Abstract
Background
Recent public health lead crises in urban areas emphasize the need to better understand exposure to environmental toxicants, particularly in higher risk groups. Although African-American children have the highest prevalence of elevated blood lead levels in the United States, little is known about when this trajectory of disproportionate burden of lead exposure first emerges.
Objective
Using tooth-matrix biomarkers that directly measure fetal and early childhood metal levels, the primary goal of this study was to determine if there were racial disparities in lead levels during fetal development and early childhood. Manganese, an essential nutrient that modifies the neurotoxic effects of lead, was also measured.
Methods
Pregnant women served by the Henry Ford Health System and living in a predefined geographic area in and around Detroit, Michigan, were recruited during the second trimester or later into the Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS), a population-based birth cohort. Offspring born between September 2003 and December 2007 were studied in childhood. Child race was parent-reported. Lead and manganese during the second and third trimesters, early postnatal life (birth through age 1 year) and early childhood (age 1 through time of tooth shedding, which ranges from 6 to 12 years) were measured via high-resolution microspatial mapping of dentin growth rings, a validated biomarker for prenatal and childhood metal exposure.
Results
African-American children (N=71) had 2.2 times higher lead levels in the second and third trimesters (both P<0.001) and 1.9 times higher lead levels postnatally in the first year of life (P=0.003) compared to white children (N=51). Lead levels in African-American children were also higher during childhood, but this effect was only marginally significant (P=0.066) and was attenuated after covariate adjustment. Additionally, we observed that African-American children had lower tooth-manganese levels during the third trimester (P=0.063) and postnatally (P=0.043), however these differences were attenuated after covariate adjustment.
Conclusion
The disproportionate burden of lead exposure is vertically transmitted (i.e., mother-to-child) to African-American children before they are born and persists into early childhood. Our results suggest that testing women for lead during pregnancy (or in pre-conception planning), may be needed to identify the risk to their future offspring, particularly for African-American women.
Keywords: Lead, manganese, racial disparity, birth cohort, environmental injustice
1. Introduction1
In utero and early-life exposure to lead, and other environmental metal toxicants, even at very low levels, has long-term health implications.1,2 Studies have demonstrated racial, geographic and socioeconomic status (SES) differences in prenatal and early childhood exposure to metals using approaches that capture exposure either at a single point in time or one cumulative measure.3–5 However, little is known about exposure patterns across early life, particularly at potential critical windows of development during prenatal and early postnatal periods.6 Consequently, results from studies examining early-life exposure to metals and future health have been inconsistent7 and hampered by lack of direct fetal markers of exposure. Recently-developed and validated tooth matrix biomarkers overcome these limitations and provide a direct measure of metal levels from the second trimester to early childhood.8 This method takes advantage of the incremental and archival nature of tooth development (similar to growth rings in trees), to reconstruct early life history of exposure to lead and other metals. Comparison with lead levels in umbilical cord blood and childhood blood have shown that teeth capture both the intensity and timing of exposure.8
Here, we study lead, an established neurotoxicant, in Detroit, Michigan (MI), USA and its surrounding suburbs, an area just 70 miles from Flint, MI, which has recently been the focus of an acute drinking water crisis that included higher than average lead levels in the drinking water.9 The primary goal of this study was to determine if there were racial disparities in lead levels during fetal development and early childhood, even after accounting for SES or residential characteristics. Lead’s effects on the nervous system can be modified by other metals, in particular manganese,10,11 thus we also sought to examine if co-exposure to manganese modified the association between race and lead exposure. We used rigorously validated tooth-matrix biomarkers to directly measure fetal and postnatal lead and manganese levels8,12–14 in a racially and socioeconomically diverse birth cohort, the Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS).15–17
2. Methods
2.1. Study Population
WHEALS recruited pregnant women with due dates from September 2003 through December 2007, and who were seeing a Henry Ford Health System (HFHS) obstetrics practitioner at one of five clinics to establish an unselected birth cohort.15–17 All women were in their second trimester or later, were aged 21–49 years, and were living in a predefined geographic area in Wayne and Oakland counties of Michigan that included the city of Detroit as well as the suburban areas immediately surrounding the city. A total of 1,258 maternal-child pairs were included in WHEALS.
WHEALS families were asked if they would donate an exfoliated primary tooth to the WHEALS Tooth Fairy Study. Between December 2011 and January 2015, 373 teeth were received from 156 participants. Teeth were selected for measurement if the child had at least some outcome data available and the tooth was relatively intact. A few children (N=17) had multiple teeth analyzed for quality control; metals levels were averaged over teeth within each child. Teeth from 152 children underwent analysis. After excluding 30 children who were non-African-American or non-white, due to small sample sizes within other groups (13 Middle Eastern, 7 Hispanic, 6 Asian and 4 Multi our final analytic sample consisted of 122 children. All participants provided written, informed consent. Study protocols were approved by the HFHS Institutional Review Board.
2.2. Covariate Measurement
Maternal race was self-reported and child race was parent-reported, usually by the mother. Household income, marital status, exposure to environmental tobacco smoke (ETS), smoking during pregnancy, exposure to indoor pets prenatally, year the residence was built and infant feeding practices at 1 month (formula feeding, exclusive breast-feeding or mixed formula/breastfeeding) were self-reported. Year residence was built was dichotomized as 1980 or after or before 1980, to indicate risk of lead exposure due to lead-based paint.18 Address during pregnancy/early childhood was recorded and used to define whether the fetus/child spent most of his/her time in an urban residence (defined as within the confines of the city of Detroit) or a suburban residence. Prenatal and delivery records for WHEALS women were abstracted to obtain antibiotic and antifungal use,19 prenatal hemoglobin levels, body mass index (BMI) at the first prenatal care visit, delivery type, birth weight and gestational age at delivery. The American College of Obstetricians and Gynecologists definitions for maternal anemia vary by trimester of measurement (11 g/dl in first and third trimester; 10.5 g/dl in second trimester)20; because some women had multiple measures of hemoglobin over the course of pregnancy and potential uncertainty in pregnancy dating, we used ever having a hemoglobin <10.5 g/dl as our definition of ever anemic. Gender- and gestational-age adjusted birth weight Z-scores were calculated using the US population as a reference.21 Children’s medical records were abstracted to obtain hemoglobin values and were used to define anemia during childhood.
2.3. Analysis of metals in tooth samples
We directly measured metals in teeth using laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) and assigned developmental times as detailed elsewhere.8,13 Teeth were sectioned and the neonatal line (a histological feature formed in enamel and dentine at the time of birth) and incremental markings were used to assign temporal information to sampling points. We used an ArF excimer laser ablation system (ESI, USA) attached to an Agilent Technologies 8800 triple-quad ICP-MS. Data were analysed as metal-to-calcium ratios (e.g. 208Pb:43Ca) to control for variations in mineral content within a tooth and between samples. Samples were analysed in two batches. Metal levels were measured at four times: second trimester, third trimester, early postnatal life (through ~age 1 year), and childhood (from ~age 1 year to time of tooth shedding which ranges from 6 to 12 years). National Institute of Standards and Technology SRM 612 was used for calibration and quality control. The detection limit was 0.05 μg/g for lead and manganese.
2.4. Statistical Analysis
For descriptive purposes, maternal and child characteristics were compared by race (African American compared to white) using a chi-square test for discrete characteristics and ANOVA for continuous characteristics. Intraclass correlation coefficients (ICC) were calculated to determine the agreement in lead and manganese levels between teeth shed from the same child.
Racial differences in the time-specific metal levels were examined using GEE22 linear regression models with the individual metal as the outcome variable and race as the predictor variable. GEE accounts for potential correlation in measurements in the same tooth at different time points; an autoregressive working correlation structure was assumed, which was selected based on the minimum QIC (Quasilikelihood under the Independence model Criterion).23 A race-by-time interaction was used to assess whether the relationship between race and metal level differs over time.24 All models were adjusted for batch, tooth type (incisor, canine, and molar) and level of tooth attrition (none, very minimal, or 1/3 or more of tooth removed). In addition to these three factors, models were further adjusted for all covariates that were a priori hypothesized potential confounders (maternal age, maternal education, household income, marital status, urban residence, prenatal antimicrobial use, prenatal ETS exposure, prenatal pet exposure, maternal anemia, maternal BMI, mode of delivery, gestational age at delivery, birthweight Z-score, year home was built, child feeding practice and child ever anemic in first year of life), and that were significantly associated with both race and metal level. Parameter estimates from models before and after adjustment for these covariates were examined, with a 20% or greater change in effect size indicating the presence of confounding. Because manganese and lead potentially interact,10,11 we explored whether racial differences in lead differed by different levels of co-exposure to manganese. Manganese tertiles were calculated and tested for interaction effects with race. Main effects were considered significant at p<0.05; interactions were considered significant at p<0.10. Analyses were conducted using SAS version 9.4 or R version 3.2.1.
3. Results
We compared the 156 children who donated a tooth to the 1,102 WHEALS children who did not donate a tooth to the study. Mothers of children who donated a tooth were more educated, more likely to be married, more likely to live in a suburban region, were slightly older at delivery, were more likely to self-report as white race and were less likely to smoke during pregnancy compared to mothers of children who did not donate a tooth (Supplemental Table 1; all p<0.05). By race, there were fewer differences in those who did and did not participate in the tooth study (Supplemental Table 2; all p<0.05). Among whites, mothers of children who donated a tooth were slightly older, more educated, less likely to have smoked during pregnancy and less likely to have a prenatal ETS exposure. Among African Americans, mothers of children who donated a tooth were slightly older and more educated.
In general, there was fairly good agreement in metal levels from teeth of the same children (n=16 children with two teeth measured in same batch). For lead, all ICC indicated moderate to excellent agreement (second trimester ICC=0.55; third trimester ICC=0.74; postnatal ICC=0.87; and childhood ICC=0.74). After excluding one individual with a potential outlier for manganese, agreement was moderate to excellent for manganese as well (second trimester ICC=0.84; third trimester ICC=0.59; postnatal ICC=0.86; and childhood ICC=0.84).
In our sample, compared to white participants, mothers of African-American children had lower levels of education and income, were less likely to keep a pet indoors, and were on average two years younger and had a higher prenatal BMI (Table 1; all p<0.05). More African-American children lived in urban areas and were exposed to ETS, while fewer were exclusively breastfed, and they tended to weigh less at birth (Table 1; all p<0.05).
Table 1.
Descriptive characteristics of study population, by child race; data are mean±standard deviation or N(%).
| Covariate | White (N=51) |
African-American (N=71) |
Pa |
|---|---|---|---|
| Maternal characteristic | |||
| Age at delivery (years) | 32.3±4.3 | 30.4±5.6 | 0.047 |
| College degree or higher | 35 (68.6%) | 20 (28.2%) | <0.001 |
| Household income | 0.001 | ||
| <$20,000 | 0 (0.0%) | 7 (9.9%) | |
| $20,000 to <$40,000 | 3 (5.9%) | 18 (25.3%) | |
| $40,000 to <$80,000 | 17 (33.3%) | 24 (33.8%) | |
| $80,000 to <$100,000 | 11 (21.6%) | 9 (12.7%) | |
| ≥$100,000 | 13 (25.5%) | 5 (7.0%) | |
| Refused to answer | 7 (13.7%) | 8 (11.3%) | |
| Married | 44 (86.3%) | 37 (52.1%) | <0.001 |
| Urban Residence | 3 (5.9%) | 51 (71.8%) | <0.001 |
| Prenatal antibiotic useb | 20 (44.4%) | 39 (61.9%) | 0.072 |
| Prenatal antifungal useb | 7 (15.6%) | 11 (17.5%) | 0.793 |
| Prenatal ETS exposure | 7 (13.7%) | 21 (29.6%) | 0.040 |
| Prenatal indoor pets | 35 (68.6%) | 22 (31.0%) | <0.001 |
| Ever anemic prenatally | 6 (11.8%) | 15 (21.1%) | 0.177 |
| First measured BMI in pregnancy (kg/m2) | 28.1±8.1 | 31.6±7.5 | 0.020 |
| Child characteristics | |||
| Cesarean section birth | 16 (31.4%) | 30 (42.3%) | 0.221 |
| Gestational age at delivery | 38.9±1.6 | 39.0±1.3 | 0.775 |
| Birthweight (g) | 3543±554 | 3370±418 | 0.059 |
| Birthweight Z-score | 0.37±1.03 | −0.07±0.88 | 0.018 |
| Male | 26 (51.0%) | 36 (50.7%) | 0.976 |
| First born | 18 (35.3%) | 27 (38.0%) | 0.758 |
| Year home was built | 0.287 | ||
| 1980 or later | 11 (21.6%) | 12 (16.9%) | |
| Before 1980 | 40 (78.4%) | 56 (78.9%) | |
| Missing | 0 (0) | 3 (4.2%) | |
| Feeding practices at 1 month | 0.025 | ||
| Formula Feeding | 13 (25.5%) | 14 (19.7%) | |
| Mixed Feeding | 22 (43.1%) | 47 (66.2%) | |
| Exclusive Breastfeeding | 16 (31.4%) | 10 (14.1%) | |
| Ever Anemic in the first year of lifeb | 2 (11.1%) | 3 (10.3%) | 0.934 |
| Ever Anemic in the first 7 years of lifeb | 4 (11.1%) | 16 (27.6%) | 0.058 |
Parametric p-value is calculated by ANOVA for numerical covariates and Chi-square test for categorical covariates
Rate of missingness>10%.
ETS: environmental tobacco smoke; BMI, body mass index.
Microspatial mapping of lead in dentin growth rings showed that racial differences in lead levels were time-dependent (interaction p<0.001); African-American children had 2.2 times higher lead levels in the second and third trimesters (both p<0.001) and 1.9 times higher lead levels postnatally in the first year of life (p=0.003) (Figure 1A; Table 2). Tooth lead levels in African-American children were also higher during childhood, but this effect only approached significance (p=0.066). Racial differences in manganese were also time-dependent (interaction p=0.091); African-American children had marginally lower tooth-manganese levels during the third trimester and significantly lower levels postnatally (p=0.063, p=0.043, respectively; Table 2; Figure 1C). Racial differences in lead were not substantially altered in models adjusted for potential confounders (Table 2; Figure 1B), with the exception of lead during childhood, which was attenuated and became non-significant. In contrast, racial differences in manganese were attenuated after adjusting for confounders (Table 2; Figure 1D). Final model results for lead were not meaningfully affected after additionally adjusting for manganese levels (data not shown). The distribution of lead and manganese are presented in Supplemental Figure 1.
Figure 1.
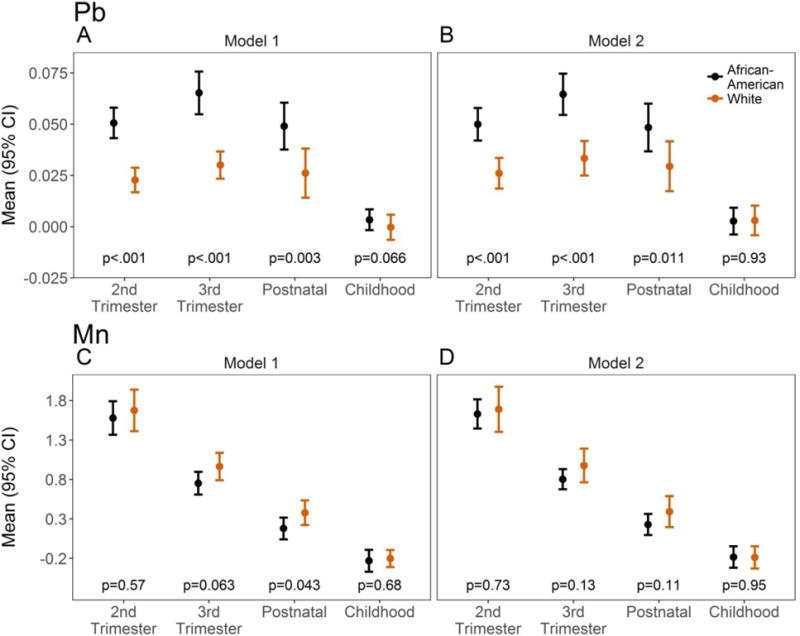
Race-specific distribution of lead (Pb) (A, B) and manganese (Mn) (C, D) by time of measurement based on GEE model results. Model 1 (A, C) is adjusted for tooth type, batch, and attrition, while Model 2 (B, D) further includes potential confounder adjustment (married, urban residence, prenatal environmental tobacco exposure, and prenatal indoor pets for Pb; prenatal indoor pets and household income for Mn). Raw data are in metal-to-calcium ratio.
Table 2.
Time-specific association of race with lead and manganese
| Lead | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjustment Covariatesa | Interaction | 2nd trimester | 3rd trimester | Postnatal | Childhood | ||||
| p-value | Est (SE)a | p-value | Est (SE) | p-value | Est (SE) | p-value | Est (SE) | p-value | |
| batch + tooth type + attrition |
<.001 | 0.028 (0.004) | <.001 | 0.035 (0.006) | <.001 | 0.023 (0.008) | 0.003 | 0.004 (0.002) | 0.066 |
|
| |||||||||
| batch + tooth type + married + urban residence + prenatal ETS + prenatal indoor pets |
<.001 | 0.024 (0.005) | <.001 | 0.031 (0.006) | <.001 | 0.019 (0.007) 0.011 | −0.001(0.004) | 0.93 | |
|
| |||||||||
| Manganese | |||||||||
| Adjustment Covariatesa | Interaction | 2nd trimester | 3rd trimester | Postnatal | Childhood | ||||
| p-value | Est (SE) | p-value | Est (SE) | p-value | Est (SE) | p-value | Est (SE) | p-value | |
|
| |||||||||
| batch + tooth type + attrition |
0.091 | −0.096 (0.170) | 0.57 | −0.212 (0.114) | 0.063 | −0.199 (0.098) | 0.043 | −0.028 (0.069) | 0.68 |
|
| |||||||||
| batch + tooth type + attrition + prenatal indoor pets household income |
0.093 | −0.058 (0.168) | 0.73 | −0.174 (0.114) | 0.13 | − 0.162 (0.101) | 0.11 | 0.005 (0.077) | 0.95 |
Estimate for mean difference in metal levels in African-American vs. white children
ETS: environmental tobacco smoke
At all time points, lead and manganese were statistically significantly and positively correlated in African-American but not white children (Table 3). The association between race and lead significantly depended on manganese (Figure 2). In the 2nd trimester, as manganese levels increased, the racial disparity in lead also increased (p=0.097); although not statistically significant, there was a similar pattern for the 3rd trimester. In early postnatal life, the racial disparity in lead was also higher for higher manganese level (p=0.01), while in childhood, the racial disparity in lead showed a U-shaped trend, being greatest for children with the lowest or highest manganese level (p=0.001).
Table 3.
Time-specific correlation between lead and manganese, overall and by race
| Correlation Coefficient | P-value | |
|---|---|---|
| Second Trimester | ||
| Overall | 0.28 | 0.002 |
| African-American | 0.49 | <0.001 |
| White | 0.11 | 0.447 |
| Third Trimester | ||
| Overall | 0.15 | 0.096 |
| African-American | 0.40 | <0.001 |
| White | 0.08 | 0.591 |
| Postnatal | ||
| Overall | 0.06 | 0.06 |
| African-American | 0.28 | 0.023 |
| White | 0.05 | 0.733 |
| Childhood | ||
| Overall | 0.47 | <0.001 |
| African-American | 0.52 | <0.001 |
| White | 0.21 | 0.146 |
Figure 2.
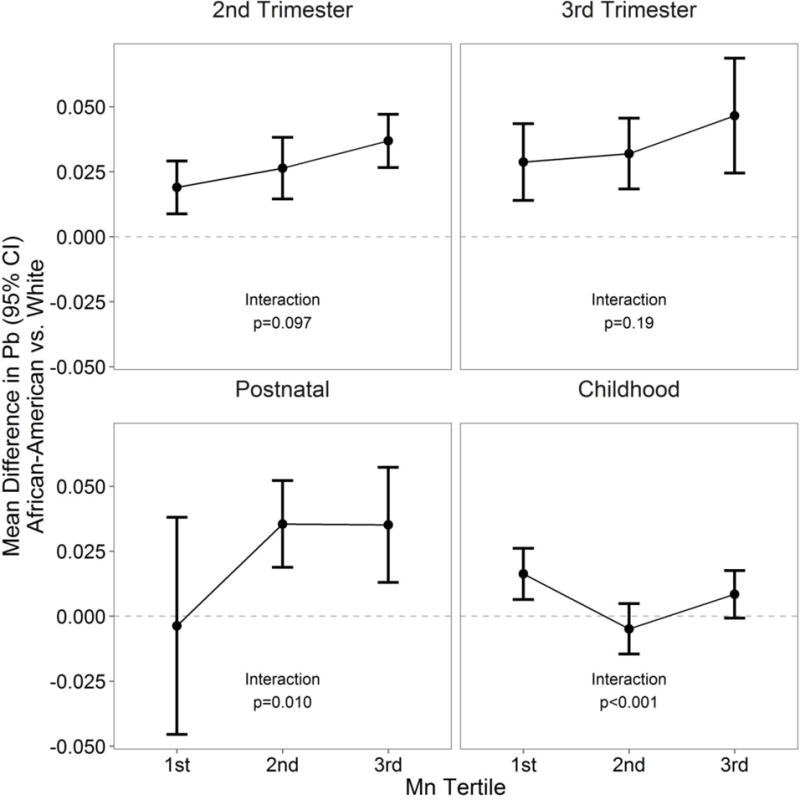
Racial difference in lead (Pb) varies as a function of manganese (Mn) level. Mn tertiles as follows: 2nd trimester: <1.25, 1.25–2.26, >2.26; 3rd trimester: <0.70, 0.70–1.28, >1.28; postnatal: <0.27, 0.27–0.56, >0.56; childhood: <0.002, 0.002–0.03, >0.003. Models are adjusted for batch, tooth type, and attrition. Raw data are in metal-to-calcium ratio.
4. Discussion
We found the disproportionate burden of lead level in teeth is vertically transmitted to African-American children before they are born, likely driven by maternal SES and geographic factors. Our results support that the environmental injustice of higher lead level in African-American communities is multi-faceted. Not only are lead exposures higher, African-Americans may be deprived of influences that may reduce lead uptake or mitigate its neurotoxic effect; notably, we found lower levels of manganese—an essential nutrient known to interact with lead levels—in African-American children pre- and postnatally, however this was attenuated after adjustment for household income. In isolation, blood and hair manganese demonstrates a U-shaped association with adverse neurodevelopment and cognitive function.25–27 Additionally, co-exposure of high lead and manganese is adversely associated with neurodevelopment and cognitive function.11,28 At appropriate levels, manganese, as an essential nutrient, is necessary for normal enzymatic function and potential protection against oxidative injury29; thus, if manganese is low, lead may have a greater detrimental effect via lead-induced oxidative stress mechanisms, for example.30 Conversely, if lead and manganese are both high, they may enhance the overall toxicity compared to either one in isolation.31 Our results suggest that further study is needed to examine how early co-exposures may interact to impact racial disparities in childhood health. This is the first evidence based on direct fetal and childhood longitudinal measures of lead and manganese levels going as far back as the second trimester in African-American children.
Although African-American children have the highest prevalence of elevated blood lead levels in the US,32,33 little is known about when this trajectory of disproportionate burden of lead exposure first emerges, which may be of concern because African-American women of childbearing age also have higher blood lead levels.34 In a study of pregnant women in Pittsburgh, Pennsylvania with up to five blood lead measurements over pregnancy, Hertz-Picciotto et al (2000) showed that black compared to white race was associated with a 11.5% higher blood lead level in pregnancy.35 However, this study did not have direct fetal measurement. We found that the racial disparity in tooth lead levels is detectable as early as the 2nd trimester and persists through the early postnatal period. This disparity was detectable even after adjusting for SES factors, including urban residence, during early life. However, after accounting for urban residence, the racial disparity in childhood tooth lead levels were attenuated. Together, this suggests that rather than racial differences in lead exposure during pregnancy it is the lifetime, cumulative exposures in the mother that may differ by race that may be the source of the racial disparity in early life lead levels when lead is mobilized from the bone during pregnancy.36 While acute lead exposures such as those in Flint have garnered both public and scientific attention9, the most common exogenous source of lead exposure in children comes from leaded paint that may remain in older homes.32,37 Public health efforts to mitigate sources of lead exposure have shown success, however, ongoing effort is still needed.18 Our results suggest that testing women during pregnancy, or even earlier as they enter child-bearing age, may be needed to identify the risk to their future offspring due to lead exposure.
Additionally, there may be racial differences in genetic factors influencing the differences in lead level shown in the current study. Haynes et al (2003) showed that children homozygous for the F allele of the Vitamin D Receptor Fok1 polymorphism had higher blood lead levels at age 24 months compared to the other genotypes; African-American children were more likely to carry the F allele than non-African American children. The authors speculate that African-Americans may have enhanced calcium absorption abilities, which would allow for increased lead absorption abilities.38 However, of note in this study, among African Americans, the relationship between house dust lead level and blood lead concentration at age 24 months did not differ by VDR Fok1 genotype, whereas this relationship did differ among non-African American children. Future studies that incorporate both maternal and child genetic information to better understand racial differences in lead and other metal exposures are needed.
Detroit and its surrounding suburbs are part of an industrialized region. We have previously shown, based on data from routine blood lead screening, that children from the WHEALS cohort have rates of elevated blood lead levels that are similar to that of the US,33,39 suggesting that our population is neither disproportionately burdened by, nor protected from, lead exposure. Compared to prenatal and postnatal tooth Mn levels measured in the CHAMACOS cohort (0.51±0.19 and 0.20±0.23, respectively), the mean prenatal and postnatal Mn tooth levels in WHEALS children (1.3±0.72 and 0.49±0.50, respectively) were somewhat higher.40
Our study has a number of strengths and limitations. Utilizing the novel tooth-matrix methodology overcomes limitations of blood lead testing, which reflects lead at a single point in time and requires venipuncture or capillary finger-prick testing, which are often undesirable for children, particularly in the context of research studies with the need for multiple measurements.41 Testing of umbilical cord blood only estimates lead exposure experienced at the end of the third trimester. We do not have information on blood or bone lead levels of the mothers during pregnancy, thus we cannot directly estimate the transmission burden from mother to child. Only a subset of the WHEALS participants donated a tooth for inclusion in the current study; the mothers of children who donated a tooth to the study were of slightly higher age and SES than the overall WHEALS cohort. In order to account for this potential participation bias, current plans to further expand tooth collection are underway. Our findings with respect to racial disparities in lead are strong and consistent with the literature, however, our results are still subject to residual confounding. Although we were able to account for maternal and child hemoglobin levels via anemia status, detailed information on maternal diet in pregnancy or diet during childhood was not available; racial differences in diet may further explain differences in lead and manganese level and should be considered in future studies.
For the majority of participants, a single tooth was used to estimate metal levels. We had at least moderate to excellent agreement in lead and manganese levels from the teeth of children who donated two teeth from the study, suggesting the single tooth measure was sufficient to estimate lead and manganese levels. Because we had relatively few children with metals measured in multiple teeth, future studies that have multiple tooth samples from the same individual may be necessary to better define reliability, although previous work suggests good reproducibility of these measurements over time.28
Conclusion
Our findings could have important implications for children who underwent gestation during recent acute urban lead exposures, not only in Flint, MI, but in areas such as Jackson, Mississppi.9,42 First, exposure effects from this contamination may be greater in children born to mothers from racial and ethnic minorities or those from socioeconomically disadvantaged areas. Second, health risk from incidents of acute lead exposure may not be limited to the current generation of children and adults; rather, increased lead burden in adult females may be transmitted to the next generation of children they have, thus continuing a vertical transfer of environmental injustice. Blood lead testing of pregnant women or women undergoing preconception counseling, may be useful to estimate risk to their offspring.
Supplementary Material
Highlights.
This study utilizes naturally shed deciduous teeth to evaluate potential racial differences in lead levels in utero through early childhood
Disproportionate burden of lead levels appears to be vertically transmitted to African-American children before they are born
Racial differences in early-life lead levels persisted even after accounting for area of residence
Acknowledgments
This study was supported by the National Institutes of Health (R01 AI050681, R01 HL113010, R21 ES022321, P01 AI089473, R00 ES019597 and DP2 ES025453) and the Fund for Henry Ford Hospital. Study sponsors had no role in the study design, data collection or interpretation, or in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations
BMI, Body Mass Index; ETS, Environmental Tobacco Smoke; GEE, Generalized Estimating Equations; HFHS, Henry Ford Health System; LA-ICP-MS, Laser ablation-inductively coupled plasma mass spectrometry; MI, Michigan; Mn, manganese; Pb, lead; QIC, Quasilikelihood under the Independence model Criterion; SES, Socioeconomic Status; WHEALS, Wayne County Health, Environment, Allergy and Asthma Longitudinal Study
References
- 1.Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6(2):55–64. doi: 10.1017/S2040174414000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heindel JJ, Balbus J, Birnbaum L, et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology. 2015;156(10):3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells EM, Jarrett JM, Lin YH, et al. Body burdens of mercury, lead, selenium and copper among Baltimore newborns. Environ Res. 2011;111(3):411–417. doi: 10.1016/j.envres.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needleman HL, Davidson I, Sewell EM, Shapiro IM. Subclinical lead exposure in Philadelphia schoolchildren. Identification by dentine lead analysis. N Engl J Med. 1974;290(5):245–248. doi: 10.1056/NEJM197401312900504. [DOI] [PubMed] [Google Scholar]
- 5.Chatman T, Wilson DJ. Lead levels in human deciduous teeth in Tennessee. Environ Lett. 1975;8(2):173–183. doi: 10.1080/00139307509437430. [DOI] [PubMed] [Google Scholar]
- 6.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grason HA, Misra DP. Reducing exposure to environmental toxicants before birth: moving from risk perception to risk reduction. Public Health Rep. 2009;124(5):629–641. doi: 10.1177/003335490912400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora M, Austin C, Sarrafpour B, et al. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9(5):e97805. doi: 10.1371/journal.pone.0097805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna-Attisha M, Kuehn BM. Pediatrician Sees Long Road Ahead for Flint After Lead Poisoning Crisis. JAMA. 2016;315(10):967–969. doi: 10.1001/jama.2016.1034. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Kim BN, Hong YC, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30(4):564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Claus Henn B, Schnaas L, Ettinger AS, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2012;120(1):126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunier RB, Bradman A, Jerrett M, et al. Determinants of manganese in prenatal dentin of shed teeth from CHAMACOS children living in an agricultural community. Environmental science & technology. 2013;47(19):11249–11257. doi: 10.1021/es4018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora M, Bradman A, Austin C, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environmental science & technology. 2012;46(9):5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin C, Richardson C, Smith D, Arora M. Tooth manganese as a biomarker of exposure and body burden in rats. Environ Res. 2017;155:373–379. doi: 10.1016/j.envres.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy-Bushrow A, Wegienka G, Barone C, et al. Race-specific relationship of birth weight and renal function among healthy young children. Pediatric nephrology. 2012;27(8):1317–1323. doi: 10.1007/s00467-012-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havstad S, Wegienka G, Zoratti EM, et al. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. 2011;128(4):880–885. doi: 10.1016/j.jaci.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegienka G, Havstad S, Joseph CL, et al. Racial Disparities in Allergic Outcomes in African Americans Emerge as Early as Age 2 Years. Clinical & Experimental Allergy. 2011;42(6):909–917. doi: 10.1111/j.1365-2222.2011.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon SL, Gaitens JM, Jacobs DE, et al. Exposure of U.S. children to residential dust lead, 1999–2004: II. The contribution of lead-contaminated dust to children’s blood lead levels. Environ Health Perspect. 2009;117(3):468–474. doi: 10.1289/ehp.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegienka G, Havstad S, Zoratti EM, Kim H, Ownby DR, Johnson CC. Combined effects of prenatal medication use and delivery type are associated with eczema at age 2 years. Clin Exp Allergy. 2015;45(3):660–8. doi: 10.1111/cea.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ACOG Practice Bulletin No. 95: Anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–207. doi: 10.1097/AOG.0b013e3181809c0d. [DOI] [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 23.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung SE, Cheong HK, Ha EH, et al. Maternal Blood Manganese and Early Neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ Health Perspect. 2015;123(7):717–722. doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollet K, Haynes EN, Dietrich KN. Manganese Exposure and Cognition Across the Lifespan: Contemporary Review and Argument for Biphasic Dose-Response Health Effects. Current Environmental Health Reports. 2016;3(4):392–404. doi: 10.1007/s40572-016-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes EN, Sucharew H, Kuhnell P, et al. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA) Environ Health Perspect. 2015;123(10):1066–1071. doi: 10.1289/ehp.1408993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora AM, Arora M, Harley KG, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finley JW, Davis CD. Manganese deficiency and toxicity: are high or low dietary amounts of manganese cause for concern? BioFactors. 1999;10(1):15–24. doi: 10.1002/biof.5520100102. [DOI] [PubMed] [Google Scholar]
- 30.Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Veterinary medicine international. 2011;2011:457327. doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CC, Chen YC, Su FC, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Lanphear BP, Weitzman M, Eberly S. Racial differences in Urban children’s environmental exposures to lead. Am J Public Health. 1996;86(10):1460–1463. doi: 10.2105/ajph.86.10.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler W, Brown M. Blood lead levels in children aged 1–5 years - United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62(13):245–248. [PMC free article] [PubMed] [Google Scholar]
- 34.Geronimus AT, Hillemeier MM. Patterns of blood lead levels in US black and white women of childbearing age. Ethnicity & Disease. 1992;2(3):222–231. [PubMed] [Google Scholar]
- 35.Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and Determinants of Blood Lead During Pregnancy. American Journal of Epidemiology. 2000;152(9):829–837. doi: 10.1093/aje/152.9.829. [DOI] [PubMed] [Google Scholar]
- 36.Silbergeld EK. Lead in bone: implications for toxicology during pregnancy and lactation. Environ Health Perspect. 1991;91:63–70. doi: 10.1289/ehp.919163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanphear BP, Weitzman M, Winter NL, et al. Lead-contaminated house dust and urban children’s blood lead levels. Am J Public Health. 1996;86(10):1416–1421. doi: 10.2105/ajph.86.10.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haynes EN, Kalkwarf HJ, Hornung R, Wenstrup R, Dietrich K, Lanphear BP. Vitamin D receptor Fok1 polymorphism and blood lead concentration in children. Environ Health Perspect. 2003;111(13):1665–1669. doi: 10.1289/ehp.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassidy-Bushrow AE, Havstad S, Basu N, et al. Detectable Blood Lead Level and Body Size in Early Childhood. Biological Trace Element Research. 2016;171(1):41–47. doi: 10.1007/s12011-015-0500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunier RB, Arora M, Jerrett M, et al. Manganese in Teeth and Neurodevelopment in Young Mexican-American Children. Environmental research. 2015;142:688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spivey A. The weight of lead. Effects add up in adults. Environ Health Perspect. 2007;115(1):A30–36. doi: 10.1289/ehp.115-a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dingle A. The Flint Water Crisis: What’s Really Going On? ChemMatters. :5–8. December 2016/January 2017. Available at: https://www.acs.org/content/dam/acsorg/education/resources/highschool/chemmatters/issues/2016-2017/December%202016/chemmatters-dec2016-flint-water-crisis.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


