Abstract
Air pollution is deleterious to human health, and numerous studies have documented racial and socioeconomic inequities in air pollution exposures. Despite the marginalized status of lesbian, gay, bisexual, and transgender (LGBT) populations, no national studies have examined if they experience inequitable exposures to air pollution. This cross-sectional study investigated inequities in the exposure of same-sex partner households to hazardous air pollutants (HAPs) in the US. We examined cancer and respiratory risks from HAPs across 71,207 census tracts using National Air Toxics Assessment and US Census data. We calculated population-weighted mean cancer and respiratory risks from HAPs for same-sex male, same-sex female and heterosexual partner households. We used generalized estimating equations (GEEs) to examine multivariate associations between sociodemographics and health risks from HAPs, while focusing on inequities based on the tract composition of same-sex, same-sex male and same-sex female partners. We found that mean cancer and respiratory risks from HAPs for same-sex partners are 12.3% and 23.8% greater, respectively, than for heterosexual partners. GEEs adjusting for racial/ethnic and socioeconomic status, population density, urban location, and geographic clustering show that living in census tracts with high (vs. low) proportions of same-sex partners is associated with significantly greater cancer and respiratory risks from HAPs, and that living in same-sex male partner enclaves is associated with greater risks than living in same-sex female partner enclaves. Results suggest that some health disparities experienced by LGBT populations (e.g. cancer, asthma) may be compounded by environmental exposures. Findings highlight the need to extend the conceptual framework for explaining LGBT health disparities beyond psycho-behavioral mechanisms translating social stress into illness to include environmental mechanisms. Because psycho-behavioral and environmental factors may together exacerbate health disparities, we call for a shift toward interdisciplinary research on LGBT health that takes into account cumulative risks, including the role of environmental exposures.
Keywords: environmental justice, LGBT populations, sexual minorities, health disparities, air pollution, United States
Introduction
Worldwide, nearly 8 million deaths per year (>14 percent of all deaths) are attributable to air pollution, which is closely linked with respiratory disease and cancer, among other conditions (World Health Organization, 2014). Studies have shown that economically and racially disadvantaged people are inequitably exposed to air pollution (Clark et al. 2014; Hackbarth et al., 2011; Jephcote and Chen, 2013), and that inequitable environmental exposures contribute to health disparities (Grineski et al., 2013; Hackbarth et al., 2011). No published studies have examined if disparate health risks from environmental exposures exist based on minority sexual orientation across the US. The presence of this knowledge gap is notable given the numerous studies of the marginalization of lesbian, gay, bisexual, and transgender (LGBT) people and the formation of gay neighborhoods in the US (and elsewhere) (Adler and Brenner, 1992; Brown, 2014; Castells, 1983; Hubbard, 2012).
Despite the lack of attention to sexual minorities by environmental health researchers, health disparities among lesbian, gay, bisexual, and transgender (LGBT) populations have been documented. Studies have characterized the increased propensity by sexual minority status (relative to heterosexual status) for mental health problems, including depression, anxiety, and suicidality (King et al., 2008; Gilman et al., 2001); tobacco, alcohol and substance use (Trocki et al., 2009; Dermody et al. 2014); HIV infection (Beyrer et al., 2012); obesity (Boehmer et al., 2007); asthma (Blosnich et al., 2013; Heck and Jacobson, 2006; Landers et al., 2011); cancer (Agénor, 2015; Boehmer et al., 2012; Cochran and Mays, 2012); and barriers in access to, as well as nonuse of, preventive healthcare (Ponce et al., 2010). These LGBT health disparities may cumulatively translate into significantly earlier all-cause mortality for sexual minorities compared to heterosexuals (Cochran et al., 2016). In terms of the etiology of LGBT health disparities, studies indicate that sexual minorities are at greater risk in part due to heightened social stress throughout the life course, in response to their experiences of antigay stigmatization, marginalization and victimization (Anderson et al., 2015; Lick et al., 2013; Meyer, 2003).
Notably, conditions with well-established links to environmental exposures (Kampa and Castanas, 2008), such as asthma and cancer, disproportionately burden LGBT populations (Agénor, 2015; Blosnich et al., 2010, 2013; Boehmer et al., 2012; Cochran and Mays, 2012; Heck and Jacobson, 2006; Landers et al., 2011). Other health problems with disparate impacts on sexual minorities—such as obesity, stress, HIV and related comorbidities—may be influenced by and/or interact with environmental exposures (Baillie-Hamilton, 2002; Chen et al., 2008; Djawe et al., 2013; Kampa and Castanas, 2008). However, only one extant study, which focused on one US metro area, has investigated disparities in environmental health risks among sexual minorities (Collins et al., 2017).
This study addresses the knowledge gap at the intersection of research on environmental health and LGBT health disparities by examining the relationship between sexual orientation and exposures to health-harming air pollution throughout the US. It addresses a recent call to improve comprehension of factors underlying health disparities within LGBT populations (Stall et al., 2016). Our specific objective is to test for disparities in exposure to hazardous air pollutants (HAPs) posing cancer and respiratory health risks based on the census tract composition of same-sex partner households across the US.
We address two questions. First, are cancer and respiratory risks from outdoor HAP exposures distributed inequitably with respect to the neighborhood composition of same-sex partner households, adjusting for confounders? The social science literature has documented the enduring tendency for dominant (heterosexist) groups to stigmatize and marginalize sexual minorities (Adler and Brenner, 1992; Brown, 2014; Castells, 1983; Hubbard, 2012). The marginalization of LGBT populations has been highlighted by heterosexist mobilizations of ‘not in my backyard’ (NIMBY) sentiments to exclude locally unwanted land uses (LULUs) associated with non-conforming sexual identities from their own neighborhoods, including businesses and community centers developed by LGBT residents (Doan, 2011). LGBT people in the US have responded to their stigmatization and marginalization over the past 50 years through the development of gay enclaves, often within neglected inner-city zones, where they have achieved substantial political empowerment. We hypothesize that this has led to higher concentrations of same-sex partner households in relatively undesirable areas that are highly polluted, because NIMBYism is predicated on the exclusion of all LULUs—including sources of pollution—to neglected zones. Second, are cancer and respiratory risks from HAP exposures distributed differently for neighborhoods with high concentrations of same-sex male vs. female partner households? Previous research indicates differences in spatial distribution between same-sex male and female partner households, with gay male partners exhibiting greater residential segregation than lesbian partners from heterosexual partners (Spring, 2013). We therefore hypothesize that exposure to cancer and respiratory risks from HAPs will differ between neighborhoods characterized by high concentrations of gay male vis-à-vis lesbian partners, with gay male partnering associated with greater risks due to increased clustering within relatively polluted central city areas.
Materials and Methods
Study Population
We conducted our investigation across all 50 states and Washington, DC using socio-demographic variables derived from 2010 Decennial Census and 2008–2012 American Community Survey (ACS) estimates at the census tract level. We included the 71,207 census tracts with at least 500 people, 200 households, and complete data to ensure that all analysis variables exhibited stability.
Outcomes: Cancer and Respiratory Risks from Exposure to Air Pollution
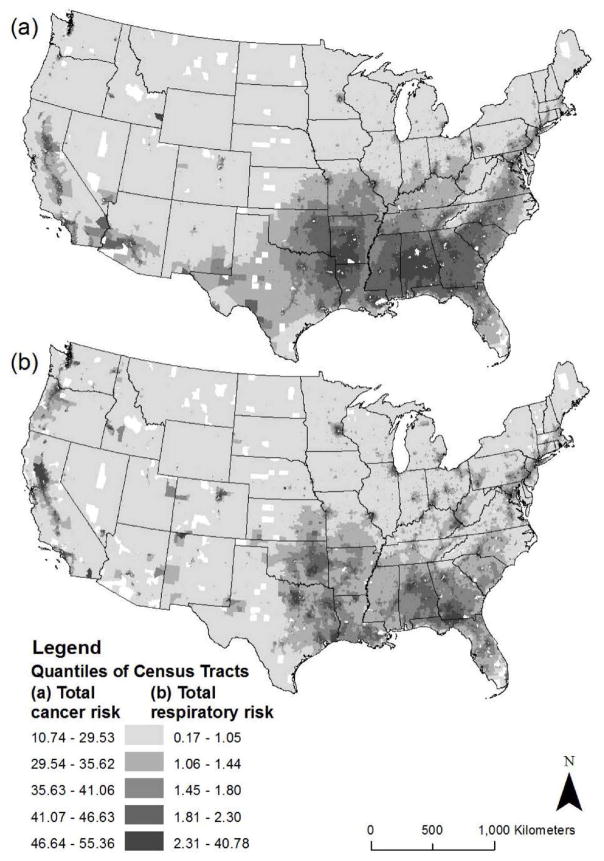
We used the US Environmental Protection Agency (EPA) National Air Toxics Assessment (NATA) for 2011 to measure census tract-level cancer and respiratory risks (Figure 1; Appendix 1). The risk estimates were obtained directly from the EPA and not transformed. The NATA includes 187 specific hazardous air pollutants (HAPs) that are known or suspected to cause cancer and respiratory health problems (Environmental Protection Agency, 2015). Information on HAP emissions from stationary, mobile and background sources come from the 2011 National Emissions Inventory, and multiple steps are used to generate estimates of cancer and respiratory risk, which are detailed elsewhere (Environmental Protection Agency, 2015). Cancer risk estimates reflect the probability that an individual will contract cancer over a 70-year lifetime as a result of exposure to HAPs. The measurement unit is excess cancer incidence attributable to HAPs, in persons per million. A lifetime cancer risk of one in a million, for example, implies that one in a million people would contract cancer if exposed continuously to that specific concentration of HAPs over 70 years. This would be excess cancer risk in addition to other cancer risks borne by a person not exposed to those HAPs. Respiratory risk estimates are scaled in terms of a hazard index (HI), which is calculated based on the concentration of each HAP and its associated inhalation reference concentration (RfC) in each census tract. The RfC is the amount of toxicity below which long-term exposure to the general population is not expected to result in adverse respiratory effects. A HI greater than 1.0 indicates the potential for adverse respiratory system effects, and, the higher the HI, the greater the estimated respiratory risk.
Figure 1.
Distribution of (a) Cancer and (b) Respiratory Risks from Hazardous Air Pollutants by Census Tract, United States, 2011.
Explanatory Variables: Same-Sex Partner Enclaves
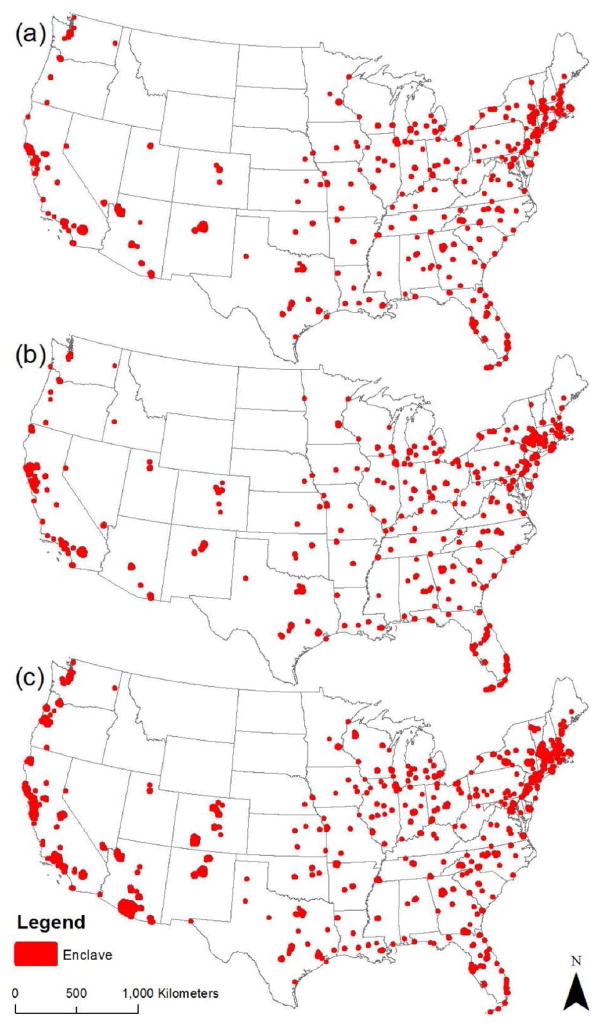
Data for the same-sex partner explanatory variables were derived from the 2010 Decennial Census, and have been used in previous demographic investigations of correlates of sexual minority status and health disparities (Baumle et al., 2009; Boehmer et al., 2012). Due to an error in the 2010 Census enumeration, an indirect correction method was developed to provide accurate estimates of same-sex partner households at the county level (O’Connell and Feliz, 2011). We used established techniques to downscale corrected county-level count estimates of same-sex male and female partner households to the census tract level (Collins et al., 2017; Gates, 2013; Poston and Chang, 2013; Spring, 2013). We then generated variables for the proportions of partner households comprised of (a) same-sex partners, (b) same-sex male partners, and (c) same-sex female partners for all US census tracts. Because each of these three proportion variables exhibited high positive skewness and kurtosis, with the majority of tracts having near zero proportions of same-sex partner households, we transformed them into dichotomous indicators. We did this by classifying all tracts into two groups—those above the 95th percentile (i.e., enclaves) and those below the 95th percentile—in terms of the proportions of (a) same-sex partner households (enclaves include 3.73 to 50.27 percent same-sex partners) (b) same-sex male partner households (enclaves include 2.03 to 44.66 percent same-sex male partners), and (c) same-sex female partner households (enclaves include 1.82 to 18.40 percent same-sex female partners). Figure 2 depicts spatial distributions of enclaves (see also Appendix 2).
Figure 2.
Distribution of (a) Same-Sex, (b) Same-Sex Male and (c) Same-Sex Female Partner Enclaves, United States, 2010. (Note: enclave boundaries are enlarged.)
Adjustment for Confounders
We adjusted for variables that have shown significant effects in prior EJ studies. For race/ethnicity, our analysis includes Decennial Census-derived variables for the proportion of individuals identified as Hispanic/Latino (of any race), black non-Hispanic, American Indian non-Hispanic, Asian non-Hispanic, Pacific Islander/Native Hawaiian non-Hispanic, and multi-/other race non-Hispanic. Henceforth, we do not add “non-Hispanic” after mention of non-Hispanic racial/ethnic groups. For socioeconomic status, we included median household income and income inequality (based on the Gini index) from the ACS, and the proportion of renter-occupied housing units from the Decennial Census. Income and housing tenure are commonly used indicators of neighborhood socioeconomic status in EJ research (Mohai and Saha, 2006). EJ studies have found positive associations between income inequality and toxic exposures (Chakraborty et al., 2014; Collins et al., 2017), and income inequality is important to adjust for here because of the links documented in the US between gay enclaves, gentrification, and neighborhood class inequalities (Brown et al., 2014; Freeman, 2009).
Due to the tendency for LGBT populations to concentrate in urban settings, we accounted for variables that reflect the level of urbanization. First, we adjusted for population density, which was calculated by dividing the total census tract population by the area of the tract in square kilometers. We also adjusted for urban vs. rural location using a dichotomous variable, which was constructed by first creating a proportion urban housing units variable for census tracts, and second by employing k-means cluster analysis to break census tracts between urban vs. rural categories. Finally, by accounting for the clustering of census tracts within counties according to the age of housing stock (see below), our models further control for urban contextual effects. Descriptive statistics for analysis variables are included in Table 1.
Table 1.
Descriptive Statistics for Variables Analyzed, United States.
| Continuous Variables | Mean | Std. Dev. | Min. | Max. |
|---|---|---|---|---|
| Dependent variables: | ||||
| NATA cancer risk (persons per million) | 40.118 | 12.545 | 10.742 | 826.309 |
| NATA respiratory risk (hazard index) | 1.846 | 1.013 | 0.173 | 40.782 |
| Adjustment variables: | ||||
| Proportion Hispanic (any race) | 0.153 | 0.209 | 0.000 | 0.994 |
| Proportion black (non-Hispanic) | 0.134 | 0.220 | 0.000 | 0.992 |
| Proportion American Indian (non-Hispanic) | 0.008 | 0.045 | 0.000 | 0.982 |
| Proportion Asian (non-Hispanic) | 0.043 | 0.083 | 0.000 | 0.898 |
| Proportion Pacific Islander/Native Hawaiian (non-Hispanic) | 0.001 | 0.009 | 0.000 | 0.716 |
| Proportion multi-/other race (non-Hispanic) | 0.002 | 0.005 | 0.000 | 0.258 |
| Median household income ($) | 56,342 | 27,463 | 4,092 | 247,064 |
| Income inequality (Gini index) | 0.414 | 0.063 | 0.141 | 0.822 |
| Proportion renter-occupied homes | 0.356 | 0.222 | 0.005 | 1.000 |
| Population density (per square km) | 2,021 | 4,555 | 0.012 | 196,419 |
| Urban location | 0.797 | 0.403 | 0 | 1 |
|
| ||||
| Dichotomous Variables | No (Non-Enclave) | Yes (Enclave) | Lower Bound | Upper Bound |
|
| ||||
| Explanatory variables: | ||||
| Same-sex partner enclave | 67,647 | 3,560 | 0.037 | 0.503 |
| Same-sex male partner enclave | 67,647 | 3,560 | 0.020 | 0.447 |
| Same-sex female partner enclave | 67,647 | 3,560 | 0.018 | 0.184 |
Note: N = 71,207 census tracts. NATA = National Air Toxics Assessment. For the dichotomous variables, Lower Bound and Upper Bound respectively denote the 95th percentile and the 100th percentile values for the census tract proportion of total partner households comprised of same-sex partners (composite, male, and female).
Statistical Analyses
We began by calculating national weighted mean cancer and respiratory risks for the total US population, as well as for opposite-sex, same-sex, same-sex male, and same-sex female partner households (Clark et al., 2014). Then, we specified four multivariate generalized estimating equations (GEEs), which accommodate clustered data (Liang and Zeger, 1986). We defined clusters of census tracts based on their county of location by median year of housing construction category (i.e., “2000 or later”, “1990 to 1999”, “1980 to 1989”, “1970 to 1979”, “1960 to 1969”, “1950 to 1959”, “1940 to 1949”, and “1939 or earlier”). This cluster definition was selected because it corresponds with spatiotemporal dimensions of the built-environment associated with the historical-geographical formation of environmental injustice (Bolin et al., 2005; Pulido, 2000), and because it enables us to adjust for contextual factors that vary substantially nationwide. Similar cluster definitions have been used previously (Collins et al., 2015a; Collins et al., 2015b; Collins et al., 2017). GEEs require specification of a within-cluster dependency correlation matrix (Liang and Zeger, 1986). We specified the exchangeable correlation matrix, which assumes constant within-cluster dependency (i.e., compound symmetry). GEE Models 1 (cancer risk) and 2 (respiratory risk) test for disparate risks from HAPs based on the same-sex partner enclave variable, while adjusting for confounders. Models 3 and 4 substitute the same-sex male and female partner enclave variables for the composite same-sex partner enclave variable. Since the GEE framework accommodates analysis of non-normally distributed data (Liang and Zeger, 1986), we conducted sensitivity analyses for Models 1–4 using continuous measures of the proportions of same-sex, same-sex male and same-sex female partner households (which exhibited high skewness and kurtosis), in place of the three corresponding dichotomous indicators. To select best fitting models, we estimated a series of GEEs using normal, gamma, and inverse Gaussian distributions with logarithmic and identity link functions. For all models, the inverse Gaussian distribution with the identity link indicated best fit by yielding the lowest quasi-likelihood under the independence (QIC) values. Based on diagnostic tests, inferences from the GEEs are not affected by multicollinearity. All independent variables were standardized before inclusion in the GEEs. IBM SPSS Statistics version 23 was used to conduct these statistical analyses.
Results
We first calculated population-weighted mean cancer and respiratory risks from HAPs in the US for the total population and by partner household type. The mean excess cancer incidence attributable to HAP exposure in the census tract of residence for same-sex partners in the US was 43.48 per million, well above the means for opposite-sex partners (38.73) and the US total population (40.01). We found a similar pattern of higher mean respiratory risk for same-sex partners (2.15) relative to opposite-sex partners (1.74) and the total population (1.84). Same-sex male partners faced greater mean cancer (45.38) and respiratory risks (2.29) from HAPs than did same-sex female partners (41.75 and 2.03, respectively), although both groups exhibited mean risks from HAPs above those of opposite-sex partners and the total population.
Results for the four GEE models are summarized in Table 2. Model 1 tests for disparate cancer risks from HAPs based on the same-sex partner enclave explanatory variable; Model 2 does the same for respiratory risks. In Models 1 and 2, the same-sex partner enclave indicator exhibits positive and statistically significant relationships with cancer and respiratory risks from HAPs, and the coefficients (3.455 and 0.212, respectively) are among the largest of the independent variables in each model, meaning it is a relatively strong predictor of cancer and respiratory risks. Adjusting for confounders, living in same-sex partner enclaves (vs. other areas) is associated with increased risks defined by 38.82 (vs. 35.37) excess cancer incidences per million and a respiratory hazard index score of 1.81 (vs. 1.60).
Table 2.
GEE Results: Predicting Cancer and Respiratory Risks from HAPs with the Same-Sex Partner Enclave Variable (Models 1–2) and Same-Sex Male vs. Female Partner Enclave Variables (Models 3–4) for Census Tracts in the United States, Adjusting for Confounders.
| Variable | B | SE | Lower CI | Upper CI | Sig. |
|---|---|---|---|---|---|
| Model 1: Cancer Risk | |||||
|
| |||||
| (Intercept) | 35.369 | 0.189 | 34.998 | 35.740 | *** |
| Same-sex partner enclave | 3.455 | 0.313 | 2.840 | 4.069 | *** |
| Proportion Hispanic | 0.936 | 0.097 | 0.746 | 1.126 | *** |
| Proportion black | 1.931 | 0.127 | 1.681 | 2.181 | *** |
| Proportion American Indian | −0.357 | 0.033 | −0.423 | −0.292 | *** |
| Proportion Asian | 0.968 | 0.093 | 0.786 | 1.150 | *** |
| Proportion Pacific Islander/Native Hawaiian | −0.084 | 0.034 | −0.150 | −0.017 | ** |
| Proportion multi-/other race | −0.090 | 0.044 | −0.176 | −0.005 | * |
| Median household income | 0.349 | 0.066 | 0.219 | 0.478 | *** |
| Income inequality (Gini index) | 0.284 | 0.037 | 0.211 | 0.357 | *** |
| Proportion renter-occupied homes | 1.138 | 0.081 | 0.980 | 1.296 | *** |
| Population density | 5.433 | 0.415 | 4.620 | 6.246 | *** |
| Urban location | 4.827 | 0.113 | 4.605 | 5.049 | *** |
| (Scale) | 0.002 | ||||
|
| |||||
| Model 2: Respiratory Risk | |||||
|
| |||||
| (Intercept) | 1.599 | 0.016 | 1.567 | 1.630 | *** |
| Same-sex partner enclave | 0.212 | 0.024 | 0.164 | 0.260 | *** |
| Proportion Hispanic | 0.090 | 0.008 | 0.074 | 0.106 | *** |
| Proportion black | 0.153 | 0.008 | 0.137 | 0.169 | *** |
| Proportion American Indian | −0.021 | 0.001 | −0.024 | −0.018 | *** |
| Proportion Asian | 0.110 | 0.011 | 0.088 | 0.132 | *** |
| Proportion Pacific Islander/Native Hawaiian | −0.002 | 0.006 | −0.013 | 0.009 | |
| Proportion multi-/other race | 0.016 | 0.007 | 0.003 | 0.028 | * |
| Median household income | 0.050 | 0.005 | 0.040 | 0.060 | *** |
| Income inequality (Gini index) | 0.011 | 0.003 | 0.005 | 0.016 | *** |
| Proportion renter-occupied homes | 0.071 | 0.006 | 0.059 | 0.082 | *** |
| Population density | 0.806 | 0.034 | 0.740 | 0.872 | *** |
| Urban location | 0.319 | 0.008 | 0.304 | 0.335 | *** |
| (Scale) | 0.118 | ||||
|
| |||||
| Model 3: Cancer Risk | |||||
|
| |||||
| Same-sex male partner enclave | 3.719 | 0.347 | 3.040 | 4.398 | *** |
| Same-sex female partner enclave | 0.406 | 0.181 | 0.052 | 0.761 | * |
|
| |||||
| Model 4: Respiratory Risk | |||||
|
| |||||
| Same-sex male partner enclave | 0.216 | 0.026 | 0.165 | 0.268 | *** |
| Same-sex female partner enclave | 0.052 | 0.018 | 0.018 | 0.087 | ** |
Note: N = 71,207 census tracts. HAPs = hazardous air pollutants. Models 1–4 uses an inverse Gaussian distribution with identity link and exchangeable correlation matrix; number of clusters = 10,455; number of measurements per cluster = 1 (min) to 749 (max). All continuous predictor variables are standardized. Models 3 and 4 also adjust for the proportions of the total population that are Hispanic, black, American Indian, Pacific Islander/Native Hawaiian, and multi-/other race; median household income; income inequality; proportion renter-occupied homes; population density; and urban location.
p<0.05;
p<0.01;
p<0.001
Models 3 and 4 substitute the same-sex male and female partner enclave variables for the same-sex partner enclave variable, and enable us test for gender differences in cancer and respiratory risk based on the census tract composition of male vs. female same-sex partner households. In Models 3 and 4, the same-sex male partner household enclave variable has strong positive associations with cancer and respiratory risks from HAPs, while the indicator for same-sex female partner household enclaves exhibits weaker positive relationships with cancer and respiratory risks (Table 2). The coefficients for the same-sex male partner enclave variable for cancer risk (3.719) and respiratory risk (0.216) are larger than those of the same-sex female partner enclave variable (0.406 and 0.052, respectively), and the same-sex male partner enclave indicator is among the strongest predictors of cancer and respiratory risks from HAPs in Models 3 and 4 (note that the other parameter estimates for Models 3 and 4 are not shown in Table 2, since they are nearly identical to those reported in Models 1 and 2, respectively).
Each other independent variable exhibits a similar relationship in terms of direction and significance with cancer risk and with respiratory risk across the four GEEs (Table 2), with the exception of the proportion multi-racial/other variable, which shows a negative association with cancer risk and positive association with respiratory risk. Across the models, as the proportion of black, Hispanic and Asian residents in a tract increases, and the proportion of American Indian residents decreases, cancer and respiratory risks from HAPs increase significantly; and, as median household income, income inequality, proportion of renter-occupants and population density increase, health risks from HAPs increase significantly.
Our sensitivity analysis used continuous variables for the proportions of same-sex, same-sex male and same-sex female partner households by census tract in place of the three corresponding dichotomous indicators of same-sex partner household enclaves. Results of the sensitivity analysis for Models 1–4 were identical to those reported in Table 2 in terms of the direction and significance of the statistical relationships, which indicates that findings for the same-sex partner variables are robust.
Discussion & Conclusions
Key Findings
In regards to our first research question, findings reveal that cancer and respiratory risks from HAPs are distributed inequitably nationwide with respect to same-sex partner households. Results of Models 1 and 2 (Table 2) indicate that the adjusted cancer and respiratory risks of residing in same-sex partner enclaves are 9.8% and 13.3% greater, respectively, than the risks of residing in other areas. This disparate pattern of risk cannot be attributed to the urban dwelling tendencies of same-sex partners. Our statistical models reveal that a pattern of environmental injustice persists even after accounting for a suite of urban contextual variables. We also considered the implications of risk disparities between same-sex partner and opposite-partner households nationwide, irrespective of their residence in or outside enclaves and without adjusting for any control variables. For same-sex partner households in the US, the population-weighted mean cancer and respiratory risks due to HAP exposures are 12.3% and 23.8% greater, respectively, than those for opposite-sex partner households.
It is notable that the effect of the same-sex partner enclave variable on health risks from HAPs is substantially stronger than the effect of either the proportion black or Hispanic variables, which have received primary focus in environmental justice research (Clark et al., 2014; Hackbarth et al., 2011; Mohai and Saha, 2006). High same-sex partner composition (an indicator of sexual minority status) in neighborhoods has a strong independent effect on exposure to health risks from HAPs, separate from the significant effects of black and Hispanic/Latino composition (indicators of racial/ethnic minority status). This suggests that sexual orientation constitutes a separate axis of sociospatial oppression associated with increased environmental health risks among same-sex partners nationwide.
In regard to our second research question, findings show that levels of exposure to cancer and respiratory risks from HAPs differ between census tracts composed of relatively high concentrations of same-sex male vs. female partner households. Gay male partnering is associated with greater environmental health risks than lesbian partnering, such that the distribution of same-sex male partner enclaves primarily accounts for the large effect size of the composite same-sex partner enclave variable on cancer and respiratory risks from HAPs. Results of Models 3 and 4 (Table 2) indicate that the adjusted cancer and respiratory risks of residing in same-sex male partner enclaves are 10.5% and 13.5% greater, respectively, than the risks of residing in non-enclaves; in contrast, the adjusted cancer and respiratory risks of residing in same-sex female partner enclaves are 1.2% and 3.3% greater, respectively, than the risks of residing in non-enclaves. In the US, the unadjusted population-weighted mean cancer and respiratory risks due to HAP exposures are respectively 17.2% and 31.6% greater for same-sex male partner households, while they are respectively 7.8% and 16.7% greater for same-sex female partner households, than for opposite-sex partner households.
The disparities in environmental health risks uncovered here are of public health significance, based on the national scope and the relatively large effect sizes of the same-sex composite and male partner enclave variables on cancer and respiratory risks from HAPs. Our results suggest that disproportionate exposures may contribute to LGBT populations experiencing disparate burdens from health conditions such as cancer and asthma (Agénor, 2015; Blosnich et al., 2010, 2013; Boehmer et al., 2012; Cochran and Mays, 2012; Heck and Jacobson, 2006; Landers et al., 2011), which have well-established links to air pollution (Kampa and Castanas, 2008). It is also possible that other health problems with disparate effects on sexual minorities—such as obesity, stress, HIV, and related comorbidities—may be influenced by and/or interact with disproportionate exposures to air pollution in a synergistic manner (Baillie-Hamilton, 2002; Chen et al., 2008; Djawe et al., 2013; Kampa and Castanas, 2008). Clearly, more research that tests for environmental health disparities based on LGBT status is needed.
Possible Explanations for Sexual Minority Environmental Injustices
Our historical-geographical understanding of LGBT populations in the context of US urbanization points toward the post-WWII clustering of sexual minorities within relatively polluted inner-city neighborhoods—due to social marginalization and the pursuit of community support and empowerment—as the primary factor shaping disparate environmental health risks experienced by same-sex partners circa 2010 (Adler and Brenner, 1992; Brown, 2014; Castells, 1983). There has been an enduring tendency for dominant groups to exclude LGBT people, businesses, and institutions from their own heteronormative residential spaces (Doan, 2011; Hubbard et al., 2015), which we hypothesize has contributed in part to the pattern of environmental injustice we uncovered.
We believe that there are instructive similarities and important distinctions between the formations of environmental injustice experienced by sexual minorities and by racial minorities in the US. Historical-geographical studies have revealed the contextual dynamics of oppression that have subjected racialized groups in the US to persistent environmental injustices (Bolin et al., 2005; Pulido, 2000). Such studies indicate that the marginalization of racial minorities and the privileging of whites have been intimately connected to the distribution of environmental risks and rewards within the urban space economy (Davis 1999; Pulido 2000). Pulido’s (2000) study of Los Angeles, for example, highlights the role of white privilege in the formation of environmental injustice. Beginning in the early twentieth century, and gaining momentum in the post-WWII period, whites were privileged in securing less polluted residential environments relative to black and Hispanic residents, by being enabled in moving to suburbs from older industrial urban cores. State and market institutions were integral to environmental inequality formation, as they subsidized suburbanization to the benefit of whites and exclusion of racial minorities (Pulido 2000), a highly discriminatory sociospatial process which took place throughout the US (Gottdiener and Hutchison, 2015). These features of urbanization have operated to continually reproduce an environmentally racist formation nationwide, constituted by the concentration of racial minority residents in polluted central city locations, with whites being preferentially afforded access to cleaner residential environments outside of urban cores.
More research is needed to adequately explain the pattern we found here, but we believe that a similar process has unfolded involving the social stigmatization of sexual minorities, their spatial exclusion with other marginalized people and LULUs in inner-city spaces across many US cities, and their unjust exposure to toxic air pollution. However, in contrast to polluted racial minority spaces in the US, which were created early in the process of industrial urbanization, evidence suggests that sexual minorities flowed into this historical-geographical process following WWII, as their marginal social status mapped their emerging enclaves within extant, highly racialized formations of environmental injustice.
The increased clustering of gay male partners within central city enclaves, and the more dispersed pattern of residence for lesbian partners (Spring, 2013), appears to be the primary reason for the gender differences in cancer and respiratory risks from HAPs we found among same-sex partners. Based on follow-up spatial statistical tests (univariate Moran’s I tests calculated using ArcGIS version 10), census tracts with a high proportion of same-sex male partners exhibit nearly twice the spatial clustering (I = 0.456; p < 0.001; distance band = 10 km) of tracts with a high proportion of same-sex female partners (I = 0.234; p < 0.001; distance band = 10 km). While we know that the development of gay enclaves in many inner-cities post-WWII was driven more by homosexual males than lesbians (Adler and Brenner, 1992; Brown, 2014; Castells, 1983; Hubbard, 2012), historical factors may not fully explain this finding. More research is needed to determine how much of this pattern is attributable to contemporary gender-specific push/pull factors, such as the privileging of masculine ideals, the greater likelihood for lesbian partners to have children and seek more child-friendly neighborhoods away from inner-city areas, and/or the economic exclusion of lesbian partners, who are of generally lower socioeconomic status, via escalating housing costs from gentrifying (albeit highly polluted) gay neighborhoods (Taylor, 2008).
Limitations
While this is the first national study of disparities in environmental health risks based on sexual orientation, it has limitations. First, the 2011 EPA NATA dataset includes risks only from direct inhalation of HAPs and excludes exposure from other pathways. The NATA risk estimates include individual and additive health effects; potentially synergistic interactions among pollutants remain unmeasured. The NATA does not include exposure to HAPs generated indoors. Interpretation of public health implications of the disproportionate health risks from HAPs quantified here should be tempered, as the NATA offers cumulative lifetime exposure measures, yet people do not remain in their 2010 census tracts of residence throughout their lifetimes. This is of particular relevance to sexual minority populations that exhibit high levels of life course mobility (Lewis, 2014). Because the NATA data inadequately characterize the range of environmental contexts that influence people’s exposures to air pollution, reliance on this dataset leads to inferences that are confounded by the uncertain geographic context problem (UGCoP) (see Kwan 2012). Future studies should seek to mitigate the UGCoP by employing measurement techniques that account for spatiotemporal variation in people’s air pollution exposure levels, daily movements and residential life course trajectories.
Second, caution should be exercised in treating the same-sex partner variable we analyzed as a proxy for LGBT populations. This variable excludes people not partnered or not living with their partners, those who do not self-report as members of a same-sex partnership, and those who identify as bisexual and/or transgender. The extent of those exclusions is notable, as Gates (2011) estimated the total population of LGBT individuals in the US circa 2010 to be 8,736,309, while the population of gay males and lesbians living with partners in households in the US based on the data we analyzed is 1,292,962. Acknowledging that, it must be recognized that same-sex partnering is a highly visible residential expression of sexual minority status and is thus an important indicator of the societal status of the LGBT people nationwide. Future research should employ data sources that provide more encompassing information on LGBT status.
Third, we focused on assessing environmental health disparities based on sexual orientation using a cross-sectional approach. While our results cannot support inferences regarding events that led to increased exposures for same-sex partner households, we can conclude that shifts toward heteronormative acceptance of alternative sexualities, societal assimilation, and spatial decentralization of LGBT residence over the past 15 years (Spring, 2013) have apparently not ameliorated the sexualized patterning of environmental injustice nationwide. Thus, while the sexualities and space literature has documented the decline of gayborhoods and dispersal of LGBT people across residential space (Brown, 2014; Hubbard et al., 2015), at a national level, same-sex partners (especially same-sex male partners) remain concentrated in more highly polluted zones. And while LGBT people have experienced social justice victories—e.g., via the legalization of civil partnership and gay marriage in some jurisdictions—struggles for equality continue. The ambivalent situation for LGBT people has been documented by some sexualities and space scholars, who have asserted that new “rights to the city” are largely limited to “homonormative LGBT people embodying middle-class and normatively gendered demeanours” (Hubbard et al. 2015), whereas those not conforming to homonormative LGBT identities (e.g., those of working class, color, and/or queer or trans identities) continue to experience barriers to mobility and exclusion (Lewis, 2016; Nash, 2011). Additionally, although some gay enclaves have experienced gentrification (and possibly improved environmental quality) over the past few decades (Brown, 2014), we detected a pattern whereby same-sex partner enclaves remained burdened by heightened risks from air pollution in 2010 nevertheless. Thus, environmental injustices based on sexual orientation produced in the US during the latter half of the twentieth century have evidently not ameliorated in recent years due to either LGBT population dispersal or gentrification of gay enclaves. It is unclear based on our cross-sectional analysis, however, whether the pattern of disproportionate risks from air pollution has become less pronounced as a result of those two processes. Future qualitative historical-geographical and quantitative longitudinal research is needed to fully explain the environmentally unjust patterns revealed here. In the context of the US, our findings represent a starting point for more detailed analysis of environmental injustices experienced by sexual minorities.
Implications for Future Research
Our findings highlight the need to expand the conceptual basis for explaining LGBT health disparities. Theory building regarding causes of LGBT health disparities has focused on individual-level psycho-behavioral mechanisms that translate stressful life course experiences of antigay stigmatization, marginalization and victimization among sexual minorities into greater risks for unhealthy behavior as well as mental and physical health problems (Anderson et al., 2015; Lick et al., 2013; Meyer, 2003). The contextual role of neighborhood social characteristics in the psycho-behavioral production of compounding health disparities among sexual minorities has only begun to be examined (Egan et al., 2011; Frye et al., 2014). The potential influence of environmental exposures on LGBT health disparities has not yet been considered, yet our findings indicate that the marginalization of sexual minorities encompasses a sociospatial dimension corresponding with heightened environmental exposures, in addition to a psycho-behavioral dimension which has been well documented in the LGBT health disparities literature. This suggests the possible existence of environmental exposure pathways whereby marginalization may contribute to and interact with health disparities in LGBT populations.
In contrast to analysts of LGBT health disparities, analysts of racial minority health disparities have conceptualized and examined the multilevel intersection of social, psycho-behavioral, environmental, and biological determinants (Hicken et al., 2012). Our study is the first to assess risks of disparate environmental exposures to the health of LGBT people across the US. More research is needed in order to clarify the nature and extent of LGBT environmental health disparities. Thus, we call for interdisciplinary research investigating the role of environmental exposures in health disparities among sexual minority populations as a first step.
Future research should examine multilevel effects of social, psycho-behavioral and environmental determinants, as they may cumulatively exacerbate respiratory diseases, cancer, and other illnesses that overburden sexual minority populations. Such research will necessitate adoption of an interdisciplinary approach integrating expertise from the socio-behavioral and environmental health sciences. Future investigations should link measures of environmental exposures with representative individual-level survey data in order to support multilevel analyses of determinants of specific health conditions in sexual minority vs. heterosexual individuals. For example, the National Health and Nutrition Examination Survey (NHANES) is nationally representative and includes detailed information on participants’ sexual orientation, specific health conditions, and a range of demographic and behavioral covariates. Restricted use NHANES files provide precise data for participants’ home locations and can be used to construct contextual environmental exposure and neighborhood social status measures. Datasets linking environmental exposure measures with individual-level variables (e.g. from the NHANES) could be used to test for environmental injustices in toxic exposures based on individuals’ sexual orientation, race/ethnicity and socioeconomic status, and by examining interaction effects—e.g., between sexual minority and racial/ethnic minority status—in exposure disparities. Analysts could characterize direct effects of environmental exposures on health outcomes with known disparate impacts in sexual minority populations, adjusting for a suite of control variables, and also determine whether effects of environmental exposures on health outcomes are modified based on individuals’ sexual orientation, race/ethnicity, sex, and socioeconomic status. Analyses of longitudinal datasets could clarify how residential mobility and life course trajectories factor into environmental exposures and, ultimately, health outcomes in LGBT populations. And to inform practical interventions, multilevel modelling of interaction effects could be employed to identify modifiable behavioral factors (e.g., smoking, diet) that might reduce detrimental effects of environmental exposures on LGBT health disparities. Because the residential pattern of same-sex partnering among males is primarily responsible for the inequitable health risks from HAPs we uncovered, future research should be attentive to the possibility that the burden of environmental health risks may be greater for gay male partners and/or other LGBT subpopulations.
Practical Implications
This study is practically relevant, since findings reveal that same-sex couples experience disparate health risks from air pollution in the US, the most important environmental determinant of health worldwide. Due to the national scope of the problem, addressing LGBT environmental health disparities presents challenges. As a first step, we believe that the research and practitioner communities need to be sensitized to the possible burden of environmental injustice on sexual minority populations nationwide. In 1994, President Clinton issued Executive Order (EO) 12898— “Federal Actions to Address Environmental Justice in Minority Populations and Low-Income Populations,” which mandates that each federal agency make achieving environmental justice part of its mission by identifying and addressing disproportionately high and adverse human health or environmental effects of its activities (Clinton, 1994). We believe that amending EO 12898 such that it explicitly mandates consideration of disparate impacts upon all socially disadvantaged groups, including sexual minority populations, might serve to catalyze more academic inquiry into environmental injustices and health inequalities based on social categories other than race and/or class as well as provide a policy basis for implementing practical interventions targeting LGBT environmental health disparities. Additionally, given the current political climate nationally, there is a critical need for the US-based academic community to lead efforts to protect public access to data on local environmental risks and population characteristics, which are currently available and fundamental to advancing knowledge of environmental and LGBT health disparities.
Supplementary Material
Appendix 1. Distribution of Cancer and Respiratory Risks from Hazardous Air Pollutants by Census Tract, (a,b) Hawaii and (c,d) Alaska, 2011.
Appendix 2. Distribution of same-sex female partner enclaves, (a) Hawaii and (b) Alaska, 2010. (Note: Hawaii and Alaska do not contain same-sex and same-sex male partner enclaves; enclave boundaries are enlarged.)
Research Highlights.
First national study of disparate environmental health risks by sexual orientation
Same-sex partners experience inequitable health risks from air pollution
Male partnering is associated with greater health risks than female partnering
LGBT health disparities may be compounded by environmental exposures
Psycho-behavioral and environmental determinants of LGBT health should be examined
Acknowledgments
Research reported in this article was supported by the U.S. National Science Foundation under award number CMMI-1129984 and by the U.S. National Institute of General Medical Sciences of the National Institutes of Health (NIH) under linked award numbers RL5GM118969, TL4GM118971, and UL1GM118970. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler S, Brenner J. Gender and space. Int J Urban Regional. 1992;16(1):24–34. [Google Scholar]
- Agénor M. What are the numbers? The epidemiology of cancer by sexual orientation and gender identity. In: Boehmer U, Elk R, editors. Cancer and the LGBT Community. Springer International Publishing; Switzerland: 2015. pp. 117–140. [Google Scholar]
- Andersen J, Zou C, Blosnich J. Multiple early victimization experiences as a pathway to explain physical health disparities among sexual minority and heterosexual individuals. Soc Sci Med. 2015;133:111–119. doi: 10.1016/j.socscimed.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Baillie-Hamilton P. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8(2):185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- Baumle A, Compton D, Poston D. Same-Sex Partners: The Demography of Sexual Orientation. State University of New York Press; Albany, NY: 2009. [Google Scholar]
- Beyrer C, Baral S, van Griensven F, Goodreau S, Chariyalertsak S, Wirtz A, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blosnich J, Lee J, Bossarte R, Silenzio V. Asthma disparities and within-group differences in a national, probability sample of same-sex partnered adults. Am J Public Health. 2013;103(9):e83–87. doi: 10.2105/AJPH.2013.301217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer U, Bowen D, Bauer G. Overweight and obesity in sexual minority women: evidence from population-based data. Am J Public Health. 2007;97(6):1134–1140. doi: 10.2105/AJPH.2006.088419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer U, Ozonoff A, Miao X. An ecological approach to examine lung cancer disparities due to sexual orientation. Public Health. 2012;126(7):605–612. doi: 10.1016/j.puhe.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin B, Grineski S, Collins T. The geography of despair: environmental racism and the making of South Phoenix, Arizona, USA. Hum Ecol Rev. 2005;12(2):155–167. [Google Scholar]
- Brown M. Gender and sexuality II: there goes the gayborhood? Prog Hum Geog. 2014;38(3):457–465. [Google Scholar]
- Castells M. The City and the Grassroots: A Cross-cultural Theory of Urban Social Movements. 7. University of California Press; Berkeley, CA: 1983. [Google Scholar]
- Chen E, Schreier H, Strunk R, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ Health Perspect. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Millet D, Marshall J. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS ONE. 2014;9(4):e94431. doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton W. Executive Order 12898 - Federal Actions To Address Environmental Justice in Minority Populations and Low-Income Populations. Federal Register. 1994;59(32) [Google Scholar]
- Cochran S, Björkenstam C, Mays V. Sexual orientation and all-cause mortality among US adults, age 18 to 59 years, 2001–2011. Am J Public Health. 2016;106(5):918–920. doi: 10.2105/AJPH.2016.303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran S, Mays V. Risk of breast cancer mortality among women cohabiting with same sex partners: findings from the National Health Interview Survey, 1997–2003. J Womens Health. 2012;21(5):528–533. doi: 10.1089/jwh.2011.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Grineski S, Chakraborty J, Montgomery M, Hernandez M. Downscaling environmental justice analysis: determinants of household-level hazardous air pollutant exposure in Greater Houston. Ann Assoc Am Geogr. 2015a;105(4):684–703. [Google Scholar]
- Collins T, Grineski S, Chakraborty J. Household-level disparities in cancer risks from vehicular air pollution in Miami. Environmental Research Letters. 2015b;10:095008. [Google Scholar]
- Collins T, Grineski S, Morales D. Sexual orientation, gender, and environmental injustice: unequal carcinogenic air pollution risks in Greater Houston. Ann Assoc Am Geogr. 2017;107(1):72–92. doi: 10.1080/24694452.2016.1218270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The Ecology of Fear: Los Angeles and the Imagination of Disaster. Metropolitan; New York: 1999. [Google Scholar]
- Dermody S, Marshal M, Cheong J, Burton C, Hughes T, Aranda F, et al. Longitudinal disparities of hazardous drinking between sexual minority and heterosexual individuals from adolescence to young adulthood. J Youth Adolescence. 2014;43(1):30–39. doi: 10.1007/s10964-013-9905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djawe K, Levin L, Swartzman A, Fong S, Roth B, Subramanian A, et al. Environmental risk factors for Pneumocystis pneumonia hospitalizations in HIV patients. Clin Infect Dis. 2013;56(1):74–81. doi: 10.1093/cid/cis841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan P, editor. Queerying Planning: Challenging Heteronormative Assumptions and Reframing Planning Practice. Ashgate; Chichester, UK: 2011. [Google Scholar]
- Egan J, Frye V, Kurtz S, Latkin C, Chen M, Tobin K, et al. Migration, neighborhoods, and networks: approaches to understanding how urban environmental conditions affect syndemic adverse health outcomes among gay, bisexual and other men who have sex with men. AIDS Behav. 2011;15(suppl 1):S35–S50. doi: 10.1007/s10461-011-9902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency. [accessed 12 September 2016];2011 National Air Toxics Assessment Web site. 2015 http://www.epa.gov/national-air-toxics-assessment/2011-national-air-toxics-assessment.
- Freeman L. Neighbourhood diversity, metropolitan segregation and gentrification: what are the links in the US? Urban Stud. 2009;46(10):2079–2101. [Google Scholar]
- Frye V, Egan J, Van Tieu H, Cerda M, Ompad D, Koblin B. “I didn’t think I could get out of the fucking park”. Gay men’s retrospective accounts of neighborhood space, emerging sexuality and migrations. Soc Sci Med. 2014;104:6–14. doi: 10.1016/j.socscimed.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates G. How many people are lesbian, gay, bisexual, and transgender? Williams Institute, University of California; Los Angeles: 2011. [accessed 12 September 2016]. https://escholarship.org/uc/item/09h684x2. [Google Scholar]
- Gates G. Geography of the LGBT population. In: Baumle A, editor. International Handbook on the Demography of Sexuality. Springer; New York: 2013. pp. 229–242. [Google Scholar]
- Gilman S, Cochran S, Mays V, Hughes M, Ostrow D, Kessler R. Risk of psychiatric disorders among individuals reporting same-sex sexual partners in the National Comorbidity Survey. Am J Public Health. 2001;91(6):933–939. doi: 10.2105/ajph.91.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottdiener M, Hutchison R. The New Urban Sociology. 5. Westview Press; Boulder, CO: 2015. [Google Scholar]
- Grineski S, Collins T, Chakraborty J, McDonald Y. Environmental health injustice: exposure to air toxics and children’s respiratory hospital admissions in El Paso, Texas. Prof Geogr. 2013;65(1):31–46. [Google Scholar]
- Hackbarth A, Romley J, Goldman D. Racial and ethnic disparities in hospital care resulting from air pollution in excess of federal standards. Soc Sci Med. 2011;73(8):1163–1168. doi: 10.1016/j.socscimed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Heck J, Jacobson J. Asthma diagnosis among individuals in same-sex relationships. J Asthma. 2006;43(8):579–584. doi: 10.1080/02770900600878289. [DOI] [PubMed] [Google Scholar]
- Hicken M, Gee G, Morenoff J, Connell C, Snow R, Hu H. A novel look at racial health disparities: the interaction between social disadvantage and environmental health. Am J Public Health. 2012;102(12):2344–2351. doi: 10.2105/AJPH.2012.300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard P. Cities and Sexualities. Routledge; Abingdon, UK: 2012. [Google Scholar]
- Hubbard P, Gorman-Murray A, Nash C. Cities and sexualities. In: DeLamater J, Plante R, editors. Handbook of the Sociology of Sexualities. Springer-Verlag; Heidelberg, Germany: 2015. pp. 287–304. [Google Scholar]
- Jephcote C, Chen H. Geospatial analysis of naturally occurring boundaries in road-transport emissions and children’s respiratory health across a demographically diverse cityscape. Soc Sci Med. 2013;82:87–99. doi: 10.1016/j.socscimed.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- King M, Semlyen J, Tai S, Killaspy H, Osborn D, Popelyuk D, et al. A systematic review of mental disorder, suicide, and deliberate self harm in lesbian, gay and bisexual people. BMC Psychiatry. 2008;8:70. doi: 10.1186/1471-244X-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan M. The uncertain geographic context problem. Ann Assoc Am Geogr. 2012;102:958–968. [Google Scholar]
- Landers S, Mimiaga M, Conron K. Sexual orientation differences in asthma correlates in a population-based sample of adults. Am J Public Health. 2011;101(12):2238–2241. doi: 10.2105/AJPH.2011.300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. Moving “out,” moving on: Gay men’s migrations through the life course. Ann Assoc Am Geogr. 2014;104(2):225–233. [Google Scholar]
- Lewis N. Canaries in the mine? Gay community, consumption and aspiration in neoliberal. Washington, DC: Urban Stud; 2017. 0042098016682418. [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lick D, Durso L, Johnson K. Minority stress and physical health among sexual minorities. Perspect Psychol Sci. 2013;8(5):521–548. doi: 10.1177/1745691613497965. [DOI] [PubMed] [Google Scholar]
- Meyer I. Prejudice, social stress, and mental health in lesbian, gay and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohai P, Saha R. Reassessing racial and socioeconomic disparities in environmental justice research. Demography. 2006;43(2):383–399. doi: 10.1353/dem.2006.0017. [DOI] [PubMed] [Google Scholar]
- Nash C. Trans experiences in lesbian and queer space. Can Geogr. 2011;55(2):192–207. [Google Scholar]
- O’Connell M, Feliz S. Same-Sex Couple Household Statistics from the 2010 Census (Working Paper Number 2011–26) U.S. Census Bureau, Social, Economic and Housing Statistics Division; Washington, DC: 2011. [Google Scholar]
- Ponce N, Cochran S, Pizer J, Mays V. The effects of unequal access to health insurance for same-sex couples in California. Health Aff. 2010;29:1539–1548. doi: 10.1377/hlthaff.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston D, Chang Y. Patterns of gay male and lesbian partnering in the metropolitan areas of the United States in 2010. Sociol Inq. 2013;63:150–165. [Google Scholar]
- Pulido L. Rethinking environmental racism: white privilege and urban development in Southern California. Ann Assoc Am Geogr. 2000;90(1):12–40. [Google Scholar]
- Spring A. Declining segregation of same-sex partners: evidence from Census 2000 and 2010. Popul Res Policy Rev. 2013;32(5):687–716. doi: 10.1007/s11113-013-9280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall R, Matthews D, Friedman M, Kinsky S, Egan J, Coulter R, et al. The continuing development of health disparities research on lesbian, gay, bisexual, and transgender individuals. Am J Public Health. 2016;106(5):787–789. doi: 10.2105/AJPH.2016.303183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Y. That’s not really my scene: working-class lesbians in (and out of) place. Sexualities. 2008;11:523–546. [Google Scholar]
- Trocki K, Drabble L, Midanik L. Tobacco, marijuana, and sensation seeking: comparisons across gay, lesbian, bisexual, and heterosexual groups. Psychol Addict Behav. 2009;23(4):620–631. doi: 10.1037/a0017334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [accessed 24 October 2016];Global Health Observatory Data. 2014 http://www.who.int/gho/phe/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Distribution of Cancer and Respiratory Risks from Hazardous Air Pollutants by Census Tract, (a,b) Hawaii and (c,d) Alaska, 2011.
Appendix 2. Distribution of same-sex female partner enclaves, (a) Hawaii and (b) Alaska, 2010. (Note: Hawaii and Alaska do not contain same-sex and same-sex male partner enclaves; enclave boundaries are enlarged.)