Abstract
Background
Shortening of gestation and intrauterine growth restriction (IUGR) are the two main determinants of birthweight. Low birthweight has been linked with prenatal arsenic exposure, but the causal relation between arsenic and birthweight is not well understood..
Objectives
We applied a quantile causal mediation analysis approach to determine the association between prenatal arsenic exposure and birthweight in relation to shortening of gestation and IUGR, and whether the susceptibility of arsenic exposure varies by infant birth sizes.
Methods
In a longitudinal birth cohort in Bangladesh, we measured arsenic in drinking water (n=1,182) collected at enrollment and maternal toenails (n=1,104) collected ≤1-month postpartum using inductively coupled plasma mass spectrometry. Gestational age was determined using ultrasound at ≤16 weeks’ gestation. Demographic information was collected using a structured questionnaire.
Results
Of 1,184 singleton livebirths, 16.4% (n=194) were low birthweight (<2500 g), 21.9% (n=259) preterm (<37 weeks’ gestation), and 9.2% (n=109) both low birthweight and preterm. The median concentrations of arsenic in drinking water and maternal toenails were 2.2 µg/L (range: bellow the level of detection [LOD]–1400) and 1.2 µg/g (range: <LOD–46.6), respectively. Prenatal arsenic exposure was negatively associated with birthweight, where the magnitude of the association varied across birthweight percentiles. The effect of arsenic on birthweight mediated via shortening of gestation affected all infants irrespective of birth sizes (β range: 10th percentile= −19.7g [95% CI: −26.7, −103.3] to 90th percentile= −10.9g [95% CI: −18.5, −5.9] per natural log water arsenic increase), whereas the effect via pathways independent of gestational age affected only the smaller infants (β range: 10th percentile= −28.0g [95% CI: −43.8, −9.9] to 20th percentile= −14.9g [95% CI: −30.3, −1.7] per natural log water arsenic increase). Similar pattern was observed for maternal toenail arsenic.
Conclusions
The susceptibility of prenatal arsenic exposure varied by infant birth sizes, placing smaller infants at greater risk of lower birthweight by shortening of gestation and possibly growth restriction. It is important to mitigate prenatal arsenic exposure to improve perinatal outcomes in Bangladesh.
1. BACKGROUND
Low birthweight (<2500 g at birth) is an important population indicator for neonatal mortality and a determinant of infant and childhood morbidity (1). Each year, an estimated 21 million infants are born with low birthweight worldwide, more than half of them are in South Asia (2). In Bangladesh, the incidence of low birthweight is estimated to be 22%, which is among the highest in the world (3), and in rural areas the estimates are as high as 31–47% (4). Low birthweight has two main causal components: preterm birth (<37 weeks of gestation) and intrauterine growth restriction (IUGR), which is commonly assessed as small for gestational age (<10th percentile of the birthweight-for-gestational age sex-specific reference population) (3). These components of low birthweight generally differ in their etiologies and risks of mortality, morbidity and impaired growth (5, 6); therefore, it is important to distinguish between them in order to identify true causal determinants of low birthweight and develop effective public health interventions (6).
An environmental factor potentially implicated in low birthweight is prenatal exposure to inorganic arsenic. Over a hundred million people worldwide are believed to be exposed to inorganic arsenic in drinking water sourced from groundwater at levels higher than the World Health Organization (WHO) recommended level of 10 µg/L.(7) But, the arsenic problem in Bangladesh is perhaps most devastating, as 40 million people, or a quarter of the country’s population, are still exposed to higher concentrations of arsenic through drinking water (8). In rural areas, where 70% of the total population live (10), the problem is much more highly prevalent, as 97% of them relies on groundwater for drinking purposes (9).
Arsenic can cross the placenta readily,(11) and prenatal arsenic exposure has been associated with spontaneous abortion,(12–14), preterm birth,(15–17) and intrauterine growth restriction (18–21). However, the epidemiological evidence for an association between prenatal arsenic exposure and birthweight is less consistent. While ten studies (22–30) reported a null or positive associations between arsenic exposure and birthweight, 14 other studies (16, 17, 20, 21, 31–41), including 6 large prospective cohort studies, reported negative associations. The negative associations reported in those studies were consistent despite the use of different exposure measures in drinking water (17, 33, 35), maternal urine (36, 38, 39, 41), toenail (35), hair (34), whole blood (21, 32, 37), soil around the residence (40), and placental tissue (20). Further, while these studies were largely conducted among populations with frequent exposure to higher levels (>10 µg/L) of arsenic through drinking water in Bangladesh (34–36), India (16), Chile (33), and Taiwan (17), several recent studies in the United States (21, 38, 39) and China (32, 37) have corroborated the negative association among populations exposed to relatively lower levels of exposure.
Most studies have also focused on birthweight as a single entity and have not used a causal framework that includes both shortening of gestation and IUGR for analysis. Mediation analysis can help identify the association between arsenic exposure and birthweight in relation to shortening of gestation and IUGR by decomposing the total effect of arsenic exposure on birthweight into indirect effect via pathways mediated through gestational age and direct effect via pathways independent of gestational age, respectively (42). Therefore, the indirect effect will estimate how much of the effect of arsenic exposure on birthweight will be via changing gestational age, whereas the direct effect will estimate how much of the effect of arsenic exposure on birthweight will be independent of gestational age. In other words, the direct effect will estimate the effect of arsenic exposure on birthweight via pathways other than changing gestational age, which will essentially include any change in birthweight that is also via intrauterine growth restriction. The total effect, estimated as the sum of the direct and indirect effect, will represent the overall effect of arsenic on birthweight. Recently, using structural equation modeling technique, our group identified that prenatal arsenic exposure was negatively associated with birthweight, which primarily mediated via shortening of gestational age. In contrast, the effect of arsenic exposure independent of gestational age was in the positive direction, and not statistically significant (35), highlighting the importance of estimating pathway-specific effects to capture the underlying heterogeneity of this complex exposure-outcome relation.
Previous studies also did not examine whether the susceptibility of arsenic exposure vary by infant birth sizes. A recent study in Mexico identified that prenatal lead exposure lowers birthweight-for-gestational age z-score, and that the magnitude of the association was larger for smaller infants (43). Both arsenic and lead has been implicated in hypoxia (44, 45) and generating oxidative stress (44, 46), which have been linked with the disruption of normal placentation, leading to adverse fetal growth outcomes (47, 48). Built upon these research, we investigated whether prenatal arsenic exposure disproportionately effect infants at the extremes of birthweight distribution. Traditional regression modeling approaches, also known as ordinal least square (OLS) regression, which have been consistently used in previous studies cannot answer this question, as OLS based regression implicitly assumes that the association between arsenic and birthweight is homogenous across birthweight percentiles. In other words, OLS based regression methods estimate the change in mean outcome variable (e.g. birthweight) in relation to the exposure of interest (e.g. arsenic exposure) and thus, summarizes the effect estimates that might have differed across the range of outcome distribution (e.g. birthweight percentiles), including those with opposing signs (49). This could potentially limit our chance to identify sensitive sub-population, who might be disproportionately affected by the exposure. For example, if arsenic exposure disproportionately affects infants at the tails of birthweight distribution, who are often at a greater risk of perinatal mortality and morbidity (50), that evidence essentially could empower public health interventions.
Quantile regression, on the other hand, allows for the effect of the exposure (e.g. arsenic) to vary across all quantiles of a response variable (e.g. birthweight) distribution and provide a more complete view of possible causal relationships between the exposure and outcome variables (49). Causal mediation modeling techniques can be combined with quantile regression to identify the causal association between prenatal arsenic exposure and birthweight in relation to gestational age across birthweight percentiles (also called quantile causal mediation analysis) (51). This method has been demonstrated previously in social science research (52). Using this modeling approach, we will be able to determine whether prenatal arsenic exposure effects birthweight via shortening gestation as well as intrauterine growth restriction and that whether infants at the tails of birthweight distribution are more susceptible to arsenic exposure. We hypothesized that arsenic exposure will be associated with lower birthweight via shortening gestational age as well as intrauterine growth restriction and that the magnitude of the association will vary by infant birth sizes.
2. METHODS
2.1 Study population
A longitudinal birth cohort was established in Bangladesh between 2008–2011. Community health care workers in the recruitment areas verbally advertised the study in the villages. Pregnant women interested in participating in the study were referred to the clinic, where their eligibility was confirmed. The details of this study, including recruitment and enrollment process were previously described (53). Briefly, women were eligible to participate if they were 18 years or older with an ultrasound confirmed singleton pregnancy of ≤16 weeks’ gestation, used a tube well as their primary source of drinking water and had been using the same drinking water source for more than six months, and intended to live in her current residence throughout her pregnancy. Of 1,613 pregnant women innitially recruited, 99 dropped out (n=99), 121 withdrawn from the study, 132 reported miscarriage, 72 reported stillbirth, and 5 reported multiple pregnancies. Complete covariate data were available for 1,180 participants in case of drinking water arsenic exposure and 1,093 participants in case of maternal toenail arsenic exposure. All subjects provided written informed consent before participation. Participants were informed and counseled on safe drinking water options if their water samples contained arsenic above Bangladeshi standard (i.e. <50µg/L). Prenatal care and multivitamins were provided to all participants. All protocols were reviewed and approved by the Human Research Committees at Harvard T.H. Chan School of Public Health and Dhaka Community Hospital Trust.
2.2 Exposure Assessment
Arsenic was measured in drinking water (n=1,182) from tubewells that women identified as their principal water source at the time of enrollment. Details of sample collection and measurement procedures have been previously described.(35) Briefly, water samples were collected in 50-ml polypropylene tubes (BD Falcon, BD Bioscience, Bedford, MA) and preserved with Reagent Grade nitric acid (Merck, Germany) to a pH<2. Field blanks were collected and analyzed for arsenic to evaluate exogenous contaminants. Samples were kept at room temperature until analysis by inductively coupled plasma mass spectrometry (ICP-MS) following US EPA method 200.8 (Environmental Laboratory Services, North Syracuse, New York). The average percent recovery of arsenic was 101% (range: 92%–110%). Samples with arsenic concentrations below the limit of detection (LOD) (n=242) ranging from 0.5–1.0 µg/L were reassigned half the value of the LOD for statistical analysis.
Arsenic was also measured in maternal toenails collected ≤1-month post-partum. We collected toenail material from all 10 toes and pooled them for analysis. Pooled toenail samples were sonicated in 1% Triton X-100 solution (Sigma-Aldrich, Inc., St. Louis, MO) and rinsed repeatedly with Milli-Q water (Millipore Corporation, Billerica, MA) to remove external contamination before microwave acid digestion using Trace Select Ultra Pure nitric acid (Fischer Scientific, Pittsburgh, PA). Digested samples were diluted with Milli-Q water before analyzing for total arsenic using ICP-MS. The reported arsenic concentrations were blank-corrected and normalized using arsenic concentration of certified human hair reference material (CRM Hair; Shanghi Institute of Nuclear Research, China). The average percent recovery and relative standard deviation of CRM hair for arsenic was 94.1% and 5.2% respectively. Toenail clippings were collected from 1,118 mothers at <1-month post-partum with a history of singleton livebirth, 1,093 of them were used in the analysis after excluding samples with toe mass ≤5mg (n=16) and/or relative standard deviation ≥25% (n=9). One samples with arsenic concentrations below the LOD ranging from 0.09–0.7 ng/L were reassigned half the value of LOD for statistical analysis.
2.3 Outcome and covariates
The study involved four scheduled visits occurring at the time of enrollment, around 28th weeks’ gestation, at the time of delivery, and ≤1 month post-partum. During those visits trained interviewers used structured questionnaires to collect demographic, medical, and environmental information. All pregnancies were dated either by crown-rump length (CRL) at 7–16 weeks or mean sac diameter (MSD) at 4–6 weeks. Fetal CRL and MSD were measured by a trained family physician by ultrasound using the formulae proposed by Robinson and Hellman, respectively (54, 55). Maternal blood samples were collected at the time of enrollment to measure hemoglobin levels. Maternal height and weight were recorded at first clinic visit and at monthly house visits following enrollment, when they also received prenatal vitamins. To ensure collection of high quality birth measurements, healthcare workers were trained following a standard protocol. All births were attended by trained healthcare workers. Birthweight was measured within 120 minutes after delivery on a pediatric scale calibrated and rounded to the nearest 10 grams before each measurement.
2.4 Statistical Analysis
Arsenic concentrations in drinking water, and maternal toenail were right skewed and subsequently transformed to their natural log. Mean birthweight across categories of all covariates were analyzed using T-test or analysis variance (ANOVA) in bivariate models. The distribution of birthweight was checked and a histogram indicated no gross violation for normality assumption.
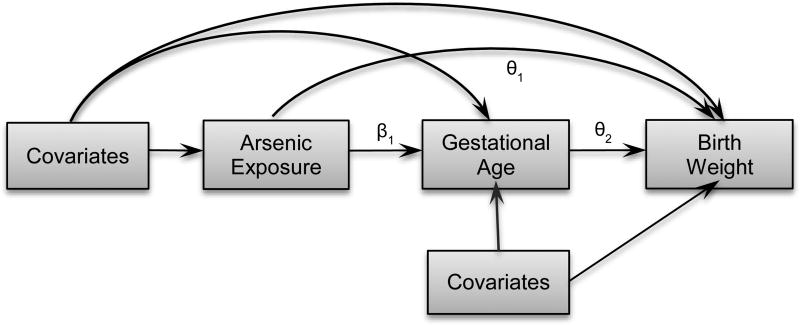
Mediation analysis for the association between prenatal arsenic exposure and birthweight was implemented considering gestational age as a mediator for 10th to 90th percentiles of birthweight following the method described by Imai et al, (51). The directed acyclic graph (DAG) in Figure 1 explains the conceptual model for causal mediation analysis (56). We hypothesized that gestational age will lie within the causal pathway between prenatal arsenic exposure and birthweight. We used quantile regressions to model percentiles of birthweight (outcome models) and linear regression to model for gestational age (mediator model). In the schema bellow, equation 1 and equation 2 represents the outcome and mediator model, respectively, where Y represents the outcome, birthweight; M denotes the mediator, gestational age; A denotes exposure, prenatal arsenic exposure; and C denotes the covariates. Following this notation, (a − a*) indicates 1 unit change in exposure from a*=0 to a level a= 1.
| (1 |
| (2) |
Figure 1.
Conceptual model for causal mediation analysis showing the relation between arsenic exposure and birthweight considering gestational age a mediator. Covariates include maternal age, education, enrollment BMI, number of past pregnancies, blood hemoglobin, secondhand smoking, and infant gender. Respective path co-efficient for the association between arsenic-birthweight, gestational age-birthweight, and arsenic-gestational age are given by θ1, θ2, and β1, respectively.
The outcome and mediator models were combined to estimate the direct, indirect and total effects of arsenic exposure on birthweight at 5th to 95th percentiles of birthweight distribution. Analyses were repeated for drinking water arsenic and maternal toenail arsenic. The natural direct effect (NDE) is given by: θ1 (τ) (a−a*), which expresses how much the birthweight would change if natural log arsenic exposure were set at level a=1 versus level a*=0, but for each individual gestational age were kept at the level it would have taken in the absence of arsenic exposure. The natural indirect effect is given by: θ2 (τ) * β1 (a−a*), which expresses how much the birth weight would change on average if natural log arsenic exposure were controlled at a=1, but gestational age were changed from the level it would take if a* = 0 to the level it would take if a = 1. The total effect (TE) is given by: NDE + NIE, which expresses how much the birth weight would change overall for a change in natural log arsenic exposure from level a* = 0 to level a = 1. Proportion mediated (PM%) was estimated using the formula, PM%=NIE/TE*100, which expresses how much of the overall effect of arsenic on birthweight is mediated via changing gestational age.
We selected a set of a priori covariates to adjust for the mediator and outcomes models that were previously found to be associated with gestational age and birthweight. Models for gestational age were adjusted for natural log transformed arsenic exposure (continuous), maternal age (continuous), education (no formal education, primary, secondary or higher), number of past pregnancies (0, 1, ≥2), enrollment BMI (<18.5, 18.5–24.9, >24.9), secondhand smoke (yes, no), and infant sex (male, female), and maternal blood hemoglobin level at enrollment (continuous). The models of birthweight were additionally adjusted for gestational age and included an interaction term between natural log arsenic exposure and blood hemoglobin level. Direct and indirect effects were averaged across all individuals. Bias corrected confidence intervals were estimated from 1000 Monte Carlo draws for nonparametric bootstrap. Analyses assume that conditional on the covariates, there is no confounding of 1) the exposure-outcome relation, 2) exposure-mediator relation, (3) mediator-outcome relation and that (4) there is no effect of the exposure that itself confounds the mediator-outcome relation (42). Analyses were implemented with R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) using the R-package “mediation” (57).
3. RESULTS
Table 1 summarizes demographic characteristics of the study population and their bivariate associations with birthweight. Average birthweight was 2837g (standard deviation: 408g; range: 800–4,800g). Nearly 22% (n=259) of the infants were born preterm (<37 weeks of gestation), 19% (n=223) small-for-gestational age,(58) 16.4% (n=194) low birthweight and 9.2% (n=109) both preterm and low birthweight. Arsenic exposures were relatively modest but spanned a wide range. The median concentrations of arsenic exposure were 2.2µg/L (range: <LOD–1400µg/L) for drinking water arsenic, and 1.2µg/g (range: <LOD–46.6µg/g) for maternal toenail arsenic. Drinking water arsenic concentration showed a modest correlation with maternal toenail arsenic (σspearman=0.48; 95% CI: 0.44–0.53).
Table 1.
Distribution of selected characteristics of study participants and their bivariate associations with birthweight [g]
| Characteristic | n (%)a | Co-efficient (95% CI) |
P-value |
|---|---|---|---|
| Maternal age, years (mean ± SD) | 23.0 ± 4.2 | −1.3 (−6.8, 4.2) | 0.75 |
| Gestational Age, weeks (mean ± SD) | 38.0 ± 1.8 | 82.5 (71.5, 93.4) | <0.001 |
| Enrollment BMI, kg/m2 | |||
| ≤18·5 | 335 (28.4) | −63.3 (−115.5, −11.1) | 0.02 |
| >18·5 to ≤25.0 | 738 (62.5) | Reference | - |
| >25.0 | 108 (9.1) | 175.0 (93.4, 256.7) | <0.001 |
| Infant Sex | |||
| Male | 598 (50.6) | 73.8 (27.3, 120.2) | 0.002 |
| Female | 583 (49.4) | Reference | - |
| No. of past pregnancies | |||
| 0 | 475 (40.2) | Reference | - |
| 1 | 353 (29.9) | −4.0 (−60.2, 52.1) | 0.90 |
| ≥2 | 353 (29.9) | −68.9 (−125.1, −12.8) | 0.02 |
| Maternal education | |||
| No formal education | 172 (14.6) | −154.6 (−223.0, −86.2) | <0.001 |
| Primary education | 380 (32.2) | −22.2 (−73.9, 29.4) | 0.40 |
| Secondary or higher | 629 (53.2) | Reference | - |
| Secondhand smoke | |||
| Yes | 495 (41.9) | −73.7 (−120.8, −26.6) | 0.002 |
| No | 685 (56.1) | Reference | - |
| Blood hemoglobin, gm/L | |||
| 7.9–10.2 | 298 (25.2) | −47.7 (−115.1, 19.7) | 0.17 |
| >10.2–11.2 | 396 (33.5) | −106.2 (−169.6, −42.8) | 0.001 |
| >11.2–12.0 | 223 (18.9) | −13.5 (−86.0, 59.1) | 0.72 |
| >12.0–16.3 | 264 (22.4) | Reference | - |
| Drinking water arsenic, µg/L | |||
| <LOD–<10 | 718 (60.8) | Reference | - |
| 10–<50 | 202 (17.1) | −115.4 (−178.8, −52.0) | <0.001 |
| 50–1400 | 261 (22.1) | −80.1 (−137.7, −22.6) | 0.006 |
| Toenail arsenic, µg/g | |||
| <LOD–<1.2 | 544 (49.8) | Reference | - |
| 1.2–<2.3 | 216 (19.8) | −76.2 (−139.4, −13.0) | 0.02 |
| 2.3–46.5 | 333 (30.4) | −4.7 (−59.4, 50.0) | 0.87 |
Values are n (%) except where indicated
Abbreviations: SD= Standard deviation; LOD= Limit of detection
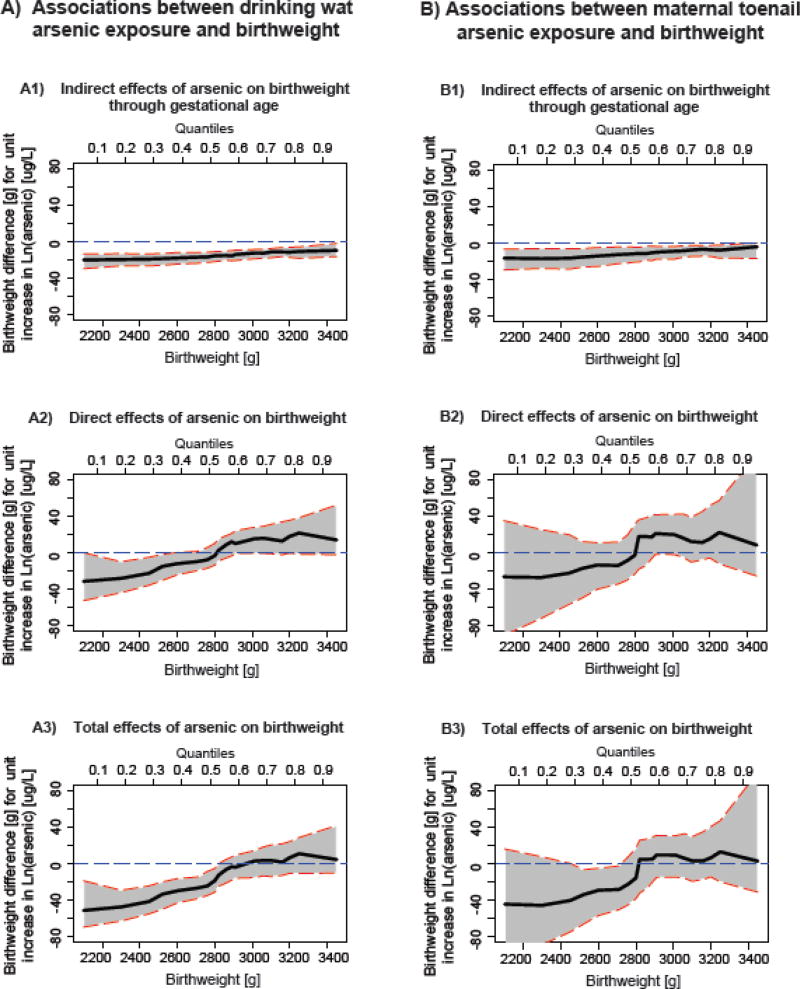
The adjusted indirect, direct, and total effects of drinking water arsenic exposure on birthweight across birthweight percentiles were presented in Figure 2 (A1–A3) and Supplemental Table 1, whereas the adjusted indirect, direct, and total effects of toenail arsenic on birthweight across birthweight percentiles were presented in Figure 2 (B1–B3) and Supplemental Table 2.. Our results suggested a heterogeneous relation between arsenic and birthweight across birthweight percentiles, which involved pathways mediated through gestational age as well as pathways independent of gestational age. The magnitude of our observed causal relations was consistent regardless of whether arsenic was measured in drinking water or maternal toenails. The indirect effects mediated through gestational age were negative and statistically significant for all infants irrespective of birth sizes, although the associations were stronger among smaller infants (Figure 2: A1, B1), suggesting a shift in the birthweight distribution curve toward the left. For instance, among infants with birthweight <2300g (i.e.10th percentile), a unit increase in natural log water arsenic exposure was associated with 19.7g (95%CI: −26.7, −13.3) lower birthweight mediated via gestational age, while among infants born with birthweight >3250g (i.e. 90th percentile), for the same exposure the change in birthweight was −10.9g (95% CI: −18.5, −5.9) (Supplemental Table 1). Similar association was observed for maternal toenail arsenic (Supplemental Table 2).
Figure 2.
Indirect, direct, and total effects of prenatal exposure to inorganic arsenic in drinking water (A1–A3) and maternal toenails (B1–B3) on birthweight considering gestational age a mediator, adjusting for maternal age, education, number of past pregnancies, secondhand smoking, enrollment BMI, blood hemoglobin level and infant gender. Solid black lines sorrounded by shadded areas represent effect estimates and 95% confidence intervals across birthweight percentiles. Horizontal blue dashed lines show reference values.
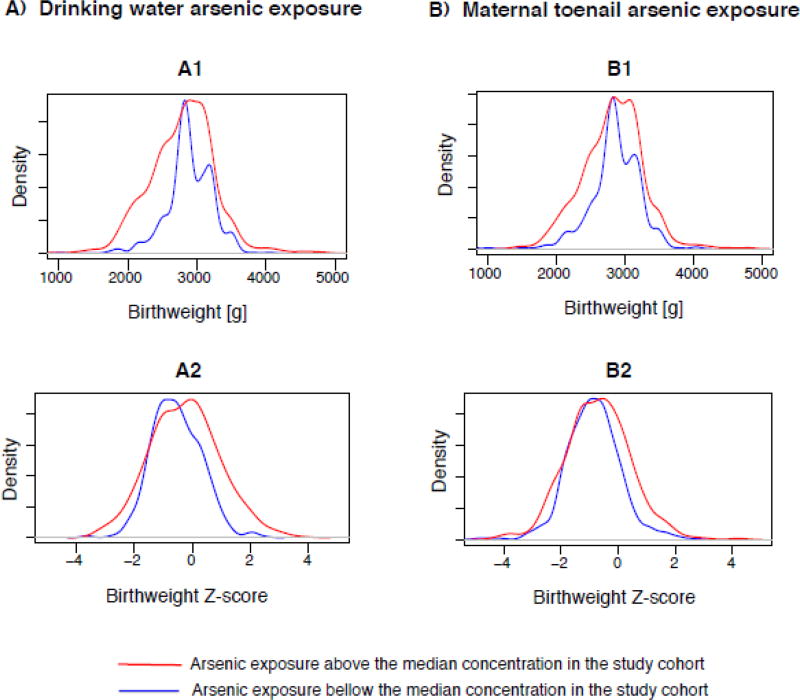
The direct effect of arsenic exposure on birthweight through pathways independent of gestational age and hemoglobin level across birthweight percentiles were heterogeneous and bidirectional (Figure 2: A2, B2). For instance, via direct pathways, each unit increase in natural log water arsenic exposure was associated with 28.0 g (95%CI: −63.1, −29.4) lower birthweight among infants with birthweight <2300g (10th percentile), whereas the reduction was 14.9 g (95%CI: −30.3, −1.7) among infants with birthweight around 2520g (20th percentile) (Supplemental Table 1). On the contrary, water arsenic exposure showed positive associations with birthweight among the heavier infants (>50th percentile), although the associations were not statistically significant. Similar pattern of associations were observed for maternal toenail arsenic, although none of the direct effect associations were statistically significant. This paradoxical change in birthweight in response to prenatal arsenic exposure via pathways independent of gestational age was supported by the shift in the birthweight-for-gestational age z-score (birthweight in units of standard deviation within strata of gestational age) curve away from the mean in arsenic exposure above the median compared to that in arsenic exposure bellow the median in our cohort (Figure 3: A2, B2).
Figure 3.
The distributions of birthweight and birthweight-for-gestational age Z-score by prenatal arsenic exposure measured in drinking water (A1–A2) and maternal toenails (B1–B2) bellow the median concentration (blue) and above the median concentration (red) in the study cohort
The direct and indirect effects were summed to obtain the total effects of arsenic exposure on birthweight. As predicted, our results showed marked heterogeneity in the total effects of arsenic exposure on birthweight across birthweight percentiles (Figure 2: A3, B3), Overall, water arsenic exposure was negatively associated with birthweight among infants <40th percentile of birthweight distribution, while the associations were strongest among the smaller infants (Supplemental Table 1). For instance, each unit increase in natural log water arsenic exposure was associated with 47.7g (95%CI: −63.1, −29.4) lower birthweight among infants with birthweight <2300g (10th percentile) compared to 18.7g (95% CI: −31.4, −5.5) decrease in birthweight among infants with birthweight around 2800g (40th percentile). The proportion of the total effects mediated by gestational age (PM%) also showed marked heterogeneity; where the contribution of the indirect pathway in overall reduction of birthweight steadily increased as birthweight percentiles increased up to 40th percentile (PM10th percentile = 41.2% vs PM40th percentile = 83.4%) (Supplemental Table 1). Similar patterns were observed for maternal toenails arsenic exposure, but the total effect associations were significant for infants at 20th and 30th percentiles of birthweight distribution (Supplemental Table 2). The overall effect of arsenic exposure on birthweight appeared to be positive among the heavier infants, but the associations were not statistically significant. These results are supported by the shift in the birthweight distribution curves away from the mean with a heavier lower tail in arsenic exposure above the median compared to that in arsenic exposure bellow the median in our study cohort (Figure 3: A1, B1).
4. DISCUSSION
This prospective cohort study was built upon our previous research that linked prenatal arsenic exposure with lower birthweight, primarily via shortening of gestational age. In this study, we expanded our previous analysis to investigate the causal relation between arsenic exposure and birthweight, and whether the susceptibility of arsenic exposure varies by infant birth sizes by implementing quantile causal mediation modeling technique. Our analyses revealed that prenatal arsenic exposure was negatively associated with birthweight, and that the magnitude of the association varied across birthweight percentiles, suggesting heightened susceptibility to arsenic exposure among smaller infants. The association between arsenic exposure and birthweight involved pathways mediated via gestational age as well as pathways independent of gestational age, indicating possible role of shortening of gestation and intrauterine growth restriction, the two main causal processes underlying low birthweight, in explaining this complex exposure-outcome relation. The negative association between arsenic and birthweight via shortening of gestation was observed among all infants irrespective of birth sizes, whereas the negative association between arsenic and birthweight via pathways independent of gestational age was observed only among the smallest infants and for drinking water arsenic exposure.
Our findings are consistent with previous epidemiological studies that also report that prenatal arsenic exposure is negatively associated with birthweight (33–37) and positively associated with preterm delivery,(15–17) and fetal growth restriction (18–20). These associations were observed in populations with very high exposure (15–17), relatively lower levels of exposure (21, 31, 37), and exposure comparable to our study population (33–36), suggesting that there is likely no safe threshold for the embryotoxic effect of arsenic. Corroborating with our study, prospective cohort studies in Bangladesh (34–36) and Chile (33) have also observed a dose-dependent relationship between arsenic exposure and birthweight. Additionally, our quantile causal mediation analysis results were fairly consistent in magnitude and direction with mean regression analysis in the same cohort (35), but captured additional shift in birthweight distribution in response to prenatal arsenic exposure. For instance, Kile et. al. previously estimated in this cohort that a unit increase in natural log drinking water and maternal toenail arsenic exposure was associated with 17.4 g (−22.8, −12.0) and 13.6 g (−22.1, −5.1) lower birthweight, respectively via shortening of gestational age (35), which were comparable to the estimates we obtained at 50th percentile of birthweight distribution (median regression) with larger sample size for drinking water (n=1,140 vs 1,181) and toenail (n= 624 vs 1,104) arsenic exposure. Additionally, our analyses revealed a significant negative association between drinking water arsenic and birthweight among the smallest infants (e.g. <20th percentile) via pathways independent of gestational age that was not captured by OLS regression technique used by Kile et al (35). This difference is likely due to the assumption of homogenous arsenic-birthweight relation made by Kile et al (35). While the heterogeneity in the associations between arsenic exposure and birthweight were identified in previous studies based on smoking,(31) infant genders,(37, 39) and maternal prepregnancy BMI status,(39) we observed disparities based on infant birth sizes. Overall, our results suggested that prenatal arsenic exposure was associated with a shift in the birthweight distribution curve away from the mean with a heavier lower tail, corresponding to a higher percentage of small preterm infants (≈9.2%) in our cohort (1). The proportion of small preterm infants in a population, which typically ranges between 2–5%, is an indicator of perinatal risk in that population (1, 59). Our findings emphasized the importance of arsenic mitigation to improve perinatal outcomes in Bangladesh.
Known biologic effects of inorganic arsenic exposure support the biological plausibility of our findings. Arsenic can generate reactive oxygen species and deplete antioxidant enzymes (e.g. glutathione) leading to oxidative stress (60). Oxidative damage in early pregnancy can disrupt placental development, function and remodeling (47), which in turn can hamper oxygen and nutrient supply to the growing fetus and production and metabolism of fetal growth regulating hormones leading to preterm delivery and IUGR (61, 62). Another plausible explanation is epigenetic alterations. Prenatal arsenic exposure has been found associated with deregulation of microRNA expression profiles in umbilical cord blood (63) and DNA methylation status in maternal and umbilical cord blood (64). MicroRNAs have important role in normal placental development; and alteration of microRNA expression profiles have been associated with abnormal placentation, preeclampsia, eclampsia, and SGA births (65, 66).
Our study has some limitations. The observed positive associations between arsenic exposure and birthweight among the heavier infants in our cohort could partly because of inadequate adjustment for maternal perinatal nutritional status. Individual’s micronutrient status (e.g. folate, antioxidants) plays an important role in arsenic detoxification, where adequate nutrition may ameliorate individual’s vulnerability to arsenic toxicity (67), resulting in heavier infants. Therefore, inadequate adjustment for perinatal nutritional status will lead to an underestimation of the negative associations between arsenic and birthweight, and the underestimation will be larger for heavier infants. Future studies to explore potential interactions between arsenic exposure and maternal nutritional status during pregnancy in relation to birthweight will be useful. Future analysis using dietary exposure as predictors of arsenic exposure will also be beneficial to estimate the relative contribution of dietary arsenic given the modest correlation (σspearman=0.48; 95% CI: 0.44–0.53) between drinking water and maternal toenail arsenic exposure in this population.
Hence, it is possible that there is error in our estimates due to unmeasured confounders. We selected a priori list of covariates that were previously found to be associated with birthweight and/or gestational age. All women were provided with free prenatal multivitamins including 400 µg of folic acid and the same level of health care during pregnancy by our community health clinics, which were among the few of healthcare providers in the catchment area. Folate supplementation has been shown to reduce blood arsenic concentration as well as the toxic effect of arsenic by increasing arsenic methylation efficiency, particularly in population with folate deficiency (68–70), and thereby may reduce the risk of embryotoxic effect of arsenic (71). The compliance of regular multivitamins intake was reported to be 99% in our cohort. Therefore, any bias associated with multivitamin supplementation for the association between arsenic exposure and birthweight will likely be non-differential and towards the null. We did not collect detail pregnancy history to adequately control for other factors that could confound our results, such as pregnancy spacing or history of adverse birth outcomes in past pregnancies. Furthermore, we were not able to test the robustness of our estimated direct and indirect effects in presence of unmeasured confounders because no such method had yet been developed for quantile causal mediation analyses technique.
It is likely that our collected toenail clippings were not in the same length for all study participants, where longer clippings would have a higher absolute arsenic concentration and reflect a greater duration of exposure (and potentially greater variability in exposure). We collected toenail clippings from all available toes and pooled them together for analysis, which would give an estimate of participant’s average duration of exposure. Furthermore, the concentrations reported in this analysis were per gram of nail, and we excluded any samples that had a toenail mass ≤ 5mg. So, we agree that there could be exposure misclassification resulting from a mixture of people with different toenail lengths (e.g. duration of exposures); however, it would likely to be non-differential.
Few women in our cohort were able to recall their date of last menstrual period (LMP), which led us to solely rely on ultrasound measurements to estimate gestational age. Pregnancy dating using ultrasound technique is considered gold standard if used in early pregnancy, but the estimations are increasingly inaccurate after the end of 1st trimester [70]. Hence, we agree that there could be some misclassification in gestational age, as we were not able to use LMP information in conjunction with ultrasound to validate pregnancy duration. In a previously published study from the same cohort [29], we demonstrated that arsenic exposure was not associated with the timing of enrollment; thus, it is likely that any misclassification of gestational age introduced by increasing variability of ultrasound measurements for women enrolled between 13–16 weeks of gestation is non-differential. Thus, this error is likely to just decrease the precision of our observed associations.
Strengths of our study include its prospective design, where we collected birth outcome data from a fairly large number of pregnant mothers. We measured arsenic exposure in drinking water that pregnant mothers identified as their primary water source early in pregnancy. Previous studies in Bangladesh demonstrated that arsenic exposure measured in drinking water show little temporal variability (72) and serve as an adequate marker for long-term exposure when collected from participant’s main water source (73). We also measured arsenic exposure in maternal toenails as a biomarker of internal dose and observed similar pattern of association. Our previous analysis demonstrated that toenail sample collected <1 month postpartum represents individual’s cumulative exposure over the past 9–12 months (74), which essentially correspond to the entire pregnancy. Our study participants also provided a toenail sample at the time of enrollment (≤ 16 weeks of gestation); hence, we are fairly confident that arsenic concentrations measured in maternal toenails collected within one-month post-partum reflects gestational exposure. Therefore, our proposed temporal relation for the causal mediation framework between the exposure, mediator and outcome is valid. Additionally, the wide range of arsenic exposure in drinking water ranging from below the LOD to 1400 µg/L in our cohort enables our study findings to be applicable to other population where exposure is modest. Moreover, our quantile causal mediation analysis technique helped us to address methodological challenges involved in the investigation of potential heterogeneous association between prenatal arsenic exposure and birthweight in relation to shortening of gestation and intrauterine growth restriction, the two main causal processes underlying low birthweight, and enabled us to identify susceptible sub-population to arsenic toxicity.
5. CONCLUSIONS
Our results showed that prenatal arsenic exposure was associated with lower birthweight, which involved shortening of gestation and possibly intrauterine growth restriction in the causal pathways and that the magnitude of the association varied across birthweight percentiles. Smaller infants, who are already at higher risk of perinatal mortality and morbidity, are more susceptible to the toxic effects of arsenic on birthweight. Thus, minimizing maternal arsenic exposure during pregnancy may significantly improve perinatal health outcomes in Bangladesh.
Supplementary Material
Highlights.
We investigated the causal relation between prenatal arsenic exposure and birthweight
Smaller infants were more susceptible to arsenic exposure.
Both shortened gestation and IUGR likely to play important role in explaining arsenic-birthweight relation.
Minimizing prenatal arsenic exposure may improve perinatal outcomes in Bangladesh
Acknowledgments
Source of Funding: The National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) supported this work [grant number: R01ES015533]
Abbreviations
- IUGR
Intrauterine Growth Restriction
- LOD
Limit of Detection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflicts of relevant to this article to disclose.
References
- 1.Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001;30(6):1233–41. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 2.WHO and UNICEF, World Health Organization. Low birthweight: country, regional and global estimates Geneva, Switzerland. 2004 [Available from: http://www.unicef.org/publications/files/low_birthweight_from_EY.pdf.
- 3.Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. The Lancet Global health. 2013;1(1):e26–36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosain GM, Chatterjee N, Begum A, Saha SC. Factors associated with low birthweight in rural Bangladesh. J Trop Pediatr. 2006;52(2):87–91. doi: 10.1093/tropej/fmi066. [DOI] [PubMed] [Google Scholar]
- 5.Kline JS, Mervyn, Stein Zena. Conception to birth : epidemiology of prenatal development. New York: Oxford University Press; 1989. [Google Scholar]
- 6.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin R, Huda NH. Arsenic poisoning in bangladesh. Oman Med J. 2011;26(3):207. doi: 10.5001/omj.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loewenberg S. In Bangladesh, arsenic poisoning is a neglected issue. Lancet. 2016;388(10058):2336–7. doi: 10.1016/S0140-6736(16)32173-0. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan SV, Johnston RB, Zheng Y. Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ. 2012;90(11):839–46. doi: 10.2471/BLT.11.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The World Bank. Bangladesh: New Life for the Rural Poor. 2014 [updated June 30, 2014; cited 2017 June 4]. Available from: http://www.worldbank.org/en/news/feature/2014/06/30/bangladesh-new-life-for-the-rural-poor.
- 11.Concha G, Nermell B, Vahter MV. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect. 1998;106(6):355–9. doi: 10.1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21(6):797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- 13.Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, et al. Chronic arsenic exposure and adverse pregnancy outcomes in bangladesh. Epidemiology. 2005;16(1):82–6. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- 14.He W, Greenwell RJ, Brooks DM, Calderon-Garciduenas L, Beall HD, Coffin JD. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion. Toxicol Sci. 2007;99(1):244–53. doi: 10.1093/toxsci/kfm162. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–31. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM, Chowdhury UK, et al. Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect. 2003;111(9):1194–201. doi: 10.1289/ehp.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CY, Chang CC, Tsai SS, Chuang HY, Ho CK, Wu TN. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res. 2003;91(1):29–34. doi: 10.1016/s0013-9351(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 18.Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ Res. 2015;140:430–9. doi: 10.1016/j.envres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LA, Raqib R, et al. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol. 2012;34(4):504–11. doi: 10.1016/j.reprotox.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Llanos MN, Ronco AM. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reprod Toxicol. 2009;27(1):88–92. doi: 10.1016/j.reprotox.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 21.Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, et al. Prenatal Arsenic Exposure and Birth Outcomes among a Population Residing near a Mining-Related Superfund Site. Environ Health Perspect. 2016;124(8):1308–15. doi: 10.1289/ehp.1510070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahamed S, Sengupta MK, Mukherjee SC, Pati S, Mukherjeel A, Rahman MM, et al. An eight-year study report on arsenic contamination in groundwater and health effects in Eruani village, Bangladesh and an approach for its mitigation. Journal of health, population, and nutrition. 2006;24(2):129–41. [PubMed] [Google Scholar]
- 23.Chakraborti D, Sengupta MK, Rahman MM, Ahamed S, Chowdhury UK, Hossain MA, et al. Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra plain. Journal of environmental monitoring : JEM. 2004;6(6):74N–83N. [PubMed] [Google Scholar]
- 24.Gelmann ER, Gurzau E, Gurzau A, Goessler W, Kunrath J, Yeckel CW, et al. A pilot study: the importance of inter-individual differences in inorganic arsenic metabolism for birth weight outcome. Environmental toxicology and pharmacology. 2013;36(3):1266–75. doi: 10.1016/j.etap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwok RK, Kaufmann RB, Jakariya M. Arsenic in drinking-water and reproductive health outcomes: a study of participants in the Bangladesh Integrated Nutrition Programme. Journal of health, population, and nutrition. 2006;24(2):190–205. [PubMed] [Google Scholar]
- 26.Mukherjee SC, Saha KC, Pati S, Dutta RN, Rahman MM, Sengupta MK, et al. Murshidabad--one of the nine groundwater arsenic-affected districts of West Bengal, India. Part II: dermatological, neurological, and obstetric findings. Clin Toxicol (Phila) 2005;43(7):835–48. doi: 10.1080/15563650500357495. [DOI] [PubMed] [Google Scholar]
- 27.Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, et al. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. J Epidemiol Community Health. 2010;64(4):325–9. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- 28.Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. Maternal exposure to low-level heavy metals during pregnancy and birth size. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2010;45(11):1468–74. doi: 10.1080/10934529.2010.500942. [DOI] [PubMed] [Google Scholar]
- 29.Vall O, Gomez-Culebras M, Garcia-Algar O, Joya X, Velez D, Rodriguez-Carrasco E, et al. Assessment of prenatal exposure to arsenic in Tenerife Island. PLoS One. 2012;7(11):e50463. doi: 10.1371/journal.pone.0050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Bi S, Xu G, Zhang Y, Mei X, Li A. Distribution and assessment of heavy metals off the Changjiang River mouth and adjacent area during the past century and the relationship of the heavy metals with anthropogenic activity. Mar Pollut Bull. 2015;96(1–2):434–40. doi: 10.1016/j.marpolbul.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Bloom MS, Neamtiu IA, Surdu S, Pop C, Anastasiu D, Appleton AA, et al. Low level arsenic contaminated water consumption and birth outcomes in Romania-An exploratory study. Reprod Toxicol. 2016;59:8–16. doi: 10.1016/j.reprotox.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan H, Piao F, Zhang X, Li X, Li Q, Xu L, et al. Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian. Biological trace element research. 2012;149(1):10–5. doi: 10.1007/s12011-012-9396-7. [DOI] [PubMed] [Google Scholar]
- 33.Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14(5):593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- 34.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49(10):1097–104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 35.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating Effects of Arsenic Exposure During Pregnancy on Perinatal Outcomes in a Bangladeshi Cohort. Epidemiology. 2016;27(2):173–81. doi: 10.1097/EDE.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–12. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Yokoyama K, Tian Y, Piao FY, Kitamura F, Kida H, et al. Decrease in birth weight and gestational age by arsenic among the newborn in Shanghai, China. [Nihon koshu eisei zasshi] Japanese journal of public health. 2011;58(2):89–95. [PubMed] [Google Scholar]
- 38.Fei DL, Koestler DC, Li Z, Giambelli C, Sanchez-Mejias A, Gosse JA, et al. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environmental health : a global access science source. 2013;12:58. doi: 10.1186/1476-069X-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. Relation between in Utero Arsenic Exposure and Birth Outcomes in a Cohort of Mothers and Their Newborns from New Hampshire. Environ Health Perspect. 2016;124(8):1299–307. doi: 10.1289/ehp.1510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott S, Bao W, Aelion CM, Cai B, Lawson AB. Does the metal content in soil around a pregnant woman's home increase the risk of low birth weight for her infant? Environ Geochem Health. 2014;36(6):1191–7. doi: 10.1007/s10653-014-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–92. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological methods. 2013;18(2):137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodosthenous RS, Burris HH, Svensson K, Amarasiriwardena CJ, Cantoral A, Schnaas L, et al. Prenatal lead exposure and fetal growth: Smaller infants have heightened susceptibility. Environment international. 2016 doi: 10.1016/j.envint.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahamed M, Siddiqui MK. Low level lead exposure and oxidative stress: current opinions. Clinica chimica acta; international journal of clinical chemistry. 2007;383(1–2):57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119(2):258–64. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jauniaux E, Burton GJ. The role of oxidative stress in placental-related diseases of pregnancy. J Gynecol Obstet Biol Reprod (Paris) 2016;45(8):775–85. doi: 10.1016/j.jgyn.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Human reproduction update. 2006;12(6):747–55. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koenker R. Quantile Regression. New York: Cambridge University Press, Cambridge; 2005. [Google Scholar]
- 50.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 51.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological methods. 2010;15(4):309–34. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 52.Shen E, Chou CP, Pentz MA, Berhane K. Quantile Mediation Models: A Comparison of Methods for Assessing Mediation Across the Outcome Distribution. Multivariate Behav Res. 2014;49(5):471–85. doi: 10.1080/00273171.2014.904221. [DOI] [PubMed] [Google Scholar]
- 53.Kile ML, Rodrigues EG, Mazumdar M, Dobson CB, Diao N, Golam M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environmental health : a global access science source. 2014;13(1):29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellman LM, Kobayashi M, Fillisti L, Lavenhar M, Cromb E. Growth and development of the human fetus prior to the twentieth week of gestation. American journal of obstetrics and gynecology. 1969;103(6):789–800. doi: 10.1016/0002-9378(69)90575-4. [DOI] [PubMed] [Google Scholar]
- 55.Robinson HP, Fleming JE. A critical evaluation of sonar "crown-rump length" measurements. Br J Obstet Gynaecol. 1975;82(9):702–10. doi: 10.1111/j.1471-0528.1975.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 56.Hernan MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health. 2004;58(4):265–71. doi: 10.1136/jech.2002.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. Journal of Statistical Software. 2013;59:1–38. [Google Scholar]
- 58.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 59.Umbach DM, Wilcox AJ. A technique for measuring epidemiologically useful features of birthweight distributions. Statistics in medicine. 1996;15(13):1333–48. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1333::AID-SIM271>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 60.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 61.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–69. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 62.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5(5):582–5. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 63.Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, et al. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2013 doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Perspect. 2012;120(7):1061–6. doi: 10.1289/ehp.1104173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. American journal of obstetrics and gynecology. 2007;196(3):261 e1–6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. American journal of obstetrics and gynecology. 2009;200(6):661 e1–7. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 67.Milton AH, Hasan Z, Shahidullah SM, Sharmin S, Jakariya MD, Rahman M, et al. Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int J Environ Health Res. 2004;14(2):99–108. doi: 10.1080/0960312042000209516. [DOI] [PubMed] [Google Scholar]
- 68.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. The American journal of clinical nutrition. 2007;86(4):1202–9. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kile ML, Ronnenberg AG. Can folate intake reduce arsenic toxicity? Nutr Rev. 2008;66(6):349–53. doi: 10.1111/j.1753-4887.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potera C. A Preventive Approach to Arsenic Toxicity: Testing Folic Acid in Bangladesh. Environ Health Perspect. 2015;123(12):A306. doi: 10.1289/ehp.123-A306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Y, Zhang C, Gao XB, Luo HY, Chen Y, Li HH, et al. Folic acid protects against arsenic-mediated embryo toxicity by up-regulating the expression of Dvr1. Scientific reports. 2015;5:16093. doi: 10.1038/srep16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slotnick MJ, Meliker JR, Nriagu JO. Effects of time and point-of-use devices on arsenic levels in Southeastern Michigan drinking water, USA. The Science of the total environment. 2006;369(1–3):42–50. doi: 10.1016/j.scitotenv.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Sohel N, Kanaroglou PS, Persson LA, Haq MZ, Rahman M, Vahter M. Spatial modelling of individual arsenic exposure via well water: evaluation of arsenic in urine, main water source and influence of neighbourhood water sources in rural Bangladesh. Journal of environmental monitoring : JEM. 2010;12(6):1341–8. doi: 10.1039/c001708f. [DOI] [PubMed] [Google Scholar]
- 74.Chen KL, Amarasiriwardena CJ, Christiani DC. Determination of total arsenic concentrations in nails by inductively coupled plasma mass spectrometry. Biological trace element research. 1999;67(2):109–25. doi: 10.1007/BF02784067. [DOI] [PubMed] [Google Scholar]
- 75.Butt K, Lim K Society of O, Gynaecologists of C. Determination of gestational age by ultrasound. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2014;36(2):171–83. doi: 10.1016/S1701-2163(15)30664-2. [DOI] [PubMed] [Google Scholar]
- 76.VanderWeele TJ, Vansteelandt S. Mediation Analysis with Multiple Mediators. Epidemiol Method. 2014;2(1):95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.