Abstract
Symptoms of anxiety are highly comorbid with major depressive disorder (MDD) and are known to alter the course of the disease. To help elucidate the biological underpinnings of these prevalent disorders, we previously examined the relationship between components of anxiety (somatic, psychic and motoric) and serotonin 1A receptor (5-HT1A) binding in MDD and found that higher psychic and lower somatic anxiety was associated with greater 5-HT1A binding. In this work, we sought to examine the correlation between these anxiety symptom dimensions and 5-HTT. Positron emission tomography with [11C]-3-amino-4-(3-dimethylamino-methylphenylsulfanyl)-benzonitrile ([11C]DASB) and a metabolite-corrected arterial input function were used to estimate regional 5-HTT binding in 55 subjects with MDD and anxiety symptoms. Somatic anxiety was negatively correlated with 5-HTT binding in the thalamus (β=−.33, p=.025), amygdala (β=−.31, p=.007) and midbrain (β=−.72, p<.001). Psychic anxiety was positively correlated with 5-HTT binding in midbrain only (β=.46, p=.0025). To relate to our previous study, correlation between 5-HT1A and 5-HTT binding was examined, and none was found. We also examined how much of the variance in anxiety symptom dimensions could be explained by both 5-HTT and 5-HT1A. The developed model was able to explain 68% (p<.001), 38% (p=.012) and 32% (p=.038) of the total variance in somatic, psychic, and motoric anxiety, respectively. Results indicate the tight coupling between the serotonergic system and anxiety components, which may be confounded when using aggregate anxiety measures. Uncovering serotonin’s role in anxiety and depression in this way may give way to a new generation of therapeutics and treatment strategies.
Keywords: anxiety, serotonin, positron emission tomography, depression
1. INTRODUCTION
Depressive and anxiety disorders have substantial comorbidity, however the shared pathophysiology between the two remains poorly understood. Studies of sequential comorbidity suggest that anxiety disorders are more often preceded by depressive disorders (Moffitt et al., 2007). Patients who are comorbid for both disorders have greater psychosocial disability and a poorer quality of life (Hirschfeld, 2001), and depression with anxious features is associated with worse treatment outcome (Fava et al., 2008).
Clinically, major depressive disorder (MDD) and generalized anxiety disorder share common core symptoms, which may reflect overlap in etiology. For example, the serotonergic (5-HT) system has been implicated in both the pathophysiology of MDD (Drevets et al., 1999), as well as modulation of anxiety symptoms (Olivier et al., 2013). Specifically, the serotonin transporter (5-HTT) is an important target for treatment of both depression and anxiety (Owens et al., 1997; Reimold et al., 2008; Tatsumi et al., 1997); selective serotonin reuptake inhibitors (SSRIs) are first-line treatments for both unipolar depression and anxiety disorders (Denys and de Geus, 2005; Feighner and Cohn, 1989; Olivier et al., 2013; Reimold et al., 2008).
The 5-HTT anxiety relationship has been explored in humans, and an association is reported between anxiety and polymorphisms of the 5-HTT (Cerasa et al., 2014; Liu et al., 2013; Pietrzak et al., 2013). Further, human PET imaging studies have consistently reported an inverse correlation between 5-HTT binding and the severity of depressive or anxiety symptoms; however, this relationship may be affected by the inclusion of heterogeneous MDD cohorts (Spies et al., 2015), particularly those with anxiety. In PTSD, lower 5-HTT binding in the midbrain and thalamus was associated with greater anxiety (Reimold et al., 2008). Similarly, an inverse correlation was found between 5-HTT expression and symptom severity in patients with PTSD (Frick et al., 2015b) But a study completed in patients with social anxiety disorder by the same group showed higher 5-HTT binding in this disorder compared to healthy control subjects (Frick et al., 2015a).
In some studies, patients with MDD exhibit lower 5-HTT binding in the amygdala (Murrough et al., 2011), midbrain (Malison et al., 1998; Parsey et al., 2006a) medial temporal lobe, and basal ganglia (Newberg et al., 2012) compared with healthy volunteers. However, using the ligand [11C]-3-amino-4-(3-dimethylamino-methylphenylsulfanyl)-benzonitrile ([11C]DASB), we previously found no differences in 5-HTT binding between MDD and healthy controls (Miller et al., 2013).
One of the challenges associated with relating neurobiology to anxiety is that the forms of anxiety co-occurring with MDD, whether subsyndromal or due to syndromal comorbid conditions (panic disorder, generalized anxiety disorder, social phobia), are heterogeneous in presentation. Therefore, breaking anxiety down into clinically distinguishable components may aid in defining its neurobiological underpinnings. This refined approach may also lead to treatments that can target specific anxiety symptoms. In a previous PET study, we used radioligand [11C]WAY-100635 [N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridil) cyclohexanecarboxyamide] to relate 5-HT1A binding to three components of anxiety derived from a large sample of MDD patients: somatic (hypochondriasis, sweating, cardiovascular, respiratory, gastrointestinal and urination symptoms), psychic (anxiousness and irritability), and motoric (agitation) components. In that study, the severity of somatic and psychic anxiety correlated with 5-HT1A binding in anterior cingulate (negatively and positively, respectively), body of cingulate, orbital prefrontal and medial prefrontal cortices, along with temporal, parietal, and occipital cortices in patients with MDD (Sullivan et al., 2005). These regional relationships may explain why buspirone, a 5-HT1A partial agonist, can improve psychic anxiety more rapidly than somatic anxiety (Feighner and Cohn, 1989).
In this present study, we estimate regional brain 5-HTT binding in MDD subjects using [11C]DASB and relate this binding to anxiety symptom dimensions. This overcomes the limitations of previous studies by specifically examining anxiety components within MDD, instead of confounding MDD group differences. To relate this analysis to our previous work examining 5-HT1A binding, we examined the correlation between 5-HTT and 5-HT1A in the regions implicated in the previous study with non-negligible levels of [11C]DASB binding (anterior cingulate cortex, amygdala, midbrain). To further conceptualize the relationship between the serotonergic system and anxiety, we developed a model in which both 5-HT1A and 5-HTT were used to predict anxiety components. This will provide a more comprehensive view of serotonergic function in major depression with co-morbid anxiety symptoms, which is needed to develop the next generation of therapeutics.
2. EXPERIMENTAL PROCEDURES
Eighty subjects between 18 and 64 years of age (mean: 34 ± 12, SD) who met the criteria for major depressive disorder in a current major depressive episode using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 2000) participated in the study. None of the subjects were in remission. Inclusion criteria required subjects to be off all psychotropic medications and to discontinue use of any medications that interact with the serotonin transporter or 5-HT1A for at least 2 weeks. Only co-medications allowed were short acting benzodiazepines or chloral hydrate for distressing anxiety of insomnia, but not within three days of the PET scans. All subjects were divided into two groups composed of 55 and 70 subjects. In Table 1, details of these groups are given.
Table 1.
Clinical Details for the two group of subjects participated in the study.
| Group 1 (N=55) | Group 2 (N=70) | |
|---|---|---|
| Age (Mean ± SD) | 40 ± 12 | 38 ± 12 |
| In/Out Patients | 13/42 | 13/57 |
| Smoking Status: | ||
| Non-smoker | 42 | 56 |
| Light smoker | 6 | 8 |
| Moderate Smoker | 5 | 4 |
| Heavy smoker | 1 | 1 |
| Unknown | 1 | 1 |
| Age of onset of first MDD episode (Mean ± SD) | 20 ± 11 | 21 ± 11 |
In the cohort used to examine the relationship between 5-HTT and anxiety, 55 subjects (31 female, 24 male) were examined (right side of Table 2). 21 subjects were recently (in the last four years) treated with antidepressants. 6 subjects’ medication history was unknown. The remaining 28 subjects were antidepressant naïve (AN) or not recently medicated (NRM). All were included in the 5-HTT analysis because a previous study from our group showed no effect from antidepressant exposure on 5-HTT binding (Miller et al., 2013). Twenty-four subjects (44%) also possessed at least one AXIS1 comorbidity, including attention deficit hyperactivity disorder (ADHD) (n=3), agoraphobia (n=1), binge eating (n=1), delusional disorder (n=1), dysthymia (n=5), generalized anxiety disorder (n=4), obsessive-compulsive disorder (OCD) (n=2), panic disorder (n=6), PTSD (n=11), specific phobia (n=3), and social phobia (n=9). All subjects received PET scans using the [11C]DASB ligand (as described below). The mean and SD of the difference between the date of psychiatric ratings and the date of scans were 2 ± 2 days, for both HDRS and BPRS. Data from 54 of the 55 subjects have been reported before (Miller et al., 2013) in a study comparing [11C]DASB binding between depressed suicide attempters, depressed non-attempters and healthy controls. However, in that study, anxiety components were not examined.
Table 2.
Demographic information for the subjects from an MDD sample (n=288) used for the principal component analysis (PCA) and the current study (n=55).
| Variable | PCA sample (n=288) | PET sample (n=55) | ||
|---|---|---|---|---|
| Sex (% female) | 60% | 56% | ||
| Mean | SD | Mean | SD | |
| HDRS-24 Score | 28.1 | 7.9 | 24.6 | 6.4 |
| BPRS Score | 35.6 | 7.5 | 32.3 | 5.5 |
BPRS – Brief Psychiatric Rating Scale
HDRS- Hamilton Depression Rating Scale
To examine the relationship between 5-HTT and 5-HT1A binding, 70 (41 female, 29 male) MDD subjects receiving both [11C]DASB (5-HTT tracer) and [11C]WAY-100635 (5-HT1A tracer) were examined. 68 of these 70 subjects have been reported before (Miller et al., 2009; Miller et al., 2013; Parsey et al., 2006a; Sullivan et al., 2005). In (Sullivan et al., 2005), the relationship between 5-HT1A binding and anxiety components was examined. In (Miller et al., 2009), authors compared 5-HT1A binding in patients with remitted MDD with currently depressed and healthy controls. In (Parsey et al., 2006a), authors evaluated the effects of antidepressant exposure and the role of C(−1019)G polymorphism on 5-HT1A in MDD. 45 of the subjects in these studies were included in the group of 55 ([11C]DASB only) subjects presented in Table 2. (The remaining 25 were not used in the anxiety component analysis because their [11C]DASB scans were not acquired within one week of clinical ratings.) Of the 70 subjects, 9 subjects were excluded from the further analysis because previous medication history, which can affect [11C]WAY-100635 binding (Gray et al., 2013), was unknown. Therefore, 61 subjects were included in analyses studying the relationship between [11C]DASB and [11C]WAY-100635 binding. Descriptive statistics for this group are given in Table 3. Two of these subjects were not included in the model where both 5-HT1A and 5-HTT binding predicted anxiety measures due to missing scale items for these subjects.
Table 3.
Descriptive statistics of the subject group who received both [11C]DASB (5-HTT tracer) and [11C]WAY-100635 (5-HT1A tracer) (AE: Exposure to prior antidepressant medication (time off medication: 2‒4 weeks), AN: Antidepressant naïve, NRM: Not recently (>4 years) medicated).
| Variables | Levels | Count | Percent (%) |
|---|---|---|---|
| Sex | Female | 36 | 59.02 |
| Male | 25 | 40.98 | |
| MedicationStatus | AE | 23 | 37.70 |
| AN & NRM | 38 | 62.30 |
In Table 3, AN and NRM groups were combined as suggested in (Gray et al., 2013; Parsey et al., 2010). Patients were off medications for at least 14 days prior to being scanned. After complete description of the study, written informed consent was obtained as approved by the Institutional Review Boards of Columbia University and New York State Psychiatric Institute for all subjects.
Clinical Assessments
Somatic, motoric and psychic anxiety factors for each subject were calculated according to a previous factor analysis (Sullivan et al., 2005) of the 24-item Hamilton Depression Rating Scale (HDRS-24) (Hamilton, 1960) and the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962). In that study, principal component analysis (PCA) was applied to HDRS-24 and BPRS items from 288 subjects (Table 2), yielding component loading factors (provided in Table 4). In Table 2, it can be seen that the sample from the previous study (Sullivan et al., 2005) and the sample in the current study (relating 5-HTT to anxiety components) are comparable in terms of sex ratio and mean HDRS and BPRS scores.
Table 4.
Rating Scale Items and related loadings generated by Principal Component Analysis (PCA). The scale items Hypochondriasis, Somatic Anxiety, Agitation, and Psychic Anxiety are HDRS-24 items 15, 2, 9, and 10, respectively. Somatic Concern, Tension, and Anxiety are BPRS items 1, 9, and 2, respectively.
| Somatic Anxiety = (0.902 × Hypochondriasis) + (0.889 × Somatic Concern) + (0.603 × Anxiety Somatic) |
| Motoric Anxiety = (0.932 × Agitation) + (0.905 × Tension) |
| Psychic Anxiety = (0.855 × Anxiety Psychic) + (0.845 × Anxiety) |
HDRS-24 and BPRS scores were used to estimate anxiety factors according to component loading factors.
Radiochemistry and Input Function Measurement
[11C]WAY-100635 and [11C]DASB were prepared as described in (Parsey et al., 2000) and (Belanger et al., 2004), respectively. Arterial input functions were collected for [11C]WAY-100635 and [11C]DASB as described in (Parsey et al., 2005; Parsey et al., 2000) and (Ogden et al., 2007; Parsey et al., 2006c), respectively. Briefly, [11C]WAY-100635 parent fraction levels were fit with the Hill function (Gunn et al., 1998) while [11C]DASB parent fraction levels were fit with a biexponential function, which was damped by a power function to take into account the low initial value. For both tracers, the input function was the product of total plasma counts and interpolated unmetabolized tracer fraction and fit with a straight line from time zero to the peak followed by the sum of three exponentials after the peak (Parsey et al., 2005). Free plasma fraction estimation was done in the same way for both tracers (Parsey et al., 2000). Scan duration was between 80 min and 120 min. The mean and the SD of the injected doses, specific activities, and the calculated masses were 16 ± 2 mCi, 1 ± 1 mCi/nmol, and 5 ± 2 ug.
Image Acquisition and Analysis
All PET scans were acquired with the same PET scanner: ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN, USA) in Kreitchman PET Center at Columbia University Medical Center. FMRIB linear image registration tool (FLIRT, FMRIB Image Analysis Group, Oxford, UK) was used to align individual PET frames, in order to correct for subject motion. The mean, motion-corrected PET image was then coregistered to the subject’s MRI to apply region of interests (ROIs) to the mean PET image, and individual PET frames. The ROI labeling process was described in (Parsey et al., 2000). Linear PET-to-MRI transformations were computed using FLIRT with a mutual information cost function, six degrees of freedom, and trilinear interpolation (DeLorenzo et al., 2009). After coregistration, time activity curves (mean activity within each ROI over time) were generated.
Modeling
For [11C]DASB, time activity curves were fit using a graphical approach, likelihood estimation in graphical analysis (LEGA), which was developed to remove the noise dependent bias inherent in the Logan graphical approach (Ogden, 2003; Parsey et al., 2003). LEGA was found to be the optimal modeling approach for [11C]DASB studies. (Ogden et al., 2007).
For [11C]WAY-100635, time activity curves were fit using a constrained two-tissue compartment model, in which the ratio of K1/k2 is constrained to that of the reference region (white matter of the cerebellum) (Parsey et al., 2000).
Outcome measures
Binding potential, BPF = (VT – VND)/fP (mL/cm3), is the closest measure of receptor density to that of in vitro studies, where VT is the total regional equilibrium distribution volume, VND is the volume of distribution of the nondisplaceable compartment (reference region) and fP, is the fraction of the ligand that is not bound to plasma proteins at equilibrium (Innis et al., 2007). For [11C]WAY-100635 ligand, this measure was used. Since an optimal estimate of VND does not exist for [11C]DASB (Parsey et al., 2006b), VT/fP, which does not depend on a reference region was used as the outcome measure for this ligand (Miller et al., 2013). Standard measurement error for calculated binding values, SE, was computed with a bootstrap algorithm that incorporates errors in metabolite, plasma, and PET image data (Ogden and Tarpey, 2006).
Statistical Analysis
Stepwise Linear Regression (SLR) for [11C]DASB VT/fP vs. Anxiety
In a previous analysis (Sullivan et al., 2005) we found a relationship between anxiety factors and [11C]WAY-100635 binding (BPF) using regression analysis. In this study, we performed the same type of regression analysis to determine the relationship between anxiety factors and [11C]DASB binding (VT/fP). As in the previous analysis, a weighted stepwise linear regression was performed between the z-values of natural logarithm of binding (in this case VT/fP) for each region and the z-values of three predictors: somatic, motoric and psychic anxiety component scores. As the scales for the predictors were different, we performed the commonly used (Huguelet et al., 2016) z-value standardization in order to ensure that the contributions of the variables to the analysis will be same. The natural logarithm of the outcome measure was applied to improve model fitting as the residuals become closer to normal distribution when this transformation is applied. Weighted regression uses information about the precision of the observations in the fitting. Weights were calculated as 1/(SE)2. We conducted this analysis in a priori ROIs of amygdala, midbrain, hippocampus, dorsal putamen, and thalamus, regions with highest 5-HTT binding. Exploratory ROIs examined were the anterior cingulate cortex and cerebellar gray matter. Sub-cortical regions were of primary interest as there is little [11C]DASB binding in the cortex.
Although the previous regression analysis (of 5-HT1A and anxiety) used sex as a covariate, previous studies have found no sex differences in [11C]DASB binding (Martinez et al., 2013). Therefore, it was not included in regression models for [11C]DASB in the current study. Regression started with the intercept value and after this, the best predictor (i.e. somatic, motoric, psychic anxiety component scores) was added to the model if the F-test for adding the predictor had p-value<0.05. When no other predictor can be added, the current predictors in the model were assessed and, if the F-test for removing the predictor had p>0.10, the predictor was removed. This process continued until no predictors could be added or removed. p-value thresholds were the default values, which are commonly used in different statistical packages and were the same thresholds used in our previous analysis (Sullivan et al., 2005). MATLAB Release 2012b (The MathWorks, Inc., Natick, Massachusetts, United States) was used for the implementation of SLR method.
[11C]WAY-100635 BPF vs. [11C]DASB VT/fP
A linear regression model incorporating measurement error in predictors was used to study the relationship between [11C]WAY-100635 BPF and [11C]DASB VT/fP, after adjusting for sex and medication status as they have been shown to affect [11C]WAY-100635 binding (Kaufman et al., 2015; Parsey et al., 2006a; Parsey et al., 2002). Both BPF and VT/fP were log-transformed in order to ensure model assumptions were met. The SE of log(BPF) was incorporated using weighted least square fit. The SE of VT was incorporated into the model by the Simulation Extrapolation (SIMEX) (Carroll et al., 2006; Cook and Stefanski, 1994), an approach to deal with measurement error in predictors. Statistical inference of such estimation is conducted via nonparametric Jackknife resampling (Lederer and H., 2006). The SIMEX method was implemented using R package “simex” version 1.5.
The relationship between [11C]WAY-100635 BPF and [11C]DASB VT/fP was evaluated in the three a priori regions: anterior cingulate, amygdala and midbrain (where the raphe is located). These regions were chosen because they were implicated in anxiety in our previous study (Sullivan et al., 2005) (anterior cingulate) and the current study (amygdala and midbrain) and they have measureable [11C]WAY-100635 and [11C]DASB uptake. We also examined this relationship between the anterior cingulate and amygdala 5-HTT and the raphe 5-HT1A because the neuronal cell bodies of serotonergic projections reside in the raphe and project broadly to the other regions of the brain (Hornung, 2003). We did not include midbrain 5-HTT because the raphe is located within the midbrain. Therefore, there are five comparisons in total.
[11C]WAY-100635 BPF and [11C]DASB VT/fP prediction of anxiety
While the above SLR technique used anxiety dimensions as the independent variable predicting 5-HTT, we also sought the examine a model predicting anxiety from 5-HTT and 5-HT1A density. A model that predicts the somatic, motoric and psychic anxiety component scores using both [11C]WAY-100635 BPF and [11C]DASB VT/fP was evaluated using the three a priori regions from above: anterior cingulate, amygdala and raphe. The methods in the calculation of the model parameters are same as the measurement error model for comparing [11C]WAY-100635 BPF with [11C]DASB VT/fP.
In all analyses above, adjusted R2 values were calculated as a measure of the explained variance.
3. RESULTS
Relationship between [11C]DASB VT/fP and Anxiety Factors
5-HTT binding in midbrain, amygdala and thalamus correlated significantly with anxiety measures (somatic and psychic) after Bonferroni correction, as shown in Table 5 and Figure 1. In thalamus, amygdala and midbrain, somatic anxiety was negatively related to 5-HTT binding, while psychic anxiety was positively related in midbrain. The highest adjusted R2 was obtained in midbrain (R2=0.54), where both psychic and somatic anxiety component scores correlated significantly with 5-HTT binding (p-value<0.001), but in opposite directions. Motoric anxiety was excluded from the model by the SLR method. There was no significant correlation in any exploratory region.
Table 5.
Weighted stepwise linear regression results for primary regions of interest (1–5) and exploratory regions (6–7). β denotes the standardized regression coefficients. The reported p-values are corrected for multiple comparisons.
| Adjusted R2 | __ANOVA__ | Remaining | ___Coefficients___ | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ROI | F p-value | Predictors | β t p-value | ||||
| 1. Amygdala | .16 | 11.4 | .007* | Somatic anxiety | −.31 | −3.36 | .007* |
| 2. Midbrain | .54 | 32.6 | <.001* | Somatic anxiety | −.72 | −7.96 | <.001* |
| Psychic anxiety | .46 | 3.71 | .0025* | ||||
| 3. Hippocampus | – | – | – | – | – | – | – |
| 4. Dorsal putamen | .08 | 5.59 | .108 | Somatic anxiety | −.24 | −2.36 | .108 |
| 5. Thalamus | .14 | 5.43 | .036* | Somatic anxiety | −.33 | −2.93 | .025* |
| Psychic anxiety | .32 | 2.51 | .076 | ||||
| 6. Anterior cingulate cortex | – | – | – | – | – | – | – |
| 7. Cerebellar gray matter | – | – | – | – | – | – | – |
p<.05. Uncorrected p-values = corrected p-values/5.
Figure 1.
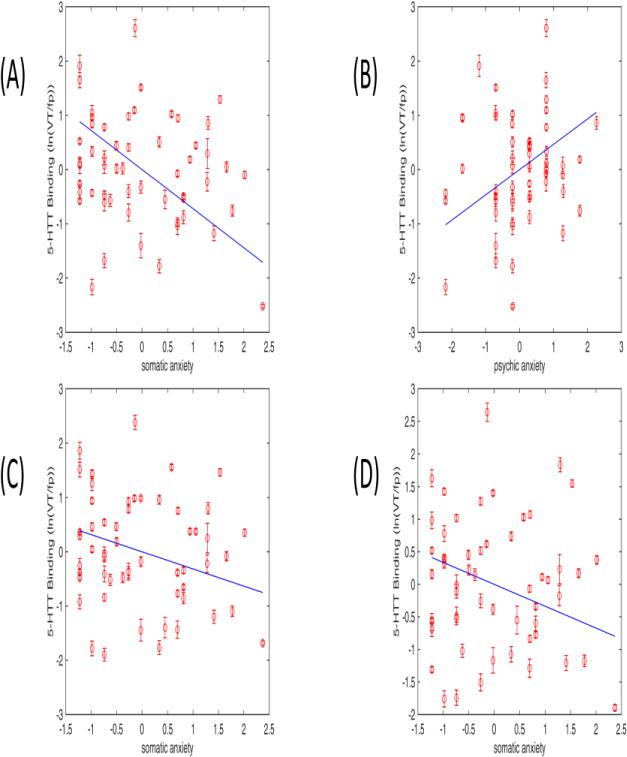
Relationship between 5-HTT binding (ln(VT/fP)) and anxiety components in midbrain (A) somatic: β=−.72; B) psychic: β=.46), amygdala (C) somatic: β=−.31) and thalamus (D) somatic: β=−.33) (z scores). The linear regression fit and standard measurement errors are indicated.
Figure 1 shows the 5-HTT binding (VT/fP) vs somatic and psychic anxieties in specific brain regions. Standard measurement errors show the reliability of the binding values.
[11C]WAY-100635 BPF vs. [11C]DASB VT/fP
log(BPF) was compared to log(VT/fP) with sex and medication status as covariates. Coefficient estimates and corresponding p-values (adjusted for multiple comparisons using Bonferroni adjustment) are shown in Table 6. No significant relationship was observed.
Table 6.
Relationship between log(BPF) and log(VT/fP) after adjusting for sex and medication status (N=61). Coefficient estimates and corresponding p values (corrected for multiple comparisons using Bonferroni adjustment) for testing whether the coefficient is different from 0. Uncorrected p-values = corrected p-values/5.
| Models 5-HT1A vs 5-HTT binding in designated brain region | Adjusted Coefficient estimate for Log(VT/fP) | p-value (Bonferroni correction) |
|---|---|---|
|
| ||
| anterior cingulate vs anterior cingulate | 0.49 | 0.0965 |
| amygdala vs amygdala | 0.37 | 0.2984 |
| raphe nuclei vs raphe nuclei | 0.36 | 0.3131 |
| raphe nuclei vs anterior cingulate | 0.55 | 0.0944 |
| raphe nuclei vs amygdala | 0.28 | 0.8845 |
[11C]WAY-100635 BPF and [11C]DASB VT/fP vs anxiety
In this model, anxiety symptom dimensions were predicted by log(BPF) and log(VT/fP) with sex and medication status as covariates. Adjusted R2 values, coefficient estimates and corresponding p-values (adjusted for multiple comparisons using Bonferroni adjustment) are shown in Table 7. 5-HTT binding and 5-HT1A binding at three brain regions (anterior cingulate, amygdala and midbrain) explained 68% (p<.001), 38% (p=.012) and 32% (p=.038) of the variance respectively for somatic anxiety, psychic anxiety and motoric anxiety symptom dimensions. For somatic anxiety, the greatest and the only significant contribution to variance in the model in terms of R2 value was the 5-HTT binding at anterior cingulate cortex (ACC) at 44%. For psychic anxiety none of the regional binding estimates alone reached significance as predictors. The greatest and the only significant contribution to variance in motoric anxiety was the 5-HTT binding at midbrain, explaining 34% of the variance in terms of R2 value (p=0.049).
Table 7.
Coefficients of log(VT/fP) and log(BPF) binding based on models: anxiety ~ log(BPF) + log(VT/fP) + Gender + Med_Status (N=59). Coefficient estimates (β) and corresponding p values (corrected for multiple comparisons using Bonferroni adjustment) for testing whether the coefficient is different from 0.
| somatic anxiety Adjusted R2 = .68 p=0.0006 |
psychic anxiety Adjusted R2 = .38 p=0.0123 |
motoric anxiety Adjusted R2 = .32 p= 0.0382 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | p-value | β | p-value | β | p-value | |
| Anterior cingulate (VT/fP) | 19.0808 | 0.0115* | 6.9681 | 0.4350 | −2.0866 | 1 |
| Amygdala (VT/fP) | −15.5454 | 0.1405 | −9.7170 | 0.2808 | −4.7272 | 0.3121 |
| Midbrain (VT/fP) | −3.0819 | 1 | 2.1035 | 1 | 6.0133 | 0.0490* |
| Anterior cingulate (BPF) | −0.9478 | 1 | −5.7782 | 0.2445 | 0.4928 | 1 |
| Amygdala (BPF) | −3.3293 | 1 | 3.1139 | 0.7378 | −0.6416 | 1 |
| Midbrain (BPF) | 3.1724 | 0.2164 | 1.5889 | 0.6484 | 0.1166 | 1 |
p<.05. Uncorrected p-values = corrected p-values/3.
4. DISCUSSION
A previous publication from our group (Sullivan et al., 2005) found that patients with MDD and comorbid panic disorder had lower 5-HT1A binding in several areas relative to those with MDD alone. Breaking anxiety down into psychic, motoric and somatic dimensions, that study predicted more than 50% of variance in 5-HT1A binding in several regions. Since 5-HT1A and 5-HTT are both extensively implicated in MDD and anxiety disorders, the natural next question was to examine correlation between 5-HTT binding and anxiety symptom dimensions in these same subjects, between 5-HTT and 5-HT1A, and to determine how much of the variance in anxiety could be explained by both the 5-HTT and 5-HT1A levels, as has been done in this study.
Interrelationship between 5-HTT and Anxiety Components in the Thalamus, Amygdala and Midbrain
Following the same approach for determining the relationship between 5-HTT binding and anxiety, in this study, we identified negative correlations between 5-HTT binding and somatic anxiety in thalamus, midbrain and amygdala, and a positive correlation between 5-HTT binding and psychic anxiety in midbrain. These results align with previous literature. Following the initial animal studies showing the role these regions play in fear, multiple pharmacological and MRI imaging studies have consistently implicated the thalamus and amygdala in anxiety (Davis, 1992) and panic disorder (Kim et al., 2012) in humans. Though the midbrain has been implicated in some studies of panic or other anxiety disorders (Damsa et al., 2009; Mochcovitch et al., 2014; Nikolaus et al., 2010; Pannekoek et al., 2013), this finding has not been consistent across imaging studies.
In preliminary PET investigations into the relationship between 5-HTT binding in MDD and anxiety symptom severity, the thalamus appears to be consistently implicated. Specifically, two studies reported negative correlations between anxiety symptom severity and the 5-HTT binding in the thalamus (Reimold et al., 2008) (Reimold et al., 2011), and one reported such correlations in the midbrain and amygdala regions as well (Reimold et al., 2008). In studies examining panic disorder, one found a positive correlation between panic disorder symptoms and binding in all areas except the hippocampus (Maron et al., 2011) and, in the other, the relationship was only seen in the anterior cingulate cortex and midbrain (Martinez et al., 2013). In both studies, this relationship was observed only in male patients and not controls or females. The inconsistencies between studies may be due to differences in cohorts (panic vs anxiety) and heterogeneity of subjects (e.g. mixing cohorts with OCD symptoms). Our study partially validates these findings, which almost all implicate the thalamus, by reporting a negative correlation in an MDD cohort between anxiety and 5-HTT binding in the thalamus (p=0.007). However, we further extend these findings by showing that this relationship is specific to somatic anxiety. Breaking anxiety into components in this way could further reduce ambiguity between studies. For example, the observed positive correlation between panic symptoms and midbrain binding is consistent with the positive correlation we observe in this brain region. However, our results suggest that this relationship is specific to psychic anxiety.
Examining the role of the midbrain in MDD, unlike previous [11C]DASB studies (Cannon et al., 2007; Meyer et al., 2004; Selvaraj et al., 2011), our previous study (Miller et al., 2013) did not report a difference in 5-HTT binding between MDD and control subjects. However, other studies showed low regional midbrain 5-HTT binding (using [11C]DASB and BPND as an outcome measure) in patients with comorbid anxiety with MDD (Martinez et al., 2013; Reimold et al., 2008). Taken together, it appears that the pathophysiology in the midbrain is inconsistently implicated in studies that examine MDD in isolation. Part of this inconsistency may stem from the inclusion of patients with anxiety symptoms in depressed cohorts and vice versa, as well as the inclusion of people with different types of anxiety. Our study identified opposite directions of the correlations in midbrain for psychic and somatic anxiety. The opposite direction of the correlation in our study could be due to the role of midbrain in both somatic (e.g. cardiovascular control) and psychic (e.g. anxiousness) symptoms. More studies are needed to replicate this finding and explain its neurobiological basis.
Choice of PET Outcome Measure
Besides evaluating specific components of anxiety to provide a more nuanced understanding of the relationship between anxiety and 5-HTT binding in MDD, another significant difference between our study and all previous imaging studies cited is that those studies used BPND as the outcome measure. BPND is defined as the ratio at equilibrium of specific binding to non-displaceable uptake (as measured in a reference region, which is assumed to be devoid of any target binding) and was estimated using the simplified reference tissue model with cerebellum as the reference region. However, as there is no ideal reference region that is completely devoid of 5-HTT and some 5-HTT binding in the cerebellum could confound study results. By using BPF or VT/fP, therefore, our study should have greater sensitivity to detect relationships with anxiety.
Relationship between 5-HT1A and 5-HTT
To gain a better understanding of the relationship between 5-HT1A and 5-HTT binding, the correlation between these entities was examined. In humans, the correlation between [11C]WAY-100635 BPF and [11C]DASB VT/fP has been examined in five previous studies, which were all done in healthy controls, with inconsistent results. Two studies found positive correlations between 5-HT1A and 5-HTT binding – one found correlations in both the raphe and the hippocampus (Lundberg et al., 2007) and the other found the correlation in the hippocampus only in women (Jovanovic et al., 2008). One study found a non-linear relationship between the 5-HT1A binding in the raphe and the 5-HTT binding in the insula/superior temporal gyrus (Bose et al., 2011). The remaining two studies did not find any correlation (Strupp-Levitsky et al., 2016; Takano et al., 2011). Of these five studies there are two studies that overlap with our methodology – in terms of outcome measures and ligands used (Bose et al., 2011; Strupp-Levitsky et al., 2016). The other studies used BPND to measure binding outcomes.
In this study, we did not find a correlation between 5-HT1A and 5-HTT binding within a region or between raphe 5-HT1A and regional 5-HTT binding. The correlation with raphe nucleus binding was examined because serotonergic projections from the raphe nucleus extend to multiple areas in the brain including the hypothalamus, brain stem, parietal and frontal cortex, hippocampus and amygdala (Hornung, 2003). However, one reason a correlation may not have been observed between these regions is because these projections are complex and the relationship between 5-HT1A binding at the raphe and 5-HTT binding at cortical and subcortical sites may be a non-linear one (Strupp-Levitsky et al., 2016). Further, all previous human studies done were in healthy controls, our study is the first one done in depressed subjects.
Incorporating 5-HT1A and 5-HTT binding into a Model of Anxiety
In a model incorporating sex, medication status and serotonin receptor binding, we found that serotonin binding (5-HT1A and 5-HTT) at three regions (anterior cingulate, thalamus and midbrain) could explain 68% of the variance (adjusted R2) in somatic anxiety symptom scores. Binding at the anterior cingulate alone could explain 44% (p=0.01) of the variance (R2) in somatic symptom severity. The anterior cingulate, which is a critical primary area, along with amygdala and the periaqueductal gray (located near the raphe in the midbrain) are implicated in the neurobiological basis of somatic symptoms (Duval et al., 2015; Graeff and Del-Ben, 2008). The ACC modulates emotional processing and as such, may trigger somatic anxiety symptom expression (Martin et al., 2009).
Interestingly, no correlation between 5-HTT binding at exploratory regions (including the ACC) and anxiety symptom severities was found. The anterior cingulate cortex’s contribution to somatic anxiety expression in this combined 5-HT1A and 5-HTT model with no correlation to 5-HTT directly seemingly indicates that the serotonergic relationship with somatic anxiety in this region is dependent on contributions from transporter binding as well as the serotonin 1A receptor and other covariates in the model.
Similarly, contributions to psychic and motoric anxiety variance are interesting to examine in this model in comparison to using 5-HTT alone as a predictor. We reported a positive correlation between psychic anxiety and midbrain transporter binding, whereas this model showed no significant contributions to the variance. The inverse is true for motoric anxiety, where our first model showed no results, and this model showed significant contributions from midbrain transporter binding. This could be due to the loss of power when using multiple predictors and covariates in the complex model. This underscores the robustness of the significant model findings.
Limitations of the study
Although the results were quite interesting, there were several limitations that should be mentioned. First, in this study anxiety was quantified using the 24-item HDRS and the BPRS. Other specific scales for anxiety like the Hamilton Anxiety Rating Scale are designed to provide greater detail on anxiety symptoms and may provide increased resolution. Second, unlike some of the previous studies, we did not analyze males and females separately because of the limited sample size. Further, a previous study from our group showed no influence of sex on 5-HTT [11C]DASB binding in an MDD cohort with no anxiety comorbidity (Miller et al., 2013). Third, our results could have been affected by the inclusion of a mixture of suicidal and non-suicidal depressed subjects. In a previous study, lower midbrain 5-HTT binding was observed in suicidal versus non-suicidal MDD and healthy controls (Miller et al., 2013). However, it is unclear whether suicidality would affect these study results. In a larger cohort, other comorbities such as this can be examined.
Conclusions & Future studies
We sought to understand the biological underpinnings of two highly comorbid disorders – anxiety and MDD. This will aid in treatment, prognosis and design of novel therapeutics (Ball et al., 2014; Evans et al., 2006). Using these sensitive divisions of anxiety, our study showed negative correlations between somatic anxiety and 5-HTT binding in thalamus, midbrain and amygdala and positive correlation between psychic anxiety and 5-HTT binding in midbrain. We hypothesize that is this due to the multiple biological roles of the midbrain, but future studies will be required to study this further. The lack of a correlation between motoric symptoms and 5-HTT suggests that inclusion of these symptoms in studying serotonin in anxiety may result in a loss of sensitivity. To determine whether 5-HTT studies would explain more of the variance in anxiety symptoms than 5-HT1A alone, we examined the 5-HT1A/5-HTT binding correlation. The lack of correlation implied that including 5-HTT in a predictive model of anxiety might explain more variance than 5-HT1A alone. Indeed, including both 5-HTT and 5-HT1A in a predictive model explains most (68%) of the variance in somatic anxiety. This finding is of paramount importance considering we have few robust correlations between biology and behavior. The results of this study indicate the tight coupling between the serotonergic system and aspects of anxiety in MDD, shedding light on the biological underpinnings of anxiety in MDD. In future studies, specific scales developed for anxiety can be used for better assessment of anxiety components.
Acknowledgments
We would like to thank Tony Jin, Hao Chen, Ruofeng Wen, Mala Ananth, Judith Crowell, Laura Kunkel, Mohammad Zia, Greg Perlman, Joshua Kaufman from Stony Brook University and Francesca Zanderigo from Columbia University for their comments and help in data acquisition and processing. We acknowledge the biostatistical consultation and support from the Biostatistical Consulting Core at the School of Medicine, Stony Brook University.
Role of the Funding Source
This study was supported with the following grants: J.J.M.: R01 MH40695, P50 MH62185, R.V.P.: R01 MH074813-01 (NIMH), NARSAD – PTSD—Serotonin and Stress System Interactions, AFSP – Suicide in Depression Comorbid with PTSD: Serotonin and Stress System Interactions, RFMH – Localization of Selective Attention Deficits in High Lethality Suicide Attempters. CD: K01 MH91354 (NIMH). Moreover, the study has been partly funded by the Russian Academic Excellence Project ‘5–100’. Funding sources had no further role.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Contributors
ZI undertook the stepwise regression analysis, contributed to the literature search and wrote the manuscript. GR helped in literature search and wrote the introduction and discussion. SR and MM helped in literature search. JY and MZ performed the remaining part of the statistical analysis. JM, GMS, PS, MAO, JJM, and RVP wrote the manuscript. CD coordinated the whole process and wrote the manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 2000. Text Revision. [Google Scholar]
- Ball TM, Stein MB, Paulus MP. Toward the application of functional neuroimaging to individualized treatment for anxiety and depression. Depression and anxiety. 2014;31:920–933. doi: 10.1002/da.22299. [DOI] [PubMed] [Google Scholar]
- Belanger MJ, Simpson NR, Wang T, Van Heertum RL, Mann JJ, Parsey RV. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nuclear medicine and biology. 2004;31:1097–1102. doi: 10.1016/j.nucmedbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, Grasby PM, Turkheimer FE, Murthy V. Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2258–2265. doi: 10.1038/npp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models: A Modern Perspective. Second. CRC Press; 2006. [Google Scholar]
- Cerasa A, Quattrone A, Piras F, Mangone G, Magariello A, Fagioli S, Girardi P, Muglia M, Caltagirone C, Spalletta G. 5-HTTLPR, anxiety and gender interaction moderates right amygdala volume in healthy subjects. Soc Cogn Affect Neurosci. 2014;9:1537–1545. doi: 10.1093/scan/nst144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JR, Stefanski LA. Simulation-Extrapolation Estimation in Parametric Measurement Error Models. Journal of the American Statistical Association. 1994;89:1314–1328. [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Current opinion in psychiatry. 2009;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Davis M. The Role of the Amygdala in Fear and Anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- DeLorenzo C, Klein A, Mikhno A, Gray N, Zanderigo F, Mann JJ, Parsey RV. In: A new method for assessing PET-MRI coregistration. Pluim JPW, Dawant BM, editors. SPIE Medical Imaging; FL, United States: 2009. pp. 72592W-72592W–72598. [Google Scholar]
- Denys D, de Geus F. Predictors of pharmacotherapy response in anxiety disorders. Current psychiatry reports. 2005;7:252–257. doi: 10.1007/s11920-005-0078-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. Pet imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and clinical risk management. 2015;11:115–126. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Dougherty DD, Pollack MH, Rauch SL. Using neuroimaging to predict treatment response in mood and anxiety disorders. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2006;18:33–42. doi: 10.1080/10401230500464661. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. The American journal of psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Cohn JB. Analysis of Individual Symptoms in Generalized Anxiety – A Pooled, Multistudy, Double-Blind Evaluation of Buspirone. Neuropsychobiology. 1989;21:124–130. doi: 10.1159/000118565. [DOI] [PubMed] [Google Scholar]
- Frick A, Åhs F, Engman J, et al. Serotonin synthesis and reuptake in social anxiety disorder: A positron emission tomography study. JAMA Psychiatry. 2015a doi: 10.1001/jamapsychiatry.2015.0125. [DOI] [PubMed] [Google Scholar]
- Frick A, Ahs F, Palmquist AM, Pissiota A, Wallenquist U, Fernandez M, Jonasson M, Appel L, Frans O, Lubberink M, Furmark T, von Knorring L, Fredrikson M. Overlapping expression of serotonin transporters and neurokinin-1 receptors in posttraumatic stress disorder: a multi-tracer PET study. Molecular psychiatry. 2015b doi: 10.1038/mp.2015.180. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Del-Ben CM. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neuroscience and biobehavioral reviews. 2008;32:1326–1335. doi: 10.1016/j.neubiorev.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang YY, Mann JJ, Parsey RV. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol Psychiatry. 2013;74:26–31. doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Hume SP, Grasby PM, Lammertsma AA. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. NeuroImage. 1998;8:426–440. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Primary care companion to the Journal of clinical psychiatry. 2001;3:244–254. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. Journal of chemical neuroanatomy. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Huguelet P, Guillaume S, Vidal S, Mohr S, Courtet P, Villain L, Girod C, Hasler R, Prada P, Olié E, Perroud N. Values as determinant of meaning among patients with psychiatric disorders in the perspective of recovery. Scientific Reports. 2016;6 doi: 10.1038/srep27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. NeuroImage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA, Mann JJ, Parsey RV, DeLorenzo C. Quantification of the Serotonin 1A Receptor Using PET: Identification of a Potential Biomarker of Major Depression in Males. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1692–1699. doi: 10.1038/npp.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Dager SR, Lyoo IK. The role of the amygdala in the pathophysiology of panic disorder: evidence from neuroimaging studies. Biology of Mood & Anxiety Disorders. 2012;2:20–20. doi: 10.1186/2045-5380-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WHK. A short introduction to the SIMEX and MCSIMEX. 2006:26–31. [Google Scholar]
- Liu B, Lavebratt C, Nordqvist T, Fandino-Losada A, Theorell T, Forsell Y, Lundberg I. Working conditions, serotonin transporter gene polymorphism (5-HTTLPR) and anxiety disorders: a prospective cohort study. Journal of affective disorders. 2013;151:652–659. doi: 10.1016/j.jad.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Borg J, Halldin C, Farde L. A PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain. Psychopharmacology. 2007;195:425–433. doi: 10.1007/s00213-007-0928-3. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Maron E, Toru I, Hirvonen J, Tuominen L, Lumme V, Vasar V, Shlik J, Nutt DJ, Helin S, Nagren K, Tiihonen J, Hietala J. Gender differences in brain serotonin transporter availability in panic disorder. Journal of psychopharmacology (Oxford, England) 2011;25:952–959. doi: 10.1177/0269881110389207. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. The Psychiatric clinics of North America. 2009;32:549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Finegersh A, Cannon DM, Dustin I, Nugent A, Herscovitch P, Theodore WH. The 5-HT1A receptor and 5-HT transporter in temporal lobe epilepsy. Neurology. 2013;80:1465–1471. doi: 10.1212/WNL.0b013e31828cf809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Hesselgrave N, Ogden RT, Sullivan GM, Oquendo MA, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287–295. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. Journal of affective disorders. 2014;167:336–342. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, et al. Depression and generalized anxiety disorder: Cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Huang Y, Hu J, Henry S, Williams W, Gallezot JD, Bailey CR, Krystal JH, Carson RE, Neumeister A. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry. 2011;70:1033–1038. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Shults J. Lower Brain Serotonin Transporter Binding in Major Depressive Disorder. Psychiatry Research. 2012;202:161–167. doi: 10.1016/j.pscychresns.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders–results from in vivo imaging studies. Reviews in the neurosciences. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Ogden RT. Estimation of kinetic parameters in graphical analysis of PET imaging data. Statistics in medicine. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [(11)C]DASB and positron emission tomography in humans: modeling considerations. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics (Oxford, England) 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Vinkers CH, Olivier B. The role of the serotonergic and GABA system in translational approaches in drug discovery for anxiety disorders. Frontiers in pharmacology. 2013;4:74. doi: 10.3389/fphar.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. The Journal of pharmacology and experimental therapeutics. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJ, Stein DJ, van der Wee NJ. Advances in the neuroimaging of panic disorder. Human psychopharmacology. 2013;28:608–611. doi: 10.1002/hup.2349. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. The American journal of psychiatry. 2006a;163:52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, Arango V, Mann JJ. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006b;59:821–828. doi: 10.1016/j.biopsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Mann JJ. Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:1471–1478. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, Mikhno A, Milak M, Zanderigo F, Sullivan GM, Oquendo MA, Mann JJ. Higher Serotonin 1A Binding in a Second Major Depression Cohort: Modeling and Reference Region Considerations. Biological psychiatry. 2010;68:170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Ojha A, Ogden RT, Erlandsson K, Kumar D, Landgrebe M, Van Heertum R, Mann JJ. Metabolite considerations in the in vivo quantification of serotonin transporters using 11C-DASB and PET in humans. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006c;47:1796–1802. [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, Guo NN, Van Heertum R, Mann JJ, Laruelle M. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Galea S, Southwick SM, Gelernter J. Examining the relation between the serotonin transporter 5-HTTPLR genotype x trauma exposure interaction on a contemporary phenotypic model of posttraumatic stress symptomatology: a pilot study. Journal of affective disorders. 2013;148:123–128. doi: 10.1016/j.jad.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R, Heinz A. Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study. Molecular psychiatry. 2008;13:606–613. 557. doi: 10.1038/sj.mp.4002149. [DOI] [PubMed] [Google Scholar]
- Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Strohle A, Zimmer A, Schonknecht P, Smolka MN, Weinberger DR, Goldman D, Machulla HJ, Bares R, Heinz A. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology. 2011;213:563–572. doi: 10.1007/s00213-010-1903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Murthy NV, Bhagwagar Z, Bose SK, Hinz R, Grasby PM, Cowen PJ. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11C]DASB. Psychopharmacology. 2011;213:555–562. doi: 10.1007/s00213-009-1660-y. [DOI] [PubMed] [Google Scholar]
- Spies M, Knudsen GM, Lanzenberger R, Kasper S. The serotonin transporter in psychiatric disorders: insights from PET imaging. The Lancet. Psychiatry. 2015;2:743–755. doi: 10.1016/S2215-0366(15)00232-1. [DOI] [PubMed] [Google Scholar]
- Strupp-Levitsky M, Miller JM, Rubin-Falcone H, Zanderigo F, Milak MS, Sullivan G, Ogden RT, Oquendo MA, DeLorenzo C, Simpson N, Parsey RV, Mann JJ. Lack of association between the serotonin transporter and serotonin 1A receptor: an in vivo PET imaging study in healthy adults. Psychiatry Res. 2016;255:81–86. doi: 10.1016/j.pscychresns.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Takano H, Ito H, Takahashi H, Arakawa R, Okumura M, Kodaka F, Otsuka T, Kato M, Suhara T. Serotonergic neurotransmission in the living human brain: A positron emission tomography study using [11C]dasb and [11C]WAY100635 in young healthy men. Synapse. 2011;65:624–633. doi: 10.1002/syn.20883. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. European journal of pharmacology. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]


