118.Inomata T, Hua J, Di Zazzo A, Dana R. Impaired Function of Peripherally Induced Regulatory T Cells in Hosts at High Risk of Graft Rejection. Sci Rep. 2016 Dec;6(1):23. 39924. doi: 10.1038/srep39924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chauhan SK, Saban DR, Dohlman TH, Dana R. CCL-21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014 Jan;192(2):15. 817–23. doi: 10.4049/jimmunol.1203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guo X, Jie Y, Ren D, Zeng H, Zhang Y, He Y, et al. In vitro-expanded CD4(+)CD25(high)Foxp3(+) regulatory T cells controls corneal allograft rejection. Hum Immunol. 2012 Nov;73(11):1061–7. doi: 10.1016/j.humimm.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 121.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005 Nov;6(11):16. 1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 122.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008 Jun;180(11):1. 7112–6. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 123.Hildebrand A, Jarsch C, Kern Y, Böhringer D, Reinhard T, Schwartzkopff J. Subconjunctivally applied naïve Tregs support corneal graft survival in baby rats. Mol Vis. 2014;20:1749–57. [PMC free article] [PubMed] [Google Scholar]
- 124.Benghiat FS, Charbonnier LM, Vokaer B, De Wilde V, Le Moine A. Interleukin 17–producing T helper cells in alloimmunity. Transplant Rev. 2009 Jan;23(1):11–8. doi: 10.1016/j.trre.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 125.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001 Jul;72(2):27. 348–50. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 126.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009 Jan;182(1):1. 309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]


