Abstract
Of the available regenerative treatment options, craniofacial tissue regeneration using mesenchymal stem cells (MSCs) shows promise. The ability of stem cells to produce multiple specialized cell types along with their extensive distribution in many adult tissues have made them an attractive target for applications in tissue engineering. MSCs reside in a wide spectrum of postnatal tissue types and have been successfully isolated from orofacial tissues. These dental-or orofacial-derived MSCs possess self-renewal and multilineage differentiation capacities. The craniofacial system is composed of complex hard and soft tissues derived from sophisticated processes starting with embryonic development. Because of the complexity of the craniofacial tissues, the application of stem cells presents challenges in terms of the size, shape, and form of the engineered structures, the specialized final developed cells, and the modulation of timely blood supply while limiting inflammatory and immunological responses. The cell delivery vehicle has an important role in the in vivo performance of stem cells and could dictate the success of the regenerative therapy. Among the available hydrogel biomaterials for cell encapsulation, alginate-based hydrogels have shown promising results in biomedical applications. Alginate scaffolds encapsulating MSCs can provide a suitable microenvironment for cell viability and differentiation for tissue regeneration applications. This review aims to summarize current applications of dental-derived stem cell therapy and highlight the use of alginate-based hydrogels for applications in craniofacial tissue engineering.
INTRODUCTION
The repair and regeneration of craniofacial tissues continue to be a challenge for clinicians and biomedical engineers.1,2 Reconstruction of pathologically damaged craniofacial tissues is often required because of tumors, trauma, or congenital malformations. The reconstructive procedures for craniofacial tissue regeneration are usually very complex as the craniofacial region is itself a complex construct, consisting of bone, cartilage, soft tissue, and neurovascular bundles. For instance, to reconstruct damaged craniofacial bones, an array of surgical procedures is available.1,2 Autologous bone grafts have been considered the gold standard for bone regenerative therapies. Together with allogenic bone grafts, this type of bone graft material comprises more than 90% of grafts performed.1–3 However, these grafting procedures have numerous disadvantages, including hematomas, donor site morbidity, inflammation, infection, and high cost. 1–3
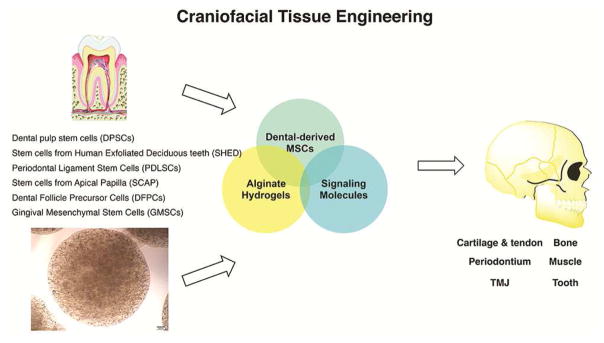
Several treatment possibilities have been introduced for articular cartilage or ligamentous tissue regeneration (grafting of autologous osteochondral tissue or the transplantation of autologous chondrocyte suspensions). However, the biomechanical properties of the tissues regenerated through these treatment options are mediocre compared with those of native articular cartilage.2,3 Furthermore, the repair and regeneration of muscle tissue (for example, tongue muscle) following traumatic injuries frequently exhibit a challenging clinical situation in the craniofacial region. Substantial esthetic and functional issues will arise if a significant amount of tissue is lost because of the inability of the native muscle tissue to regrow and fill the defect site. To find an alternative treatment option for the reconstruction of craniofacial tissue, clinicians and scientists have been analyzing new approaches in craniofacial tissue regeneration to maximize patient benefit and minimize related complications. Craniofacial tissue regeneration using mesenchymal stem cells (MSCs) presents an advantageous alternative therapeutic option.4–7 MSCs are multipotent cells that are capable of multiple lineage differentiation based on the presence of inductive signals from the microenvironment.7–10 MSCs reside in a wide spectrum of postnatal tissue types10–15 and have been successfully isolated from several orofacial tissues.12–18 Studies have confirmed the self-renewal and multilineage differentiation capacities of orofacial-derived MSCs and have shown that they have better growth properties than bone marrow mesenchymal stem cells (BMMSCs).12–23 Therefore, dental MSCs are attractive for craniofacial applications as they may be better at differentiating into craniofacial tissues (Fig. 1).12–29
Figure 1.
Craniofacial tissue regeneration based on dental-derived mesenchymal stem cells encapsulated in 3-dimensional alginate hydrogel microspheres.
Biomaterials are widely used to engineer the physiochemical properties of the extracellular cell microenvironment to tailor niche characteristics and direct cell phenotype and differentiation. Such interactions between stem cells and biomaterials have largely been studied by introducing the cells into 2- or 3-dimensional scaffolds, or by encapsulating the cells within hydrogel biomaterials.30–32 Alginate hydrogel has been used extensively as a vehicle for stem cell delivery in tissue regeneration.31,32 The ability to control the spatial presentation of alginate enables the examination of the effects of alginate hydrogel on stem cell differentiation in a systematic way.30–33 In the current review, the application of dental-derived MSCs and alginate hydrogel for potential applications in craniofacial tissue regeneration is emphasized.
Dental-derived mesenchymal stem cells
Harvesting and using a sample of autologous cells from the diseased organ/tissue is the major contemporary approach for tissue engineering. However, this process might not yield sufficient cells for implantation procedures, especially in patients with extensive end-stage organ failure. In addition, from organs such as the pancreas, the isolation and expansion of primary autologous human cells might not be feasible. In these instances, other sources of cells for cell therapy, including pluripotent human embryonic stem cells or mesenchymal stem cells, might be a promising alternative. The combination of novel stem cell sources for cell therapy applications and concepts of tissue engineering can introduce novel treatment options for organ replacement. The presence of MSCs in a wide range of adult tissues and their multilineage differentiation capability have made them an attractive source for tissue regeneration applications.34,35 A “stem cell” refers to a clonogenic, undifferentiated cell that is capable of self-renewal (the ability to go through numerous cell division cycles while maintaining the undifferentiated state) and multilineage differentiation (potency or plasticity, which is the capacity to differentiate into specialized cell types). Stem cells can be totipotent, which means they can recreate the entire organism; pluripotent, which means they can create tissues of all 3 germ layers (ectoderm, mesoderm, and endoderm); or multipotent, which means they can produce cell types from more than one (but not all) lineage.34–37
Embryonic stem cells (derived from inner cell mass of preimplantation embryo) have been categorized as predominately pluripotent.38 Adult stem cells (derived from many ectodermal and mesodermal organs in adults), however, are mainly multipotent. Compared with the pluripotent and almost immortal nature of embryonic stem cells, adult stem cells appear more mature with a finite lifespan and only multipotent differentiation capacity.38 Adult stem cells (also known as somatic stem cells) are undifferentiated cells found in specialized tissues and organs of adults. In particular, bone marrow contains hematopoietic stem cells (HSCs). These were the first type of adult stem cells identified as also being able to form all types of blood cells. Bone marrow mesenchymal stem cells (BMMSCs) can generate bone, cartilage, fat, and fibrous connective tissue. Friedenstein et al39 first identified MSCs in human bone marrow. These cells have been defined as a population of postnatal stem cells hierarchically organized with the capacity to differentiate into specialized cells of at least one mesenchymal lineage such as bone, cartilage, fat, muscle, or neuronal cells.40–42
Currently, multipotent mesenchymal stem cells (MSCs) from bone marrow (BM) and MSC-like populations are considered to be the gold standard for cell therapies. Populations of MSCs derived from adipose tissues and umbilical cord blood have been shown to be promising alternative multipotent MSC sources. Studies have confirmed that these MSCs have multilineage differentiation capacity.42,43 In the past decades, the search for MSC-like cells in different tissues has led to the discovery of a wide range of stem cells in almost every organ and tissue in the body. For instance, multipotent MSCs have been identified and extracted in organs such as the liver, placenta, lung, and skin.40–43
MSC derived from dental and orofacial tissues are another example of stem cells residing in specialized tissues that have recently been isolated and characterized.12 The first type of dental stem cell was isolated from the human pulp tissue and termed postnatal dental pulp stem cells (DPSCs). 12 Subsequently, more types of dental-MSC-like populations were isolated and characterized, namely, stem cells from exfoliated deciduous teeth (SHED),13 periodontal ligament stem cells (PDLSCs),14 stem cells from apical papilla (SCAP),15,16 dental follicle precursor cells (DFPCs),17 and more recently gingival mesenchymal stem cells (GMSCs).18,19 The detailed relationship among these types of stem cell populations is still unclear. Initially, it was hypothesized that because dental and orofacial tissues are specialized tissues that do not undergo continuous remodeling as do osteogenic tissues, MSCs derived from them may be more committed or restricted in their differentiation potency than hBMMSCs. Several studies have compared the properties of dental- and orofacial-derived mesenchymal stem cells with those of BMMSCs and have confirmed the multidifferentiation capacity of dental- and orofacial-derived MSCs.19–24 Moreover, a subpopulation of stem cells derived from human dental pulp has been identified with osteogenic differentiation capacity that can form bone-like tissues in vivo.20 In contrast with hBMMSCs, dental-derived MSCs are more committed to be differentiated to odontogenic tissues rather than osteogenic tissues.22–24 In addition to their osteogenic potential, several studies have reported that subpopulations of dental-derived MSCs have adipogenic and neurogenic differentiation potential as they exhibit adipocyte- and neuronal-like cell morphologies and high expression levels for related gene markers.18–25 More recent studies have confirmed that dental-derived MSCs have better growth properties than hBMMSCs.22–24
Among dental-derived stem cells, PDLSCs and GMSCs are of special interest as they are easily accessible and can be found in the oral environment. Similar to dental pulp tissue, they can also be obtained as a discarded biological sample. Several research groups have identified and isolated gingival mesenchymal stem cells (GMSCs) from human gingival tissues. GMSCs have several advantages over BMMSCs; for example, GMSCs are easy to isolate, homogenous, and proliferate faster than BMMSCs. 18–23 In addition, harvesting gingival tissue is more straightforward than harvesting bone marrow and the healing of the donor site is faster without any morbidity or scar formation.18,19 GMSCs display stable morphology and do not lose MSC characteristics after multiple cell division cycles, maintaining normal karyotype and telomerase activity in long-term cultures.18 Other studies have confirmed that GMSCs exhibit stem cell-like properties and immunomodulatory characteristics similar to human bone marrow MSCs.19,21–23 In vivo studies have demonstrated that GMSCs could repair the mandibular and calvaria defects in a rat surgical reconstruction model showing that GMSCs could be a promising source for stem cell-mediated bone tissue engineering application.27,28 Several other studies have confirmed multilineage differentiation capacity and the self-renewal capacity of GMSCs.18–23 Mitrano et al27 reported that mesenchymal gingival stem cells can be differentiated to osteogenic, chondrogenic, and adipogenic tissues under specific differentiation in the same way as the multilineage adult BMSCs. They concluded that the GMSCs might have therapeutic applications in tissue regeneration.
Periodontal ligament (PDL) contains cell populations that can differentiate into cementoblasts or osteoblasts. 21–23 MSCs derived from periodontal ligament tissues can regenerate periodontal tissue.21–23 Studies have confirmed that MSCs retrieved from PDL tissues exhibit postnatal stem cell properties, confirming periodontal ligament tissue as a promising source of cells with stem cell-like regenerative characteristics that can be successfully used for periodontal tissue engineering.21–23 Human PDLSCs have been successfully isolated from extracted human teeth and ex vivo-expanded PDLSCs are capable of regenerating a typical cementum/periodontal ligament-like structure when transplanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier.29
Alginate hydrogel as scaffold for tissue engineering
Microscopically, a hydrogel is a network of cross-linked polymer chains interspersed with water molecules. These polymer chains can have a low modulus of elasticity and exhibit tissue-like flexibility because of their high water content.44,45 Macroscopically, a hydrogel is a porous material. The pore size can selectively allow only certain sizes of soluble molecules or cells to go through and promote angiogenesis.46 Hydrogel pore size can be altered by using different methods, including gas foaming, electrospinning, and solvent casting.46 Microscopic manipulations of cross-link density and backbone hydrophilicity, in addition to macroscopic modulation of pore size, can alter both the physical and biological properties of synthetic hydrogels.47,49
Hydrogels are capable of forming 3-dimensional matrices for the encapsulation of cells or sensitive bioactive molecules. Many natural and synthetic hydrogel biomaterials have been used for craniofacial tissue regeneration and repair. The most frequently used natural biomaterials are alginate, gelatin, collagen, and fibrin, while synthetic biomaterials, including the synthetic polyesters PLA and PLGA, are the most frequently used in biomedical applications.45
Alginates are natural heteropolysaccharides isolated from brown sea algae. Alginate belongs to a family of linear block polyanionic copolymers composed of (1–4)-linked -L-guluronic acid (G units) and (1–4)-linked -D-mannuronic acid (M units) that differ in amount and serial distribution along the polymer chain depending on the source of the alginate.48 Alginate hydrogel forms stable hydrogels in the presence of certain divalent cations, including Ca2+ and Ba2+, through the ionic interaction between the cation and the carboxyl functional group of G units located on the polymer chain. The composition and sequential structure of alginate are often a key functional attribute, and variations in the composition and/or the sequential structure may change the performance of an alginate for a particular end use. As alginates are macromolecules of a heterogeneous structure with no regular repeating unit, the alginate molecule cannot be described by the monomer composition alone. Because of its inherent biocompatibility, alginate hydrogel has been recognized as a promising biomaterial for biomedical applications.49 Other unique properties of alginates include modifiable immunosuppression50 and degradation51 properties, making alginate an FDA approved biomaterials.52
Alginate presents a large number of pendant carboxylic acid groups, which provide sites for heterogeneous mineral nucleation. Furthermore, the gel properties of alginate allow for diffusion of biologically active species both into and out of the matrix, ensuring cell viability and allowing the secretion of pharmacological molecules as well as the proper excretion of waste molecules. While allowing for fast diffusion, alginate gels maintain sufficient mechanical strength and stability to house cells. Studies have confirmed that alginate hydrogel can be chemically altered to modify its degradation profile, enhance cell binding,53 or to incorporate several growth factors, making it a drug delivery vehicle.
The alginate gels can be prepared by ionic crosslinking, the diffusion method, or the internal gelation method. These methods differ in the way the crosslinking ion is introduced and hence the gelling kinetics of the 2 methods is very different.54,55 The diffusion method is characterized by allowing calcium (or other divalent ions) to diffuse from an outer reservoir into the alginate solution. Although this method is widely used in the food industry, it has also become popular as an immobilization technique. The method is rapid and results in an inhomogeneous distribution of alginate as discussed in the section covering alginate beads. Furthermore, the internal gelation method is based on the mixing of an inactive form of the crosslinking ion with alginate solution.53–55
Two different types of degradation processes have been shown for hydrogel networks, surface erosion, and bulk erosion.56 Usually, surface erosion occurs when the rate of water diffusion into a specimen is slower than the hydrolysis reaction; then water will be adsorbed on the surface before it can diffuse into the bulk of the hydrogel specimen. Bulk erosion occurs when the rate of water diffusion into the specimen is much faster than the hydrolysis reaction.56,57
Hydrogels can mimic the mechanical properties of the extracellular matrix because of the low elastic modulus of alginate. In addition, modifying the mechanical strength of hydrogel biomaterials based on the concentration and type of crosslinking ion and the gelling environment is relatively easy. For instance, hydrogels that are rich in G units exhibit greater elasticity and mechanical strength than alginate hydrogels that are rich in M unit content.
Because of the unique properties of alginates, such as hydrophilicity, biocompatibility, and low production cost, they are widely used in biomedical applications (wound dressings, drug delivery devices, cell encapsulations, angiogenesis, and tissue engineering). 56,57 The degradation of alginate hydrogels can be modified and controlled by oxidizing the alginate with sodium periodate. By modifying the chemistry of the alginate hydrogels and, therefore, the degradation of the biomaterial, they can be considered as promising cell and drug delivery devices.55–57
Several types of cells have been reported to maintain their morphology in alginate hydrogel scaffolds.56 Chondrocytes have been successfully encapsulated in alginate scaffolds and have been differentiated toward chondrogenic tissues.57 Additionally, hepatocytes have been encapsulated in alginate hydrogel as a 3-dimensional scaffold and encapsulated cells were found to grow in alginate scaffolds. The results of these studies showed that hepatocytes encapsulated in alginate hydrogel had the ability to secrete albumin, which indicates proper cell function.58 Other studies59,60 have reported the encapsulation of different types of cells such as cardiomyocytes, rat marrow cells, and Schwann cells in hydrogel scaffolds. Also, fetal rat myocytes cultured on porous alginate hydrogel disks have been used for successful cardiac tissue regeneration without any immunorejection.60
Microencapsulation systems are one of the most popular strategies in tissue engineering. Hydrogels and specifically alginate are quite promising because of the ease of infusion of nutrients and oxygen between encapsulated stem cells within the alginate hydrogels and the microenvironment while the encapsulated stem cells stay within the 3-dimensional matrix. The alginate microspheres can be injected at the time of transplantation and can be used as drug delivery devices, making them the materials of choice for many biomedical applications.61–63
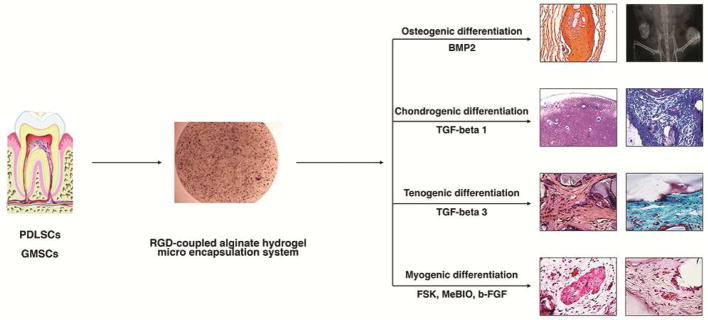
Our group and others64–70 have confirmed that alginate hydrogel scaffold can be considered as a promising encapsulating biomaterial for dental- and orofacial-derived MSCs, including GMSCs and PDLSCs (Fig. 2). Alginate hydrogel microspheres encapsulating dental-derived MSCs are a promising and effective treatment in craniofacial tissue engineering. Alginate microspheres can fill the gaps of irregularly shaped defects, enabling minimally invasive surgical procedures. Moshaverinia et al65–67 used alginate hydrogel for the first time as a scaffold for PDLSCs and GMSCs. In their studies, they developed an injectable alginate hydrogel delivery system for PDLSCs and GMSCs. Encapsulated dental-derived MSCs encapsulated in alginate hydrogel microspheres exhibited high levels of viability and capacities for differentiation toward osteogenic tissues in vitro and in vivo. Because of the biodegradability of alginate hydrogel, an additional clinical visit to remove the scaffold can be eliminated. Alginate hydrogel scaffold is capable of enhancing hard and soft tissue regeneration to accomplish minimally invasive dental and orthopedic surgeries.
Figure 2.
Appropriate signaling molecules and microenvironment (scaffold) differentiate dental-derived mesenchymal stem cells (PDLSCs and GMSCs) toward osteogenic, chondrogenic, tenogenic, and myogenic tissues for high quality tissue engineering.
Additionally, alginate microspheres can be successfully applied in the controlled delivery of signaling molecules and growth factors. Moshaverinia et al68 developed a novel codelivery system based on TGF-b1-loaded alginate microspheres encapsulating PDLSCs and GMSCs for cartilage regeneration applications. These studies showed in in vitro and in vivo models that codelivery alginate microencapsulation systems are promising for high quality cartilage regeneration. The developed system is a straightforward and readily manipulated means to encapsulate dental MSCs in alginate hydrogel. They provide a 3-dimensional, injectable, and biodegradable cell delivery scaffold for cartilage tissue engineering. Moreover, Moshaverinia et al69 confirmed that PDLSCs and GMSCs encapsulated in biodegradable and injectable alginate hydrogel scaffold containing appropriate inductive signals (TGF-b3) can be used as an alternative treatment in tendon tissue engineering. MSC and alginate constructs were able to effectively differentiate and organize their extracellular matrix into tendon tissue.
More recently, Ansari et al70 confirmed that GMSCs, when encapsulated in an alginate hydrogel microencapsulation system with multiple growth factor delivery capacity (loaded with myogenic growth factors), can be used in the repair and regeneration of muscular tissues.
They also reported that the GMSC/alginate hydrogel construct could be considered a promising candidate for vascularized tissue engineering. Based on the results of this study, GMSCs appear be an advantageous alternative therapeutic approach for tongue muscle repair and regeneration.
CONCLUSIONS
Stem cell-mediated tissue regeneration presents an immense opportunity for the entire medical field. It can lead to the regeneration of damaged tissues, aid in tissue grafting, or assist with bone reconstruction if grafting is not feasible. The applications of hydrogel-delivered mesenchymal stem cells for craniofacial tissue regeneration are wide, ranging from regenerating bone lost to oral disease to tissue reconstruction if the patient does not want a graft. The advantages of the application of the alginate microencapsulation system are the straightforward chemistry, ease of use for dental-derived MSC encapsulation, injectability and biodegradability, introducing a 3-dimensional, cell delivery scaffold for craniofacial tissue engineering. However, these applications come with the challenges inherent in any new clinical procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monaco E, Bionaz M, Hollister SJ, Wheeler MB. Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology. 2011;75:1381–99. doi: 10.1016/j.theriogenology.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffold. Eur Cell Mater. 2003;5:29–39. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 3.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019. 20102. [DOI] [PubMed] [Google Scholar]
- 4.Smith A. A glossary for stem-cell biology. Nature. 2006;441:1060–77. [Google Scholar]
- 5.Salinas CN, Anseth KS. Mesenchymal stem cells for craniofacial tissue Regeneration. J Dent Res. 2009;88:681–92. doi: 10.1177/0022034509341553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltz A, Kandalam U. Mesenchymal stem cells and alginate microcarriers for craniofacial bone tissue engineering: A review. J Biomed Mater Res A. 2016;104:1276–84. doi: 10.1002/jbm.a.35647. [DOI] [PubMed] [Google Scholar]
- 7.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu N, Lyu X, Fan H, Shi J, Hu J, Luo E. Animal models for craniofacial reconstruction by stem/stromal cells. Curr Stem Cell Res Ther. 2014;9:174–86. doi: 10.2174/1574888x09666140213150811. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Zhang H, Gangolli R. Advances of mesenchymal stem cells derived from bone marrow and dental tissue in craniofacial tissue engineering. Curr Stem Cell Res Ther. 2014;9:150–61. doi: 10.2174/1574888x09666140213142258. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Feng G, Wei X, Huang L, Ren A, Dong N, et al. The effects of mesenchymal stem cells in craniofacial tissue engineering. Curr Stem Cell Res Ther. 2014;9:280–9. doi: 10.2174/1574888x09666140213204202. [DOI] [PubMed] [Google Scholar]
- 11.Machado E, Fernandes MH, de Gomes PS. Dental stem cells for craniofacial tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:728–33. doi: 10.1016/j.tripleo.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 13.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo B-M, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 15.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:70–9. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–83. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carinci F, Piattelli A, Guida L, Perrotti V, Laino G, Oliva A, et al. Effects of Emdogain on osteoblast gene expression. Oral Dis. 2006;12:329–42. doi: 10.1111/j.1601-0825.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Pan J, Wright JT, Bencharit S, Zhang S, Everett ET, et al. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod. 2010;36:820–5. doi: 10.1016/j.joen.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, et al. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A. 2014;20:611–21. doi: 10.1089/ten.tea.2013.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, et al. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed Mater Res A. 2013;101:3285–94. doi: 10.1002/jbm.a.34546. [DOI] [PubMed] [Google Scholar]
- 24.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–51. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 25.McCulloch CA, Bordin S. Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res. 1991;26:144–54. doi: 10.1111/j.1600-0765.1991.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 26.Lindroos B, Mäenpää K, Ylikomi T, Ylikomi T, Oja H, Suuronen R, et al. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329–35. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 27.Mitrano TI, Grob MS, Carrion F, Nova-Lamperti E, Luz PA, Fierro FS, et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–25. doi: 10.1902/jop.2010.090566. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, et al. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011;20:2093–102. doi: 10.1089/scd.2010.0523. [DOI] [PubMed] [Google Scholar]
- 29.Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, et al. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37:1088–99. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 30.Man Y, Wang P, Guo Y, Xiang L, Yang Y, Qu Y, et al. Angiogenic and osteogenic potential of platelet-rich plasma and adipose-derived stem cell laden alginate microspheres. Biomaterials. 2012;33:8802–11. doi: 10.1016/j.biomaterials.2012.08.054. [DOI] [PubMed] [Google Scholar]
- 31.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization of the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159–71. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 32.Tour G, Wendel M, Tcacencu I. Cell-derived matrix enhances osteogenic properties of hydroxyapatite. Tissue Eng Part A. 2011;17:127–36. doi: 10.1089/ten.TEA.2010.0175. [DOI] [PubMed] [Google Scholar]
- 33.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368–93. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CJ1, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–22. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggeri L, Capanni M, Martelli MF, Velardi A. Cellular therapy: exploiting NK cell alloreactivity in transplantation. Curr Opin Hematol. 2001;8:355–9. doi: 10.1097/00062752-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 38.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–4. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 41.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 42.Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J. Enhanced setting standards for human embryonic stem cells. Science. 2003;300:913–6. doi: 10.1126/science.1082940. [DOI] [PubMed] [Google Scholar]
- 43.Richards M, Fong C-Y, Chan W-K, Wong P-C, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–6. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. J Adv Res. 2015;6:105–21. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010;16:371–83. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotlarchyk MA, Botvinick EL, Putnam AJ. Characterization of hydrogel microstructure using laser tweezers particle tracking and confocal reflection imaging. J Phys Condens Matter. 2010;22:194121. doi: 10.1088/0953-8984/22/19/194121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poncelet D, Lencki R, Beaulieu C, Halle JP, Neufeld RJ, Fournier A. Production of alginate beads by emulsification/internal gelation. I. Methodology. Appl Microbiol Biotechnol. 1992;38:39–45. doi: 10.1007/BF00169416. [DOI] [PubMed] [Google Scholar]
- 48.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–99. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Weir MD, Xu HH. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomater. 2010;6:4118–26. doi: 10.1016/j.actbio.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smidsrød O, Skja G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–8. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 51.Orive G, Santos E, Poncelet D, Hernández RM, Pedraz JL, Wahlberg LU, De Vos P, Emerich D. Cell encapsulation: technical and clinical advances. Trends Pharmacol Sci. 2015;36:537–46. doi: 10.1016/j.tips.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashi T, Nagamori E, Sone T, Matsunaga S, Fukui K. A novel transfection method for mammalian cells using calcium alginate microbeads. J Biosci Bioeng. 2004;97:191–5. doi: 10.1016/S1389-1723(04)70189-9. [DOI] [PubMed] [Google Scholar]
- 54.Bünger C, Tiefenbach B, Jahnke A, Gerlach C, Freier T, Schmitz KP, et al. Deletion of the tissue response against alginate-pll capsules by temporary release of co-encapsulated steroids. Biomaterials. 2005;26:2353–60. doi: 10.1016/j.biomaterials.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 55.Boontheekul T, Kong H-J, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 56.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–50. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 57.Perng CK, Kao CL, Yang YP, Lin HT, Lin WB, Chu YR, et al. Culturing adult human bone marrow stem cells on gelatin scaffold with pNIPAAm as transplanted grafts for skin regeneration. Journal of Biomedical Materials Research Part A. 2008;84:622–30. doi: 10.1002/jbm.a.31291. [DOI] [PubMed] [Google Scholar]
- 58.Nunamaker EA, Purcell EK, Kipke DR. In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res A. 2007;83:1128–37. doi: 10.1002/jbm.a.31275. [DOI] [PubMed] [Google Scholar]
- 59.Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules. 2000;33:4291–4. [Google Scholar]
- 60.Strand B, Gåserød O, Kulseng B, Espevik T, Skjåk-Bræk G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul. 2002;19:615–30. doi: 10.1080/02652040210144243. [DOI] [PubMed] [Google Scholar]
- 61.Metters A, Anseth K, Bowman C. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer. 2000;41:3993–4004. [Google Scholar]
- 62.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 63.Kang SW, Cha BH, Park H, Park KS, Lee KY, Lee SH. The effect of conjugating RGD into 3D alginate hydrogels on adipogenic differentiation of human adipose-derived stromal cells. Macromol Biosci. 2011;11:673–9. doi: 10.1002/mabi.201000479. [DOI] [PubMed] [Google Scholar]
- 64.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moshaverinia A, Chen C, Xu X, Ansari S, Zadeh HH, Schricker SR, et al. Regulation of the stem cell–host immune system interplay using hydrogel coencapsulation system with an anti-inflammatory drug. Adv Funct Mater. 2015;25:2296–307. doi: 10.1002/adfm.201500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galler KM, Cavender A, Yuwono V, Dong H, Shi S, Schmalz G, et al. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A. 2008;14:2051–8. doi: 10.1089/ten.tea.2007.0413. [DOI] [PubMed] [Google Scholar]
- 67.Moshaverinia A, Ansari S, Chen C, Xu X, Akiyama K, Snead ML, et al. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials. 2013;34:6572–9. doi: 10.1016/j.biomaterials.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moshaverinia A, Xu X, Chen C, Akiyama K, Snead ML, Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9:9343–50. doi: 10.1016/j.actbio.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moshaverinia A, Xu X, Chen C, Ansari S, Zadeh HH, Snead ML, et al. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials. 2014;35:2642–50. doi: 10.1016/j.biomaterials.2013.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, et al. Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann Biomed Eng. 2016;44:1908–20. doi: 10.1007/s10439-016-1594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]