Abstract
Dopamine (DA) neurotransmission within the brain’s reward circuit has been implicated in the pathophysiology of depression and in both, cognitive and pharmacological mechanisms of treatment response. Still, a direct relationship between measures of DA neurotransmission and reward-related deficits in patients with depression has not been demonstrated. To gain insight into the symptom-specific alterations in the DA system in patients with depression, we used positron emission tomography (PET) and the D2/3 receptor-selective radiotracer [11C]raclopride in twenty-three non-smoking un-medicated Major Depressive Disorder (MDD) patients and sixteen healthy controls (HC). We investigated the relationship between D2/3 receptor availability and baseline measures of depression severity, anxiety, anhedonia, and cognitive and pharmacological mechanisms of treatment response. We found that, compared to controls, patients with depression showed greater D2/3 receptor availability in several striatal regions, including the bilateral ventral pallidum/nucleus accumbens (vPAL/NAc), and the right ventral caudate and putamen. In the depressed sample, D2/3 receptor availability in the caudal portion of the ventral striatum (NAc/vPAL) correlated with higher anxiety symptoms, whereas D2/3 receptor availability in the rostral area of the ventral striatum correlated negatively with the severity of motivational anhedonia. Finally, MDD non-remitters showed greater baseline anxiety, greater D2/3 availability in the NAc/vPAL, and greater placebo-induced DA release in the bilateral NAc. Our results demonstrate abnormally high D2/3 receptor availability in the ventral striatum of patients with MDD, which seem to be associated with comorbid anxiety symptoms and lack of response to antidepressants.
Keywords: Depression, Anhedonia, Anxiety, Dopamine, PET, Treatment Response
INTRODUCTION
Multiple lines of evidence have implicated dopamine (DA) dysregulation with the brain’s reward neural circuit in the pathophysiology and treatment of depression. A direct relationship between striatal DA receptor availability and reward related deficits and their contribution to treatment response in patients with depression has not been investigated yet.
Clinically, DA dysregulations in depression have been linked to anhedonia (Nestler and Carlezon, 2006), one of the two cardinal symptoms of Major Depressive Disorder (MDD). Initially, the DA deficiency hypothesis of anhedonia (Wise, 1980) was thought to be related to reductions in the subjective experience of pleasure (consummatory anhedonia). However, this conceptualization has been largely abandoned in favor of a pivotal role of DA in the motivation to pursue meaningful rewards (motivational anhedonia) (Berridge and Robinson, 2003; Salamone, 2007). In humans, current evidence suggests that blunted processing of incentive salience, incentive motivation, and reinforcement learning might precede depressive symptoms. However, when these abnormalities are worsened by recurrences and emerge coupled with abnormalities in regions implicated in coding the hedonic value of stimuli, these disruptions might lead to more tenuous anticipatory reward-related associations, and ultimately anhedonic symptoms (Whitton et al., 2015).
From the treatment perspective, pharmacological enhancement of DA signaling with DA agonists, such as bromocriptine or pramipexole (Bouras and Bridges, 1982; Cassano et al., 2005; Shopsin and Gershon, 1978) and DA transporter (DAT) inhibitors, such as amineptine and bupropion, exhibit varying degrees of antidepressant effects (Stahl et al., 2004). Deep brain stimulation affecting the nucleus accumbens (NAc)—a key region implicated in reward processing— has also demonstrated evidence of sustained efficacy in patients diagnosed with treatment-resistant depression in an open trial (Bewernick et al., 2012). Furthermore, previous evidence suggests that current first-line pharmacotherapies (e.g., SSRIs) do not adequately address motivational and reward-processing deficits in depression (Dunlop and Nemeroff, 2007; McCabe et al., 2009; McCabe et al., 2010; Price and Hotopf, 2009), and that anhedonia is generally a predictor of poor treatment response (Spijker et al., 2001).
DA has also been associated with the formation of placebo-induced positive expectations and subsequent treatment responses in clinical trials, introducing variability and confounding the interpretation of potential drug effects (Enck et al., 2013). It has been shown that the strength of beliefs of improvement directly modulate dopamine release in patients with Parkison’s Disease (de la Fuente-Fernandez et al., 2001), and in the context pain (Scott et al., 2007, 2008). Broadly, expectations are fundamental in all emotional processes, and allow an individual to interact with an upcoming emotional or motivational situation before it actually occurs (Petrovic et al., 2005). The neural correlates of expectations have been shown in several functional neuroimaging studies of emotion in general and of reward processing specifically (Ernst et al., 2004; Knutson et al., 2001a; Knutson et al., 2001b). Overall, the dopaminergic system seems to be involved in the complex interaction among the pathophysiology of MDD, the mechanisms of action of some antidepressant treatments and the patients’ expectations of improvement, all of which contribute to treatment response variability.
Human in vivo neuroimaging studies in depressed samples have aimed to clarified these questions. Studies using 123I-iodobenzamide single-photon emission computed tomography ([123I]-IBZM SPECT) initially reported increased binding of striatal D2 receptors compared to controls (D’Haenen H and Bossuyt, 1994; Shah et al., 1997), which were thought to reflect either an up-regulation of D2/3 receptors, increased affinity of the receptor for the radioligand, decreased synaptic dopamine concentrations or potentially a combination of these mechanisms (Dunlop and Nemeroff, 2007). These initial findings were followed by a number of negative (Ebert et al., 1996; Hirvonen et al., 2011; Klimke et al., 1999; Montgomery et al., 2007; Parsey et al., 2001; Yang et al., 2008) or even opposite (Busto et al., 2009) studies using 123I-IBZM SPECT or [11C]-raclopride positron emission tomography (PET).
Some of the inconsistencies described above might be explained by the lack of approaches that specifically investigate DA-related symptoms of depression, such as anhedonia, and their relationship with sub-regions within the basal ganglia (e.g NAc). Here we used positron emission tomography (PET) and the D2/3 selective radiotracer [11C]raclopride to examine the relationships among D2/3 receptor availability, symptoms of anhedonia, and the response to antidepressants and placebo treatments. For this purpose, we utilized a design identical to one recently utilized to examine endogenous opioid mechanisms of the placebo response in MDD (Peciña et al., 2015). In the present report, we also aimed to dissect the motivational and consummatory components of the overall anhedonia construct (Treadway and Zald, 2011), using two different self-reported questionnaires: the Apathy Evaluation Scale (motivational anhedonia) (Marin et al., 1991) and the Snaith-Hamilton Pleasure Scale (SHAPS) (consummatory anhedonia) (Snaith et al., 1995). We hypothesized that in patients with MDD, primary DA function deficits within the ventral striatum (e.g. a presynaptic reduction in function) would result in observable, compensatory up-regulation of striatal D2/3, lower placebo-induced DA release, and greater motivational (but not consummatory) anhedonia. Secondly, it was expected that higher motivational anhedonia scores would be linked to poor clinical response to both open-label antidepressant medication and a placebo intervention designed to increase positive expectations of improvement.
EXPERIMENTAL PROCEDURES
1. Subject Characterization
Sixteen healthy controls (HC, females= 9) and twenty-six patients meeting DSM-5 criteria for moderate-severe MDD (females=10), aged 18 to 56 years (HC=40±8.7, MDD=37±13.8, mean ± SD), were recruited through local advertisement. In addition to completing physical and neurological examinations, study participants were screened using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). For the MDD group, inclusion criteria included the diagnosis of MDD, 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) scores >12 and excluded suicidal ideation, comorbid psychiatric conditions (except for anxiety disorders), the use of psychotropic agents or recreational drugs, left-handedness and pregnancy. Average disease duration (current episode – disease onset age) was 11.5 years (Range: 0–44 y). Subjects were not taking any psychotropic medications at the time of the study for at least 6 months, except for occasional use of sleep aids (frequency of less than 2 per week), and none for at least a week prior to the study. Additional information about lifetime exposure to antidepressant medication is reported in Supplemental Table 1. Of interest, we found no significant differences in baseline D2/3 binding potential between those previously exposed to antidepressant medication (including one subject with previous exposure to aripiprazole), and those who were antidepressant naïve. Three patients with MDD were currently smokers, and were excluded from the analyses because of the documented relationship between smoking status and DA receptor availability measures. Drug abuse or dependence history were not collected, but drug test were performed before each scanning procedures and negative results were require for participation in the study. None of the participants had taken antidepressant medications for at least 6 months prior to enrollment in the study. Written informed consent was obtained and all of the procedures were approved by the University of Michigan Investigational Review Board for Human Subject Use and the Radioactive Drug Research Committee. Data were collected and stored using Research Electronic Data Capture (REDCap) (Harris et al., 2009).
2. Trial Design
After the initial screening, MDD patients entered a clinical trial, which has been described in detail elsewhere (Pecina et al., 2015). The study had two phases, a 2-week placebo single-blind RCT and a 10-week open-label flexible-dose antidepressant treatment. This study had been specifically designed to investigate changes in opioid (Pecina et al., 2015) and dopamine receptor availability in response of expectations of mood improvement in patients with MDD (“the placebo effect”), as well as the role of inter-individual variability in receptor availability in the response to open-label antidepressant treatments. All subjects in this study (n=23) were also studied with [11C]carfentanil, a μ-opioid receptor radiotracer, as previously described (Pecina et al., 2015) (Fig.1).
Figure 1. Experimental Design.
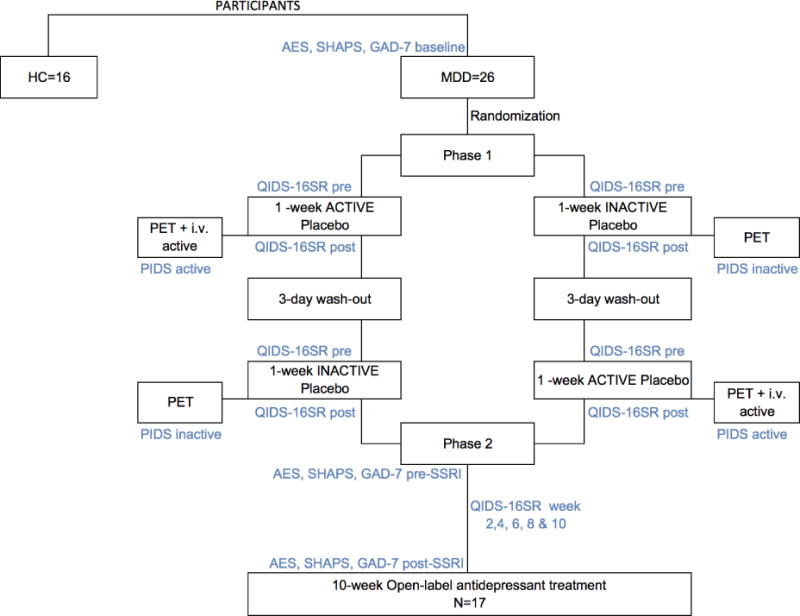
Abbreviations: PET: Positron Emission Tomography; i.v.: intravenous; QIDS-16SR: Quick Inventory of Depression Symptomatology; PIDS: Patient’s Impression of Depression Severity; HRDS: Hamilton Rating Depression Scale; Snaith: Snaith-Hamilton Pleasure Scale; NEO-PI-R: NEO Personality Inventory Revised.
2.1. Placebo Phase
During the first phase, subjects were randomized to (1) 1-week “active” oral placebo treatment (2 pills/day), with expectations that it represented a fast-acting antidepressant agent (“active” placebo condition), or (2) 1-week “inactive” oral placebo with disclosure that it was an inactive control (“inactive” placebo condition). After a 3-day “washout” period without pills, participants were crossed over into the group to which they were not previously assigned. After each placebo week, participants underwent a PET scanning session. As a challenge to induce endogenous DA system activation and to determine acute placebo effects, the PET session following the 1-week “active” oral placebo included the administration of an i.v. “active” placebo. This consisted of 1mL of 0.9% isotonic saline introduced i.v. every 4 minutes during 20 minutes, starting at minute 42 and lasting for 15 seconds each time. Subjects were aware that the study drug was to be administered through a computer-generated human voice recording, followed by a second-by-second count of the infusion timing (15 seconds). No i.v. placebo followed the “inactive” placebo condition. The post-inactive PET scan was considered a baseline condition. While open-label placebos have been associated with symptom relief under expectations of improvement (Kaptchuk et al., 2010), and could potentially lead to changes in DA neurotransmission, this has not been the case when placebos are administered in the absence of such expectations, and therefore no changes in DA binding should be expected under such manipulations. Reductions in the in vivo availability of receptors after an acute neurotransmitter releasing challenge (i.e., placebo administration) are thought to reflect processes, such as competition between radiotracer and endogenous ligand, associated with neurotransmitter release (Narendran and Martinez, 2008).
Clinical Assessments during the Placebo Phase
Depression symptoms were assessed using the Quick Inventory of Depressive Symptomatology (QIDS-SR16) (Rush et al., 2003) at pre- (baseline) and post-each placebo treatment. A single measure of sustained placebo response was created by subtracting the changes in QIDS-SR16 reductions from “active” and “inactive” placebo treatments [(QIDS-SR16 pre - post) “active” placebo—(QIDS-SR16 pre - post) “inactive” placebo]. In addition, patients’ impression of depression severity (PIDS) ratings (“from 0 to 100 how depressed do you feel now?) were acquired every 4 minutes during the 2 PET scans, in the presence and absence of the i.v. placebo. Acute, i.v. placebo responses were assessed by the subtraction PIDS no i.v. – PIDS active i.v.
2.2. Antidepressant Phase
Following the placebo phases and the two PET sessions, participants were invited to participate in an un-blinded 10-week open-label trial with an FDA-approved antidepressant, in most cases citalopram (starting at 20 mg/day and up to 40 mg/day in 92% of cases). Two patients who did not tolerate citalopram switched to another antidepressant (duloxetine 60 mg/day or bupropion 150 mg/day) at week 4. Two patients with a prior history of non-response to citalopram were treated with an alternative antidepressant (mirtazapine 30 mg/day and fluoxetine 20 mg/day respectively).
Clinical Assessments during the Antidepressant Phase
At screening and during each clinical visit, participants’ depressive symptoms were assessed using the QIDS-SR16 (Rush et al., 2003). At screening and immediately before (week 0) and after the trials (week 10) we collected information regarding: anxiety levels (Generalized Anxiety Disorder: GAD-7), consummatory anhedonia (SHAPS) and motivational anhedonia (AES) (Fig.1). Remission rates were established using the QIDS-SR16 (<5) and changes in the clinical assessments in response to the antidepressant treatment were examined against the remission rates, after controlling for baseline severity, using ANCOVA models.
3. Neuroimaging Methods
Immediately after each 1-week of placebo treatment, participants were positioned in the PET scanner gantry (Siemens HR+, Knoxville, Tennessee) and 2 i.v. (antecubital) lines were placed. A light forehead restraint was used to eliminate intrascan head movement. Four 90 min PET scanning sessions, with and without i.v. placebo administration, were completed, but only those two using [11C] raclopride are reported here. Images were acquired in 3-dimensional mode (reconstructed full-width/half-maximum resolution, approximately 5.5 mm in plane and 5.0 mm axially), with the septa retracted and scatter correction. [11C] Raclopride was synthesized at high specific activity by the reaction of O-desmethyl raclopride with [11C] methyl triflate. 15.0 ± 2.2 (mean ± SD) mCi were administered in each of the imaging procedures, with a mass of raclopride of 0.20 ± 0.15 (mean ± SD) μg per scan. Fifty percent of the radiotracer dose was administered as an initial bolus and the remaining 50% by continuous infusion for the remainder of the study to more rapidly achieve steady-state levels. For each study, 21 sets of dynamic scans were acquired with an increasing duration (four 30-second frames, three 1-minute frames, two 2.5-minute frames, eight 5-minute frames, and four 10-minute frames). Images were reconstructed using iterative algorithms (brain mode; Fourier rebinding algorithm with ordered-subsets expectation maximization, 4 iterations, and 16 subsets; no smoothing) into a 128×128-pixel matrix in a 28.8-cm-diameter field of view. Attenuation correction was performed through a 6-minute transmission scan (Ge68 source) obtained before the PET study and with iterative reconstruction of the blank/transmission data, followed by segmentation of the attenuation image. Small head motions during PET were corrected by an automated computer algorithm for each subject before analysis, and the images were co-registered with the same software (Minoshima et al., 1993). Time points were then decay corrected during reconstruction of the PET data. Image data were then transformed on a voxel-by-voxel basis into 2 sets of parametric maps, a tracer transport measure (K1 ratio) and a receptor-related measure (non-displaceable binding potential, BPND, or receptor availability in vivo) (Innis et al., 2007). To avoid the need for arterial blood sampling, these measures were calculated using a modified Logan graphical analysis (Logan et al., 1996), using the cerebellum as reference region. Using the bolus-continuous infusion protocol described above, the slope of the Logan plot becomes linear ~5 min post-tracer administration and is proportional to the receptor concentration divided by its affinity for the radiotracer [BPND + 1, or (f2Bmax/Kd) +1] (Mintun et al., 1984). Bmax is the receptor concentration and Kd, the receptor-ligand dissociation constant. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value.
Anatomical MRI studies were acquired on a 3-T scanner (Philips Achieva, Best, Netherlands). A high resolution structural image was obtained for anatomic normalization using a T1-weighted, gradient echo (MPRAGE) sequence (220 slices, slice thickness = 1mm, echo time = 4.6 msec, repetition time = 9.8 msec, flip angle = 8°, field of view =240 mm2).
3.1 Data Analysis
All preprocessing and data analyses were performed using the SPM8 toolbox (Wellcome Department of Cognitive Neurology, University College, London, England) for Matlab (MathWorks, Natick, Massachusetts). For each experimental period, the anatomical MRI was coregistered to the K1 PET image and then warped to Montreal Neurological Institute (MNI) space using the voxel-based morphometry (VBM) toolbox. The resulting deformation fields were used to warp the PET images to MNI space. To compensate for small residual anatomic variations across subjects and to improve signal-to-noise ratios, the warped PET images were smoothed with a 6-mm3 Gaussian kernel.
Group analyses of PET images were performed with mass univariate general linear models. Due to raclopride’s specific binding in the striatum, no global normalization was applied to the data, and therefore the calculations presented herein are based on absolute BPND (f2 Bmax/Kd) estimates (Carson et al., 1997). A mask was applied so that only regions with specific D2/3 receptor binding were included in the analyses (voxels with BPND >0.1) (Wager et al., 2007). Baseline (inactive placebo condition) and subtraction analyses (inactive-active placebo condition) were performed on D2/3 receptor images to assess main effects of the placebo intervention. For each analysis, 1-sample, 2-tailed t values were calculated for each voxel. A bilateral nucleus accumbens region of interest (ROI) map was created using the Harvard Oxford subcortical atlas in FSL (Jenkinson et al., 2012). Regional BPND values were obtained by applying this ROI to the individual parametric [11C]raclopride images. We used MarsBaR (Brett et al., 2002) to extract average NAc BP values for quantification of regional changes in BPND, graphing and determination of correlation coefficients. Other regions within the striatum outside of the NAc were considered significant at p<0.05 FWE-corrected voxel-wise (Friston et al., 1994). FWE-corrected individual parametric maps were also extracted for quantification of regional changes in BPND, graphing and determination of correlation coefficients. Measures of D2/3 availability in vivo (Innis et al., 2007) at baseline, and the reductions in BPND during i.v. placebo administration (reflecting activation of dopamine neurotransmission) were then related to the clinical measures and placebo and antidepressant responses. Because of our interest in three different clinical domains: anhedonia (SHAPS and AES), overall depression (QIDS-16SR) and anxiety scores (GAD-7), and one measure of subjective expectations of improvement, Pearson/Spearman correlations between clinical measures and DA baseline binding and release measures were considered significant at p<0.0125 after Bonferroni correction (p<0.05/4). All statistical analyses were controlled by age and QIDS-16SR scores. Additional nuisance variable for each particular analysis are described in the results section.
RESULTS
Clinical Measures of depression, anxiety, motivational and consummatory anhedonia and treatment response
Average clinical depression [QIDS-16SR (mean ± SD): 16 ± 5], anhedonia (SHAPS: 29.4 ± 9.6; AES: 5.88 ± 3.67) and anxiety (GAD-7: 10.05 ± 5.1) scores were computed. QIDS-16SR and SHAPS scores were significantly correlated, and so were SHAPS and AES scores (for all r>0.5, p<0.05). Anxiety scores were not significantly correlated with measures of depression severity or anhedonia. There were no significant sex effects on any of the clinical measures used (QIDS-16SR, HDRS, SHAPS, AES and GAD-7). Baseline measures of depression severity, anxiety, consummatory and motivational anhedonia were not significantly correlated with depression symptom improvement in response to i.v. or oral placebo (changes in PIDS or QIDS-16RS respectively) or 10 weeks of antidepressant medication treatment (changes in QIDS-16RS, GAD-7, SHAPS and AES scores).
Expectations of improvement were not correlated with depression severity, anxiety, consummatory anhedonia, or apathy scores, nor with the improvement of these symptoms in response to i.v. or oral placebo (changes in PIDS or QIDS-16RS respectively) or 10 weeks of antidepressant treatment (changes in QIDS-16RS, GAD-7, SHAPS and AES).
Effect of MDD diagnosis on D2/3 BPND
We first examined the effect of group (healthy controls versus MDD) on baseline D2/3 BPND. Mean D2/3 receptor BPND in several regions within the striatum was significantly higher in subjects with MDD compared to the healthy control group [MNI coordinates: bilateral NAc/ventral pallidum (vPAL): −14, 6, 6 and 12, 2 −8; right ventral caudate (vCAU): 18, 8, 8; right putamen (PUT): 30, −16, −6; for all regions p < 0.05 FWE-corr., K>10 voxels, Fig. 2]. No effects were observed for the opposite contrast.
Figure 2. Group effect on striatal D2/3 receptor availability at baseline (MDD>HC).
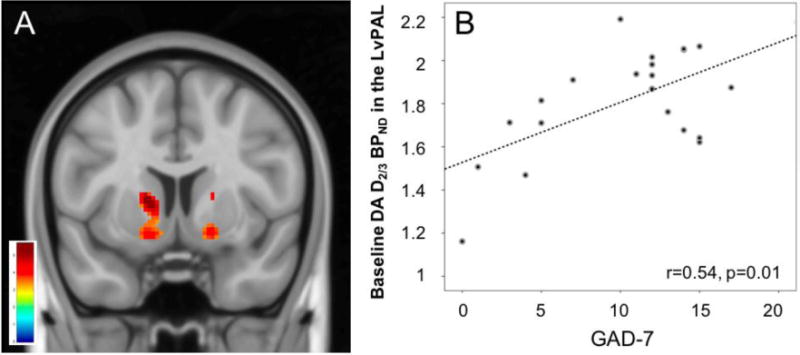
A) MDD patients had greater D2/3 receptor binding in the ventral striatum, including the left nucleus accumbens/ventral pallidum (NAc/vPAL) and the left ventral caudate (vCAU), which are thought to reflect either an up-regulation of D2/3 receptors, increased affinity of the receptor for the radioligand or a decreased synaptic dopamine concentration (p<0.05 FWE-corr, displayed at p<0.001). B) Correlation between GAD-7 anxiety scores and measures of baseline D2/3 receptor availability in the left NAc/vPAL.
Baseline D2/3 BPND and Depression, Anxiety, Anhedonia Symptoms and Expectations of Improvement
Within the MDD sample, we examined the relationship between D2/3 BPND in the NAc bilateral ROIs and the regions that resulted significant in the analysis above, and symptoms of anhedonia, as well as overall depression and anxiety severity. Considering our interest in three different clinical domains and the subjective expectations of improvement, these correlations were deemed significant at p<0.0125, after Bonferroni correction. Each model included the variable of interest and age as a nuisance variable. We found a significant positive correlation between D2/3 receptor availability in the left NAc/vPAL (−14, 6, 6) and GAD-7 scores (r=0.54, p=0.01) (Fig. 2). We also found a negative correlation between D2/3 receptor availability in the NAc ROI bilaterally and AES scores (r=−0.5, p=0.01) (Fig. 3). These results remain significant after controlling for AES scores and GAD-7 scores respectively. We found no significant association between baseline measures of D2/3 receptor availability and SHAPS consummatory anhedonia scores or overall depression severity (QIDS-16SR scores).
Figure 3.
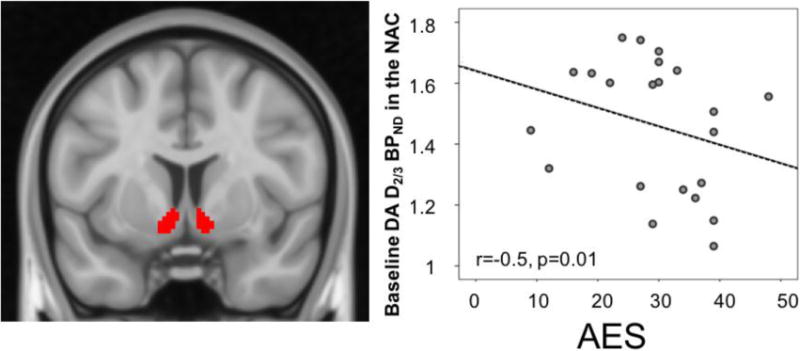
Baseline D2/3 receptor availability in nucleus accumbens region of interest (ROI) (left) correlation with AES apathy scores (right).
Patient’s expectations of recovery were positively correlated with baseline D2/3 BPND in the NAc bilaterally (p=0.046, p=0.05), although these effects did not survive Bonferroni correction.
Placebo-induced changes in D2/3 BPND and the clinical responses to Placebo and Antidepressants
The administration of the i.v. placebo, compared to no administration, resulted in significant activation of DA neurotransmission (acute reductions in BPND) in the bilateral NAc ROI (mean change=0.08, SD=0.15, t=2.5, p=0.02). Placebo-induced changes in BPND were not significantly correlated with the participant’s levels of expectations of improvement or the i.v. or oral response to placebo or 10 weeks of antidepressant treatment.
Effect of Remission Group on Clinical Measures, Striatal D2/3 BPND, Placebo-induced ΔD2/3 BPND
Based on the evidence provided above we conducted an exploratory analysis to examine whether remitters, compared to non-remitters, showed significant differences in baseline D2/3 receptor availability or placebo-induced DA release. GAD-7 scores at baseline, but no other clinical measures, were significantly higher in non-remitters [GAD-7 remitters: (mean ± SD) 5.43 ± 5.1; GAD-7 non-remitters: 12.6 ± 3.1; F=11.14, p=0.005], therefore the following analyses were controlled by baseline QIDS-16SR, GAD-7 scores and age (Fig. 4).
Figure 4. Univariate analysis of the effect of remission on.
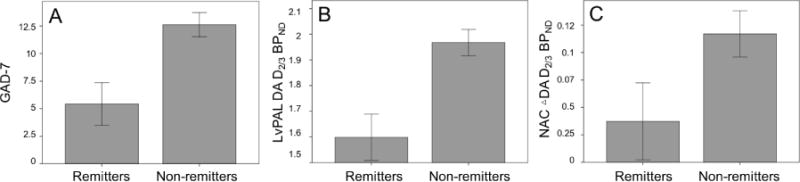
GAD-7 scores (A), baseline measures of DA D2/3 BPND (B) and placebo-induced changes in DA D2/3 BPND (C), after controlling for age, GAD-7 and baseline QIDS-16SR (B & C) (for all, p<0.05).
MDD remitters (QIDS-16SR<5, n=7), compared to non-remitters (n=9), showed significantly greater clinical responses to placebo (decreases in QIDS-16SR after one week of placebo pills) [ΔQIDS-16SR remitters: (mean ± SD) 5 ± 5; ΔQIDS non-remitters: −2.7 ± 5; F=9, p=0.012]. As previously described in a larger sample (Pecina et al., 2015), the response to one week of placebo was significantly correlated to the response to 10 weeks of antidepressants (r=0.7, p=0.003).
Non-remitters, compared to remitters, had significantly higher baseline D2/3 BPND in the left NAC/vPAL (NAC/vPAL remitters: 1.5 ± 0.23; NAC/vPAL non-remitters: 2 ± 0.1; F=7, p=0.02). Unexpectedly, MDD non-remitters, compared to remitters, also showed significantly greater DA activation in the NAc bilaterally in response to the i.v. placebo (ΔNAc remitters: 0.01 ± 0.03; ΔNAc non-remitters: 0.15 ± 0.03; F=6.8, p= 0.02) (Fig. 4).
DISCUSSION
In this study, we aimed to identify in vivo DA receptor availability differences in patients with MDD compared to healthy controls, as well as to relate measures of DA function to depression severity, anxiety, motivational and consummatory anhedonia, and the response to placebo and SSRI treatment. We found that compared to controls, patients with MDD showed greater DA D2/3 receptor availability in several regions within the striatum, including the NAc/vPAL bilaterally, the right vCAU, and the right PUT. In the MDD patient sample, DA D2/3 BPND measures in the NAc/vPAL were significantly correlated with higher anxiety symptoms, as measured by higher baseline GAD-7 scores. The NAc ROI DA D2/3 BPND measures, bilaterally, correlated negatively with the severity of the patient’s apathy scores and at a trend level with the subject’s levels of expectations of improvement with the “active” placebo treatment. Furthermore, the administration of the “active” placebo, compared to the “inactive” placebo, was associated with greater DA release in the ventral striatum. Finally, compared to MDD remitters, the non-remitter patients showed significantly greater baseline GAD-7 scores and DA D2/3 availability in the NAc/vPAL. Non-remitters also showed greater placebo-induced DA release in the NAc bilaterally compared to remitters.
The existence of DA deficits in patients with MDD remains controversial. As summarized in the introduction, in vivo neuroimaging studies using [123I]-iodobenzamide single-photon emission computed tomography ([123I]-IBZM SPECT) or [11C]-raclopride positron emission tomography (PET) have reported increased (D’Haenen H and Bossuyt, 1994; Meyer et al., 2006; Shah et al., 1997), no change (Ebert et al., 1996; Hirvonen et al., 2011; Klimke et al., 1999; Montgomery et al., 2007; Parsey et al., 2001; Yang et al., 2008) or even decreased (Busto et al., 2009) binding to striatal DA D2/3 receptors, compared to controls. A number of potential confounders can explain these differences (e.g. medication status, heterogeneity of MDD, differences in neuroimaging methods). Still, the hypothesis that DA deficits in depression resulting in up-regulation of DA receptor sites remains plausible and is supported by the results presented here. We show that increments in baseline DA D2/3 BPND in the more caudal portion of the NAc were associated with higher anxiety levels in MDD patients. These effects are consistent with the notion that caudal ventromedial striatal regions, which receive inputs from several “limbic” brain regions, including the amygdala and anterior cingulate cortex (Fudge et al., 2004; Russchen et al., 1985; Shammah-Lagnado et al., 2001), are involved in fear generation. In fact, preclinical evidence suggests that fear generation requires simultaneous stimulation of D1 and D2 receptors at caudal sites of the NAc (Richard and Berridge, 2011). On the contrary, the rostral ventral striatum is known to be a substrate for goal-directed behaviors based on its inputs from multiple brain regions mediating motivation and reward (Haber et al., 2000). We found a negative association between motivational, but not consummatory, anhedonia, and DA D2/3 receptor binding in the ventral striatum. Extensive animal literature has elegantly related the DA system to incentive salience (“wanting”) through the mesolimbic DA projections (Berridge and Robinson, 1998; Pecina et al., 2003), and the opioid system (and others, such as cannabinoid and GABA systems) to the hedonic impact of reward (“liking”), through what has been defined as the hedonic hotspots (e.g. the NAc rostrodorsal medial shell) (Pecina and Berridge, 2005). We originally hypothesized that higher D2/3 BPND in the NAc will be associated with higher apathy scores, consistently with a lower DA tone. A negative relationship, as observed here, might potentially be explained by the paradoxical positive incentive effects of higher anxiety levels. For example, it has been shown that corticotropin-releasing factor (CRF), a neuropeptide released in response to acute stressors and other arousing environmental stimuli, acts in the NAc of naive mice to increase DA release through co-activation of the receptors CRFR1 and CRFR2 (Lemos et al., 2012). Furthermore, CRF injections in NAc shell have shown to amplify positive motivation for cued rewards, in particular by magnifying incentive salience (Pecina et al., 2006).
The administration of i.v. placebo in the context of a therapeutic agent was associated with increased DA neurotransmission in the NAc. Placebo treatments in humans were initially hypothesized to be associated with the activity of mesolimbic DA cells during reward anticipation and their capacity to adapt to environmental information (Fields, 2004; Irizarry and Licinio, 2005). This hypothesis was first confirmed in patients with Parkinson’s disease (de la Fuente-Fernandez et al., 2002) and subsequently during placebo analgesia experiments (Scott et al., 2008). Furthermore, our group demonstrated that NAc BOLD signal during monetary reward anticipation was correlated with placebo-induced DA release as measured with PET (Scott et al., 2007). In our sample, DA release during placebo administration was not associated with the improvement of depressive symptoms after the i.v. or the oral placebo (changes in PIDS or QIDS-16RS) nor 10 weeks of antidepressant treatment (changes in QIDS-16RS, GAD-7, SHAPS and Apathy scores). On the contrary, and in a previously published study, we have shown that endogenous opioid mediated neurotransmission during placebo administration in a sample that included the volunteers whose data is reported here, was associated with both, improvement of depressive symptoms in response to 1 week of placebo treatment and 10 weeks of antidepressant treatment (changes in QIDS-16RS), and explained up to 43% of the variance in the response to the antidepressants (Pecina et al., 2015). This evidence supports a greater role of the opioid system in the actual “effectiveness” of the placebo, whereas the dopamine system might be involved in processing the “saliency” of the treatment itself or learning of the reward experience, as it has been supported extensively by the preclinical literature (Berridge and Robinson, 1998; Pecina and Berridge, 2005; Pecina et al., 2003).
Studies investigating the relationship between baseline D2/3 binding measures and response to antidepressant treatment have revealed conflicting results. Ebert et al showed that 150 mg of amitriptyline/daily for three weeks led to a decrease in IBZM binding to DA D2 receptors in five treatment responders, while it remained unchanged in non-responders (Ebert et al., 1996). Kimke et al also demonstrated increased striatal IBZM binding in responders to a selective serotonin reuptake inhibitor (SSRI), but instead binding was decreased in non-responders (Klimke et al., 1999). Contrary, striatal IBZM binding was significantly lower in patients who responded to a selective serotonin reuptake inhibitor (SSRI) compared to non-responders and control subjects (Klimke et al., 1999). Finally, Hirvonen et al found that fluoxetine, but not psychotherapy, increased [11C]raclopride binding in the thalamus, but not within the striatum, although this increase was not correlated with clinical improvement (Hirvonen et al., 2011). Here we found that compared to MDD remitters, MDD non-remitters showed significantly greater baseline DA D2/3 receptors in the NAc/vPAL area. These results are consistent with previous evidence, where age-corrected baseline IBZM binding in the striatum was significantly lower in treatment responders than in depressed non-responders and control subjects (Klimke et al., 1999). In that study there was a significant linear correlation between treatment response and change of D2 receptor binding during treatment in the basal ganglia. We also showed that non-remitter MDD patients showed significantly higher GAD-7 scores and greater placebo-induce DA release. This increased dopaminergic responsiveness may again be explained by the paradoxical effect of high CRF levels in patients with higher anxiety which might result in greater DA release(Lemos et al., 2012). It is also well documented that MDD patients with high anxiety symptoms are less likely to respond to antidepressant treatment (Davidson et al., 2002) and that concomitant use of anxiolytics/hypnotics is a significant predictor of treatment resistance in older adults with depression (Bosworth et al., 2002).
Our results point to baseline DA striatal abnormalities in patients with MDD which seem to be positively associated to symptoms of anxiety (NAc/vPAL) and negatively associated with motivational anhedonia (NAc). Furthermore, this work supports the existence of a DA-mediated endophenotype of lack of remission in response to SSRI treatment in depressed patients with high levels of anxiety, which will need to be confirmed in larger samples. While not directly linked to clinical improvement on subjective depression severity measures, the i.v. placebo intervention was associated with increased DA release in the ventral striatum, which was greater in MDD non-remitters, and might be attributed to the saliency of the stimuli. The results from this study, in addition to clarifying pathophysiological mechanisms associated with MDD, a heterogenous diagnostic cluster, could potentially be used to stratify patients who might initially benefit from 2nd line treatment options or augmentation pharmacotherapy targeting DA mechanisms. Increasingly personalized treatment decisions may reduce the overall lack of response to first line antidepressant treatments as well as to improve overall outcomes for MDD, a frequent and disabling disease process.
Supplementary Material
Acknowledgments
We would also like to acknowledge the contribution of the technologists of the PET Center and the Department of Radiology at the University of Michigan.
FUNDING AND DISCLOSURE:
This work was supported by the NIH R01 MH086858, (JKZ), K23 MH108674 (MP), the Phil F. Jenkins Foundation, the Michigan Institute for Clinical & Health Research grant support (CTSA: UL1RR024986).
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Drs. Peciña and Zubieta had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors have no interests to disclose that are or might be perceived to be in conflict with the work reported in this study.
CONTRIBUTORS
Marta Peciña M.D. Ph.D:
Data collection, interpretation and data analysis.
Manuscript preparation.
Magdalena Sikora Ph.D.:
Data analysis.
Erich T. Avery B.A:
Data collection.
Joseph Heffernan B.S.:
Data analyses.
Susana Peciña Ph.D.:
Data interpretation and manuscript editing.
Brian J. Mickey M.D. Ph.D:
Data collection, interpretation and manuscript editing.
Jon-Kar Zubieta M.D. Ph.D:
Study design, data collection, interpretation and manuscript editing.
References
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research. Brain research reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosworth HB, McQuoid DR, George LK, Steffens DC. Time-to-remission from geriatric depression: psychosocial and clinical factors. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2002;10:551–559. [PubMed] [Google Scholar]
- Bouras N, Bridges PK. Bromocriptine in depression. Current medical research and opinion. 1982;8:150–153. doi: 10.1185/03007998209112376. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. 2002. p. 16. [Google Scholar]
- Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA. Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse. 2009;63:681–689. doi: 10.1002/syn.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Cassano P, Lattanzi L, Fava M, Navari S, Battistini G, Abelli M, Cassano GB. Ropinirole in treatment-resistant depression: a 16-week pilot study. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2005;50:357–360. doi: 10.1177/070674370505000612. [DOI] [PubMed] [Google Scholar]
- D’Haenen H A, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biological psychiatry. 1994;35:128–132. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Meoni P, Haudiquet V, Cantillon M, Hackett D. Achieving remission with venlafaxine and fluoxetine in major depression: its relationship to anxiety symptoms. Depression and anxiety. 2002;16:4–13. doi: 10.1002/da.10045. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ, Stoessl AJ. Dopamine release in human ventral striatum and expectation of reward. Behavioural brain research. 2002;136:359–363. doi: 10.1016/s0166-4328(02)00130-4. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of general psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression–striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology. 1996;126:91–94. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature reviews. Neuroscience. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. The Journal of comparative neurology. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Hietala J, Kajander J, Markkula J, Rasi-Hakala H, Salminen JK, Nagren K, Aalto S, Karlsson H. Effects of antidepressant drug treatment and psychotherapy on striatal and thalamic dopamine D2/3 receptors in major depressive disorder studied with [11C]raclopride PET. Journal of psychopharmacology. 2011;25:1329–1336. doi: 10.1177/0269881110376691. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Irizarry KJ, Licinio J. An explanation for the placebo effect? Science. 2005;307:1411–1412. doi: 10.1126/science.307.5714.1411. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, Kowalczykowski M, Miller FG, Kirsch I, Lembo AJ. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5:e15591. doi: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimke A, Larisch R, Janz A, Vosberg H, Muller-Gartner HW, Gaebel W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry research. 1999;90:91–101. doi: 10.1016/s0925-4927(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological psychiatry. 2010;67:439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, McNeely HE, Sagrati S, Boovariwala A, Martin K, Verhoeff NP, Wilson AA, Houle S. Elevated putamen D(2) receptor binding potential in major depression with motor retardation: an [11C]raclopride positron emission tomography study. Am J Psychiatry. 2006;163:1594–1602. doi: 10.1176/ajp.2006.163.9.1594. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Annals of neurology. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Stokes P, Kitamura Y, Grasby PM. Extrastriatal D2 and striatal D2 receptors in depressive illness: pilot PET studies using [11C]FLB 457 and [11C]raclopride. Journal of affective disorders. 2007;101:113–122. doi: 10.1016/j.jad.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, Cooper TB, Van Heertum R, Mann JJ, Laruelle M. Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biological psychiatry. 2001;50:313–322. doi: 10.1016/s0006-3223(01)01089-7. [DOI] [PubMed] [Google Scholar]
- Pecina M, Bohnert AS, Sikora M, Avery ET, Langenecker SA, Mickey BJ, Zubieta JK. Association Between Placebo-Activated Neural Systems and Antidepressant Responses: Neurochemistry of Placebo Effects in Major Depression. JAMA Psychiatry. 2015;72:1087–1094. doi: 10.1001/jamapsychiatry.2015.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing–induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Price A, Hotopf M. The treatment of depression in patients with advanced cancer undergoing palliative care. Current opinion in supportive and palliative care. 2009;3:61–66. doi: 10.1097/SPC.0b013e328325d17a. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain research. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology. 2007;191:389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of general psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychological medicine. 1997;27:1247–1256. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. The Journal of comparative neurology. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Shopsin B, Gershon S. Dopamine receptor stimulation in the treatment of depression: piribedil (ET-495) Neuropsychobiology. 1978;4:1–14. doi: 10.1159/000117615. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry: the journal of mental science. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta psychiatrica Scandinavica. 2001;103:122–130. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Primary care companion to the Journal of clinical psychiatry. 2004;6:159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and biobehavioral reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. The Dopamine Synapse and the Notion of Pleasure Centers in the Brain. Trends in neurosciences. 1980;3:91–95. [Google Scholar]
- Yang YK, Yeh TL, Yao WJ, Lee IH, Chen PS, Chiu NT, Lu RB. Greater availability of dopamine transporters in patients with major depression–a dual-isotope SPECT study. Psychiatry research. 2008;162:230–235. doi: 10.1016/j.pscychresns.2007.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


