Abstract
HIV-infected smokers lose more years of life to tobacco-related disease than HIV. Since neurocognitive deficits are common among those with HIV and are associated with smoking persistence, these deficits may be a unique barrier to smoking cessation among HIV-infected smokers. Documenting unique differences in and correlates of cognition among HIV-infected smokers is a critical step towards developing a population-specific tobacco cessation treatment. We compared neurocognitive function between HIV-infected (n=103) and HIV-uninfected smokers (n=70), accounting for demographic and smoking-related variables. We also evaluated whether HIV-related health outcomes (e.g., CD4 count, viral load, depression ratings, quality of life [QoL]) and HAART adherence were associated with cognition. Participants completed neurocognitive tasks (N-back and Continuous Performance Task [CPT]) measuring working memory, attention, and processing speed, and intra-individual variability. Stepwise regression models were conducted and validated with resampling techniques. HIV-infected smokers performed worse than HIV-uninfected smokers on working memory, processing speed, and intra-individual variability (all p<0.01). ROC analysis for the model including cognitive measures demonstrated 85% area under the curve, which indicates “good prediction” for distinguishing between HIV-infected and HIV-uninfected smokers. This was a significant improvement over the model including demographic and smoking-related variables only (p=0.0003). Among HIV-infected smokers, neurocognitive performance was negatively associated with QoL and depression ratings. Smoking cessation interventions for HIV-infected smokers should consider cognitive neurorehabilitation as a potential strategy to decrease the likelihood of nicotine relapse and decrease tobacco-related morbidity in this population.
Keywords: Smoking cessation, HIV, Cognition, HIV-associated neurocognitive disorder, Tobacco Use
Introduction
Highly active antiretroviral therapy (HAART) has substantially improved survival rates among individuals with HIV (Deeken et al, 2012). Between 2001 and 2008, the percentage of adults living with HIV who are aged 50 or older increased from 17% to 31% and the rate continues to rise (Mahy et al, 2014). Although HAART enhances life expectancy and quality of life (QoL), HIV-infected individuals are increasingly vulnerable to non-AIDS-related diseases including cardiovascular disease, pulmonary disease, and non-HIV-associated malignancies (Rubinstein et al, 2014). Thus, addressing modifiable risk factors, such as tobacco use, among those with HIV/AIDS is a critical priority (Nahvi and Cooperman, 2009). In addition to cardiovascular disease, pulmonary disease and cancer, tobacco use increases the risk of opportunistic infections, and undermines HAART efficacy (Shirley et al, 2013). Indeed, HIV-infected smokers lose more life-years due to tobacco use than to HIV infection (Helleberg et al, 2013). Unfortunately, the smoking rate among HIV-infected individuals is more than twice the rate observed in the general population (Pacek and Cioe, 2015). Consequently, the development of smoking cessation interventions for HIV-infected smokers is an important priority.
To this end, assessing the role of neurocognitive deficits that may be unique to this population and pose a barrier to quitting smoking is essential. In the general population, smokers are at greater risk for neurocognitive deficits (Durazzo et al, 2012; Paul et al, 2006) and subsets of smokers, particularly those with psychiatric comorbidities characterized by neurocognitive deficits, may use nicotine to enhance cognitive function or attenuate withdrawal-related cognitive deficits (Ashare et al, 2014b; Lieberman et al, 2013). Indeed, resuming smoking ameliorates abstinence-induced cognitive deficits (Ashare et al, 2014a; Evans and Drobes, 2009). Importantly, deficits in cognitive function predict relapse above and beyond clinical measures (Loughead et al, 2015).
Cognitive deficits are a hallmark of HIV-1 infection (Weber et al, 2013) and are often referred to as HIV-associated neurocognitive disorder (HAND), which is classified as: HIV-associated dementia (HAD), mild neurocognitive disorder (MND), and asymptomatic neurocognitive impairment (ANI) (Antinori et al, 2007; Robertson and Yosief, 2014). While HAART has significantly reduced the incidence of HAD, MND and ANI persist. Indeed, 25–47% of HIV-infected people exhibit deficits in multiple cognitive domains including memory, verbal fluency, processing speed, and executive function (Heaton et al, 2015; Sacktor et al, 2016), which are associated with functional disabilities including unemployment, difficulty driving, and poor HAART adherence (Doyle et al, 2013). Although smoking may be negatively associated with cognition among HIV-infected individuals (Bryant et al, 2013; Schouten et al, 2014), few studies have assessed whether HIV-infected smokers experience greater cognitive deficits than their non-smoking counterparts.
The effects of HIV-1 infection on cognition may make HIV-infected smokers uniquely less able to quit smoking compared to HIV-uninfected smokers. Therefore, nicotine dependence interventions in the HIV-infected community may need to address cognitive impairment. This study was designed to: (1) examine whether neurocognitive performance differed between HIV-infected and HIV-uninfected smokers, accounting for demographic and smoking-related variables; and (2) identify which cognitive domains exhibit the greatest difference between the two samples. Secondarily, we evaluated whether HIV-related health outcomes (e.g., CD4 count, viral load, depression ratings, QoL) and HAART adherence were associated with cognition. Documenting unique differences in and correlates of cognition among HIV-infected smokers is a critical step towards developing a population-specific tobacco cessation treatment.
Methods
Samples
HIV-infected Participants
Male and female participants over the age of 18 were recruited for a placebo-controlled clinical trial analyzing the effectiveness of varenicline for smoking cessation among those with HIV. Participants were recruited via media advertisements and from clinical sites, and all study visits were conducted at the University of Pennsylvania. For the present study, baseline data from 103 HIV-infected treatment-seeking cigarette smokers (≥ 5 cigarettes/day) from this parent trial were analyzed. Participants must have been diagnosed with HIV, have viral loads <1000 copies/mL, and CD4+ counts >200 cells/mm3, ALT/AST <2 times upper limit, and creatinine clearance >50 mL/min. Lifetime history of psychosis, suicide attempt, current or planned pregnancy, current use of smoking cessation medications, unstable or untreated alcohol/substance abuse, or non-fluency in English were exclusionary.
HIV-uninfected Participants
Male and female treatment-seeking smokers (≥ 10 cigarettes/day), ages 18–60 years old, were recruited from the community via advertisements for a placebo-controlled trial evaluating the effects of galantamine on short-term smoking cessation. After providing written informed consent, participants completed an in-person eligibility screen including an expired breath carbon monoxide (CO) reading to confirm smoking status (≥ 10 ppm). Exclusion criteria included: current or planned pregnancy; low or borderline intelligence [Shipley IQ score <90, (Zachary, 2000)]; untreated alcohol/substance abuse; heart, kidney, or liver disease; uncontrolled hypertension; Alzheimer’s disease; lifetime history of psychosis or bipolar disorder; suicide attempt; current major depression; and current use of smoking cessation or psychotropic medications, or contraindicated medications for galantamine.
Measures
Demographic and Smoking-related Measures
Self-reported demographics (e.g., age, sex, race) and smoking characteristics (e.g., cigarettes per day, number of years smoked, age at initiation) as well as the Fagerström Test for Nicotine Dependence [FTND (Heatherton et al, 1991)]) were collected from both samples.
Cognitive Tasks
Continuous Performance Task (CPT)
The Penn Continuous Performance Task (CPT) measures visual attention and vigilance (Kurtz et al, 2001). In this task, a series of red vertical and horizontal lines (7-segment displays) flash in a digital numeric frame (like a digital clock). The participant must press the spacebar whenever the lines form complete numbers or letters. The task is divided into two parts, each consisting of 180 trials (60 targets): first, the participant is asked to respond to numbers and then to letters. The discrimination index (i.e., the difference between the hit rate and the false alarm rate), which reflects the ability to distinguish between targets and foils (Snodgrass and Corwin, 1988), was calculated. The second outcome was median reaction time (RT) to correct targets (i.e., processing speed). We also computed the coefficient of variation (CV), which is the standard deviation (SD) of RT divided by the mean RT and reflects intra-individual variability in RT.
Visuospatial Working Memory N-Back Task
The current study employed a visuospatial working memory task based on prior research (Green et al, 2005). During the N-back, participants are instructed to remember the location of a stimulus, a gray circle approximately 5 cm in diameter, as it appears randomly in eight possible locations around the perimeter of a computer screen. The stimulus appears for 200 ms, followed by an interstimulus interval (ISI) of 2800 ms. A cross-hair remains visible during the stimulus presentation to cue participants to look at the center of the screen. The N-back includes four conditions of varying difficulty presented in pseudorandomized counterbalanced order: the 0-back, 1-back, 2-back, and 3-back. Each block consists of 50 trials with 15 targets per block. Participants respond only to targets (30% of stimuli) by pressing the spacebar. During the 0-back, participants are instructed to respond if the stimulus appears in a predetermined location (upper left corner of the screen). During the 1-back, participants are instructed to respond whenever the stimulus appears in the same location as the stimulus that immediately preceded it. During the 2- and 3-back conditions, participants are instructed to respond whenever the stimulus appears in the same location as the stimulus that preceded it by 2 or 3 trials, respectively. The primary outcomes for this task are the discrimination index (described above), median RT during correct targets, and CV (described above).
HIV-related Health Outcomes (HIV-infected Sample Only)
For the HIV-infected sample, HIV-transmission category, HAART type and adherence information was collected. HAART adherence was a binary variable indicating whether any doses were missed in the past month (yes/no). Because efavirenz has documented adverse effects on cognition (Ma et al, 2016), we included a binary variable indicating current use of efavirenz. Laboratory values (HIV-RNA, CD4 count) were extracted from medical records. The HIV/AIDS-Targeted QoL (HAT-QoL) is a 34-item instrument assessing nine dimensions: overall function, sexual function, disclosure worries, health worries, financial worries, HIV mastery, life satisfaction, medication worries, and provider trust. All items use a “past 4 weeks” timeframe and a Likert-type scale from 1 (“all of the time”) to 5 (“none of the time”). Lower scores indicate lower QoL (Holmes and Shea, 1998). The Hospital Depression and Anxiety Scale (HADS) (Zigmond and Snaith, 1983), a 14-item self-report measure, assessed depression and anxiety ratings.
Data Analysis
Descriptive statistics were used to characterize the demographic, smoking history, disease-specific data, and neurocognitive performance for each sample. First, we conducted a forward stepwise regression (p-value≥0.2 for removal, and ≤0.1 for entry) to select predictors for a logistic regression model (STATA logistic) to correctly classify group (HIV-uninfected vs. HIV-infected). Demographic variables (e.g., sex, age, race, and education) and smoking-related measures (FTND, cigarettes per day) were entered as baseline predictors. Neurocognitive measures (e.g., discrimination index, RT, and CV for the N-back and the CPT) were also included.
Following logistic regression, receiver operating characteristic (ROC) analysis was used to assess the predictive potential of the models (Steyerberg, 2008). Estimates of predictive accuracy are expressed as area under the ROC curve (AUC). The ROC curve is a plot of the sensitivity versus 1-specificity, representing the accuracy of a classification system, and provides a comparable metric across experiments (Bradley, 1997). Per ROC standards (Harrell et al, 1996), an AUC of .70–.80 indicates fair prediction; .80–.90 good prediction; and .90–1.0 excellent prediction. We then sought to validate the predictive models using two resampling methods. First, bootstrap procedures were used to create 1000 replicates of the data (random with replacement) and a stepwise regression (see above) was applied to assess model stability (Austin and Tu, 2004). The frequency of a variable’s inclusion in a model is considered an index of robustness, with those selected most often considered to be least influenced by outliers and error. To examine the model’s potential for prediction in new cases, we performed leave-one-out cross-validation (LOOCV) using root mean square error (RMSE) and mean absolute error (MAE) as indicators of goodness-of-fit (Steyerberg, 2008).
Secondarily, we sought to evaluate the association among HIV-related outcomes and neurocognition among HIV-infected smokers. We conducted separate stepwise regression models for each of the following outcomes: CD4 count, viral load, HAART adherence, the nine HAT-QoL domains, and HADS depression anxiety. Neurocognitive measures, demographic and smoking-related variables were included as predictors (see above). Current use of efavirenz was also included as a potential covariate.
Results
Sample Characteristics
Demographic and smoking-related characteristics for both samples are presented in Table 1. The samples were comparable with regard to sex, FTND, and cigarettes per day (p’s>0.10). The HIV-infected sample was significantly older, started smoking at a younger age, exhibited lower baseline CO, was more likely to be African American, and reported lower levels of education and income (p’s<0.10). All HIV-infected participants were stable on HAART and the most commonly reported medications were ritonavir (39%), emtricitabine/tenofovir disoproxil fumarate (TDF) (43%), darunavir (25%), and efavirenz/emtricitab/TDF (21%).
Table 1.
Demographic and smoking characteristics of HIV-uninfected and HIV-infected samples.
| Variable | HIV-uninfected (n=70) | HIV-infected (n=103) | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 42.7 | 11.0 | 47.9 | 9.6 | 0.001 |
| Cigarettes per day | 16.4 | 6.0 | 14.7 | 8.0 | 0.12 |
| FTND | 5.2 | 1.8 | 5.0 | 1.8 | 0.35 |
| CO at baseline (ppm) | 21.1 | 9.8 | 15.8 | 10.0 | 0.001 |
| Age smoking initiation (years) | 18.0 | 4.3 | 16.4 | 0.51 | 0.03 |
| Longest quit attempt (days)a | 277 | 63 | 307 | 63 | 0.75 |
| Plasma HIV RNA level [viral load (copies/mL)]b | N/A | N/A | 1.5 | 0.30 | |
| Plasma CD4 Count (cells/mm3) | N/A | N/A | 689.9 | 323.7 | |
| Time since HIV diagnosis (months) | N/A | N/A | 183.3 | 100.1 | |
| % | Count | % | Count | ||
|
|
|||||
| Sex (Female) | 36% | 25 | 27% | 28 | 0.23 |
| Mode of Transmission | N/A | ||||
| Sex | N/A | N/A | 91% | 98 | |
| Injection Drug Use | N/A | N/A | 5% | 5 | |
| Unknown | N/A | N/A | 4% | 4 | |
| HAART Adherence | N/A | ||||
| Missed a dose in last month | N/A | N/A | 35% | 35 | |
| Current use of efavirenz | N/A | N/A | 22% | 23 | N/A |
| Race | 0.05 | ||||
| African American | 60% | 42 | 79% | 81 | |
| White | 29% | 20 | 17% | 17 | |
| More than one race | 7% | 5 | 2% | 2 | |
| Unknown | 4% | 3 | 3% | 3 | |
| Education | 0.007 | ||||
| Some high school | 3% | 2 | 18% | 19 | |
| High school or GED | 29% | 20 | 33% | 34 | |
| Some college/technical school | 47% | 33 | 30% | 31 | |
| College graduate or beyond | 21% | 15 | 18% | 19 | |
| Incomec | < 0.001 | ||||
| < $20,000 | 41% | 29 | 74% | 76 | |
| $20,000 – $35,000 | 21% | 15 | 16% | 16 | |
| $35,001 – $50,000 | 21% | 15 | 5% | 5 | |
| $50,001 – $75,000 | 7% | 5 | 5% | 5 | |
| > $75,000 | 6% | 4 | 1% | 1 | |
| 24-hour quit attempt (yes) | 74% | 52 | 73% | 75 | 0.8 |
Note.
Only individuals who reported having a quit attempt lasting at least 24 hours were included (HIV- n=52, HIV+ n=75);
based on log 10 transformation;
Two individuals in the HIV-uninfected sample refused to answer income question.
Differences in Neurocognitive Function between HIV-infected and HIV-uninfected Smokers
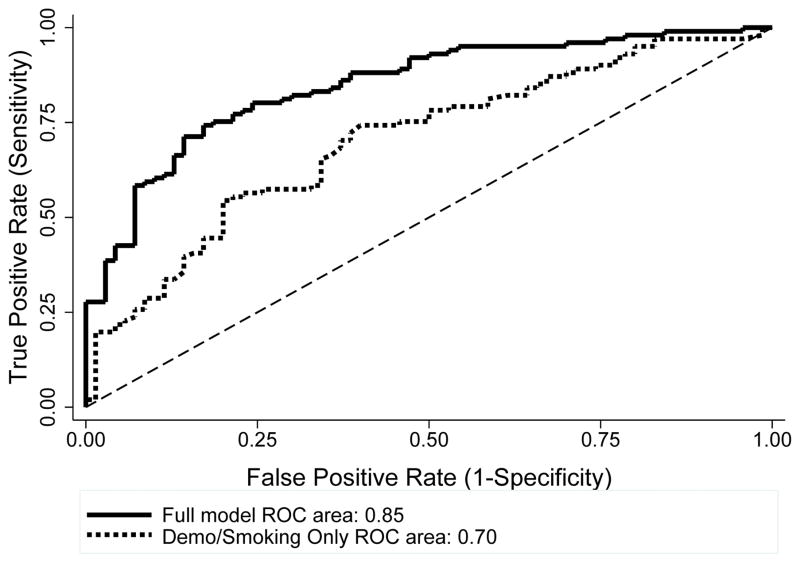
Table 2 contains the means and SDs for all neurocognitive outcomes. The forward stepwise model retained education (OR=0.42, p=0.05, 95% CI: 0.18–0.98) and FTND (OR=0.78, p=0.04, 95% CI 0.63–0.98), indicating that the HIV-infected sample reported lower levels of education and FTND scores. For neurocognitive measures, the stepwise model retained the N-back discrimination index (OR=0.001, p=0.001, 95% CI: 0.0002–0.05) and RT (OR=1.01, p<0.001, 95% CI: 1.0–1.01), and the CPT CV (OR=2.1x108, p=0.002, 95% CI: 1419–3.2x1013), indicating worse cognitive performance among the HIV-infected sample, compared to the HIV-uninfected sample. For the ROC curve analysis, the model including all demographic and neurocognitive variables, produced a relatively high AUC of 85% corresponding to 76% correct classification rate (Figure 1). This model represents a significant improvement over demographic and smoking behavior predictors (age, FTND, race, education, sex, cigarettes per day) alone (AUC 70%, X2(1)=12.8, p=0.0003).
Table 2.
N-back and CPT Performance for HIV-infected and –uninfected Samples
| Measure | HIV-uninfected | HIV-infected | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| N-back Discrimination Index | 0.72 | 0.09 | 0.62 | 0.14 |
| N-back RT | 616.8 | 111.7 | 728.2 | 174.5 |
| N-back CV | 0.25 | 0.05 | 0.24 | 0.07 |
| CPT Discrimination Index | 0.85 | 0.15 | 0.81 | 0.12 |
| CPT RT | 452.8 | 52.5 | 488.8 | 49.6 |
| CPT CV | 0.18 | 0.03 | 0.21 | 0.03 |
Note. Raw values are depicted for each task. CV = Coefficient of Variation
Figure 1.
ROC curves for two predictive models of HIV status. The full model (blue) includes demographic, smoking, and neurocognitive variables, yielding an AUC of 85%. Smoking and demographic predictors alone (red) achieved an AUC of 70% (red).
Bootstrapping generated 1000 replicates of the data and conducted model selection on each replicate (Austin and Tu, 2004). The most frequent variables selected were (i.e., more than 500 times; number of times selected in parentheses): N-back RT (992), N-back discrimination index (934), CPT CV (893), education (661), and FTND (620). Notably, these were the same variables identified by the stepwise regression model. LOOCV further evaluated model performance. The RMSE and MAE for the full model (0.43 and 0.33, respectively) were lower than for the model including only demographic and smoking variables (0.48 and 0.44, respectively), indicating the full model yielded lower predictor error. LOOCV procedures yielded 85.6% AUC, corresponding to 79% correct classification. Thus, performance of both the training data and the LOOCV model would be characterized as “good prediction” (Hosmer and Lemeshow, 2000).
Association of Neurocognition with HIV-related Measures
For the model predicting CD4 count, being female (b=213.5, p=0.003) and having a lower discrimination index on the N-back (b=-567.1, p=0.01) were associated with higher CD4 count. For HADS depression, a lower discrimination index on the N-back was associated with higher depression ratings (b=-5.6, p=0.002). Likewise, being Caucasian (b=1.2, p=0.03), having a higher discrimination index on the N-back (b=3.6, p=0.02), and longer RT on the CPT (b=0.01, p=0.03) were associated with better HAT-QoL Health. For HAT-QoL Satisfaction, fewer cigarettes per day was associated with higher QoL (b=-0.10, p=0.006). Lastly, lower education was associated with greater odds of missing a dose of HAART in the past month (OR=3.1, p=0.01, 95% CI 1.33–7.4). There were no significant predictors of viral load, HADS anxiety, or other domains of the HAT-QoL.
Discussion
This study compared cognitive performance between HIV-infected and HIV-uninfected smokers and identified the association of neurocognitive performance with HIV-related health outcomes. Previous studies with HIV-infected smokers and heavy drinking HIV-infected individuals showed that cigarette smoking was negatively associated with cognitive functioning (Bryant et al, 2013; Monnig et al, 2016). The present study extends these findings by demonstrating that, even after accounting for demographic and smoking-related characteristics, HIV-infected smokers performed worse on multiple cognitive domains. Moreover, among HIV-infected smokers, neurocognitive performance may be related to important health outcomes, including QoL and depression ratings.
First, compared to HIV-uninfected smokers, smokers with HIV performed worse on measures of working memory, processing speed, and intra-individual variability. This is consistent with evidence that, in the post-HAART era, the cognitive domains affected by HIV-infection are learning, memory, and executive function (Heaton et al, 2015; Sacktor et al, 2016). While the observed differences are modest, they may be important clinically. Indeed, slower processing speed is prospectively associated with greater cognitive decline (Cysique et al, 2010) and those who exhibit ANI may be more likely to progress to symptomatic HAND (Grant et al, 2014; Heaton et al, 2015). Additionally, intra-individual variability is higher among older HIV-infected individuals (Morgan et al, 2011). Thus, our finding that HIV-infected smokers exhibited greater intra-individual variability builds on evidence that inconsistent responding may be an important marker of cognitive decline (Hilborn et al, 2009) and highlights unique cognitive factors that may be important to address among HIV-infected smokers in conjunction with a smoking cessation intervention.
The current findings also suggest that several cognitive factors may be associated with HIV-related health outcomes. For instance, lower working memory performance was associated with higher depression ratings, lower health-related QoL, and higher CD4 count. Previous studies have found positive associations between successful cognitive aging and QoL (Moore et al, 2014). Although our findings are cross-sectional, it would be important to examine whether interventions to improve working memory could have downstream effects on QoL and depression ratings. Although lower nadir CD4 is reliably associated with worse cognitive function (Ellis et al, 2011), the relationship between neurocognition and current CD4 count is equivocal (Heaton et al, 2011). The current study did not assess nadir CD4, but our data suggest that better working memory may be associated with a lower current CD4 count. Although somewhat counterintuitive, some HAART medications (e.g., efavirenz) may have beneficial effects on CD4 count, while having adverse effects on neurocognition (Ma et al, 2016). Although we controlled for current use of efavirenz, the current sample was not large enough to evaluate differences in duration of use or by HAART type. This question should be addressed in future studies.
Because we controlled for demographic and smoking-related variables, the observed differences in cognitive performance may be caused by HIV-1 infection and the consequent neuronal damage via neurobiological mechanisms (Lindl et al, 2010). In the post-HAART era, persistent inflammation that does not completely reverse with viral suppression is thought to contribute to HAND (Hunt et al, 2016; Lederman et al, 2013). Tobacco smoking also induces inflammatory markers implicated in HAND (e.g., CRP, IL-6, MCP-1) (Stampfli and Anderson, 2009) and may compromise the integrity of the blood-brain barrier increasing the likelihood of monocyte transmigration into the brain and thus, enhancing the risk for neurodegeneration (Manda et al, 2010). Moreover, brain regions critical for neurocognitive function (e.g., prefrontal cortex and medial temporal lobe) are impacted by HAND (Hakkers et al, 2016) and smoking (Weiland et al, 2015). Thus, interactive effects between smoking and HIV-infection on inflammation and neural pathways suggest a potential mechanism underlying the differences in cognition between HIV-infected and HIV-uninfected smokers.
Controlling for other demographic and smoking-related variables, only education and nicotine dependence significantly differed between the groups. Notably, cognitive variables significantly improved the model’s predictive validity to distinguish between HIV-infected and uninfected smokers, over and above demographic and smoking-related variables alone. With respect to HIV-related outcomes, women had a higher current CD4 count and Caucasians reported higher health-related QoL. Although previous studies found associations between cognition and HAART adherence (Thaler et al, 2015), in our study only lower education was associated with lower HAART adherence. Extending findings from the general population (Coste et al, 2014), we found that a higher smoking rate was associated with lower life satisfaction.
Several limitations warrant mention. First, these data were cross-sectional and potential mechanisms (e.g., neural substrates and inflammation) require further investigation. Second, including HIV-infected non-smokers would have allowed us to evaluate whether our findings were specific to smoking or HIV-infection. Third, although none of the HIV-uninfected smokers self-reported an HIV diagnosis, we did not have biological confirmation that these smokers were HIV negative, yet the <2% prevalence of HIV in Philadelphia makes it unlikely we misclassified HIV status (Philadelphia Department of Public Health, 2015). Lastly, even though we controlled for demographic and smoking-related differences, the samples were drawn from two clinical trials and some inclusion criteria differed between studies. Nevertheless, both trials only included daily smokers seeking treatment to quit smoking and both samples had comparable levels of nicotine dependence and smoking rate.
In summary, this study shows that HIV-infected smokers exhibit significantly worse working memory and processing speed and greater variability in response time compared to HIV-uninfected smokers. These cognitive measures may be related to important HIV-related health outcomes and may help identify smokers most likely to benefit from a treatment targeting cognition. Cognition has been identified as an important target for smoking cessation, particularly for those vulnerable to cognitive deficits (Ashare and Schmidt, 2014). Cognitive neurorehabilitation interventions (Weber et al, 2013) and cognitive enhancing medications (e.g., varenicline) (Patterson et al, 2009) might be beneficial additions to tobacco cessation programs for HIV-infected smokers, perhaps with extended treatment to ensure enhanced cognitive function and reduced relapse risk. It is imperative that future studies with HIV-infected smokers examine treatment-related and withdrawal-related changes in cognition and relapse rates to further improve the health of this population.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (R01 DA033681 and K23 DA035295) and through core services and support from the Penn Center for AIDS Research (P30 AI045008) and Penn Mental Health AIDS Research Center (P30 MH097488).
Footnotes
Conflict of Interest
Dr. Schnoll receives medication and placebo free from Pfizer and has provided consultation to Pfizer and GlaxoSmithKline. These companies had no involvement in this study.
Dr. Siegel has received grants and/or has acted as a consultant to Astellas, Merck, and Zynerba that is unrelated to the content of this manuscript.
Dr. Gross sits on a Data Safety and Management Board for a Pfizer drug treating inflammatory bowel disease that is unrelated to the contents of this manuscript.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014a;76(Pt B):581–91. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin Drug Discov. 2014;9:579–94. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Strasser AA, Wileyto EP, Cuevas J, Audrain-McGovern J. Cognitive Deficits Specific to Depression-Prone Smokers During Abstinence. Exp Clin Psychopharmacol. 2014b;22:323–331. doi: 10.1037/a0037072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Tu JV. Bootstrap methods for developing predictive models. American Statistician. 2004;58:131–137. [Google Scholar]
- Bradley AP. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern recognition. 1997;30:1145–1159. [Google Scholar]
- Bryant VE, Kahler CW, Devlin KN, Monti PM, Cohen RA. The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS. AIDS Care. 2013;25:1308–16. doi: 10.1080/09540121.2013.764965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste J, Quinquis L, D’Almeida S, Audureau E. Smoking and health-related quality of life in the general population. Independent relationships and large differences according to patterns and quantity of smoking and to gender. PLoS One. 2014;9:e91562. doi: 10.1371/journal.pone.0091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, Shi C, Yu X, Wu Z, Abramson IS, Grant I, Heaton RK. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010;24:983–90. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, Dezube BJ. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55:1228–35. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KL, Morgan EE, Morris S, Smith DM, Little S, Iudicello JE, Blackstone K, Moore DJ, Grant I, Letendre SL, Woods SP. Real-world impact of neurocognitive deficits in acute and early HIV infection. J Neurovirol. 2013;19:565–73. doi: 10.1007/s13365-013-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–11. doi: 10.1016/j.drugalcdep.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS (London, England) 2011;25:10. doi: 10.1097/QAD.0b013e32834a40cd. 1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–62. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Phan KL, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81:575–84. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hakkers CS, Arends JE, Barth RE, Du Plessis S, Hoepelman AI, Vink M. Review of functional MRI in HIV: effects of aging and medication. J Neurovirol. 2016 doi: 10.1007/s13365-016-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Health PDoP. AIDS Activities Coordinating Office Surveillance Report, 2014. Philadelphia, PA: City of Philadelphia; 2015. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60:473–80. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727–34. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. J Clin Exp Neuropsychol. 2009;31:412–24. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Holmes WC, Shea JA. A new HIV/AIDS-targeted quality of life (HAT-QoL) instrument: development, reliability, and validity. Med Care. 1998;36:138–54. doi: 10.1097/00005650-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. Wiley-Interscience; 2000. [Google Scholar]
- Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016;214(Suppl 2):S44–50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, Duan N, Hosford DA. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–75. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–20. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Vaida F, Wong J, Sanders CA, Kao YT, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, Morgello S, Simpson DM, Heaton RK, Grant I, Letendre SL. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2016;22:170–8. doi: 10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS (London, England) 2014;28:S453–S459. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda VK, Mittapalli RK, Geldenhuys WJ, Lockman PR. Chronic exposure to nicotine and saquinavir decreases endothelial Notch-4 expression and disrupts blood-brain barrier integrity. J Neurochem. 2010;115:515–25. doi: 10.1111/j.1471-4159.2010.06948.x. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA, Monti PM. Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men. AIDS Care. 2016;28:300–5. doi: 10.1080/09540121.2015.1093595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav. 2014;18:1186–97. doi: 10.1007/s10461-014-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–54. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Cioe PA. Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV. Curr HIV/AIDS Rep. 2015;12:413–20. doi: 10.1007/s11904-015-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline Improves Mood and Cognition During Smoking Abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, Clark CR, Gunstad J, Gordon E. Cognitive status of young and older cigarette smokers: data from the international brain database. J Clin Neurosci. 2006;13:457–65. doi: 10.1016/j.jocn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Robertson K, Yosief S. Neurocognitive assessment in the diagnosis of HIV-associated neurocognitive disorders. Semin Neurol. 2014;34:21–6. doi: 10.1055/s-0034-1372339. [DOI] [PubMed] [Google Scholar]
- Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS. 2014;28:453–65. doi: 10.1097/QAD.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86:334–40. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, Prins M, Reiss P. Cross-sectional Comparison of the Prevalence of Age-Associated Comorbidities and Their Risk Factors Between HIV-Infected and Uninfected Individuals: The AGEhIV Cohort Study. Clinical Infectious Diseases. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- Shirley DK, Kaner RJ, Glesby MJ. Effects of Smoking on Non-AIDS-Related Morbidity in HIV-Infected Patients. Clinical Infectious Diseases. 2013;57:275–282. doi: 10.1093/cid/cit207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology-General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–84. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- Steyerberg E. Clinical prediction models: a practical approach to development, validation, and updating. Springer Science & Business Media; 2008. [Google Scholar]
- Thaler NS, Sayegh P, Arentoft A, Thames AD, Castellon SA, Hinkin CH. Increased neurocognitive intra-individual variability is associated with declines in medication adherence in HIV-infected adults. Neuropsychology. 2015;29:919–25. doi: 10.1037/neu0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: a qualitative review and call to action. Neuropsychol Rev. 2013;23:81–98. doi: 10.1007/s11065-013-9225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE. Reduced executive and default network functional connectivity in cigarette smokers. Hum Brain Mapp. 2015;36:872–82. doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RS. Shipley Institute of Living Scale - Revised Manual. Western Psychological Services; 2000. [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]