Summary
Background
Food allergy is an increasingly common health problem in Western populations. Epidemiological studies have suggested both positive and negative associations between food allergy and infection with the gastric bacterium Helicobacter pylori.
Objective
The objective of this work was to investigate whether experimental infection with H. pylori, or prophylactic treatment with H. pylori-derived immunomodulatory molecules, affects the onset and severity of food allergy, either positively or negatively.
Methods
We infected neonatal C57BL/6 or C3H mice with H. pylori or treated animals with H. pylori components (bacterial lysate or the immunomodulator VacA) and subsequently subjected them to four different protocols for food allergy induction, using either ovalbumin or peanut extract as allergens for sensitization and challenge. Readouts included anaphylaxis scoring, quantification of allergen-specific serum IgE and IgG1 and of the mast cell protease MCPT1, as well as splenic T-helper-2 cell-derived cytokine production. Mesenteric lymph node CD4+FoxP3+ regulatory T-cells were subjected to flow cytometric quantification and sorting followed by qRT-PCR, and to DNA methylation analyses of the Treg-specific demethylated region (TSDR) within the FOXP3 locus.
Results
Mice that had been infected with H. pylori or treated with H. pylori-derived immunomodulators showed reduced anaphylaxis upon allergen sensitization and challenge, irrespective of the allergen used. Most of the immunologic assays confirmed a protective effect of H. pylori. CD4+FoxP3+ T-cells were more abundant in protected mice and exhibited a stable Treg phenotype characterized by FOXP3 TSDR demethylation.
Conclusions and Clinical Relevance
H. pylori confers protection against the anaphylaxis associated with ovalbumin and peanut allergy and affects the epigenome of T-cells, thereby promoting stable Treg differentiation and functionality. Prophylactic treatment with H. pylori-derived immunomodulators appears to be a promising strategy for food allergy prevention.
Keywords: anaphylaxis, bacterial immunomodulators, epigenetics, food allergy
Introduction
Food allergy is an increasingly common condition in Western countries as well as Asia, affecting ~6–8 % of children and ~4 % of adults [1, 2]. It is caused by inappropriate T-cell-driven immune responses to harmless food components of milk, eggs, peanuts, tree nuts, seafood, shellfish, soy, wheat (the “big eight”) and other foods. Food allergy often co-occurs with other atopic diseases such as allergic rhinitis and conjunctivitis, eczema and allergic asthma and is believed to result mechanistically from the breakdown of the T-cell suppressive mechanisms conferred by oral immune tolerance. Symptoms of food allergy usually occur with a fast onset (from seconds to one hour) and include rashes, hives, swelling and itching of parts or the whole face, wheezing, diarrhea, nausea and, in severe cases, anaphylaxis. The mainstay of treatment is the strict avoidance of food allergens; accidental intake requires epinephrine injections in severe and anti-histamines and steroids in mild cases. The benefits of allergen immunotherapy for food allergies are currently unclear and the procedures are thus not (yet) recommended.
The hygiene [3] and disappearing microbiota [4] hypotheses postulate that the reduced exposure to environmental and pathogenic microbes early in life and/or the loss of ancestral indigenous microbes colonizing various niches of the human body contribute to an increased allergy risk. Both hypotheses have served to explain the dramatic increase of the prevalence of food allergy in Western countries. The paradigmatic example of a microbe that is increasingly lost from human populations is H. pylori. Chronic infection with this gastric colonizer on the one hand represents an important risk factor for the development of gastritis, ulcers and gastric cancer [5, 6] but on the other hand appears to confer protection against esophageal disorders such as gastro-esophageal reflux disease and esophageal cancer, as well as asthma, allergy and inflammatory bowel diseases [7, 8]. Whereas the inverse association of H. pylori with allergic asthma, rhinitis and eczema is well supported by large epidemiological studies and meta-analyses [9–12], the effects of H. pylori positivity on the risk of developing food allergy are controversial. Few reports are available and the existing studies suffer from small sample sizes, heterogeneous populations and non-standardized methodologies [13]. Several studies have found a positive association of H. pylori, especially of strains harboring the virulence factor CagA, with food allergy in children [14, 15] or adults [16]. Other studies have found no association [17], or a negative association [12]. The latter study reported both a decreased sero-prevalence of H. pylori in food allergy patients relative to controls (33% vs. 40% as determined by urea breath test and serology) as well as a reduced production of allergic mediators (such as eosinophilic cationic protein and mast cell tryptase) in H. pylori-positive relative to H. pylori-negative food allergy patients [12]. Mechanistically, positive associations with food allergy were explained by a breakdown of the epithelial barrier to food allergens due to the chronic inflammation of the H. pylori-infected gastric mucosa, whereas negative associations were attributed to the immunomodulatory activity of H. pylori on the activation and polarization of T-cell responses.
We have previously examined the role of H. pylori in various experimental models of allergic asthma and have consistently detected a strong protective effect, especially of neonatal exposure to the bacteria, on the development of allergic asthma in response to various allergens [18–20]. Given the controversial results from observational studies in humans with food allergy, the strongly increasing prevalence of food allergy in children and adults, and the robust effects observed in our allergic asthma models, we asked whether experimental H. pylori infection would alleviate the clinical and immunological symptoms of food allergy induced by two different common food allergens. We used recombinant ovalbumin and peanut extract, administered via various routes, to trigger anaphylaxis symptoms in two strains of mice. When administered intraperitoneally for the purpose of allergen challenge, peanut extract was superior to ovalbumin in inducing swelling and edema of the mucosal surfaces of the face, as well as a variety of systemic parameters related to Th2, B- and mast cell activity. H. pylori infection and H. pylori extract treatment, and also the administration of several doses of purified VacA, reduced clinical symptoms of food allergy in all or some of the four examined models and decreased Th2 cytokine production, mast cell protease secretion, and allergen-specific serum IgG1 levels. The same treatments could be shown to promote Treg numbers, regulatory activity and stability by demethylating the TSDR of the FOXP3 locus in regulatory T-cells (Tregs) of mesenteric lymph nodes. Our results thus suggest that H. pylori down-modulates immune responses to common food allergens by affecting the epigenome of Tregs and promoting their stable lineage differentiation, thereby conferring protection against various clinical and immunological hallmarks of food allergy.
Methods
Animal experimentation
C57BL/6 and C3H mice were purchased from Janvier and included in allergy experiments at 5–7 weeks of age. For the induction of ovalbumin (OVA)-induced food allergy, C57BL/6 mice were sensitized twice i.p. with 50 µg OVA (Sigma A5503-5G) emulsified in aluminum hydroxide (Alum Imject, Thermo Scientific 77161) on day 0 and 14, followed by challenge via oral gavage on days 28, 29, 30 and 31 with 60 mg OVA. Symptoms were scored after each of the first three challenges; mice were sacrificed by CO2 inhalation 30–45 min after the last challenge and blood and tissue samples were collected. For the induction of peanut extract (PE)-induced food allergy, C57BL/6 mice were sensitized orally once a week for four weeks with 2 mg PE adjuvanted with 20 µg cholera toxin (List Biologicals 101B) followed by either four oral challenges on four consecutive days with 10 mg PE (in this model, symptoms were scored after the first three challenges) or two i.p. challenges (with a two-day break) with 1 mg PE (in that case, symptoms were scored after the first challenge). Scoring was done for 40 min beginning right after challenge, with scores indicating the following: 0, no sign of reaction; 1, repetitive scratching and rubbing around the nose/mouth and head, ear canal digging with hind legs; 2, decreased activity with an increased respiratory rate, pilar erecti and/or puffing around the eyes and/or mouth; 3, labored respiration and cyanosis around the mouth and tail and/or periods of motionless for more than 1 min; lying prone on stomach; 4, slight or no activity after prodding/whisker stimuli or tremors and convulsion; 5, death. In the oral challenge models, cumulative scores were calculated by adding up three individual scores per mouse. C3H mice were sensitized orally once a week for five weeks with 2 mg PE adjuvanted with 20 µg cholera toxin followed by two oral challenges on two consecutive days with 14 mg PE (symptoms were scored after the first challenge). The processing and analysis of animal tissues is described below; MCPT1 in serum was quantified by ELISA (88-7503-88, eBioscience) according to the manufacturer’s instructions. All animal experimentation was reviewed and approved by the Zurich Cantonal Veterinary Office (license 170/2014 to A.M.). The H. pylori strain PMSS1 was cultured as previously described [21]; mice were infected by oral gavage on day 6 and 7 after birth with 107 to 108 bacteria. For the production of H. pylori extract, bacterial cultures were pelleted, washed with PBS, and subjected to three freeze-thaw cycles and homogenization using a pressure cell homogenizer (Stansted SPCH-18). The homogenate was centrifuged at 3000× g, the resulting supernatant was sterile filtered, and the protein concentration was determined by BCA Protein Assay Kit (Thermo Scientific 23227). Oligomeric s1m1 type VacA was purified from H. pylori strain 60190 as described previously [22]. The dosage of extract and VacA was adjusted to the age of the mice and the application mode: extract i.p. 5–100 µg, extract p.o. 50–200 µg, VacA i.p. 5–20 µg. Mice were treated with VacA or extract once a week. For the production of peanut extract, partially defatted peanut flour (Golden Peanut Company) was extracted overnight in 10× PBS, solid parts were removed by centrifugation, and extract was concentrated using Amicon Ultracel 3K centrifugal filters (Merck Millipore UFC900324). The final protein concentration was determined by BCA Protein Assay Kit (Thermo Scientific 23227).
Allergen-specific ELISAs
For OVA-specific IgG1 ELISA, high affinity plates were coated with 10 µg OVA in carbonate-bicarbonate coating buffer (pH 9.6) overnight at 4° C. After washing and blocking with 2 % BSA in PBS, serum samples were diluted in 1 % BSA/PBS and added for 2 hours at 37°C. After further washing, the plates were incubated for 1 h at 37°C with HRP-coupled anti-mouse IgG1 (eBioscience 18-4015-82). Wells were washed again before adding HRP substrate and measuring absorbance in a Spectromax plate reader. OVA-specific IgE was measured using the same procedure as described for IgG1, except that HRP-coupled anti-mouse IgE (GeneTex GTX77227) was used for detection. For PE-specific IgE ELISA, high affinity plates were coated with 500 µg PE overnight as described above. After washing and blocking with 5 % gelatine in PBS at 37°C for 1 hour, serum samples diluted in 2 % BSA/PBS were added to the plate for 2 hours at 37°C. After further washing, the plates were incubated with anti-mouse IgE-biotin (BD Biosciences 553419) and washed before avidin-HRP (Thermo Scientific 21130) was added. Wells were washed again before addition of HRP substrate and absorbance detection in a plate reader.
Re-stimulation of splenocytes and cytokine ELISAs
Spleens were pushed through a 40 µm cell strainer and washed with PBS prior to red blood cell lysis. Splenocytes were seeded into 96 well plates in RPMI 1640 medium (Gibco 21875-034 plus FCS and Penicillin-Streptomycin) supplemented with 200 µg/ml PE or OVA. After 4 days in culture, supernatants were collected and stored at −20°C until cytokines were quantified by IL-5 (88-7054-88) and IL-13 (88-7137-88) ELISA according to the manufacturer’s instructions (eBioscience).
TSDR methylation analysis
Total mesenteric lymph node cells of male C57BL/6 mice were isolated by means of collagenase type IV (Sigma C5138) digestion and pushing through a cell strainer. After fixation, permeabilization and washing, the cells were stained with anti-mouse CD4-FITC (Biolegend 100510) and Foxp3-APC antibodies (eBioscience 17-5773-82). CD4+Foxp3+ and CD4+Foxp3− cells were sorted on a FACS Aria. Genomic DNA was isolated from sorted cell subsets using the NucleoSpin® Tissue kit (Macherey-Nagel). An additional step was added to the manufacturer’s protocol to remove formaldehyde-induced crosslinking. Briefly, Chelex-100 beads (Biorad) were added after the lysis step and incubated at 95°C for 15 min in a shaker. Chelex-100 beads were spun down and the supernatant was transferred to a fresh tube. After addition of an adjusted amount of 100 % ethanol the following purification steps were performed according to the manufacturer’s protocol. Genomic DNA was converted with bisulfite using the EZ DNA Methylation Kit (Zymo Research) according to the manufacturer’s instructions. The Treg-specific demethylated region (TSDR) was amplified by PCR and analyzed by pyrosequencing on a PSQ96MA (Qiagen) as described recently [23]. Primers for sequencing were (in 5’ to 3’ direction), S1: CCATACAAAACCCAAATTC, S2: ACCCAAATAAAATAATATAAATACT, S3: ATCTACCCCACAAATTT, S4: AACCAAATTTTTCTACCATT), which cover CpG motifs 3–12 of the TSDR core region.
Flow cytometric analysis and cell sorting for qRT-PCR
Total mesenteric lymph node cells of male C57BL/6 mice were isolated by pushing through a cell strainer. After fixation, permeabilization and washing, the cells were stained with anti-mouse CD4 PerCP/Cy5.5 (Biolegend 116012), anti-mouse CD45 BV650 (Biolegend 103151), anti-mouse TCR β chain PE/Cy7 (Biolegend 109222), anti-mouse CD8α BV510 (Biolegend 100752), anti-mouse Foxp3 BV421 (Biolegend 126419), the fixable viability dye eFluor780 (eBioscience 65-0865-14) and flow cytometrically measured using a LSR II Fortessa instrument followed by a detailed analysis using FlowJo software. For FACS-sorting of regulatory T-cells, mesenteric lymph node cells were stained with anti-mouse CD45 BV650 (Biolegend 103151), anti-mouse TCR β chain PE/Cy7 (Biolegend 109222), anti-mouse CD4 BV711 (Biolegend 100550), anti-mouse CD25 PE (Biolegend 101903), fixable viability dye eFluor780 (eBioscience 65-0865-14) and sorted on a FACS Aria. RNA of sorted cells was isolated using the RNeasy Mini Kit (Qiagen 74106), converted into cDNA, and subjected to TaqMan Real-Time PCR assay using the primers Mm03024075 (Hprt), Mm00475162 (Foxp3), Mm01178820 (Tgfb1) and Mm01288386 (IL10; all from Thermo Fisher Scientific). Samples were run on a Light Cycler 480 and normalized to the house-keeping gene Hprt.
Statistical analysis
GraphPad Prism 6 was used for all statistical analyses. In all graphs each symbol represents an individual animal and horizontal lines indicate medians. The Kruskal-Wallis test followed by Dunn's multiple comparisons test was used throughout. Stars are used to indicate the level of significance according to the p-value: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Results
Live H. pylori infection or treatment with H. pylori whole cell extract alleviates symptoms in an ovalbumin-induced food allergy model
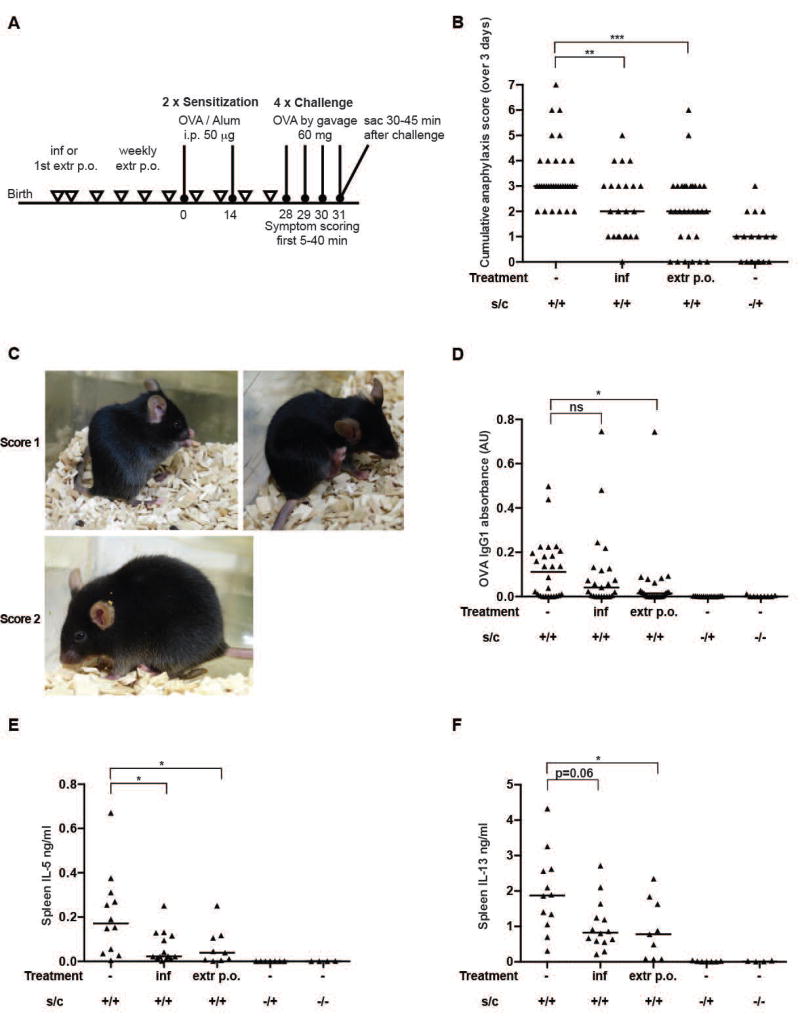
To assess whether infection with H. pylori or treatment with whole cell extract protects against allergic responses to ingested ovalbumin, we either infected neonatal C57BL/6 mice with live H. pylori on days 6 and 7 after birth or intragastrically administered weekly doses of whole cell extract from day 7 onwards. As adults, mice were subjected to intraperitoneal ovalbumin (OVA, adjuvanted with alum) sensitization followed by intragastric OVA challenges (see schematic in Figure 1A). Mice were scored for anaphylactic symptoms such as scratching around the nose, mouth and ears, decreased activity and increased respiratory rate and puffy eyes for 40 min after each challenge. At the study endpoint, serum levels of OVA-specific IgE and IgG1 antibodies, serum levels of mast cell protease 1 (MCPT1), and the splenic production of the Th2 cytokines IL-5 and IL-13 were quantified by ELISA. Mice that had been sensitized and challenged with OVA (positive controls) developed mild to moderate anaphylactic symptoms accompanied by high serum titers of OVA-specific IgE and IgG1 and elevated levels of MCPT1, a systemic marker of mast cell degranulation (Figure 1B–D, suppl. Figure 1A,B). The re-stimulation of splenic single cell preparations with OVA revealed a clearly elevated production of IL-5 and IL-13 relative to control groups that had not been sensitized (but challenged) or had never been exposed to OVA (Figure 1E,F). In contrast, mice that were infected with H. pylori or had received H. pylori whole cell extract were assigned significantly lower anaphylaxis scores, exhibited reduced serum levels of OVA-specific IgG1, and produced lower amounts of splenic Th2 cytokines upon re-stimulation (Figure 1B–F); the assessment of serum MCPT-1 levels confirmed these trends for the extract treatment, but not for the live infection, and serum IgE levels were not reduced by either extract treatment or live infection (suppl. Figure 1A,B). Overall, the results indicate that H. pylori infection and extract treatment efficiently reduce anaphylaxis symptoms and most other hallmarks of ovalbumin-specific allergy; the effects on anaphylaxis scores in particular, the most relevant readout from a clinical perspective, are strong and robust.
Figure 1. OVA- induced food allergy is ameliorated by neonatal H. pylori infection or weekly extract treatment.
(A) Schematic of OVA-induced food allergy and H. pylori-specific interventions. Mice were sensitized twice intraperitoneally (i.p.) with 50 µg of alum-adjuvanted OVA and orally challenged four weeks after the first sensitization on four consecutive days with 60 mg OVA (s/c; positive controls). Negative control animals were challenged but not sensitized, or neither sensitized nor challenged. One group of mice was neonatally infected on day 6 and 7 after birth with the H. pylori strain PMSS1 (inf), and another group was treated weekly by oral gavage with 100–200 µg (adjusted to body weight) whole cell H. pylori extract starting on day 6 or 7 after birth (extr p.o.). (B) Cumulative anaphylaxis score assigned upon challenge (sum of three scores). s/c, sensitization and challenge. (C) Representative pictures showing mice with a score of 1 (repetitive scratching around nose and head, and hind-leg-ear-digging) and a score of 2 (puffing around eyes, pilar erecti, decreased activity). (D) Serum OVA-specific IgG1, as determined by ELISA. (E,F) Cytokine production, as determined by IL-5 and IL-13 ELISA, of splenocytes that had been re-stimulated in vitro with OVA for four days. In B,D,E and F, each symbol represents one mouse. Graphs show pooled data from 5 (B), 4 (D) and 2 (E,F) experiments. Horizontal lines indicate medians; the Kruskal-Wallis test followed by Dunn's multiple comparisons test was used throughout for the calculation of p-values.
Food allergy symptoms induced by peanut extract are reduced by H. pylori infection and treatment with H. pylori extract or the H. pylori immunomodulator VacA
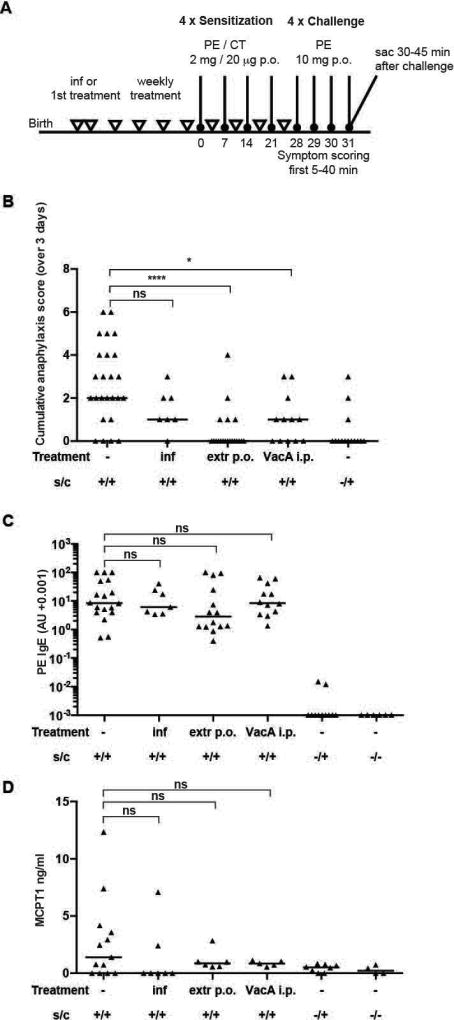
We next sought to determine if these results could be corroborated in additional models of food allergy. As proteins contained in peanuts are among the most common food allergens in Western societies, we opted for peanut-specific sensitization and challenge. Mice received four intragastric weekly doses of cholera toxin-adjuvanted peanut extract (PE) for the purpose of sensitization, followed by intragastric challenge with PE on four consecutive days beginning one week after the last sensitization (Figure 2A). Neonatally infected mice and mice treated with either H. pylori extract or the purified H. pylori immunomodulator VacA were sensitized and challenged alongside a group of positive controls that received no intervention whatsoever. The food allergy symptoms elicited by intragastric sensitization and challenge with peanut extract were relatively mild and comparable to symptoms induced with OVA (Figure 2B–D). Similar to the OVA model, regular treatment with extract or the purified immunomodulator VacA effectively reduced the anaphylaxis scores, but failed to affect peanut-specific serum IgE levels; live infection had no significant effect on any of the parameters but showed a trend towards lower anaphylaxis scores (Figure 2B,C). MCPT serum levels were modestly reduced by all three interventions (Figure 2D), albeit not significantly. In summary, we observe in two complementary food allergy models that H. pylori-based prophylactic interventions reduce clinically relevant symptoms of food allergy and modulate immunologic correlates of the disease.
Figure 2. Amelioration of allergic symptoms in an oral challenge peanut allergy model upon H. pylori specific treatments.
(A) Schematic of PE-induced food allergy and H. pylori interventions. Mice were sensitized four times by oral gavage (p.o.) with 2 mg of peanut-extract (PE) adjuvanted with 20 µg cholera toxin (CT), followed four weeks later by oral challenges on four consecutive days with 60 mg OVA (positive controls). Negative control animals were challenged but not sensitized, or neither sensitized nor challenged. One group of mice was neonatally infected on day 6 and 7 after birth with the H. pylori strain PMSS1 (inf), and another group was treated weekly by oral gavage with 100–200 µg (adjusted to body weight) whole cell H. pylori extract starting on day 6 or 7 after birth (extr p.o.). A final group received once-weekly increasing doses (adjusted to body weight) of 5–20 µg purified VacA (VacA i.p.) starting on day 6 or 7 after birth. (B) Cumulative anaphylaxis score assigned upon challenge (sum of three scores). (C,D) Serum PE-specific IgE and MCPT1 levels, as determined by ELISA. In B–D, each symbol represents one mouse. Graphs show pooled data from 4 (B), 3 (C) and 2 (D) experiments. Horizontal lines indicate medians; the Kruskal-Wallis test followed by Dunn's multiple comparisons test was used throughout for the calculation of p-values.
Severe anaphylaxis induced by peanut extract is alleviated by H. pylori
Both above described models of food allergy produce relatively mild symptoms. Mice rarely develop systemic symptoms such as decreased activity or effects on respiration rates. To mimic more severe anaphylactic reactions, which can be life-threatening in peanut-allergic children, we devised a model that produced significantly higher anaphylaxis scores, accompanied by higher serum MCPT levels and stronger cytokine production by restimulated splenocytes relative to the other two models. In this model, mice were sensitized orally by four CT-adjuvanted doses of peanut extract followed by two intraperitoneal challenges with the same extract (Figure 3A). Most mice of the positive control group were assigned anaphylaxis scores of three (defined as labored respiration and cyanosis around the mouth and tail and/or periods of motionless for more than 1 min, lying prone on stomach) or four (slight or no activity after prodding/whisker stimuli or tremors and convulsion; Figure 3B,C) already after the first challenge dose. Anaphylaxis scores were strongly reduced by treatment with VacA, but not so much by the other examined interventions (Figure 3B); moreover, the Th2 cytokine production of re-stimulated splenocytes (Figure 3D,E) and MCPT and IgE levels measured in serum showed only modest trends (suppl. Figure 2A,B). Finally, we switched to a fourth model of food allergy, this time using particularly anaphylaxis-sensitive C3H mice [24]. These mice exhibited anaphylaxis scores of up to three upon oral sensitization and challenge, and thus indeed proved to be more susceptible to peanut extract than C57BL/6 mice also in our hands (suppl. Figure 3A–C). Regular H. pylori extract treatment had modest, but not statistically significant, effects on anaphylaxis scores, serum MCPT levels and spleen weights (suppl. Figure 3B–E). Treatment with VacA had similar effects, but too few mice were analyzed to draw definitive conclusions (suppl. Figure 3B–E). In summary, the severe anaphylaxis induced by the intraperitoneal administration of allergen to sensitized C57BL/6 mice, or the oral challenge of C3H mice, can be reduced somewhat by prior infection with H. pylori, or the regular exposure to H. pylori extract or VacA.
Figure 3. Systemically induced peanut food allergy is reduced upon neonatal H. pylori infection or weekly treatment with extract or VacA.
(A) Schematic of PE-induced food allergy and H. pylori interventions. Mice were sensitized four times by oral gavage (p.o.) with 2 mg of peanut-extract (PE) adjuvanted with 20 µg cholera toxin (CT), followed one week later by two i.p. challenges (with a three day interval) with 1 mg PE (positive controls). Negative control animals were challenged but not sensitized, or neither sensitized nor challenged. One group of mice was neonatally infected on day 6 and 7 after birth with the H. pylori strain PMSS1 (inf), and another group was treated weekly by oral gavage or i.p. with whole cell H. pylori extract starting on day 6 or 7 after birth (extr p.o./i.p.). A final group received weekly increasing doses (adjusted to body weight) of 5–20 µg purified VacA (VacA i.p.) starting on day 6 or 7 after birth. (B) Anaphylaxis score assigned at first challenge. (C) Representative pictures showing mice with a score of 3 (lying prone on stomach for more than one minute). (D,E) Cytokine production, as determined by IL-5 and IL-13 ELISA, of splenocytes that had been re-stimulated in vitro with PE for four days. In B,D, and E, each symbol represents one mouse. Graphs show pooled data from 8 (B) and 6 (D,E) experiments. Horizontal lines indicate medians; the Kruskal-Wallis test followed by Dunn's multiple comparisons test was used throughout for the calculation of p-values.
H. pylori infection, as well as extract and VacA treatment induce demethylation of the Treg-specific demethylated region (TSDR) in FoxP3+ Tregs, promoting their lineage commitment and suppressive activity
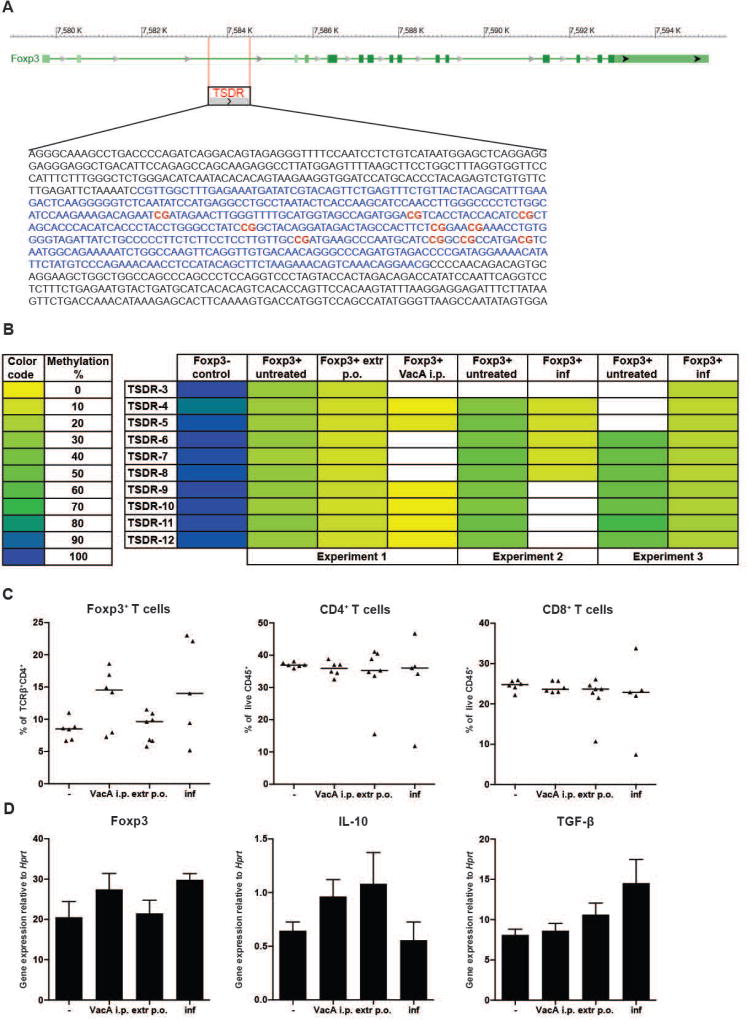
Tregs have been implicated in asthma protection in H. pylori-infected mice and are numerically and functionally different in naïve and infected animals [18–20]. To assess whether the interventions shown here to confer food allergy protection affect the differentiation and stability of Tregs, we set out to quantify the methylation status of CpG motifs (Figure 4A) localized within a region of the FOXP3 locus termed TSDR because of its selective demethylation in lineage-committed (stable) Tregs [25, 26]. Tregs from pooled mesenteric lymph nodes of 6–8 male mice per group were FACS-sorted based on their CD4 and FoxP3 expression, and subjected to genomic DNA extraction, bisulfite conversion and TSDR-specific pyrosequencing. CD4+ FoxP3− T-cells were sorted and analyzed in parallel. FoxP3− T-cells exhibited a more or less complete methylation of the TSDR, with on average 96 % methylated CpG motifs (Figure 4B and data not shown); their methylation did not change upon intervention (data not shown). In contrast, FoxP3+ T-cells exhibited strong differences in their methylation status: whereas FoxP3+ T-cells that had been harvested from naive mice showed methylation levels varying between 25–50 % depending on the experiment, this level decreased to 10 % upon extract treatment, 10–17 % upon live infection and down to 1 % upon VacA treatment (Figure 4B). As reported previously [25, 26], the CpG motifs within the TSDR showed very consistent methylation patterns within one sample, indicating an all-or-nothing mechanism of demethylation that encompasses the entire locus (not all regions could be analyzed in all samples though, Figure 4B). Furthermore, flow cytometric analysis of MLN T-cells revealed somewhat higher frequencies of FoxP3+ cells among all CD4+ T-cells in VacA-treated and neonatally infected mice, whereas overall CD4+ and CD8+ frequencies were similar (Figure 4C). CD4+CD25+ Tregs sorted from the MLNs of the same mice receiving interventions expressed modestly more FoxP3 and IL-10 and/or TGF-β than Tregs from untreated controls, confirming that TSDR demethylation has functional consequences with respect to Treg expansion and regulatory cytokine expression. We conclude from these data that epigenetic marks leading to stable expression of FoxP3, and to the definitive lineage commitment of Tregs, reflect the suppressive effects of H. pylori-specific treatments on food allergy in the models described above.
Figure 4. Decreased methylation of the Treg-specific-demethylated-region (TSDR) and increase in regulatory cytokine expression upon H. pylori-specific treatment.
(A) Schematic overview of the FOXP3 locus with the TSDR upstream of the TSS (retrieved using BLAST). The CG-containing region is marked in blue and CG motifs covered by pyrosequencing are marked in bold red. (B) Methylation pattern of 10 CG dinucleotides within the TSDR, of FACS-sorted MLN-derived CD4+Foxp3+ and CD4+FoxP3− T-cells. Animals were treated as indicated. The methylation status of the individual CGs is color-coded (left panel). White cells indicate sequences that failed to yield interpretable results due to technical problems. (C) Frequencies of FoxP3+ Tregs among all TCRβ+ CD4+ T-cells, and of CD4+ and CD8+ T-cells among all CD45+ leukocytes in the MLNs of mice treated from 7 days of age onwards with either VacA, or H. pylori extract, or live bacteria. Horizontal lines represent medians. (D) Expression of FoxP3, IL-10 and TGF-β by sorted CD4+CD25+ Tregs, as assessed by qRT-PCR and normalized to Hprt, of the mice shown in C. Means +SEM are shown.
Discussion
Although food allergy represents an important human disorder of increasing prevalence in most parts of the world and H. pylori is known to be protective against allergies with respiratory tract manifestations [10, 18–20, 27, 28], little experimental or epidemiological evidence is available that addresses a possible inverse correlation, or protective effect, of H. pylori in food allergy. One of the reasons for this lack of data probably lies in the fact that no universally accepted food allergy model exists that would faithfully reflect all or most aspects of the human condition. We have adapted and optimized four complementary food allergy models, using various routes of sensitization and challenge, two different common food allergens and two mouse strains, to investigate in a comprehensive manner whether the presence of H. pylori affects clinically relevant food allergy symptoms, in either a beneficial or detrimental way. We found no strong evidence for a promoting role of H. pylori in food allergy, as had been suggested in two observational studies in humans [14, 15]. In contrast, live H. pylori infection or extract or VacA treatment conferred detectable protection against anaphylactic symptoms in several of the models; the effects on clinical symptoms were associated with protective effects on systemic parameters of food allergy, such as mast cell protease levels in serum and allergen-specific IgG1 levels in some but not all models. The effects of H. pylori on food allergy were not as impressive as its beneficial effects in models of allergic asthma in which neonatal exposure to the bacteria essentially reduces parameters of airway inflammation, airway hyper-responsiveness and goblet cell metaplasia to almost background levels [18–20]. The stronger effects in the asthma models may have to do with the so-called “gut-lung axis”, the idea of a special connection between the mucosal immune systems of the two organs that has been put forward to explain the effects of (deliberate or pathogenic alterations of) the gut microbiota on lung diseases [29]. We were able to link the protection against the airway hyper-reactivity, inflammation, eosinophilia and excessive mucus production that are hallmarks of allergic asthma to the strong suppressive activity of H. pylori-induced Tregs [19, 20]. Here, we present a possible epigenetic correlate of the suppressive/protective activity of Tregs in allergy, as these cells- coming from an H. pylori-infected host- exhibit an epigenetic signature at their FOXP3 locus that is consistent with stable expression of FoxP3 and stable lineage differentiation and functionality [25, 26]. We have shown in earlier work that the induction of Tregs depends on the H. pylori virulence factor and immunomodulator VacA; VacA mutants fail to induce suppressive Tregs and to confer asthma protection, and VacA alone, administered in purified form, recapitulates many of the beneficial effects of live infection [22, 30]. H. pylori VacA mutants fail to colonize persistently and at wild type levels, and rather are cleared effectively by an overshooting T-helper-1 response. The immunomodulatory effects of VacA were confirmed in the food allergy models presented here, and were reflected in the epigenetic signature of Tregs isolated from VacA-treated mice. The rationale for applying preventive treatments in contrast to therapeutic treatments (which would start after sensitization) with VacA was governed by our prior experience in asthma models, in which we reported preventive but not therapeutic efficacy of H. pylori-specific tolerization. In conclusion, H. pylori possesses strong immunomodulatory properties, mediated at least in part by the VacA protein, that allow it to modulate T-cell responses directed at the infection itself, as well as at conspicuous food and environmental antigens. These properties are evident not only locally in the gastric mucosa, but systemically, and strongly affect the individual carrier’s allergy risk.
Supplementary Material
Acknowledgments
This work was funded by the Swiss National Science Foundation (grants BSCGIO_157841/1 and SNF 310030-143609 to A.M.), the National Institutes of Health (AI039657 and CA116087) and the Department of Veterans Affairs.
Footnotes
Author contributions
AK and AM contributed to study concept and design. AK conducted all animal experiments with help from SU and AD. AK, SF, JH performed analyses and interpretation of data. TLC provided critical materials. All authors critically revised the manuscript for accuracy. AK and AM wrote the paper.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124:1549–55. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. The Journal of allergy and clinical immunology. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. quiz 8. [DOI] [PubMed] [Google Scholar]
- 3.Strachan DP. Hay fever, hygiene, and household size. Bmj. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England journal of medicine. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 6.Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–44. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Kyburz A, Muller A. The Gastrointestinal Tract Microbiota and Allergic Diseases. Dig Dis. 2016;34:230–43. doi: 10.1159/000443357. [DOI] [PubMed] [Google Scholar]
- 8.Koch KN, Muller A. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes. 2015;6:382–7. doi: 10.1080/19490976.2015.1105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Yu C, Sun Y. The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter. 2013;18:41–53. doi: 10.1111/hel.12012. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luther J, Dave M, Higgins PD, et al. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077–84. doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konturek PC, Rienecker H, Hahn EG, et al. Helicobacter pylori as a protective factor against food allergy. Med Sci Monit. 2008;14:CR452–8. [PubMed] [Google Scholar]
- 13.Ma ZF, Majid NA, Yamaoka Y, et al. Food Allergy and Helicobacter pylori Infection: A Systematic Review. Front Microbiol. 2016;7:368. doi: 10.3389/fmicb.2016.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrado G, Luzzi I, Lucarelli S, et al. Positive association between Helicobacter pylori infection and food allergy in children. Scand J Gastroenterol. 1998;33:1135–9. doi: 10.1080/00365529850172467. [DOI] [PubMed] [Google Scholar]
- 15.Corrado G, Luzzi I, Pacchiarotti C, et al. Helicobacter pylori seropositivity in children with atopic dermatitis as sole manifestation of food allergy. Pediatr Allergy Immunol. 2000;11:101–5. doi: 10.1034/j.1399-3038.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 16.Figura N, Perrone A, Gennari C, et al. CagA-positive Helicobacter pylori infection may increase the risk of food allergy development. J Physiol Pharmacol. 1999;50:827–31. [PubMed] [Google Scholar]
- 17.Kolho KL, Haapaniemi A, Haahtela T, et al. Helicobacter pylori and specific immunoglobulin e antibodies to food allergens in children. J Pediatr Gastr Nutr. 2005;40:180–3. doi: 10.1097/00005176-200502000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Koch KN, Hartung ML, Urban S, et al. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest. 2015;125:3297–302. doi: 10.1172/JCI79337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold IC, Dehzad N, Reuter S, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–93. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oertli M, Sundquist M, Hitzler I, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–96. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold IC, Lee JY, Amieva MR, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engler DB, Reuter S, van Wijck Y, et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci U S A. 2014;111:11810–5. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang BH, Hagemann S, Mamareli P, et al. Foxp3(+) T cells expressing ROR gamma t represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal immunology. 2016;9:444–57. doi: 10.1038/mi.2015.74. [DOI] [PubMed] [Google Scholar]
- 24.Berin MC, Zheng Y, Domaradzki M, et al. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61:64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 25.Toker A, Engelbert D, Garg G, et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol. 2013;190:3180–8. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- 26.Polansky JK, Kretschmer K, Freyer J, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 27.Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561–7. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. doi: 10.1371/journal.pone.0004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2016 doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 30.Oertli M, Noben M, Engler DB, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A. 2013;110:3047–52. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.