Abstract
Recent reports support higher than expected rates of binge alcohol consumption among women and girls. Unfortunately, few studies have assessed the mechanisms underlying this pattern of intake in females. Studies in males suggest that alcohol concentrations relevant to the beginning stages of binge intoxication may selectively target tonic GABAergic inhibition mediated by GABAA receptor subtypes expressing the δ-subunit protein (δ-GABAAR). Indeed, administration of agonists that interact with these δ-GABAAR prior to alcohol access can abolish binge drinking behavior in male mice. These δ-GABAAR have also been shown to exhibit estrous-dependent plasticity in regions relevant to drug taking behavior, like the hippocampus and periaqueductal grey. The present experiments were designed to determine whether the estrous cycle would alter binge drinking, or our ability to modulate this pattern of alcohol use with THIP, an agonist with high selectivity and efficacy at δ-GABAAR. Using the Drinking-in-the-Dark (DID) binge-drinking model, regularly cycling female mice were given 2 hours of daily access to alcohol (20%v/v). Vaginal cytology or vaginal impedance was assessed after drinking sessions to track estrous status. There was no fluctuation in binge drinking associated with the estrous cycle. Both Intra-posterior-VTA administration of THIP and systemic administration of the drug was also associated with an estrous cycle dependent reduction in drinking behavior. Pre-treatment with finasteride to inhibit synthesis of 5α-reduced neurosteroids did not disrupt THIP’s effects. Analysis of δ-subunit mRNA from posterior-VTA enriched tissue samples revealed that expression of this GABAA receptor subunit is elevated during diestrus in this region. Taken together, these studies demonstrate that δGABAARs in the VTA are an important target for binge drinking in females and confirm that the estrous cycle is an important moderator of the pharmacology of this GABAA receptor subtype.
Keywords: GABAA, extrasynaptic, binge drinking, THIP, females, mouse, alcohol, delta, tonic inhibition, Estrous, C57BL/6J, Ethanol, Binge Drinking, Drinking in the Dark, THIP, ventral tegmental area, VTA, diestrus, extrasynaptic, GABA
INTRODUCTION
Binge drinking behavior among women and girls has increased dramatically in recent years (Keyes, Grant, Hasin 2008). In the United States, a recent report from the Center for Disease Control and Prevention highlights this “under-recognized problem,” finding that almost 14 million women aged 18 through 34 binge drink an average of 3 times per month (CDC, 2013). This trend is also seen elsewhere; for example, in the United Kingdom binge-drinking rates among young women have doubled in the past decade (Smith and Foxcroft, 2009). The prevalence of this risky pattern of alcohol intake among women may be of concern given clinical reports suggesting that women display a telescoped development of addiction upon initial drug use (Randall et al., 1999; Hernandez-Avila, 2004; Johnson et al., 2005). Even when sex differences in the rate of the development of problems from alcohol use are challenged (Keyes et al., 2010; Lewis and Nixon, 2014), clinical findings continue to support differences in the maladaptations that occur from alcohol use (Squeglia et al., 2011; Smith et al., 2015) and in the mechanisms that may drive problematic use (Perry et al., 2013; Foster et al., 2015), alcohol seeking (Cyders et al., 2016) and binge alcohol intake (Skinner, Kristman-Valente and Herrenkohl, 2015). Unfortunately, few preclinical studies have specifically explored the neurochemical mediators of binge alcohol intake in females.
A growing number of reports support inhibition mediated by extrasynaptic GABAA receptors as a target for the effects of binge alcohol intoxication and consumption (Nie et al., 2011; Ramaker et al., 2011, 2012, 2014a, 2014b; Fritz and Boehm, 2014). Unlike the classic synaptic subtype of GABAA receptors that mediate phasic inhibition following vesicular release of GABA, extrasynaptic GABAA receptors mediate a constant tonic inhibition, responding to the low to moderate concentrations of ambient GABA found in the extrasynaptic space (Wei et al., 2003). Many of these extrasynaptic GABAA receptors express the δ subunit protein (δ-GABAAR; Mody and Pearce, 2004). Inclusion of this subunit appears to alter lateral diffusion of the receptor across the membrane and its ability to cluster at the synapse (Jacob, Moss and Jurd, 2008). The tonic inhibition mediated by these δ-GABAAR subtypes has been shown to be enhanced by the 17–30mM alcohol concentration range achieved during a binge drinking session (Glykys et al., 2007; Olsen et al., 2007). Indeed, genetic inactivation of δ-GABAAR in the nucleus accumbens reduces alcohol-drinking behavior in male mice (Nie et al., 2011). Further, pharmacological manipulation using 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP/gaboxadol), an agonist with high efficacy and selectivity for δGABAARs (Boehm et al., 2006; Bhattarai, 2011; Meera, 2011; Mortensen 2010), reduces binge alcohol consumption in mice (Moore et al., 2007; Fritz and Boehm, 2014; Quoilin and Boehm, 2016). However, no study has explored the effect that estrous cycle may have on the role that δ-GABAARs play in mediating binge drinking in females.
Findings thus far suggest important interactions between extrasynaptic GABAA receptors and gonadal hormones. Many neurosteroids-in particular the progesterone derived allopregnanolone and THDOC as well as the androgen derived androstanediol-work as positive allosteric modulators, increasing efficacy of GABA at GABAA receptors, especially at δ containing GABAA receptors, where GABA acts as a weak agonist. Thus, the δ subunit may be said to confer sensitivity to the neurosteroid-induced enhancement of GABAergic inhibition (Belelli 2002; Brown 2002; Mihalek 1999; Spigelman 2003; Wohlfarth 2002). Previous reports demonstrate that allopregnanolone may be altered following voluntary consumption of alcohol (Finn et al., 2004b). As GABA has low efficacy at δ containing GABAA receptors, low doses of allopregnanolone act as positive allosteric modulators by increasing the efficacy of GABA for opening δ containing channels (Bianci and MacDonald, 2003). High doses of allopregnanolone have also been shown to work as direct agonists at GABAA receptors (Hosie et al., 2006). This relationship between neurosteroid level and alcohol intake may be bidirectional. In particular, pretreatment of mice with finasteride, a compound that blocks activity of 5α reductase and synthesis of 5α-reduced neurosteroids like allopregnanolone, dampens alcohol drinking behaviors (Ford, Nickel and Finn, 2005; ). Although this effect might be sex specific (see Ford et al., 2008; Finn et al., 2010), it is possible that neurosteroid availability and potential changes to the expression and function of δ-GABAA receptors that parallel changes in neurosteroid levels are relevant to binge drinking behavior in females. Indeed, recent findings suggest that neurosteroids acting at δ-GABAARs can drive synaptic plasticity of dopaminergic neurons at the start of the reward circuit, at the level of the ventral tegmental area (Vashchinkina et al., 2014). Availability of neuroactive steroids oscillates across the estrous cycle much the same as their hormone precursors. For example, allopregnanolone shows a dip in availability during diestrus (Koonce, Walf and Frye, 2012). This reduction in the levels of neuroactive steroids that can act as positive allosteric modulators of δ containing GABAA receptors-like allopregnanolone-would be associated with an apparent drop in the efficacy of drugs that target this receptor during diestrus. For example, we could expect that a particular dose of THIP-a compound that works as a super-agonst at δ-GABAA receptors-would be more effective at producing GABAergic inhibition at δ-GABAA receptors at times when neuroactive steroids that act as positive allosteric modulators of δ-GABAA receptors are at their highest circulating levels, like proestrus and estrus when compared to its effects at diestrus. However, diestrus is also a time when the expression of δ-GABAA receptors peaks in various regions. Thus, it is possible that the contrasting shifts in availability of neuroactive steroids and expression of δ-GABAA receptors would result in maintenance of sensitivity to compounds acting on this receptor subtype.
Given the important role that GABAergic signaling in the VTA plays in alcohol intake generally (Nowak et al., 1998) and in binge consumption specifically (Moore and Boehm, 2009, Melón and Boehm, 2011), estrous-dependent changes in neurosteroid availability also suggests interactions between estrous cycle, neurosteroidogenesis and the mechanisms that underlie binge alcohol consumption.
In addition to oscillations in the synthesis and availability of neurosteroids, expression of δ-GABAARs fluctuates across the ovarian cycle. Changes in the expression of δ subunit protein occur across the estrous cycle in the hippocampus (Magiure et al., 2005) and periaqueductal gray (Griffiths and Lovick, 2005; Lovick et al., 2005). Transcription of Gabrd mRNA in the hippocampus also alternates across the ovarian-cycle in a subregion selective manner (Wu et al., 2013). Of course, changes in these extrasynaptic δ-GABAARs may be driven by the neurosteroid fluctuations described earlier (Maguire et al., 2005; Wu et al., 2013). Still, compounds that target this GABAA subtype in order to modulate alcohol-related behaviors may suffer from ovarian-related changes in their efficacy. Furthermore, behaviors that may be driven by activity of δ-GABAARs may be altered across the estrous cycle (Barth, Ferando and Mody 2014; Cushman et al, 2014; Sabaliauskas et al., 2015).
The goal of this series of experiments was to determine whether the estrous cycle affects our ability to modulate binge drinking by targeting the extrasynaptic GABAA receptor system. Additionally, we wanted to clarify whether the ovarian cycle would change characteristics of this specific pattern of alcohol consumption in female mice and whether changes in the expression of extrasynaptic GABAA receptors was associated with any of the estrous-dependent effects found. Finally, to clarify whether changes in availability of the 5alpha reduced pregnane-derived neurosteroids also played a role in any changes in sensitivity to THIP across the cycle, we preteated a cohort of animals with finasteride. We hypothesized that the murine estrous cycle would be an important regulator of both baseline binge drinking behavior and the ability to pharmacologically disrupt this pattern of intake in a high-bingeing inbred strain, due to estrous-associated changes in the expression of δ-containing GABAA receptors.
METHODS AND MATERIALS
Animals
Female C57BL/6J inbred mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained at the Indiana University-Purdue University-Indianapolis (IUPUI) School of Science Animal Resource Center. Mice were 70–80 days old at the start of each experiment. Animals were individually housed in standard shoebox cages and habituated to a 12-hour reverse light/dark schedule for at least 7 days prior to initiation of experiments. The temperature of the colony room was maintained around 21°C. In order to preserve regular estrous cycling in the females, non-manipulated, socially-housed males were also housed in the colony room. Food was available ad libitum in the home cage. Water was available ad libitum in the home cage except when ethanol was made available as per the Drinking-in-the-Dark (DID) protocol (see below). The IUPUI School of Science Institutional Animal Care and Use Committee approved all procedures prior to initiation of experiments.
Drugs and Drinking Solutions
Ethanol (190 proof) was obtained from Pharmco, Inc (Brookfield, CT). Ethanol solutions (20% v/v) were made with tap water. THIP, Finasteride and β-cyclodextrin were obtained from Sigma Aldrich (St. Louis, MO). THIP was made fresh daily in physiological saline (0.9% NaCl) and administered at 0.1ml/10 grams of body weight (Experiment 2 and 3) or at 25–50ng/200nL per microinfusion site (Experiment 1). Finasteride was made fresh daily in 20% β-cyclodextrin and distilled water and administered at 0.1 ml/10 grams of body weight. Control animals, administered the appropriate vehicle, were included on each test day.
Estrous Status
Cells from vaginal lavages were sampled daily (between zeitgeber time 15 and 18; ZT 15 and 18), beginning 6–10 days before mice had access to alcohol and continuing throughout the drinking period. Mice were removed from their cages and lightly immobilized against their cage tops. Sterile saline (~ 10 μL) was introduced into the vaginal opening and gently flushed back and forth, using a plastic transfer pipette (Globe Scientific, Paramus, NJ). The fluid was collected, placed onto a clean glass slide (VWR, West Chester, PA) and cover-slipped. Pictures of each slide were taken at 10× and 40× objectives so that two independent investigators could verify estrous status based on established criteria for vaginal cytology (Caligioni 2009). Only animals that demonstrated at least two consecutive regular cycles (moving through all 4 phases of estrous and with no extended diestrus: >4days diestrus smears) day before drug manipulation day were included in the statistical analyses. For experiment 3, prior to vaginal lavages, vaginal impedance was also recorded using a digital Estrous Monitor (EC40, Fine Science Tools) as a secondary method of categorizing estrous phase. The individually calculated average cycle length for mice in our studies ranged from 4.5–6 days.
Drinking-in-the-Dark
Three hours following lights out, water bottles were removed from each animal/cage and replaced with modified sipper tubes for alcohol drinking. These drinking tubes were made from 10mL serological pipettes fitted with double ball bearing sippers (Ancare, Belmore, NY). Animals were given access to unsweetened ethanol (20%, v/v) with these drinking tubes for 2 hours each day. There was no access to regular water bottles during this limited ethanol access period. Fluid intake was recorded as the change in fluid level along the drinking tube graduations.
Homecage locomotor monitoring system
On the drug test day, cages were placed on a rack containing photocell-based activity monitors (Opto M3, Columbus Instruments Inc, Columbus, OH) as previously described (Linsenbardt and Boehm, 2012). Briefly, this system was designed to accommodate the standard sized shoebox mouse cage (18.4 cm wide × 29.2 cm long × 12.7 cm tall). The device interfaced with a laptop (Dell, Round Rock, TX) running manufacturer provided software (version 1.4.0) that translated photocell beam breaks into ambulatory activity counts in 5-minute epochs. Photocell emitters were 2.54 cm apart (12 along long walls and 8 along short walls).
Experiment 1: Does estrous status modulate the effect of intra-ventral tegmental area THIP on binge drinking and locomotor activity?
Eighty-six mice received bilateral stereotaxic implants of stainless steel 25-gauge guide cannulae, as previously described (Moore and Boehm, 2009; Linsenbardt and Boehm, 2009; Melón and Boehm, 2011; Fritz and Boehm, 2014, Kasten and Boehm, 2015). Coordinates for the anterior-VTA targeted implants (AP, −3.16mm; ML + 0.5mm; DV, −2.0mm) and the posterior-VTA targeted implants (AP, −3.64 mm; ML + 0.5mm; DV, −2.0mm) were adjusted for each mouse based on ratio between the published bregma-lambda distance and the distance measured for the individual mouse (Franklin and Paxinos, 1997). Mice had at least 1 week of recovery before initiation of alcohol access using the DID protocol described above. For the six days of alcohol consumption preceding drug microinfusion, mice were gently restrained for 30–60 seconds prior to their alcohol access. This served to habituate the animals to the handling required for drug delivery. On the 2 days prior to the drug microinfusion, this habituation handling included introduction of mock microinjectors extending 0.5mm beyond the tip of the guide cannulae. THIP or vehicle (200nL saline/side) was infused at a rate of 382nl/min and administered as 50ng/200nL/side or 25ng/200nL/side.
Immediately following drug challenge and subsequent assessment of drinking on day 7, estrous status was determined (estrus or non-estrus), and brains were harvested for histological verification of correct microinjection placements.
Experiment 2: Does estrous status influence mRNA expression of GABAA subunits in the VTA?
Quanitative real-time reverse transcriptase PCR (qPCR) was performed to compare mRNA levels of three GABAA receptor subunits, as previously described (Linsenbardt and Boehm, 2010), across the estrous cycle. The δ and α4 subunits are exclusively expressed by extrasynaptic GABAA receptors, whereas ɣ2 is often found in synaptic GABAA receptors. Immediately following vaginal smears, naive C57BL/6J mice were euthanized via cervical dislocation and brains were rapidly removed, snap frozen within 1 minute and stored at −80°C until harvesting of VTA and hippocampal (HPC) tissue by laser microdissection. HPC was included as a positive control, given previously published findings on estrous changes in δ transcription in this region (Maguire et al., 2005; Wu et al., 2013). For laser microdissection, tissue was first cryosectioned (14 μm) and transferred to polyethaline naphthalate (PEN) slides (Leica Microsystems, Wetzler, Germany), before microscope assisted dissection (Leica LMD6500 Microdissection System; Buffalo Grove, IL). RNA was isolated using Qiagen RNeasy mini kit (Qiagen, Valencia, CA) with additional DNase I treatment and stored at −80°C until further processing. RNA integrity and quality was determined using the Experion RNA highsens chip in conjunction with the Experion automated electrophoresis system and included software (Biorad, Hercules, CA). cDNA was generated with 20ng starting material for VTA and 30ng starting material for HPC using the Biorad iScript kit (Biorad, Hercules, CA). The Biorad CFX96 detector and C1000 thermal cycler were used to perform qPCR using a SYBR-Green RT-PCR mastermix. Results were analyzed using the comparative CT method with GAPDH used as the housekeeping gene. Analysis (t-test) of 2−CT values of GAPDH across estrous from HPC and VTA enriched samples confirmed its appropriate use as an internal control. Transcript levels are expressed as a percent change from estrus values.
Experiment 3. Does systemic THIP have estrous-dependent effects on binge drinking and locomotor activity in estrus vs nonestrus females?
We wanted to determine whether systemic THIP would have an estrous cycle-dependent effect on alcohol consumption and determine whether any reduction in intake noted after this GABAA agonist was due to reductions in locomotor behavior. Thus, a cohort of animals (n=32) received limited access to ethanol for 6 days, with THIP (8 mg/kg) or vehicle administered immediately prior to ethanol intake on the seventh day. Unlike experiment 2, these mice received ethanol access using a volumetric drinking monitor (Columbus Instruments Inc., Columbus, OH) so that other features of drinking (i.e. time to first drink) could be monitored across estrous. Time to first drink was recorded as the first contact with the sipper tube that resulted in the release of the drop of fluid that closes the circuit. Further details of this volumetric drinking system have been previously described (Linsenbardt and Boehm, 2014). Additionally, cages were located in a home cage activity monitoring system to monitor the locomotor response to THIP.
To replicate the above in a cohort of mice that had long term stable intake of alcohol, females (n=48) were given access to 20% alcohol in modified sipper tubes using the methods described earlier. After 3 weeks of drinking, mice were administered THIP (0, 4 or 8mg/kg) immediately prior to ethanol intake on the final day of access. Vaginal impedance was measured at the end of drinking session and vaginal lavages were then administered to assess vaginal cytology.
Experiment 4: Does diestrus phase alter drinking or effects of systemic THIP on drinking?
4a Experiment 4a: Does diestrus phase affect alcohol drinking behavior?
For this experiment, 60 mice received daily access to ethanol as per DID drinking procedures (see above). At the end of each 2-hour access period, mice were removed from their cages and vaginal lavages were administered to determine estrous status.
Experiment 4b: Does diestrus phase alter the effect of THIP on alcohol drinking behavior?
This experiment used the same 60 mice as experiment 3a. Immediately preceding access to ethanol (3 hours into the dark cycle), mice were injected with vehicle (saline) or THIP (4 or 8 mg/kg) and returned to their home cage. These THIP doses were chosen based on previous work in our lab (Moore et al., 2007).
Experiment 4c: Does blocking synthesis of 5α-reduced neurosteroids disrupt estrous-dependent effect of THIP on alcohol drinking behavior?
One hundred and fifty mice were used for this experiment, using the same procedures as experiment 4b with two exceptions. First, only the 8mg/kg THIP dose was used as this was the only dose that produced significant reductions in binge drinking in experiment 4b. Second, mice were injected with vehicle or finasteride (100mg/kg) the evening before the eighth day of ethanol access. This dose of finasteride and schedule of pretreatment (22 hours prior to THIP treatment) was chosen based on others’ published efforts demonstrating the dose and treatment schedule of finasteride associated with the greatest reduction in brain neurosteroid levels (Finn et al., 2004a; Ford et al., 2008). On test day, the effect of this finasteride pretreatment on THIP’s actions on binge-drinking and associated locomotor activity was assessed for one hour. Final groups in this experiment are represented by the following terms-vehicle x vehicle; finasteride x vehicle; vehicle x THIP and finasteride x THIP- to clarify their treatment schedule.
Statistics
All data were analyzed using IBM SPSS version 22 (IBM). For experiment 1, ethanol intake on the drug microinjection day was compared separately for mice with posterior-VTA or anterior-VTA indwelling cannulae, using a two-way ANOVA of estrous status (estrus vs non-estrus) and drug dose (vehicle, 50ng THIP, 100ng THIP for posterior VTA and vehicle, 100ng THIP for anterior-VTA). Bonferroni adjusted pairwise comparisons were used to clarify any significant interactions. For homecage activity, one-way ANOVA was used to compare drug dose for posterior VTA infusions collapsed across cycle, with Dunnet’s posthoc used to compare drug infusion dose to vehicle where appropriate. Homecage activity following anterior VTA infusions were compared using Student’s t-test (vehicle, 100ng THIP). In experiment 2, Student’s t-tests were used for each subunit (δ, α4, γ2) to assess transformed (2−ΔΔ CT) mRNA expression levels between diestrus or estrus females. For experiment 3, t-test or One-Way ANOVA were used as appropriate to assess effects of systemic THIP on intake, time to sipper tube or locomotor activity across estrous status (estrus vs. non-estrus). For experiment 4a, an average ethanol consumed was calculated for each mouse for each phase of its cycle and compared using a One-way ANOVA of Estrous (Proestrus, Estrus, Metestrus, Diestrus). For experiment 4b, ethanol intake (g/kg in 1 hour) on the day of THIP administration was compared across the stages of estrous in a two-way ANOVA of estrous status (Proestrus, Estrus, Metestrus, Diestrus) and drug dose (vehicle, 4mg/kg THIP, 8mg/kg THIP). For experiment 4c, ethanol intake and homecage activity was compared across the stages of estrous in a three-way ANOVA of estrous status, finasteride pretreatment (vehicle and 100mg/kg FIN) and THIP dose (vehicle, 8mg/kg THIP). For main effects across drug dose, Dunnett’s was used to compare drug group against vehicle controls. For all experiments, Grubb’s test was used to identify any statistical outlier. All figures are group means and error bars represent standard error of the mean. Eta squared (η2) is reported for all significant findings following ANOVAs to give an estimate of the percent variance in the data that may be attributed to a particular variable or interaction between variables. For all pairwise comparisons (Student’s t-tests, Bonferroni adjusted comparisons, Dunnet’s posthoc), Cohen’s d is reported to estimate the effect size differences between the two means (Lakens, 2013).
RESULTS
Experiment 1: Does estrous status alter the efficacy of intra-VTA THIP to temper binge drinking?
Experiment 1a
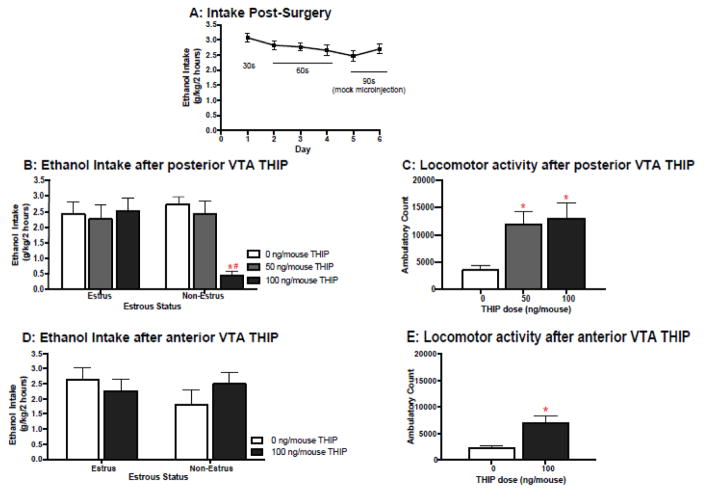
After recovery from stereotaxic surgery, mice had 7 days of limited access to alcohol using the DID protocol. Following histological verification of correct microinjection placements, only 71 of the 86 original mice (Posterior=41, Anterior=30) were included in the statistical analyses that follow. These mice consumed an average of 2.75 g/kg of ethanol across the first 6 days of once daily 1hr access (Figure 1A). On day 7 mice received a microinjection of THIP immediately prior to ethanol access. For intra-posterior VTA infusions, mice received vehicle, 50 or 100 ng of THIP (25 or 50 ng/side). We found a significant interaction of estrous status and THIP dose [F(2,35)=4.02, p<0.05; η2=.17; Figure 1B]. Bonferroni adjusted pairwise comparisons clarified that the effective THIP dose (100ng/mouse) tempered drinking for non-estrus females only (p<0.01; d=1.99). A follow up group of mice with cannulae targeting the anterior-VTA showed no effect of this THIP dose on intake, regardless of estrous-status (Figure 1D).
Figure 1.
Estrous status and effect of Intra-VTA THIP on ethanol intake. A) Following recovery from surgery, mice consumed an average of 2.75g/kg ethanol. B) THIP microinjection (50ng/200uL/side or 100ng/mouse) into the Posterior or Anterior VTA on day 7 significantly increased locomotion (*p’s<0.05). C) Intra-anterior VTA THIP at that same dose was not associated with a change in binge drinking. D)Intra-posterior VTA THIP at that dose was associated with an attenuation of binge drinking for non-estrus females only (*p<0.05, as compared to vehicle, #p<0.05, as compared to similarly treated estrus females; n’s=5–10/estrous status/region/dose).
During this final day of alcohol access, mouse cages were placed within home-cage activity monitoring frames to assess home-cage activity after drug manipulation. Intra-posterior VTA THIP significantly increased home-cage locomotion [F(2,35)= 4.07, p<0.05, η2= 0.17]. Dunnet’s post hoc clarified that both the 100 ng (p<0.05; d=1.2) and 50ng doses (p<0.05; d=1.3) were associated with significant locomotor activation (Figure 1C). Unlike ethanol consumption, there was no interaction of estrous status and dose on locomotor activity. Additionally, a significant increase in activity was also noted following intra-anterior VTA administration of 100 ng THIP [F(1,26)=7.54, p<0.05; η2= 0.06; Figure 1E].
Experiment 2: Does estrous status influence mRNA expression of GABAA subunits in the VTA?
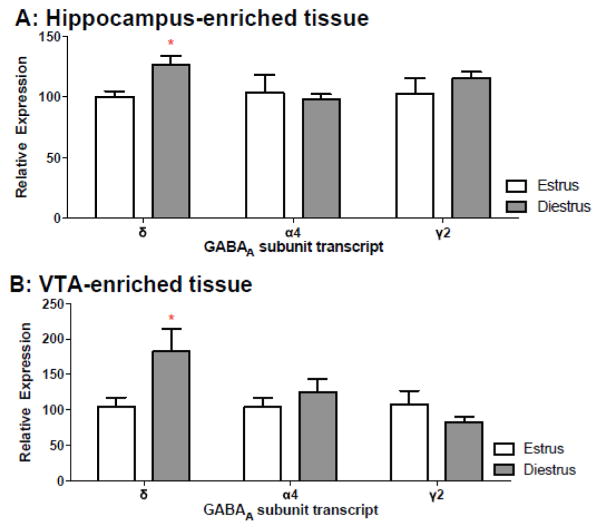
To determine whether cyclic changes in the expression of GABAA receptor subunits might underlie the estrous-dependent effect of THIP on binge drinking, RNA was isolated from ventral tegmental area (VTA) and hippocampal (HPC) enriched tissue of ethanol naïve females exhibiting diestrus- or estrus-positive vaginal smears. Analysis of δ GABAA subunit mRNA levels from HPC tissue showed that diestrus females express greater levels of δ GABAA subunit transcript in this region when compared to estrus females [t(8)=3.2, p<0.05; d=2.3; Figure 2A], confirming published findings (Maguire et al., 2005; Wu et al., 2013). Expression of δ GABAA subunit mRNA levels in the VTA was ~77% greater in diestrus as compared to estrus mice [t(8 )=2.6, p<0.05; d=1.9; Figure 2B]. For both the VTA and HPC, α4 and γ2 mRNA levels remained fairly stable across diestrus and estrus (although γ2 in the VTA was reduced by almost 24%, this finding was not significant: t(10)=1.13, p=0.28). These data suggest that cyclical changes in the availability of δ-containing GABAA receptors may underlie differences in the efficacy of THIP to temper binge drinking in our model.
Figure 2.
Estrous status and changes in GABAA subunit mRNA. A) Hippocampal (HPC) δ GABAA subunit expression is ~27% greater in diestrus females when compared to estrus females (*p<0.05). B) In tissue laser microdissected from the VTA, δ GABAA mRNA expression is ~77% greater in diestrus females when compared to estrus females (*p<0.05; n’s=4–6/estrous status).
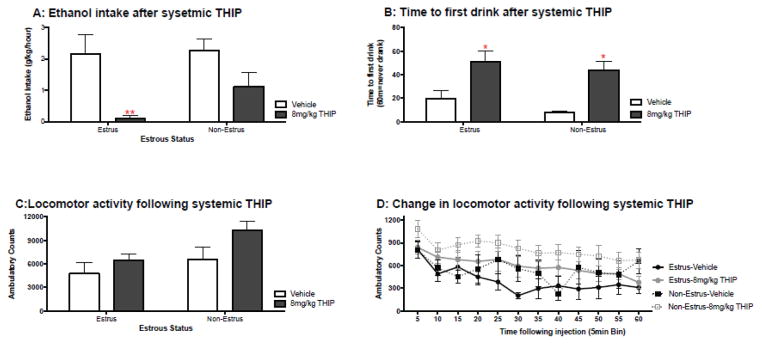
Experiment 3: Does estrous status alter the efficacy of systemic THIP to temper binge drinking?
To determine whether systemic THIP will still show an estrous-dependent effect on binge drinking, females had access to ethanol using a volumetric drinking monitor. Estrous cycle did not affect total amount of ethanol consumed during a binge session, although this volumetric drinking system did slightly reduce intakes compared to what we typically find using modified drinking tubes (3.5±0.3g/kg/2hours). Estrous cycle did not have a significant effect on time to the sipper tube on either the 1st (estrus= 13.3±5.6min, n=10; nonestrus=15.9±5.1 min, n= 22), or 7th day of access (estrus= 3.6±1.3min, n=15; nonestrus=3.2±0.7min, n= 17).
On the 8th day of access, mice were administered THIP (8mg/kg) or vehicle (saline) and returned to the homecage for limited access to ethanol (Figure 2A). THIP was associated with a significant reduction in intake for estrus mice [t(8.4)=3.4, p<0.01, d=1.6]. Non-estrus mice had a marginal reduction in intake following THIP [t(14.4)=2.0, p=0.06; d=1.0 ]. Unlike amount of alcohol consumed, THIP administration was associated with a significant delay in time to first drink (Figure 2B) for both estrus [t(13)=2.87, p<0.05, d=1.6] and non-estrus mice [t(15)=4.31, p<0.01, d=2.3]. The effect of THIP on intake does not seem to be confounded by depressed locomotion as the compound had no significant effect on locomotor activity for either group (Figure 2C). Furthermore, the time course of locomotor activity does not appear to have contributed to the delay in time to approach sipper tube noted following THIP (Figure 2D).
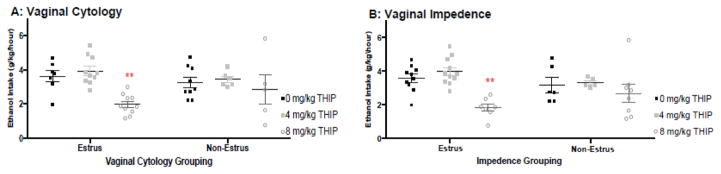
In a separate cohort of mice, long term access (3 weeks) to alcohol was initiated in their home-cage using regular modified drinking tubes to determine whether high and stable intakes of alcohol will also be tempered by THIP in an estrous-dependent manner. During the final week of access, females were consuming 5.3–5.5 g/kg of ethanol. As multiple proxy-methods exist to determine estrous cycle, and the relationship between these methods and cycling of hormones that characterize estrous are often contested (Weixelbaumer et al., 2014), we used both impedance and vaginal cytology to group mice as estrus or non-estrus to establish whether our findings would be maintained using two independent methods.
Following the characterization of estrous status via vaginal cytology, as was used in experiment 1, we found that estrus mice had a dose dependent reduction in alcohol consumption following THIP [F(2,27)=18.8; p<0.01; η2= .61], as 8mg/kg THIP significantly reduced intake of alcohol for estrus females (p<0.01; d=2.5). There was no effect of either dose of THIP on intake for non-estrus females.
Using impedance characterization, mice with vaginal wall impedance measures of 2kOhms were included as “estrus” and mice with impedance measures below 2kOhms were characterized as “non-estrus”. Estrus mice had an average impedance of 3.1±0.22 KOhms. Non-estrus mice had an average impedance of 1.3±0.22 KOhms. These values concur with changes in vaginal wall impedance across estrous reported in the literature (Weixelbaumer et al., 2014). The use of vaginal impedance to characterize estrus and non-estrus females resulted in re-characterization of some mice when compared to the cytology analysis (n=3 estrus to non-estrus and 2 non-estrus to estrus). Still, using this characterization, estrus females again showed a dose dependent response to THIP [F(2,28)=19.25; p<0.01; η2=.61], as the highest dose of THIP (8mg/kg) significantly reduced intakes for these females (p<0.01; d=2.6). For non-estrus mice, no dose of THIP was effective in altering intake of alcohol.
Experiment 4: Does diestrus phase alter drinking or effects of systemic THIP on drinking?
Experiment 4a: Does diestrus phase affect alcohol drinking behavior?
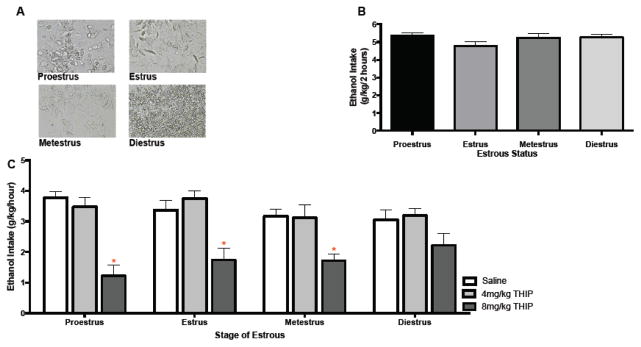
As diestrus females showed increased δ mRNA in both the hippocampus and VTA, and our data from experiments 1 and 3 suggest that altering activity of δ-GABAAR can effect alcohol intake, we wanted to determine whether baseline intake of alcohol in this limited access paradigm would be shifted for mice across these four stages of the cycle. Vaginal lavages were taken immediately following limited access alcohol drinking (see Figure 5A for representative photomicrographs). In order to aid in the analysis, daily ethanol intake was collapsed across each cycle such that every animal had an average value of ethanol consumed during each of their four stages of the estrous cycle. Average daily ethanol consumed across each phase of the estrous cycle is shown in Figure 5B. These mice did not display significant changes in the amount of alcohol consumed across the cycle.
Figure 5.
Estrous status and sensitivity to THIP’s effect on binge drinking. A) Photomicrographs of vaginal smears used to assess estrous. B) Average ethanol (g/kg) intake at each stage of estrous C) THIP significantly reduced intake at 8mg/kg (i.p) for females that were proestrus, estrus or metestrus at the time of drug administration (* p<0.05, as compared to vehicle; n’s = 6–13/estrous status/dose).
Experiment 4b: Does diestrus phase alter the effect of THIP on alcohol drinking behavior?
Animals had access to alcohol for 7 days prior to THIP administration. Two of the original 150 mice were not included because they had extended (>4 days) diestrus smears, leaving 148 mice in this study. Immediately preceding the 8th day of access, mice were administered THIP or vehicle. Vaginal lavages were taken following 1 hour of alcohol access on this drug manipulation day. A two-way ANOVA of drug dose (0, 4 or 8 mg/kg) and status (proestrus, estrus, metestrus or diestrus) revealed a significant interaction of drug dose and estrous status [F(6, 143) = 2.315, p<0.05; η2= 0.06; Figure 5c]. Bonferroni adjusted pairwise comparison revealed that the highest dose of THIP decreased drinking for mice in the proestrus (p<0.0001; d=2.8), estrus (p<0.001; d=1.3) and metestrus (p<0.01, d=1.6) phases of the estrous cycle. Mice in the diestrus phase did not decrease drinking after administration of this effective dose of the drug (p=0.139).
Experiment 4c: Does blocking synthesis of 5α-reduced neurosteroids disrupt estrous-dependent effect of THIP on alcohol drinking behavior?
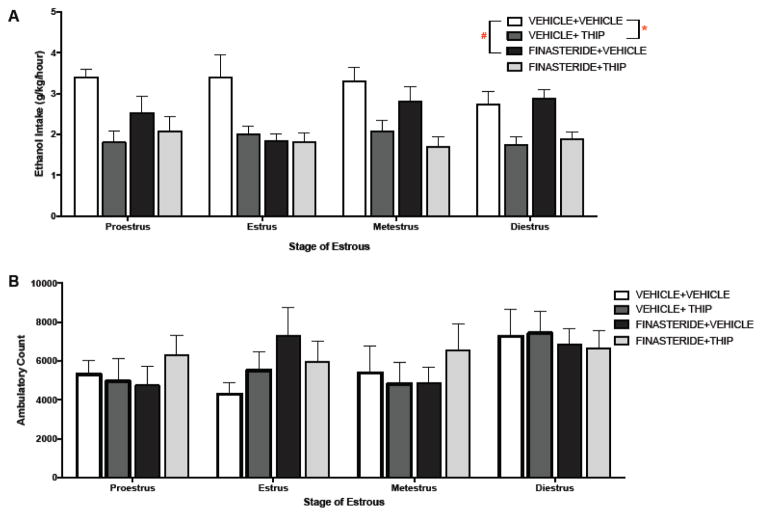
Animals had access to alcohol for 7 days prior to THIP administration. Due to a behavioral testing equipment failure during activity recording for the final cohort of mice, activity analysis includes data for only 119 of the 148 mice included in the drinking analysis. Immediately following the end of the 7th day of drinking, mice were administered 100 mg/kg of finasteride or vehicle (20% Beta-cyclodextrin), a dose and time frame demonstrated to decrease synthesis of 5α-reduced neurosteroids (Finn et al., 2004a). Immediately preceding ethanol access on the 8th day, mice were administered THIP (8 mg/kg) or vehicle (saline). Vaginal lavages were taken following 1 hour of alcohol access on this drug manipulation day to verify the stage of estrous. Both ethanol consumption and simultaneous locomotor activity were analyzed using a three-way ANOVA of finasteride dose (0, 100 mg/kg), THIP dose (0, or 8mg/kg) and estrous status (proestrus, estrus, metestrus or diestrus). The analysis on ethanol consumption following drug manipulation revealed a main effect of THIP dose [F(1, 132) = 43.163, p<0.0001, η2=0.21], as mice reduced their intake following THIP administration (Figure 6A). However, unlike experiment 1b, there was no significant interaction of THIP dose and estrous status, as the drug significantly reduced drinking for mice in all four phases. We also found a significant main effect of finasteride, [F(1, 132) = 6.285, p<0.05, η2= 0.04], as pretreatment with this 5α-reductase inhibitor the evening before the test day reduced drinking for females (Figure 6A). There was a marginal interaction of finasteride and estrous phase. However, because this only approached significance, we were not justified in performing post hoc analyses to determine the stage(s) driving the finasteride effect. Lastly, there was a significant interaction of finasteride dose and THIP dose [F(3, 132) =4.978, p<0.05, η2=0.03]. Bonferroni adjusted pairwise comparison clarified that control mice (not receiving THIP) decreased their intake following pretreatment with finasteride (p<0.001; d=0.65).
Figure 6.
Pretreatment on day 7 with finasteride or vehicle and sensitivity to THIP treatment on day 8. A) THIP significantly reduced intakes in all females following either day 7 vehicle or finasteride pretreatment, regardless of estrous status (*p<0.05, compared to vehicle+vehicle group). Similarly, pretreatment with finasteride on day 7 reduced intakes on day 8 (#p<0.05, compared to vehicle+vehicle group; n’s = 6–16/estrous status/dose). B) No significant change in homecage activity confounded the effect of these drugs on alcohol intake
During the final drug manipulation day, mouse cages were re-located to the home cage locomotor activity frames to get a measure of locomotion during drinking and following drug administration. A three-way ANOVA on total (1 hour) home cage activity did not reveal any significant effects of finasteride or THIP. There was, however, a marginal effect (p=0.07) of estrous status on home cage activity during this ethanol drinking (Figure 6B).
DISCUSSION
The findings presented herein confirm that THIP, a GABAA agonist with high selectivity at low doses for extrasynaptically located δ-GABAARs, can modulate alcohol consumption. These data extend current knowledge, showing for the first time that the efficacy of this compound to temper drinking is modulated by estrous status in naturally cycling female mice. Additionally, we show that intra-posterior VTA administration of THIP significantly reduces alcohol intake during diestrus, when expression of δ mRNA in the VTA is increased by over 70%. Together, our findings add to the growing body of evidence supporting a role for δ-GABAARs in the maintenance of binge alcohol intake and suggest that estrous dependent fluctuations in availability of these extrasynaptically located receptors may significantly impact the ability of compounds targeting this receptor population to modulate ethanol usage.
Previous studies from our lab (Moore et al., 2007; Fritz and Boehm, 2014; Quoilin and Boehm, 2016) and others (Ramaker et al., 2011, 2012, 2014a, 2014b) have shown that THIP can significantly reduce voluntary ethanol consumption and operant responding for the drug. Given THIP’s high affinity for δ-GABAARs, these data support a possible role for these extrasynaptically located GABAA receptors in mediating this pattern of ethanol use. Indeed, viral mediated knockdown of δ-GABAARs in the nucleus accumbens shell results in the reduction of ethanol consumption and preference in male Long-Evans rats (Nie et al., 2011). Furthermore, a naturally occurring point mutation in α6, the α subunit found preferentially expressed with δ in the cerebellum, has been shown to be important for the motor-incoordinating effects of ethanol, a characteristic thought to negatively modulate consumption of intoxicating levels of ethanol (Hanchar et al., 2005). Extrasynaptically located δ-GABAARs respond to low to moderate concentrations of GABA. Similarly, the tonic inhibition mediated by these δ-GABAARs has been shown to be enhanced the low-to-moderate doses of ethanol relevant to brain concentrations achieved during an active binge drinking session (17–30mM; Glykys et al., 2007). This sensitivity suggests that δ-containing GABAARs may play a significant role in mediating the pharmacological consequences of binge drinking associated with intoxication. If these effects drive this pattern of consumption, it is plausible that pretreatment with a compound that targets this receptor population may alter subsequent drinking. Indeed, in all analyses of THIP’s ability to reduce intake for a given receptive estrous group, effect size estimates supported large effects (Cohen’s d> 0.8, Lasken, 2011). This suggests that the mechanism by which THIP administration produces a reduction in consumption plays a significant role in drinking behavior, as alcohol intake for groups where THIP was effective were often a full standard deviation unit lower than control drinkers.
It should be noted that THIP can have effects on the intake of non-alcoholic fluids (Moore et al., 2007). Though these could be related to non-specific effects on locomotor activity our data suggests no sedative effects at our effective dose. There is the additional possibility that the dose that is effective in reducing intake (8mg/kg THIP) targets both synaptic and extrasynaptic GABAA receptors. However, we found that THIP’s effects on intake of a non-alcohol fluid (10 % sucrose) was also estrous dependent, with estrus females (M=1.1 ± 0.24 mL) showing a greater reduction in intake of sucrose after THIP than non-estrus females (M=1.8± 0.3 mL) when compared to vehicle treated females (M=2.14 ± 0.14 mL; data not shown). Still, the possibility remains that THIP could be acting on δ-mediated mechanisms that are both directly and indirectly related to alcohol’s actions. For example, systemic THIP (6 mg/kg) has been shown to induce conditioned place aversion in mice (Vashchinkina et al., 2012). It is therefore possible that aversive effects of THIP could underlie the reduced drinking seen following systemic administration of the drug.
Given the suggested involvement of δ-GABAARs in binge drinking behavior and previous reports demonstrating oscillations in the expression of this receptor population throughout the estrous cycle (Maguire et al., 2005; Griffiths and Lovick, 2005; Lovick et al., 2005; Wu et al., 2013), we expected estrous-dependent changes in this pattern of ethanol use. However, our data do not support any shifts in binge drinking across the 4 stages of estrous in female C57Bl/6J mice. It is possible that our crude measurement of total ethanol (g/kg) consumed failed to capture changes in the other more fundamental characteristics of the drinking behavior that takes place during the entire 1-hour limited access period. For example, it may be that mice change the intensity of their bouts or their time to initiate drinking/total time drinking which would support estrous-associated changes in the processes that underlie this ethanol use behavior. Indeed, an elegant analysis of the microarchitecture of drinking behavior in freely cycling female rats demonstrated estrous-dependent changes in intake behaviors, like increased bout frequency during metestrus (diestrus 1) alongside no measurable shift in the total amount of ethanol consumed (Ford et al., 2002). Therefore, additional work needs to be done to determine whether other processes associated with binge drinking behavior in freely cycling female mice shift across their estrous cycle. In our initial attempt to understand differences in the pattern of intake in these mice using a volumetric sipper system, our data on time to initiate drinking did not support an effect of estrous status on this measure at either the start or end of the week of access to alcohol. Further efforts are needed to more finely determine whether estrous cycle status influences the microarchitecture of binge alcohol drinking.
When using the volumetric drinking system, THIP administration was associated with a complete abolishment (rather than temperance) of drinking behavior for estrus females and only a marginal reduction (p=0.06) of drinking for non-estrus mice. Though a calculation of the effect size for the latter trend suggests a large effect (d=1.0), variability within the data (potentially caused by the limited design inherent in the inclusion of two phases-metestrus and diestrus- into one “nonestrus” group) results in large sample size requirements (>23mice/group) to pull out statistically significant findings using null-hypothesis testing. The variability was greater in the volumetric drinking system experiments than all others, and this may be due to additional exogenous variables (i.e. noise as the system refills the tube and registers each lick) associated with the use of the volumetric system. Interestingly, the variability was larger for the nonestrus vs estrus grouping, suggesting that physiological differences in the phases included in these pairings (diestrus and metestrus for the first; proestrus and estrus for the latter) that drive the estrous dependent effects of THIP are greater within the nonestrus group.
Although the effects of intra-posterior VTA THIP were also estrous dependent, intra-posterior VTA THIP was only able to reduce intake for non-estrus mice (diestrus and metestrus), unlike systemic THIP which was most effective for estrus mice. It is not surprising that systemic and central administrations of a compound would have different effects. Still, the data suggest that the intra-posterior population of THIP sensitive receptors may not mediate the effect that systemic THIP has on binge drinking. These intra-VTA data add to growing findings showing neuroanatomical hetereogeneity of the GABAergic system in the VTA (examples in rats: Arnt and Scheel-Kruger, 1979 Ikemoto, Murphy and McBride, 1998; Rodd-Henricks et al., 2000; examples in mice: Boehm et al., 2002; Bechtholt and Cunningham, 2005; Moore and Boehm, 2009; Melón and Boehm, 2011; for review see Sanchez-Catalan et al., 2014) as the reduction in binge drinking for nonestrus females was only noted after intra-posterior administration of the compound. Unlike drinking, locomotor activity increased following both anterior and posterior administration of THIP. Given the long known heterogeneity of the locomotor response to GABAA agonists into the anterior versus posterior VTA (Arnt and Scheel-Kruger, 1979) we were surprised to find this effect. A plausible explanation is that the increased activity noted following anterior-VTA THIP is due to diffusion of the compound to the posterior VTA. However, as there was no change in drinking in these same animals, we do not think that is the case. Furthermore, this hyperlocomotion was not estrous dependent. Collectively, these results suggest a possible mechanistic disconnect between the locomotor effects of THIP and its role in reducing ethanol drinking. Indeed, evidence supports neurochemical and physiological heterogeneity of VTA GABAergic neurons (Margolis et al., 2012; Morales and Margolis, 2017). In particular, GABAA receptors may be expressed by VTA DA neurons as well as local GABAergic interneurons (Edwards et al., 2017). Therefore, depending on the population of intra-VTA GABAA receptors activated, one can have an inhibitory or permissive effect on firing of VTA DA neurons.
Both systemic and posterior-VTA administration of THIP reduced intake in our study. Though this suggests that the effects of THIP at the posterior VTA may underlie our systemic effects, the opposing role that estrous plays in sensitivity to this THIP effect following central vs. peripheral administration, alongside evidence that pharmacological activation of THIP in the nucleus accumbens (NAc) also reduces intake complicates this interpretation. Interestingly, in the NAc, both pharmacological activation of δ GABAARs (Ramaker et al., 2015) and reduction of their expression via genetic manipulation (Nie et al., 2011) results in depressed intake. In the case of the pharmacological activation, the expected leftward shift in the ethanol dose response curve may result in reduced intake by mice in the limited access (2hour) paradigm used in that study because less alcohol may be needed to produce the effects associated with drinking pharmacologically relevant doses of alcohol in that short paradigm. In the case of genetic downregulation of δ in the NAc, the expected rightward shift in alcohol’s dose response curve following lower levels of δ GABAARs could result in an increase in the threshold of alcohol intake necessary for the drug to achieve effects that would engender continued drinking (i.e. reinforcement) in this 24hr-access paradigm, causing reduced drive to drink. Lastly, differences in the species used across all of these studies could play a role in contradictory results. Indeed, systemic THIP administration (16mg/kg) has been shown to enhance acquisition and total intake of ethanol in rats as well as increase bout size while the same dose reduces intake in mice, with no change in bout size (Boyle, Smith and Amit, 1992; Ramaker et al., 2015). It remains that our findings, along with longstanding work of others using either pharmacological or genetic manipulation of δ containing GABAA receptors in rats or mice support changes in ethanol consumption following manipulation of this receptor subtype.
To understand our estrous dependent pharmacological effects, we explored two possibilities. First, that changes in availability of progesterone-derived neurosteroids, which can act as positive allosteric modulators of GABA and whose levels would change across the estrous cycle could modulate the efficacy of THIP and second, that previously supported changes in the availability of δ containing GABAA receptors in could underlie the estrous-dependent effects of this δ-specific compound. To test the first hypothesis, finasteride was administered in order to block neurosteroidogenesis. As finasteride is a general inhibitor of 5α-reductase, in addition to reductions in allopregnanolone and THDOC, we would inhibit synthesis of androgens-like DHT-that may also be playing a role in the estrous-specific effects of THIP. Indeed, Nilsson et al. (2015) have shown that serum levels of DHT in female mice may be reduced as much as 70% during diestrus vs. metestrus. This drop in DHT during this final phase of diestrus would result in reduced availability of androstanedione, a DHT-derived neuroactive steroid that, like allopregnanolone, acts as a positive allosteric modulator of GABAA receptors. Interestingly, when the animals were given multiple injection experiences-as in this experiment (experiment 4) where the systemic THIP injection is preceded by an injection of finasteride or vehicle the evening prior-diestrus no longer confers insensitivity to THIP’s effects on drinking behavior. Injection experience is the only variable that differentiates experiment 4 from the multiple systemic THIP experiments presented herein. This surprising finding highlights the important role that stress or state of the animal plays in the expression of estrous-dependent effects. As all mice in our drinking studies were individually housed, it is additionally possible that compounded stress from single housing, estrous status and injection experience influenced our findings. It remains, however, that blocking synthesis and reducing levels of neurosteroids that act as positive allosteric modulators at GABAA receptors did not influence THIP’s ability to temper alcohol drinking in our model.
To test the second hypothesis, qPCR was used to assess changes in expression of GABAA subunit transcripts in the hippocampus and VTA. Previous studies confirm estrous-associated oscillations in the expression of δ at both the mRNA (Wu et al., 2013) and protein (Maguire et al., 2005) levels in the hippocampus. These findings were supported by our qPCR analysis of δ mRNA in laser microdissected hippocampal tissue, with diestrus females showing a 27% greater δ expression than estrus females. The behavioral relevance of these changes in δ range from estrous-dependent changes in anxiety-like behavior and seizure susceptibility (Maguire et al., 2005), network activity during sleep (Barth, Verando and Mody, 2014) and learning (Sabaliauskas et al., 2015), with recent findings demonstrating that estrous dependent changes in hippocampal dependent learning is abolished in δ-GABAAR knockout mice (Cushman et al., 2014). Although our goal for the hippocampal analysis was limited to its use as a positive control for our investigation of δ changes in the VTA, these studies showing the impact that estrous-dependent changes in δ expression across the hippocampus can have on a variety of behaviors warrant further investigation of the potential role that δ-GABAAR in this region may play in estrous-dependent effects on binge drinking in females. For the VTA, we harvested tissue from both the anterior and posterior regions (in order to improve the amount of RNA we isolated from each animal so that samples did not need to be pooled). Still, the data suggests that δ expression in the VTA increases by over 70% during diestrus compared to estrus. In fact, effect size estimates of the changes in δ expression in both the hippocampus and VTA support large effects sizes for such changes across both regions.
Although we only assess changes in the expression of δ at two points throughout the estrous cycle (estrus and diestrus), two seminal studies have demonstrated that diestrus (diestrus II) may be unique in its higher expression of both δ mRNA and protein when compared to all other phases in the cycle (Lovick et al., 2005; Griffiths and Lovick, 2005). This may explain the surprising differences in the sensitivity to THIP at metestrus vs. diestrus noted in our work. We did not find a complementary change in the mRNA expression of the α4 subunit, the preferential partner of δ in cortical extrasynaptic GABAA receptors, suggesting that the potential GABAA receptors that assemble with the increased δ subunit available include alternative α subunits. Further, we did not find a concomitant change in the γ2 subunit mRNA. In both cases, we caution that changes in mRNA levels do not necessarily translate to functional changes in protein availability or receptor subtype levels. Still, as γ2 appears to often be found in GABAA receptors recruited to the synapse, our findings suggest that diestrus could be a time of selective increase in only the tonic component of GABA-mediated inhibition. If these δ-containing GABAA receptors are located on interneurons targeting dopamine (DA) receptors in this region, as suggested by Vashchinkina et al. (2012), we believe our data suggests that DA receptors in the VTA may be under reduced inhibitory constraint during the diestrus phase. Whether this reduced inhibitory drive is selective for either accumbal or prefrontal cortex projecting DA neurons (Lammel et al., 2011) may have interesting implications for potential changes in reward and/or aversion sensitivity across estrous.
CONCLUSION
In these experiments we demonstrated for the first time that in female mice (1) transcription of the δ GABAA subunit in the VTA is significantly increased during diestrus (as compared to estrus) and (2) THIP, a compound with high affinity for δ containing GABAA receptors, has estrous-dependent effects on ethanol consumption when administered systemically or into the posterior-VTA, and estrous independent effects on locomotor activity when administered into the posterior or anterior VTA. These data suggest that the estrous cycle may be a significant factor in the pharmacological modulation of binge alcohol drinking using δ GABAA-specific compounds in female mice.
Figure 3.
Drinking behaviors and activity after THIP. A) Estrus (proestrus and estrus) mice show significant reduction in intake after THIP (**p<0.01) whereas non-estrus (metestrus and diestrus) mice had a marginal reduction in intake (p=0.06). B) THIP administration significantly delayed the time to first drink for both groups (*p’s>0.05, compared to relevant vehicle group). C) This delay in time to first drink does not correspond to any significant reduction in activity across time D) The effect of THIP on intake are not due to competing changes in locomotor behaviors, as THIP only marginally increased activity for non-estrus mice (p=0.07) and had no effect on activity for estrus mice. n’s=6–9/estrous status/dose.
Figure 4.
Drinking behavior after THIP in mice categorized using vaginal cytology or vaginal impedance. Mice were given 3 weeks of access to alcohol to induce stable and high limited-access consumption. A) Using vaginal cytology to characterize estrous status, estrus females consumed significantly less alcohol following THIP administration (**p<0.01, compared to vehicle). B) Using vaginal impedance to characterize estrous status in these females, we similarly find that estrus females consumed significantly less alcohol following THIP and see no reduction in intake for THIP treated non-estrus mice (**p<0.01, compared to vehicle; n’s = 5–11/estrous status/dose).
HIGHLIGHTS.
Intra- posterior ventral tegmental area administration of THIP has estrous dependent effects on drinking
Intra- posterior and anterior ventral tegmental area administration of THIP have estrous-independent effects on homecage locomotor activity
Expression of δ GABAAR mRNA oscillates across the estrous cycle in VTA enriched tissue
Systemic administration of THIP, an agonist with high affinity for δ-GABAAR, fails to inhibit binge drinking behavior for diestrus females.
Acknowledgments
This work was supported by the National Institutes of Health [grant AA016789 to SLB, F31AA018910 to EMM, T32 AA07462 and K12GM074869 to LCM]; the IUPUI Undergraduate Research Opportunity Program to ZTN; and the Indiana Alcohol Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnt J, Scheel-Krüger J. GABA in the ventral tegmental area: differential regional effects on locomotion, aggression and food intake after microinjection of GABA agonists and antagonists. Life Sci. 1979;25(15):1351–1360. doi: 10.1016/0024-3205(79)90402-8. [DOI] [PubMed] [Google Scholar]
- Barth AM, Ferando I, Mody I. Ovarian cycle-linked plasticity of δ-GABA A receptor subunits in hippocampal interneurons affects γ oscillations in vivo. Front Cell Neurosci. 2014:8. doi: 10.3389/fncel.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA A receptors. Neuropharmacology. 2002;43(4):651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bhattarai JP, Park SA, Park JB, Lee SY, Herbison AE, Ryu PD, Han SK. Tonic extrasynaptic GABA(A) receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology. 2011;152(4):1551–61. doi: 10.1210/en.2010-1191. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low-to high-efficacy gating patterns. J Neurosci. 2003;23(34):10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Homanics GE, Blednov YA, Harris RA. δ-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol. 2006;541(3):158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Smith BR, Amit Z. Microstructural analysis of the effects of THIP, a GABAA agonist, on voluntary ethanol intake in laboratory rats. Pharmacol Biochem Behav. 1992;43(4):1121–1127. doi: 10.1016/0091-3057(92)90491-w. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136(7):965–74. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009:A-4I. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Binge Drinking: A Serious, Under-recognized Problem Among Women and Girls (CDC Vital Signs) Atlanta, GA: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- Cherpitel CJ. Alcohol and violence- related injuries: an emergency room study. Addiction. 1993;88(1):79–88. doi: 10.1111/j.1360-0443.1993.tb02765.x. [DOI] [PubMed] [Google Scholar]
- Cook RL, Clark DB. Is There an Association Between Alcohol Consumption and Sexually Transmitted Diseases? A Systematic Review. Sex Transm Dis. 2005;32(3):156. doi: 10.1097/01.olq.0000151418.03899.97. [DOI] [PubMed] [Google Scholar]
- Cushman JD, Moore MD, Olsen RW, Fanselow MS. The role of the δ GABA (A) receptor in ovarian cycle-linked changes in hippocampus-dependent learning and memory. Neurochem Res. 2014;39(6):1140–1146. doi: 10.1007/s11064-014-1282-6. [DOI] [PubMed] [Google Scholar]
- Cyders MA, VanderVeen JD, Plawecki M, Millward JB, Hays J, Kareken DA, O’Connor S. Gender Specific Effects of Mood on Alcohol Seeking Behaviors: Preliminary Findings Using Intravenous Alcohol Self Administration. Alcohol Clin Exp Res. 2016;40(2):393–400. doi: 10.1111/acer.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Bortolato M, Finn DA, Ramaker MJ, Barak S, Ron D, Liang J, Olsen RW. Recent advances in the discovery and preclinical testing of novel compounds for the prevention and/or treatment of alcohol use disorders. Alcohol Clin Exp Res. 2013;37(1):8–15. doi: 10.1111/j.1530-0277.2012.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: At risk for what? Drug Alcohol Depend. 2008;95(1–2):62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards NJ, Tejeda HA, Pignatelli M, Zhang S, McDevitt RA, Wu J, Bass CE, Bettler B, Morales M, Bonci A. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat Neuro. 2017 doi: 10.1038/nn.4482. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Therapeut. 2004a;101(2):91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004b doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of Ethanol Self Administration: Estrous Cycle Phase Related Changes in Consumption Patterns. Alcohol Clin Exp Res. 2002;26(5):635–643. [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32(8):1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M. Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med. 2015;45(14):3047–3058. doi: 10.1017/S0033291715001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. Mouse brain in stereotaxic coordinates. Academic press; 1997. [Google Scholar]
- Fritz BM, Boehm SL., II Site-specific microinjection of THIP into the infralimbic cortex modulates ethanol intake in male C57BL/6J mice. Behav Brain Res. 2014;273:8–15. doi: 10.1016/j.bbr.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10(1):40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gorin-Meyer RE, Wiren KM, Tanchuck MA, Long SL, Yoneyama N, Finn DA. Sex differences in the effect of finasteride on acute ethanol withdrawal severity in C57BL/6J and DBA/2J mice. Neuroscience. 2007;146(3):1302–1315. doi: 10.1016/j.neuroscience.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express α4, β1 and δ GABAA receptor subunits: Plasticity of expression during the estrous cycle. Neuroscience. 2005;136(2):457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8(3):339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Guan Y, Xiao C, Krnjevic K, Xie G, Zuo W, Ye JH. GABAergic Actions Mediate Opposite Ethanol Effects on Dopaminergic Neurons in the Anterior and Posterior Ventral Tegmental Area. J Pharmacol Exp Ther. 2012;341(1):33–42. doi: 10.1124/jpet.111.187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depen. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Richter L, Kleber HD, McLellan AT, Carise D. Telescoping of drinking-related behaviors: gender, racial/ethnic, and age comparisons. Substance Use & Misuse. 2005;40(8):1139–1151. doi: 10.1081/JA-200042281. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Boehm SL. Intra-nucleus accumbens shell injections of R (+)-and S (−)-baclofen bidirectionally alter binge-like ethanol, but not saccharin, intake in C57Bl/6J mice. Behav Brain Res. 2014;272:238–247. doi: 10.1016/j.bbr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depen. 2008;93(1–2):21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry. 2010;167(8):969–976. doi: 10.1176/appi.ajp.2009.09081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Nixon SJ. Characterizing gender differences in treatment seekers. Alcohol Clin Exp Res. 2014;38(1):275–284. doi: 10.1111/acer.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164(2):424–434. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Ethanol-induced locomotor sensitization in DBA/2J mice is associated with alterations in GABA(A) subunit gene expression and behavioral sensitivity to GABA(A) acting drugs. Pharmac Biochem Behav. 2010;95(3):359–366. doi: 10.1016/j.pbb.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Role of Novelty and Ethanol History in Locomotor Stimulation Induced by Binge Like Ethanol Intake. Alcohol Clin Exp Res. 2012;36(5):887–894. doi: 10.1111/j.1530-0277.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Boehm SL. Alterations in the rate of binge ethanol consumption: implications for preclinical studies in mice. Addict Biol. 2014;19(5):812–825. doi: 10.1111/adb.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SMJ, Martin IL. Changes in GABAA receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131(2):397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8(6):797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PloS one. 2012;7(7):e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA(A) receptors. J Neurophysiol. 2011;106(4):2057–64. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón L, Boehm SL., II GABAA receptors in the posterior, but not anterior, ventral tegmental area mediate Ro15-4513-induced attenuation of binge-like ethanol consumption in C57BL/6J female mice. Behav Brain Res. 2011;220(1):230–237. doi: 10.1016/j.bbr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96(22):12905–10. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA A receptors. Trends Neurosci. 2004;27(9):569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-line ethanol intake in C57BL/6J mice. Pharmac Biochem Behav. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Boehm SL., II Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123(3):555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 2010;588(Pt 8):1251–68. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal KJ, Kawachi I, Miller M, Rimm EB. Drinking frequency and quantity and risk of suicide among men. Social Psychiatry and Psychiatr Epidemiol. 2007;42(2):153–160. doi: 10.1007/s00127-006-0144-1. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge Drinking Among US Adults. JAMA. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter. PubsNiaaaNihGov. Winter; Retrieved April 28, 2013, from http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf.
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA. 2011;108(11):4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABAA receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 1998;139(1–2):108–116. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABA A receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41(3):201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW. Extrasynaptic GABAA receptors in the nucleus accumbens are necessary for alcohol drinking. Proc Natl Acad Sci USA. 2011;108(12):4699–4700. doi: 10.1073/pnas.1102818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BL, Pescosolido BA, Bucholz K, Edenberg H, Kramer J, Kuperman S, Schuckit MA, Nurnberger JI., Jr Gender-specific gene–environment interaction in alcohol dependence: the impact of daily life events and GABRA2. Behav Genet. 2013;43(5):402–414. doi: 10.1007/s10519-013-9607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ian Ragan C, McKernan RM. Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur J Pharm-Mol Ph. 1995;290(3):175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Boehm SL. Involvement of the GABAA Receptor in Age Dependent Differences in Binge Like Ethanol Intake. Alcohol Clin Exp Res. 2016;40(2):408–417. doi: 10.1111/acer.12953. [DOI] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of Ethanol Drinking in Mice via Modulation of the GABAA Receptor with Ganaxolone, Finasteride, and THIP. Alcohol Clin Exp Res. 2011;35(11):1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA. Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology. 2012;63(4):555–564. doi: 10.1016/j.neuropharm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Phillips TJ, Finn DA. Differences in the reinstatement of ethanol seeking with ganaxolone and THIP. Neuroscience. 2014a;272:180–187. doi: 10.1016/j.neuroscience.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong-Kaufman MN, Ford MM, Phillips TJ, Finn DA. Effect of nucleus accumbens shell infusions of ganaxolone or THIP on ethanol consumption in mice. Psychopharmacology (Berl) 2014b:1–12. doi: 10.1007/s00213-014-3777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60(2):252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at α4βδ GABA A receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2015;1621:170–186. doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Catalan MJ, Kaufling J, Georges F, Veinante P, Barrot M. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282:198–216. doi: 10.1016/j.neuroscience.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Serras A, Saules KK, Cranford JA, Eisenberg D. Self-injury, substance use, and associated risk factors in a multi-campus probability sample of college students. Psychology of Addictive Behaviors. 2010;24(1):119–128. doi: 10.1037/a0017210. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Kristman-Valente AN, Herrenkohl TI. Adult binge drinking: childhood sexual abuse, gender and the role of adolescent alcohol-related experiences. Alcohol Alcohol. 2015:agv093. doi: 10.1093/alcalc/agv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Foxcroft D. Drinking in the UK. An exploration of trends. Joseph Rowntree Foundation; 2009. [Google Scholar]
- Smith KW, Gierski F, Andre J, Dowell NG, Cercignani M, Naassila M, Duka T. Altered white matter integrity in whole brain and segments of corpus callosum, in young social drinkers with binge drinking pattern. Addict Biol. 2015 doi: 10.1111/adb.12332. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J Neurophysiol. 2003;90(2):903–10. doi: 10.1152/jn.01022.2002. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35(10):1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100(24):14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchinkina E, Panhelainen A, Vekovischeva OY, Aitta-Aho T, Ebert B, Ator NA, Korpi ER. GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J Neuroscience. 2012;32(15):5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchinkina E, Manner AK, Vekovischeva OY, Den Hollander B, Uusi-Oukari M, Aitta-aho T, Korpi ER. Neurosteroid agonist at GABAA receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacology. 2014;39(3):727–737. doi: 10.1038/npp.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56(1):182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23(33):10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22(5):1541–9. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]