Abstract
Amputees who wish to rid themselves of a phantom limb must weaken the neural representation of the absent limb. Conversely, amputees who wish to replace a lost limb must assimilate a neuroprosthetic with the existing neural representation. Whether we wish to remove a phantom limb or assimilate a synthetic one, we will benefit from knowing more about the developmental process that enables embodiment. A potentially critical contributor to that process is the spontaneous activity—in the form of limb twitches—that occurs exclusively and abundantly during active (REM) sleep, a particularly prominent state in early development. The sensorimotor circuits activated by twitching limbs, and the developmental context in which activation occurs, could provide a roadmap for creating neuroprosthetics that feel as if they are part of the body.
Keywords: body schema, REM sleep, myoclonic twitching, development, brain-machine interface, neural circuit, motor cortex, cerebellum
Our Bodies, Our Selves
Embryonic limbs begin as small buds protruding from the body wall. Over time, these buds differentiate into limbs that enable walking, running, reaching, grasping, hugging, and touching. To support such diverse functions, fully developed limbs are highly complex systems composed of multiple joints set in motion by distinct muscles, a proprioceptive sense provided by muscle spindles and Golgi tendon organs, and diverse receptors for touch and vibration [1]. Moreover, the machinery of the limb is integrated at many levels with the even more complex neural circuitry of the spinal cord and brain. When this process of integration is done well, we accept each limb as a part of us. We sense that we “own” it. This sense of body ownership and the neural machinery that supports it constitutes a body schema (see Glossary) [2].
Inherent in the notion of a body schema is the intertwining of mind and body. In contrast with the view that the mind can be understood without consideration of the body, an embodied perspective emphasizes the critical contributions to mind of such factors as sensory-motor coupling, body morphology, and physical constraint [3]. In other words, although minds control aspects of the body, bodies also shape the mind [4,5].
Today, the concept of embodiment is informing the creation of biologically inspired robots [6]. But even the most sophisticated robots still do not experience one of the most foundational of all biological processes—development. Although development may not seem like a particularly important process for a robot, the central idea behind the emerging field of developmental robotics is that a contemporary robot—precisely because it does not develop—can never achieve the flexibility and adaptability required for life in a growing, ever-changing body [5,7–9]. Accordingly, for a robot to be as flexible and adaptable as a human, it must experience a developmental process.
If development can inform the creation of better robots, then perhaps it can also inform our understanding of other similar problems, including efforts aimed at integrating synthetic and biological body parts. But for development to be a guide for understanding these sorts of problems, we must first understand the process by which developing limbs become incorporated into the body schema.
Sleep, Myoclonic Twitching, and Embodiment
Long before limbs are used for moving infants around a room or reaching for objects, they move spontaneously and with a seeming lack of purpose. From the earliest stages of embryonic development, spontaneous movements are necessary for normal limb development, including bone, cartilage, and joint formation [10]. Such movements can be caused by factors within the muscle (i.e., myogenic) or from signals provided by the spinal cord and brain (i.e., neurogenic); over development, myogenic movements fade as neurogenic movements gain in importance.
Two types of neurogenic movement emerge over development. The first type occurs when infants are awake and comprises large (i.e., high-amplitude), continuous, or sustained movements of the limbs, such as stretching, kicking, and yawning. The second type of movement occurs during active sleep, which is most prevalent early in development [11–13], and comprises discrete, brief, and jerky movements of the limbs, digits, face, eyes, and, in rodents and many other mammals, whiskers and tail (Figure 1A). These movements are called myoclonic twitches. Twitching continues into adulthood in select muscle groups, depending on species-typical morphology and functional importance (Box 1).
Figure 1.
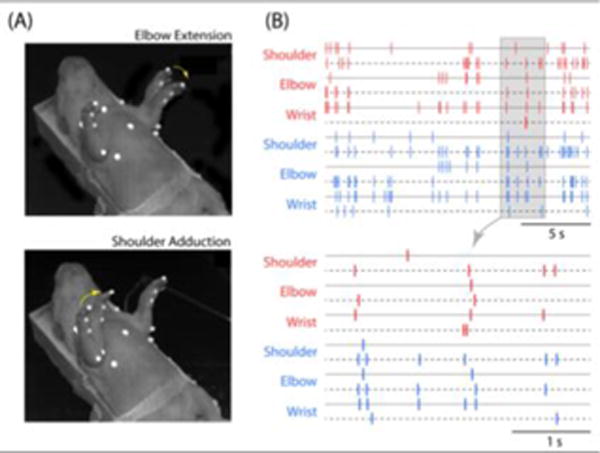
Twitches are Produced Discretely and are Spatiotemporally Organized. (A) Sequential high-speed video frames in an 8-day-old rat to show the discrete nature of twitching, illustrated here using the left elbow (top) and right shoulder (bottom). The yellow arrows indicate the direction in which the limb is moving and the white markers were used for motion tracking of limb movements. (B) Records of limb twitching in an 8-day-old rat at a long (top) and short (bottom) timescale to illustrate the discreteness and spatiotemporal organization of twitching; the segment denoted by the gray box is expanded below. Each tick mark indicates a single twitch at the shoulder, elbow, or wrist in the right (red) or left (blue) forelimb. Solid and dashed lines indicate the direction of movement at each joint (i.e., flexion or extension, adduction or abduction). Adapted from [16].
Box 1. Myoclonic twitching Across Species and Across the Lifespan.
Because twitching has traditionally been interpreted as a by-product of other brain processes (see [63]), there has been little motivation to assess the quantity and patterning of twitching in infants or adults [16]. But without even the most basic descriptive and quantitative data about twitching, we cannot fully evaluate its possible significance.
To help remedy this situation, we created a website to serve as a clearing-house for video recordings of sleep-related twitching in all its forms (http://www.twitchsleep.net). The website links to the multitudes of videos available on YouTube from people across the globe who are fascinated by the behavior of sleeping animals (including humans) at home, in zoos, and in the wild. The website also includes videos donated by sleep scientists and laypeople. Although just beginning, the site contains numerous videos representing 40 different species—from aardvarks to wild boars, and even some insects—across the lifespan.
What emerges from viewing this collection of videos is the sheer diversity and ubiquity of twitching. Also, certain patterns begin to emerge. For example, although twitching is similarly abundant in the limbs and face of kittens, puppies, piglets, ferret kits, and rat pups, twitching is more heterogeneous in adults, with some body parts twitching much more than others. This seems particularly clear in those species that have highly specialized morphologies. For example, twitches of the eyes (i.e., rapid eye movements) occur in humans, dogs, capybaras, and many other species, but so do twitches of the whiskers in cats and rats, the snout in pigs, the digits of ferrets, the ears of bats, the claws of aardvarks, the neck of giraffes, and the trunk of elephants. Thus, in addition to the general maintenance of sensorimotor function in adults, twitching may be especially important for the calibration of those specialized parts of the body that are involved in active sensing [64]—that is, the process by which animals direct movable sensors toward objects of interest so as to maximize the precision of stimulus detection, enhance perception, and achieve behavioral ends with greater efficiency.
Traditionally, twitching was considered a by-product of a dreaming brain (see [14]) or a sign of incomplete motor inhibition [15]. The former view is negated by an absence of evidence for cortical involvement—and an abundance of evidence for brainstem involvement—in the production of twitches. The latter view has more merit, but cannot easily explain the persistence of twitching into adulthood in various muscle groups across a diverse array of species. In contrast, when considered in its own right within the context of development, we find that twitching limbs exhibit complex spatiotemporal patterns across multiple joints, reflecting the emergence of motor synergies (Figure 1B) [16,17]. But for twitching to actually contribute to—rather than merely reflect—the development of the sensorimotor system, it is necessary that the twitch movements themselves trigger sensory feedback (i.e., reafference) and that this feedback, in turn, activates sensorimotor structures.
Sensorimotor processing depends on a complex network of structures at each level of the neuraxis (see Figure 2A), and now substantial evidence supports the idea that reafference from twitching limbs triggers neural activity at each of those levels: from the spinal cord [18,19] to the dorsal column nuclei (which include the external cuneate nucleus [20]), cerebellar cortex and deep cerebellar nuclei [21–23], thalamus [24,25], cerebral cortex [20,25–28], and hippocampus [29,30]. (Activity in the basal ganglia is also likely to be influenced by twitch-related reafference, but this possibility has not yet been investigated.) Moreover, the red nucleus, which is part of a group of premotor structures in the mesodiencephalic junction [31], contributes to the production of twitches while also receiving reafference, suggesting that the integration of motor output and sensory feedback occurs even within this so-called motor nucleus [32]. It also notable that the nuclei within the mesodienchephalic junction are associated with the evolution of limbs and flapping fins, and appear to have been differentially recruited across species for the control of highly specialized structures such as the human hand and the elephant’s trunk [33]. Twitching, it seems, is enmeshed within the neural machinery that gives limbs their purpose.
Figure 2.
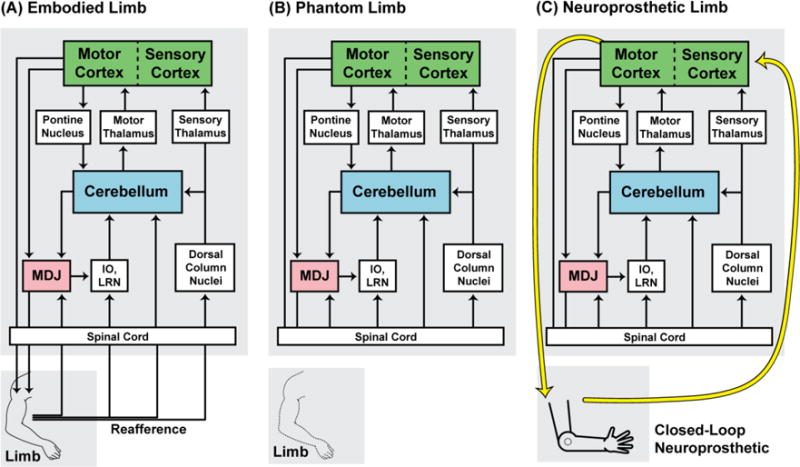
Circuit Diagram Illustrating the Web of Neural Structures that Support Embodiment. (A) Some major sensorimotor structures involved in the production of movement and the processing of reafferent signals. Reafference from twitching limbs triggers neural activity within all of the sensorimotor loops shown: These loops include afferent and efferent projections from nuclei in the mesodiencephalic junction (MDJ; red), cerebellum (blue), and sensorimotor cortex (green). Importantly, the limb receives motor commands from both motor cortex and nuclei in the MDJ (including the red nucleus), and the reafferent pathways complete each loop, allowing each structure to monitor and update its representation of the limb. IO, inferior olive; LRN, lateral reticular nucleus. (B) A phantom limb is perceived when the limb is absent, but the sensorimotor structures representing the limb remain intact. Without reafference to update the limb’s neural representation, the phantom limb persists. (C) A closed-loop neuroprosthetic limb interfaces directly with motor cortex and sensory cortex (yellow arrows), providing reafference that bypasses subcortical sensorimotor structures. By interacting exclusively or primarily with sensorimotor cortex, the rest of the existing limb representation is unaltered, thereby limiting the incorporation of the neuroprosthetic limb into the body schema.
Just as the red nucleus is more than merely a motor nucleus, the functions of motor cortex go beyond motor control. We have seen this quite clearly by recording neural activity in this region of cortex in sleeping infant rats and noting the prominent neural activation that follows the onset of even the most delicate twitch. In marked contrast, during wake movements in the same pups—and even as the limbs move vigorously—the motor cortex is often silent [28]. These observations led to two conclusions. First, the motor cortex plays a negligible role in the production of movement—whether sleep or wake movement—at these early ages. This conclusion is wholly consistent with what is known about the brainstem generators of twitches [32] and the development of the cortical control of wake movements [34].
Second, the motor cortex begins its existence primarily as a recipient and processor of sensory input. In fact, even in adults, the sensory function of motor cortex is well established [35] (see also [36] for evidence that sensory cortex can play a role in motor control). In infants, the sensory drive to motor cortex is much more salient because its motor function has yet to emerge. Moreover, the relative absence of reafferent activation of motor cortex during wake movements suggests the presence of a state-dependent gating mechanism that selectively passes twitch-related reafference but blocks wake-movement-related reafference. We recently identified such a gating mechanism in a medullary structure—the external cuneate nucleus—that receives direct proprioceptive input from muscles in the forelimbs and neck [20]. The demonstration of a mechanism that preferentially passes twitch-related reafference throughout the infant brain supports the idea that twitching represents a unique mechanism for developing and refining sensorimotor circuits and somatotopic maps (see Box 2).
Box 2. Assessing the Functional Importance of Twitching.
Active sleep has been consistently implicated in the consolidation of memory, especially motor memories (for review, see [62]). Importantly, a recent study using adult mice trained on a motor task provided direct evidence that active sleep strengthens and maintains newly formed dendritic spines in primary motor cortex [65]; in three-week-old mice, which are undergoing rapid spine formation, active sleep exerted similar effects in motor cortex. Such studies, however, have not yet considered the possibility that twitching contributes to neural plasticity.
The sheer quantity of twitch-related reafference during active sleep in early infancy points to an important role in driving activity-dependent processes that are necessary for sensorimotor development. What is still lacking, however, is direct evidence for such a role. The difficulty thus far in demonstrating the functional contributions of twitching stems, in large part, from the fact that it is not possible to simply lesion a “twitch center” in the brainstem: The structures that produce twitching also produce wake behavior. One way to solve this problem—especially in adults—is to use optogenetics to precisely control in a state-dependent manner the neural structures that produce twitches or those that convey sensory feedback from twitches.
If the contributions of active sleep to sensorimotor plasticity and consolidation of motor memories are modulated by reafference from twitches, then specific changes in twitching should be observed after wake-related learning tasks. Although such studies have not yet been conducted using limbs or digits, they have been conducted using rapid eye movements (REMs). REMs result from twitches of the extraocular muscles [66] (although they are not always interpreted as such; see [14]). Several studies in human and non-human primates manipulated the quantity or patterning of eye movements during wake and found complementary relations with subsequent REMs during sleep [67–69]. For example, using prisms to reduce the field of view in humans, Herman and Roffwarg found an increase in the density of REMs without changes in the duration of active sleep [67].
Interestingly, Huber and colleagues immobilized the arm of human adults and reported increased cortical plasticity and topographically restricted changes in cortical slow-wave activity during quiet (non-REM) sleep [70]. Neither active sleep nor twitching was assessed in that study. Thus, in addition to investigating whether twitching contributes to sensorimotor learning, future studies should also seek to understand how active and quiet sleep interact to promote plasticity.
One critical upshot of the foregoing discussion is that the motor functions of the brain—including motor cortex—are built on a foundation of sensation. Thus, sensorimotor integration is a concept that goes to the heart of what behavioral competencies require: A complex network of inextricable links within and among sensorimotor—not sensory and motor—structures. Thus, whatever a body schema is, it is the product of the highly distributed network illustrated in Figure 2A, including the sensorimotor cortex, cerebellum, and associated structures. But also, the body schema is the product of a developmental process through which limbs are fully assimilated with the nervous system. By this account, twitches both enable the development of embodiment and provide a window onto the architecture of embodiment.
Mimicking Development to Enhance Recovery of Sensorimotor Function
Because neural plasticity is so clearly heightened in early development, one key to rewiring the adult brain is to “remove the brakes” on plasticity [37]. But development is characterized not only by the absence of brakes on plasticity, but also the presence of spontaneous activity—in the visual, auditory, and sensorimotor systems—that promotes it [38]. And if twitching contributes to developmental plasticity in the sensorimotor system, then perhaps we can use this knowledge to heighten plasticity in adults.
As one striking example of developmental plasticity, consider the corticorubral tract, which connects the motor cortex to the ipsilateral red nucleus. In response to unilateral damage to the motor cortex in infant rats, the undamaged motor cortex not only retains its connections with the ipsilateral red nucleus, but also sprouts new connections with the contralateral red nucleus, thus giving the undamaged cortex effective control over both sides of the body [39]. Critically, this spontaneous rewiring occurs only if the cortical damage occurs before 10 days of age in rats. Given that this period of heightened plasticity corresponds with the developmental period of maximal active sleep and twitching, it may be that twitching directly contributes to both the typical development of the corticorubral system and its ability to rewire after early injury.
Although rewiring of the adult rat corticorubral system does not occur spontaneously after injury, recent studies suggest that rewiring is possible when a stimulation protocol is used that mimics some of the features of twitching [40,41]. For these studies, the brain damage entails unilateral transection of the corticospinal tract at the level of the medulla, which causes profound unilateral deficits in forelimb control. To promote recovery after the transection, the investigators electrically stimulated the unaffected motor cortex 6 hours a day for 10 days. This protocol induced sprouting of connections from the stimulated motor cortex to the contralateral red nucleus and other structures, once again allowing one side of the brain to gain control over both sides of the body. Moreover, forelimb motor function was restored.
For the present purposes, what is most striking about this protocol is how the motor cortex was stimulated to induce rewiring and recovery: The researchers carefully calibrated their stimulation protocol so as to induce discrete movements of the elbow, wrist, or digits of the forelimb; notably, stimulations occurred during the day when rats spend most of their time asleep. Accordingly, it is possible—although still untested—that this stimulation protocol, by inadvertently mimicking some aspects of twitching, owes its effectiveness to its ability to discretely activate motor and sensory circuits involved in forelimb control.
It should be noted that a stimulation protocol that more closely mimics twitching would entail stimulation of the red nucleus and other brainstem premotor nuclei that are known to produce twitching [32]; however, these brainstem nuclei are much less accessible than motor cortex. But such details may not matter so long as the stimulation protocol results in the activation of sensorimotor circuits throughout the neuraxis in a discrete and somatotopically precise way.
Phantom Limb As a Window Onto The Origins of Embodiment
The sensorimotor system’s heightened developmental plasticity is powerfully illustrated by the behavioral feats of humans born without limbs—from hand-walking in people born without legs to the remarkably dexterous feet and toes of so-called “armless wonders.” These individuals exemplify how humans and other animals learn to use the body they have, not the body they were “supposed” to have [42]. Although the developmental plasticity inherent in the sensorimotor system enables functionality in individuals with atypically formed bodies, it is also essential for the everyday process of adapting to the increases in limb size, shape, and strength that characterize typical development across a diversity of species, including those with extreme morphological and behavioral specializations [43].
When a limb is removed, either through accident or surgery, there remains in over 90% of amputees a vivid sense that the limb remains exactly where it was. Such phantom limbs are fascinating, but they are also instructive about the neural circuits that are devoted to each limb and make it possible for the limb to function as part of an integrated whole. As illustrated in Figure 2B, the loss of a limb leaves behind these neural circuits, thereby providing the material basis for the phantom. Thus, to accomplish a truly comprehensive amputation, one would have to remove not only the limb, but also the web of limb-devoted sensorimotor structures that, in their entirety, encompass the schema for that limb.
Phantom limb most commonly arises in adults after trauma or surgery. Moreover, the likelihood of phantom limb depends on the age at which a limb was lost. According to one study [44], limb amputation at 4–6 years of age produced a phantom in 91% of cases, whereas the incidence dropped to about 62% at 2–4 years of age and to 33% at 0–2 years of age; moreover, the incidence was still 20% in those children born with missing limbs (see also [45,46]).
The fact that phantom limb arises in cases of congenital limb loss—no matter how low the incidence—led some to conclude that the neural system that enables phantom limb is, at least in part, innate or, in other parlances, hardwired, genetically determined, or pre-programmed [44,47–49]. However, as is typically the case with such claims of innateness [50], empirical evidence argues for an alternative, developmental explanation.
Ten years ago, in a reexamination of phantom limb associated with early limb loss, Price [51] proposed that limbs are incorporated into the body schema through a process that begins in utero with “spontaneous muscle activity.” He suggested that spontaneous limb activity gives rise to sensory feedback, thereby driving the development of the neural networks that underlie the perception of a phantom limb. Although this proposal did not consider a role for sleep and sleep-related twitches (indeed, the proposal focused on spontaneous movements that are “initiated within the muscles themselves”), it is easy to imagine how twitches could serve the purpose of establishing a phantom limb in the minority of cases involving congenital amputation—especially given the fact that the active sleep is even more abundant in preterm than full-term human infants [52]. Accordingly, the odds of developing a phantom may depend on whether a fetus’s limb failed to develop at all or was amputated (e.g., due to amniotic band syndrome; [53]) after having developed and begun to move.
Beyond Repair: Fulfilling the Promise of Neuroprosthetics
The ultimate test of our understanding of embodiment is playing out today within the burgeoning field of brain-machine interfaces (BMI). Fulfilling BMI’s promise of achieving the complete assimilation of a neuroprosthetic limb with an existing nervous system will require advances in many fields, including computational neuroscience, sensor technology, and materials science [54]. It is also possible that this promise will depend upon the incorporation of knowledge about the developmental process by which natural limbs are assimilated.
Researchers widely recognize that only a closed-loop BMI, in which the motor control of a neuroprosthetic limb is accompanied by some form of sensory feedback, will achieve the most complete assimilation [54,55]. To close the loop, sensory feedback from vision can be used to adjust the movements of a prosthetic toward a goal. But to achieve maximum functionality and a stronger sense of assimilation, diverse sensory feedback—especially proprioceptive and tactile feedback—will likely be critical [56,57].
As illustrated in Figure 2C, closed-loop neuroprosthetic control is most readily achieved using implanted devices that translate signals from motor cortex into output commands to a prosthetic and convey sensory signals from the prosthetic to sensory cortex [54,55]. However, this approach fails to engage most of the sensorimotor network normally involved in limb control.
Therefore, as prosthetic sensor technology improves, the incorporation of a neuroprosthetic limb into the body schema may require maximal engagement of the cortical and subcortical components that constitute the sensorimotor system. To accomplish this, sensory input from the neuroprosthetic might be routed into the nervous system through peripheral nerves (e.g., in the stump) or through the spinal cord or brainstem. Moreover, drawing inspiration from development and the stimulation experiments described earlier, a brain stimulation protocol that drives movement in the neuroprosthetic and triggers associated sensory feedback throughout the sensorimotor system may be most effective for inducing plasticity and enabling somatotopic remapping.
Concluding Remarks
Developmental scientists emphasize the daily quantity of input that enables and supports the development of such skills as walking [58] and word learning [59]. Twitching fits squarely within this perspective: The many thousands of discrete twitches that are produced each day by the human infant provide ample opportunity for the activity-dependent development of the sensorimotor system and the assimilation of growing limbs into the nervous system.
The ideas put forward here about the possible value of leveraging understanding of development, sleep, and twitching to improve recovery of function after injury and improving neuroprosthetics are speculative. The ideas rest on the simple fact that the muscles and sensory receptors of developing limbs establish functional connectivity at every level of the sensorimotor system from the spinal cord to the forebrain. In addition, sleep-related twitches provide ample opportunity for limbs to form those functional connections via discrete activations during active sleep, a state that provides an ideal low-noise environment for the transmission of high-fidelity signals. Whether these considerations can help to improve the assimilation of neuroprosthetics into the nervous system of children or adults remains an open question (see Outstanding Questions).
Outstanding Questions.
Does reafference from twitches contribute to the plasticity that occurs after tasks or manipulations that engage the sensorimotor system, such as limb immobilization, finger-sequence learning, and the wearing of eye prisms?
Is it possible to enhance recovery of sensorimotor function in humans after stroke or injury through the use of therapies that explicitly mimic twitching? If so, which features of twitching (e.g., discrete stimulation, occurrence during active sleep, site of stimulation) are most effective for fostering recovery?
Which structures or circuits within the sensorimotor system are most important for modifying a body schema? Studies of phantom limb and neuroprosthetic assimilation in brain-damaged populations may help to answer this question.
Can sleep—and sleeping with a neuroprosthetic limb—enhance the assimilation of that limb into an existing nervous system? How does brain activity during active and quiet (non-REM) sleep change throughout the process of assimilation?
Do individual differences in the ability to assimilate a neuroprosthetic relate to individual differences in sleep and twitching?
The likelihood that a phantom limb arises after an amputation increases through the first several postnatal years. Do individual differences in sleep and twitching help to explain individual differences in the occurrence of a phantom limb?
Oliver Sacks observed that all amputees understand “that a phantom limb is essential if an artificial limb is to be used” (p. 66) [60]. In other words, the neural machinery that produces a phantom is the same machinery with which a fully functional neuroprosthetic must be assimilated. Our claim here is that limbs assimilate with the nervous system through a developmental process, and that a fuller understanding of that process will help us design more lifelike neuroprosthetics. In turn, improvements in neuroprosthetic technology will afford new opportunities for addressing basic questions in sensorimotor control [61], including questions about the roles that active and quiet (non-REM) sleep play in the consolidation of motor memories [62]. Ultimately, this interplay between basic science and advanced technology will help us answer some of the most ancient questions concerning the origins of our sense of self and the mechanisms that sustain it.
Trends.
Myoclonic twitches, the discrete and spontaneous limb movements that occur exclusively during active sleep, are not mere by-products of a dreaming brain.
Recent research in infant rats suggests that sensory feedback from twitching limbs, by sequentially activating sensorimotor circuits in the spinal cord and brain, contributes to the assimilation of growing limbs into the developing nervous system.
Such insights into the origins of embodiment help us understand a phenomenon like phantom limb, especially in those cases where phantoms occur despite the loss of a limb at or before birth.
If we fully understand the developmental process by which limbs are normally assimilated, we will be better able to design neuroprosthetics for adult amputees that feel like natural limbs.
Acknowledgments
Preparation of this paper was made possible by a grant from the National Institutes of Health (R37-HD081168) to M.S.B. We thank Greta Sokoloff and Karen Adolph for many helpful comments.
Glossary
- Active sleep
Also called rapid eye movement (REM) sleep, this stage of sleep is abundant in early development and is characterized by such behavioral and electrophysiological components as suppressed muscle tone, myoclonic twitching (which includes rapid eye movements), and an activated “wake-like” electroencephalogram. The second major stage of sleep is called quiet (non-REM) sleep
- Body schema
The totality of neural information about the body, provided in part by tactile and proprioceptive inputs, that enables such sensorimotor functions such as postural control, locomotion, and goal-directed action
- Brain-machine interface (BMI)
Also called a brain-computer interface, BMIs are devices that enable functional interactions between a neural system and an externally controlled object, such as a neuroprosthetic limb
- Reafference
Sensory stimulation that occurs in response to self-generated actions, as when you wave an arm and produce proprioceptive and visual feedback. In contrast, exafference refers to sensory stimulation that is generated by an external source, as when light shines in your eyes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proske U, Gandevia SC. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 2.Marshall PJ, Meltzoff AN. Body maps in the infant brain. Trends Cogn Sci. 2015;19:499–505. doi: 10.1016/j.tics.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeifer R, et al. Cognition from the bottom up: on biological inspiration, body morphology, and soft materials. Trends Cogn Sci. 2014;18:404–413. doi: 10.1016/j.tics.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer R, Bongard J. How the body shapes the way we think: a new view of intelligence. MIT Press; 2007. [Google Scholar]
- 5.Byrge L, et al. Developmental process emerges from extended brain–body– behavior networks. Trends Cogn Sci. 2014;18:395–403. doi: 10.1016/j.tics.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeifer R, et al. Self-organization, embodiment, and biologically inspired robotics. Science. 2007;318:1088–1093. doi: 10.1126/science.1145803. [DOI] [PubMed] [Google Scholar]
- 7.Lungarella M, et al. Developmental robotics: a survey. Connect Sci. 2003;15:151–190. [Google Scholar]
- 8.Oudeyer PY. WIREs Cogn Sci. 2016. What do we learn about development from baby robots? [DOI] [PubMed] [Google Scholar]
- 9.Adolph KE, Robinson SR. Motor development. In: Lerner RM, et al., editors. Handbook of child psychology and developmental science. 7. Vol. 2. 2015. pp. 114–157. [Google Scholar]
- 10.Müller G. Embryonic motility: environmental influences and evolutionary innovation. Evol Dev. 2003;5:56–60. doi: 10.1046/j.1525-142x.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- 11.Kayser MS, Biron D. Sleep and development in genetically tractable model organisms. Genetics. 2016;203:21–33. doi: 10.1534/genetics.116.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jouvet-Mounier D, et al. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg MS, Rattenborg NC. Decomposing the evolution of sleep: Comparative and developmental approaches. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 3. Elsevier; 2017. pp. 523–545. [Google Scholar]
- 14.Blumberg MS, Plumeau AM. A new view of “dream enactment” in REM sleep behavior disorder. Sleep Med Rev. 2016;39:34–42. doi: 10.1016/j.smrv.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohyama J, et al. Brainstem control of phasic muscle activity during REM sleep: a review and hypothesis. Brain and Development. 1994;16:81–91. doi: 10.1016/0387-7604(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg MS, et al. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumberg MS, et al. Development of twitching in sleeping infant mice depends on sensory experience. Curr Biol. 2015;25:656–662. doi: 10.1016/j.cub.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inácio AR, et al. Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nature Comm. 2016;7:13060. doi: 10.1038/ncomms13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersson P, et al. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 20.Tiriac A, Blumberg MS. Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep. eLife. 2016;5 doi: 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokoloff G, et al. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Dev Neurobiol. 2015;75:1140–1153. doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokoloff G, et al. Twitch-related and rhythmic activation of the developing cerebellar cortex. J Neurophys. 2015;114:1746–1756. doi: 10.1152/jn.00284.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Rio-Bermudez C, et al. Spontaneous activity and functional connectivity in the developing cerebellorubral system. J Neurophysiol. 2016;116:1316–1327. doi: 10.1152/jn.00461.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiriac A, et al. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khazipov R, et al. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 26.Milh M, et al. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- 27.McVea DA, et al. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J Neurosci. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiriac A, et al. Self-generated movements with “unexpected” sensory consequences. Curr Biol. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. J Neurosci. 2008;28:10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks TP, Onodera S. The mammalian red nucleus and its role in motor systems, including the emergence of bipedalism and language. Prog Neurobiol. 2012;96:165–175. doi: 10.1016/j.pneurobio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Del Rio-Bermudez C, et al. Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci. 2015;35:8322–8332. doi: 10.1523/JNEUROSCI.0564-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onodera S, Hicks TP. Evolution of the motor system: Why the elephant“s trunk works like a human”s hand. Neuroscientist. 1999 doi: 10.1177/107385849900500411. [DOI] [Google Scholar]
- 34.Martin JH. The corticospinal system: From development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 35.Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. 2011;72:477–487. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matyas F, et al. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- 37.Bavelier D, et al. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkby LA, et al. A role for correlated spontaneous activity in the assembly of neural circuits. 2013;80:1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nah SH, Leong SK. Bilateral corticofugal projection to the red nucleus after neonatal lesions in the albino rat. Brain Res. 1976;107:433–436. doi: 10.1016/0006-8993(76)90242-0. [DOI] [PubMed] [Google Scholar]
- 40.Carmel JB, et al. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmel JB, et al. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur J Neurosci. 2013;37:1090–1102. doi: 10.1111/ejn.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumberg MS. Freaks of nature: What anomalies tell us about development and evolution. Oxford University Press; 2009. [Google Scholar]
- 43.Krubitzer L, Dooley JC. Cortical plasticity within and across lifetimes: how can development inform us about phenotypic transformations? Front Hum Neurosci. 2013;7:620. doi: 10.3389/fnhum.2013.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melzack R, et al. Phantom limbs in people with congenital limb deficiency or amputation in early childhood. Brain. 1997;120:1603–1620. doi: 10.1093/brain/120.9.1603. [DOI] [PubMed] [Google Scholar]
- 45.Simmell ML. The reality of phantom sensations. Social Research. 1962;29:337–356. [Google Scholar]
- 46.Weinstein S, et al. Phantoms and somatic sensation in cases of congenital aplasia. Cortex. 1964;1:276–290. [Google Scholar]
- 47.Ramachandran VS, Hirstein W. The perception of phantom limbs. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 48.Brugger P, et al. Beyond re-membering: phantom sensations of congenitally absent limbs. Proc Natl Acad Sci USA. 2000;97:6167–6172. doi: 10.1073/pnas.100510697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallagher S, et al. Hand-mouth coordination, congenital absence of limb, and evidence for innate body schemas. Brain and Cognition. 1998;38:53–65. doi: 10.1006/brcg.1998.1020. [DOI] [PubMed] [Google Scholar]
- 50.Blumberg MS. Development evolving: the origins and meanings of instinct. WIREs Cogn Sci. 2016 doi: 10.1002/wcs.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price EH. A critical review of congenital phantom limb cases and a developmental theory for the basis of body image. Conscious Cogn. 2006;15:310–322. doi: 10.1016/j.concog.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Parmelee A, et al. Sleep states in premature infants. Dev Med Child Neurol. 1967;9:70–77. doi: 10.1111/j.1469-8749.1967.tb02212.x. [DOI] [PubMed] [Google Scholar]
- 53.Cignini P, et al. Epidemiology and risk factors of amniotic band syndrome, or ADAM sequence. J Prenat Med. 2012;6:59–63. [PMC free article] [PubMed] [Google Scholar]
- 54.Lebedev M, Nicolelis M. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29:536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249–266. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dadarlat MC, et al. A learning-based approach to artificial sensory feedback leads to optimal integration. Nature Neuro. 2015;18:138–144. doi: 10.1038/nn.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suminski AJ, et al. Incorporating feedback from multiple sensory modalities enhances brain-machine interface control. J Neurosci. 2010;30:16777–16787. doi: 10.1523/JNEUROSCI.3967-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolph KE, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psych Sci. 2012;23:1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Paul H. Brookes Publishing ompany; 1995. [Google Scholar]
- 60.Sacks O. The Man Who Mistook His Wife for a Hat. HarperPerennial. 1990 doi: 10.1192/bjp.166.1.130. [DOI] [PubMed] [Google Scholar]
- 61.Golub MD, et al. Brain–computer interfaces for dissecting cognitive processes underlying sensorimotor control. Curr Opin Neurobiol. 2016;37:53–58. doi: 10.1016/j.conb.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 63.Blumberg MS, et al. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeder CE, et al. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, et al. REM sleep selectively prunes and maintains new synapses in development and learning. Nature Neurosci. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seelke AMH, et al. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herman J, Roffwarg H. Modifying oculomotor activity in awake subjects increases the amplitude of eye movements during REM sleep. Science. 1983;220:1074–1076. doi: 10.1126/science.6844929. [DOI] [PubMed] [Google Scholar]
- 68.De Gennaro L, et al. The complementary relationship between waking and REM sleep in the oculomotor system: an increase of rightward saccades during waking causes a decrease of rightward eye movements during REM sleep. Electroencephalogr Clin Neurophysiol. 1995;95:252–256. doi: 10.1016/0013-4694(95)00090-l. [DOI] [PubMed] [Google Scholar]
- 69.Berger RJ. Characteristics of REM sleep following different conditioned rates of waking eye movement in the monkey. Percept Mot Skills. 1968;27:99–117. doi: 10.2466/pms.1968.27.1.99. [DOI] [PubMed] [Google Scholar]
- 70.Huber R, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]


